The Study of Composite Materials Properties Based on Polymers and Nano-Additives from Industrial Wastes from Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Materials for Epoxy Resin Composites
2.1.2. Materials for Polystyrene-Based Composites
2.2. Methods
- -
- ED-20 pre-dosing;
- -
- Microspheres and microsilica dosing as filler and their further addition to epoxy oligomer in the amount of 2, 5, 10, 15, 20 wt%;
- -
- adding PEPA in the ratio of resin:hardener (1:10) and then mixing the composition
- -
- the atomic, molecular, and crystal structure of various substances and their optical properties;
- -
- the structure of polymers and their isotropic and isomeric modifications, as well as the structure of intermediate formations, such as chemical radicals and molecular associations;
- -
- the chemical composition of an unknown sample by the presence of certain spectral lines (or groups of lines) and their intensity;
- -
- the band gap energy, etc.
3. Results and Discussion
3.1. Epoxy Resin-Based Materials
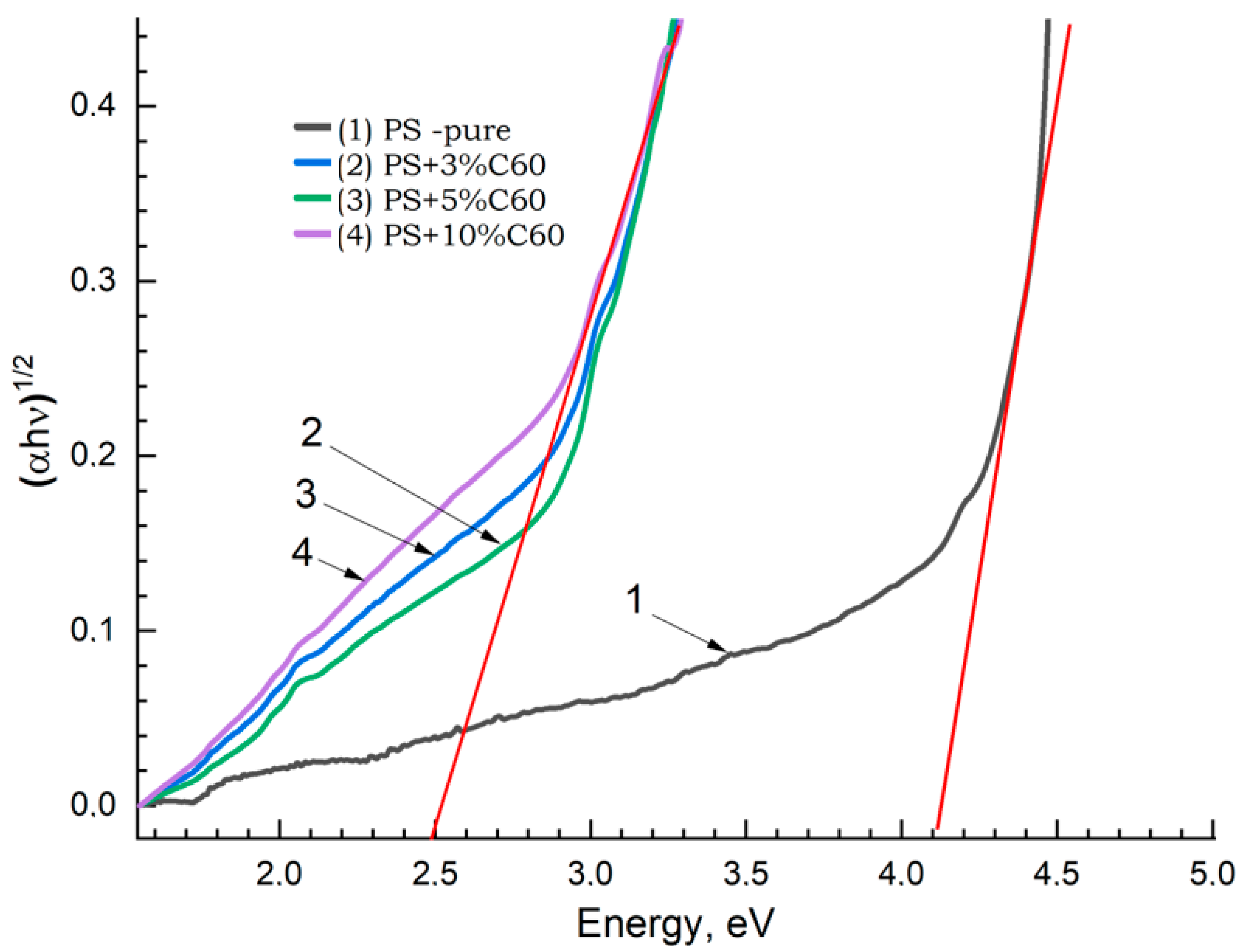
3.2. PS (Polystyrene)-Based Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soney, C.G.; Sam, J.; Sreelakshmi, R. Polymer Nanocomposites in Supercapacitors; CRC Press: Boca Raton, FL, USA, 2023; 284p. [Google Scholar]
- Sabu, T.; Didier, R.; Deepalekshmi, P. Spectroscopy of Polymer Nanocomposites; William Andrew: Norwich, NY, USA, 2016; 484p. [Google Scholar]
- Joseph, N.; Mathew, A.A.; Daniel, E.C.; Balachandran, M. Polymer-Carbon nanocomposite: Synthesis, optical and biocidal properties. Chemistry 2023, 5, 100826. [Google Scholar] [CrossRef]
- Bokobza, L. Spectroscopic Techniques for the Characterization of Polymer Nanocomposites, Review. Polymers 2018, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Litina, K.; Miriouni, A.; Gournis, D.; Karakassides, M.A.; Georgiou, N.; Klontzas, E.; Ntoukas, E.; Avgeropoulos, A. Nanocomposites of polystyrene-b-polyisoprene copolymer with layered silicates and carbon nanotubes. Eur. Polym. J. 2006, 42, 2098–2107. [Google Scholar] [CrossRef]
- Kamal, K.K.; Jitendra, K.P.; Sravendra, R. Handbook of Polymer Nanocomposites. Processing, Performance and Application; Volume B: Carbon Nanotube Based Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2015; 577p. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Serekpayeva, M.; Ibzhanova, A.; Niyazbekova, R.; Kokayeva, G.; Aldabergenova, S. Properties of Epoxy Resins-Based Composite Materials with the Addition of Microspheres. Chem. Eng. Technol. 2023, 46, 1170–1175. [Google Scholar] [CrossRef]
- Karami, P. Polymer/nanodiamond composites—A comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef]
- Imran, K.A.; Lou, J.; Shivakumar, K.N. Enhancement of electrical and thermal conductivity of polypropylene by graphene nanoplatelets. J. Appl. Polym. Sci. 2018, 135, 45833. [Google Scholar] [CrossRef]
- Niranjan, K. Nanomaterials and Polymer Nanocomposites: Raw Materials to Applications; Imprint Elsevier: Tezpur, India, 2019; 410p. [Google Scholar]
- Allemand, P.M.; Khemani, K.C.; Koch, A.; Wudl, F.; Holczer, K.; Donovan, S.; Grüner, G.; Thompson, J.D. Organic molecular soft ferromagnetism in a fullerene C60. Science 1991, 253, 301–303. [Google Scholar] [CrossRef]
- Niyazbekova, R.; Mukhambetov, G.; Tlegenov, R.; Aldabergenova, S.; Shansharova, L.; Mikhalchenko, V.; Bembenek, M. The Influence of Addition of Fly Ash from Astana Heat and Power Plants on the Properties of the Polystyrene Concrete. Energies 2023, 16, 4092. [Google Scholar] [CrossRef]
- Massana, J.; Reyes, E.; Bernal, J.; Leon, N.; Sanchez-Espinosa, E. Influence of nano- and micro-silica additions on the durability of a high-performance self-compacting concrete. Constr. Build. Mater. 2018, 165, 93–103. [Google Scholar] [CrossRef]
- Radoman, T.S.; Dzunuzovic, J.V.; Jeremic, K.B.; Grgur, B.N.; Milicevic, D.S.; Popovic, I.G.; Dzunuzovic, E.S. Improvement of epoxy resin properties by incorporation of TiO2 nanoparticles surface modified with gallic acid esters. Mater. Des. 2014, 62, 158–167. [Google Scholar] [CrossRef]
- Serekpayeva, M.A.; Niyazbekova, R.; Negim, A.E. Investigation of the Properties of Metallurgical Slags and Dust of Electro Filters to Obtain Protective Anticorrosive Coatings. Int. J. Technol. 2022, 13, 544–552. [Google Scholar] [CrossRef]
- Niyazbekova, R.; Serekpayeva, M.A.; Kokayeva, G.A.; Kardybai, S. Investigation of the properties of composite materials based on epoxy resins with microsilica additives. Complex Use Miner. Resour. 2021, 318, 63–70. [Google Scholar] [CrossRef]
- Gladchenko, S.V.; Polotskaya, G.A.; Gribanov, A.V.; Zgonnik, V.N. Study of solid-phase polystyrene-fullerene compositions. J. Eng. Phys. 2002, 47, 105–109. [Google Scholar] [CrossRef]
- Bagrovskaya, N.A.; Alekseeva, O.B. Effect of solvent on the optical properties of fullerene in solutions and polymer composites based on polystyrene. Liq. Cryst. Their Pract. Use 2013, 3, 22–28. [Google Scholar]
- Hossain, F.M.; Sheppard, L.; Nowotny, J.; Murch, G.E. Optical properties of anatase and rutile titanium dioxide: Ab initio calculations for pure and anion-doped material. J. Phys. Chem. Solids 2008, 69, 1820–1828. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A.; Wood, D.L. Optical and magnetic investigations of the localized states in semiconducting glasses. Phys. Rev. Lett. 1970, 25, 749–752. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, S.; Hussein, A.M.; Saeed, S.R. Optical Characteristics of Polystyrene Based Solid Polymer Composites: Effect of Metallic Copper Powder. Int. J. Met. Hindawi Publis. Corp. 2013, 2013, 123657. [Google Scholar] [CrossRef]
- Kokayeva, G.; Niyazbekova, R.; Serekpayeva, M.; Ibzhanova, A.; Bekeshev, A. Using of microsilica for improvement of physical and mechanical properties of epoxide-based composite material. East.-Eur. J. Enterp. Technol. 2023, 4, 18–25. [Google Scholar] [CrossRef]
- Micah, M.D.; Jasim, U.M.; Okenwa, I.O. Synthesis and characterization of polystyrene carbon nanotube nanocomposite for utilization in the displaced foam dispersion methodology. Compos. Part B Eng. 2016, 98, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Haridas, M.; Basu, J.K. Optical properties of polymer nanocomposites. Bull. Mater. Sci. 2008, 31, 213–217. [Google Scholar] [CrossRef]
- Sangawar, V.S.; Golchha, M.C. Evolution of the optical properties of Polystyrene thin films filled with Zinc Oxide nanoparticles. Int. J. Sci. Eng. Res. 2013, 4, 2700–2705. [Google Scholar]
- Ismail, R.A.; Almashhadani, N.J.; Sadik, R.H. Preparation and properties of polystyrene incorporated with gold and silver nanoparticles for optoelectronic applications. Appl. Nanosci. 2017, 7, 109–116. [Google Scholar] [CrossRef]
- Ahang, M.H.; Elham, M.A.D.; Shujahadeen, B.A. Steps Toward the Band Gap Identification in Polystyrene Based Solid Polymer Nanocomposites Integrated with Tin Titanate Nanoparticles. Polymers 2020, 12, 2320. [Google Scholar] [CrossRef] [PubMed]
- Yellampalli, S. Carbon Nanotubes—Polymer Nanocomposites; InTech: Rijeka, Croatia, 2011; p. 410. [Google Scholar] [CrossRef]
- Bembenek, M.; Makoviichuk, M.; Shatskyi, I.; Ropyak, L.; Pritula, I.; Gryn, L.; Belyakovskyi, V. Optical and Mechanical Properties of Layered Infrared Interference Filters. Sensors 2022, 22, 8105. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, O.V.; Kechek’yan, A.S.; Shevchenko, V.G. A Study of Oriented Conductive Composites with Segregated Network Structure Obtained via Solid-State Processing of UHMWPE Reactor Powder and Carbon Nanofillers. Polym. Compos. 2019, 40, 146–155. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Yablokov, M.Y.; Mukhortov, L.A.; Goncharuk, G.P.; Ozerin, A.N. Migration of carbon nanoparticles to the surface of the melt of polymer composite material. Dokl. Chem. 2019, 489, 283–287. [Google Scholar] [CrossRef]
- Ahmad, F.I.; Pei, S.G. Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; 632p. [Google Scholar]
- Noskov, A.V.; Alekseeva, O.V.; Kraev, A.S.; Agafonov, A.V. Dielectric spectroscopy of polystyrene films modified with fullerenes. J. Chem. Chem. Technol. 2013, 56, 40–45. [Google Scholar] [CrossRef]
- Tuichiev, S.; Ginzburg, B.M.; Rashidov, J.; Tabarov, S.; Muhamad, A. Influence of small additions of multilayered carbon nanotubes on the structure and physical properties of polymers. Rep. Natl. Acad. Sci. Tajikistan 2010, 53, 627–632. [Google Scholar] [CrossRef]
- Hasan, S.J. Electrical and optical properties modification of polystyrene by Vanadium (V) ion-pair organometallic complex. Adv. Appl. Sci. Res. 2015, 6, 1–6. [Google Scholar]
- GOST 20282-86; General Purpose Polystyrene. (USSR State Standard Specification). Standards Publishing House: Moscow, Russia, 1991; p. 36.
- Tuychiev, L. Structural, Morphological, Mechanical and Thermal Studies of polymeric Nanocomposites. Ph.D. Thesis, Higher Attestation Commission of RF, Dushanbe, Tajikistan, 2017; p. 150. [Google Scholar] [CrossRef]
- Tuychiev, S.; Sharifov, D.M.; Aknazarova, S.; Rashidov, D.; Tabarov, S.; Tuichiev, L.; Aloviddinov, A. Research of thermal and relaxation properties fullerene-containing polymers. Bull. Tajik Natl. Univ. 2013, 1, 102–105. [Google Scholar] [CrossRef]
- Qaism, A.L.-B.; Alsaad, A.M.; Ahmad, A.A.; Al-Sawalmih, A. Structural, Electronic and Optical Characterization of ZnO Thin Film-Seeded Platforms for ZnO Nanostructures: Sol–Gel Method Versus Ab Initio Calculations. J. Electron. Mater. 2019, 48, 5028–5038. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Liqaa, H.A.; Ahmed, A.A.; Rasheed, N.A. Optical properties of polystyrene with carbon nanotube and carbon nano incorporated and surface morphology studies. Int. Nano Lett. 2023, 13, 165–176. [Google Scholar] [CrossRef]

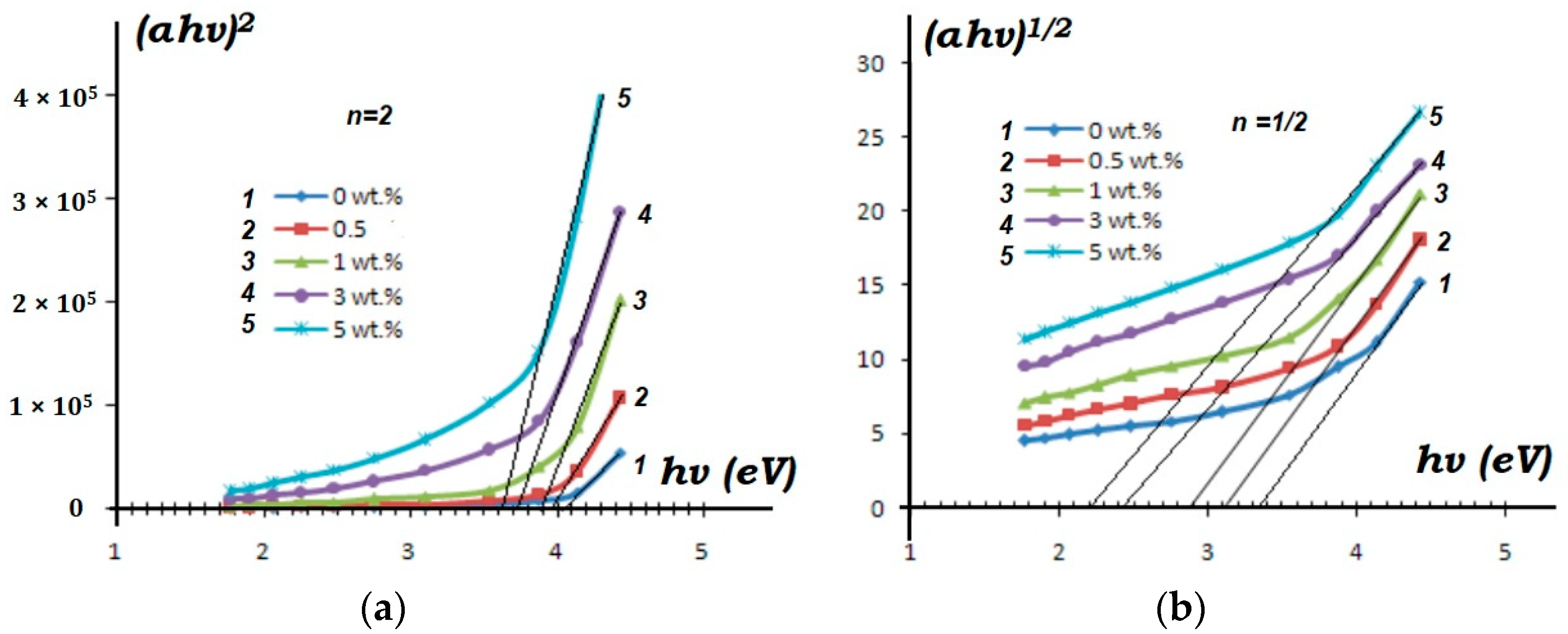


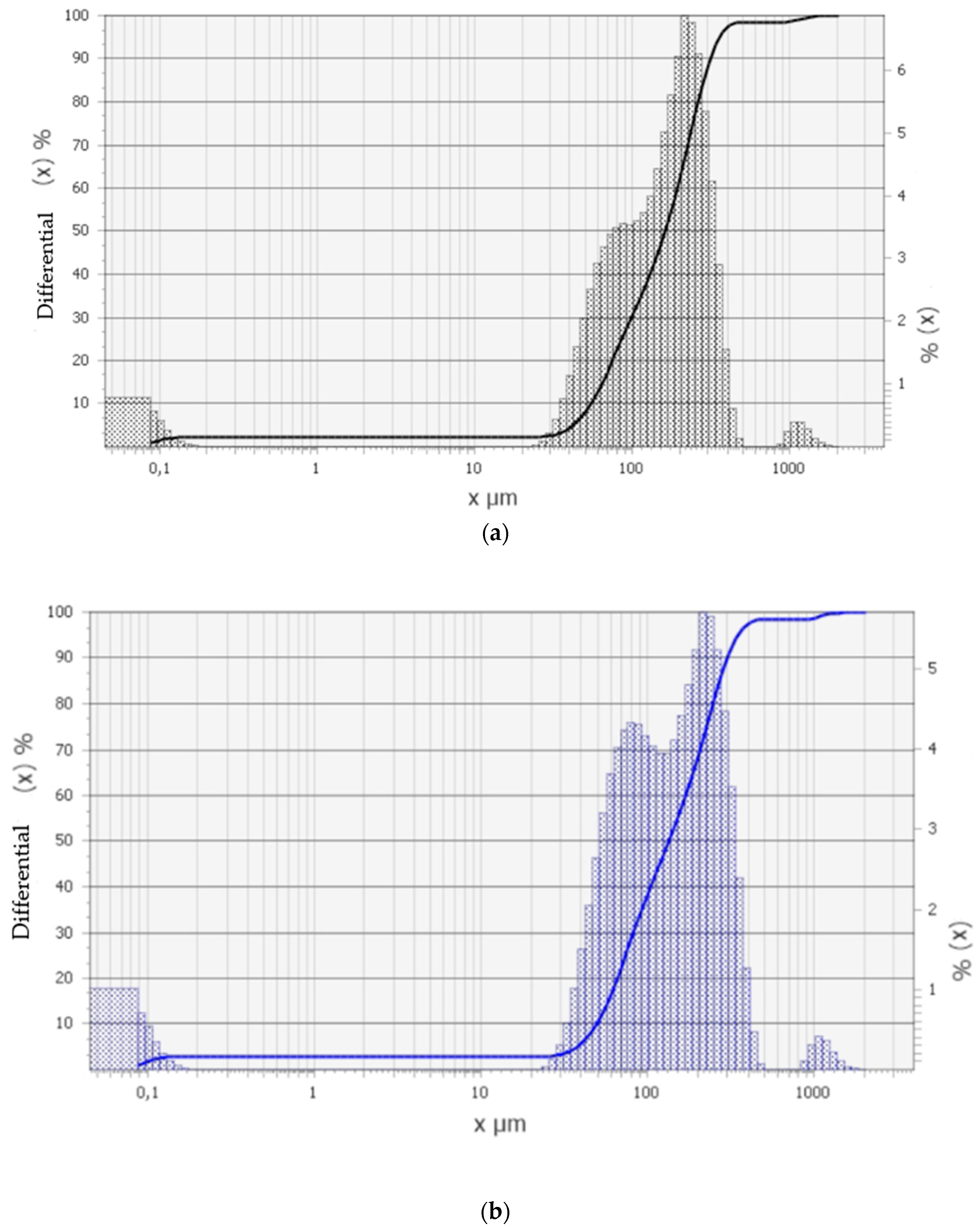
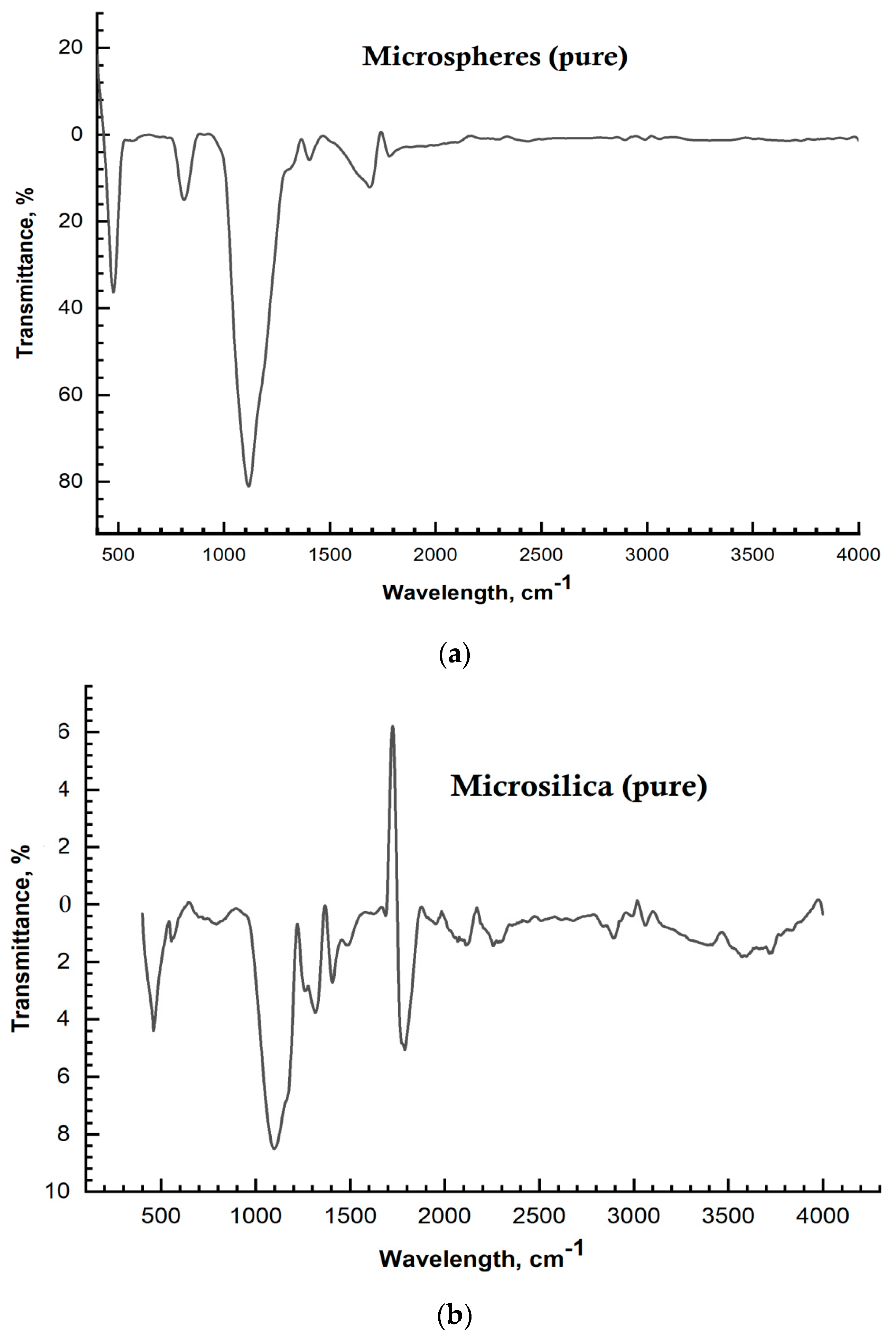
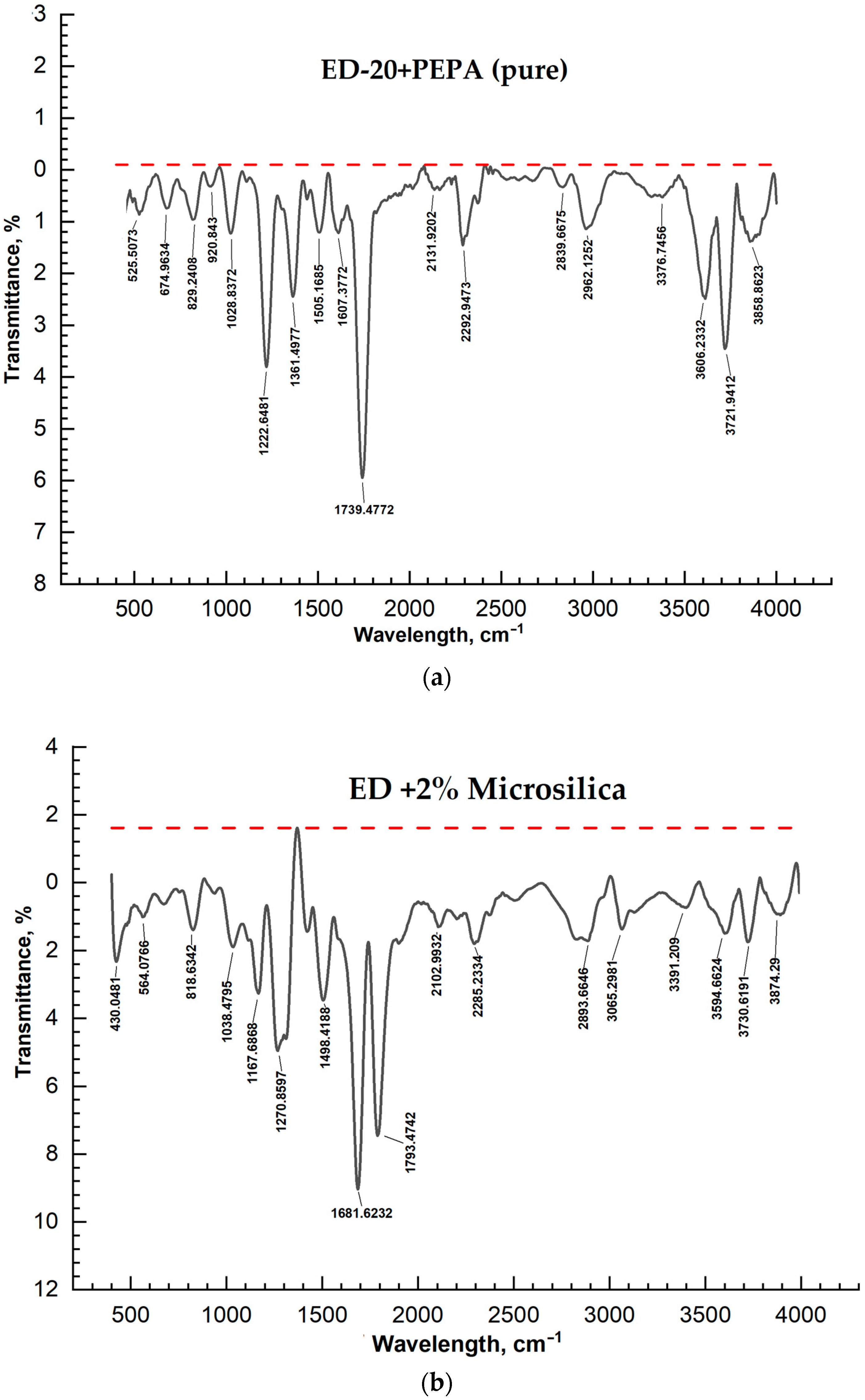



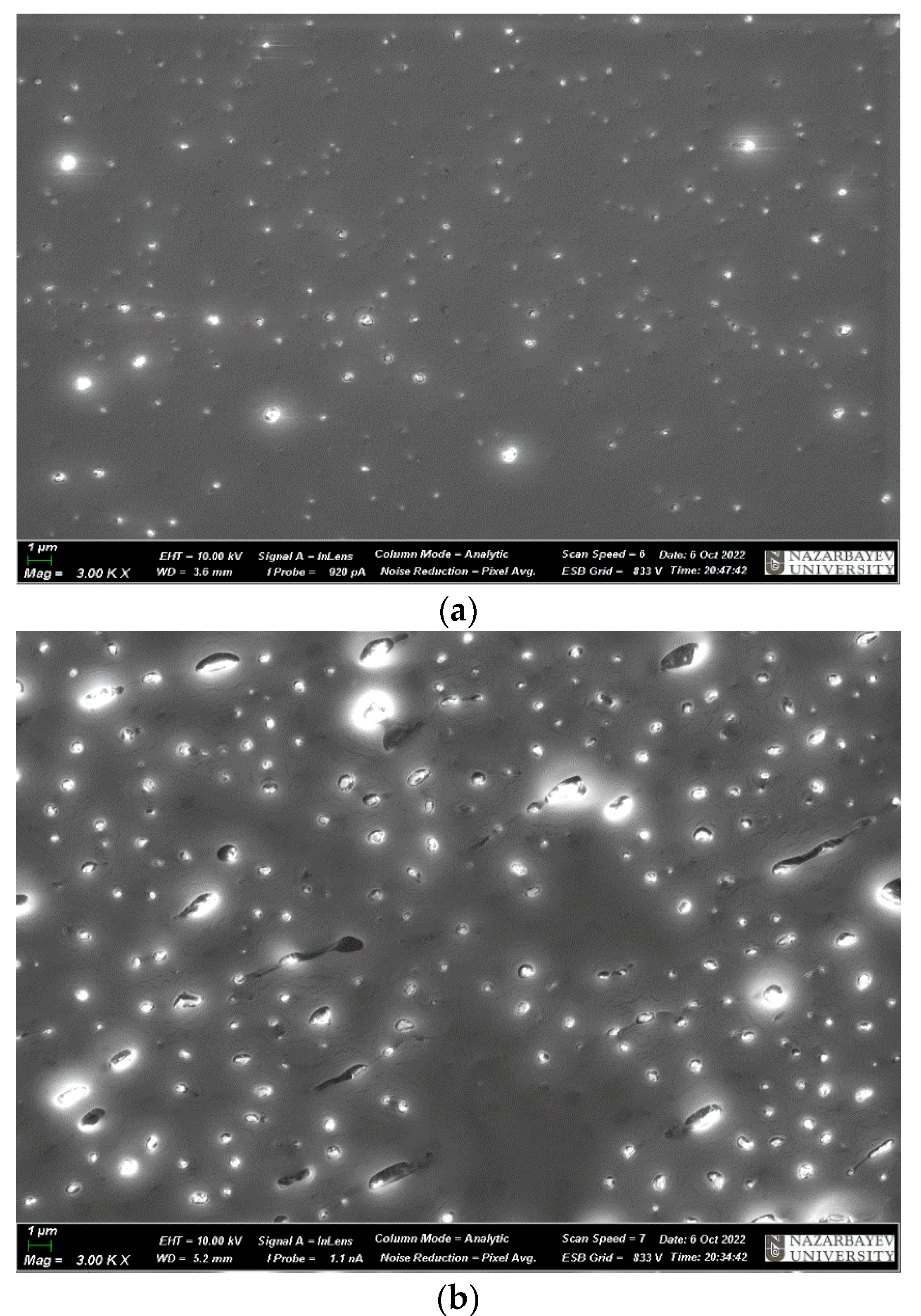
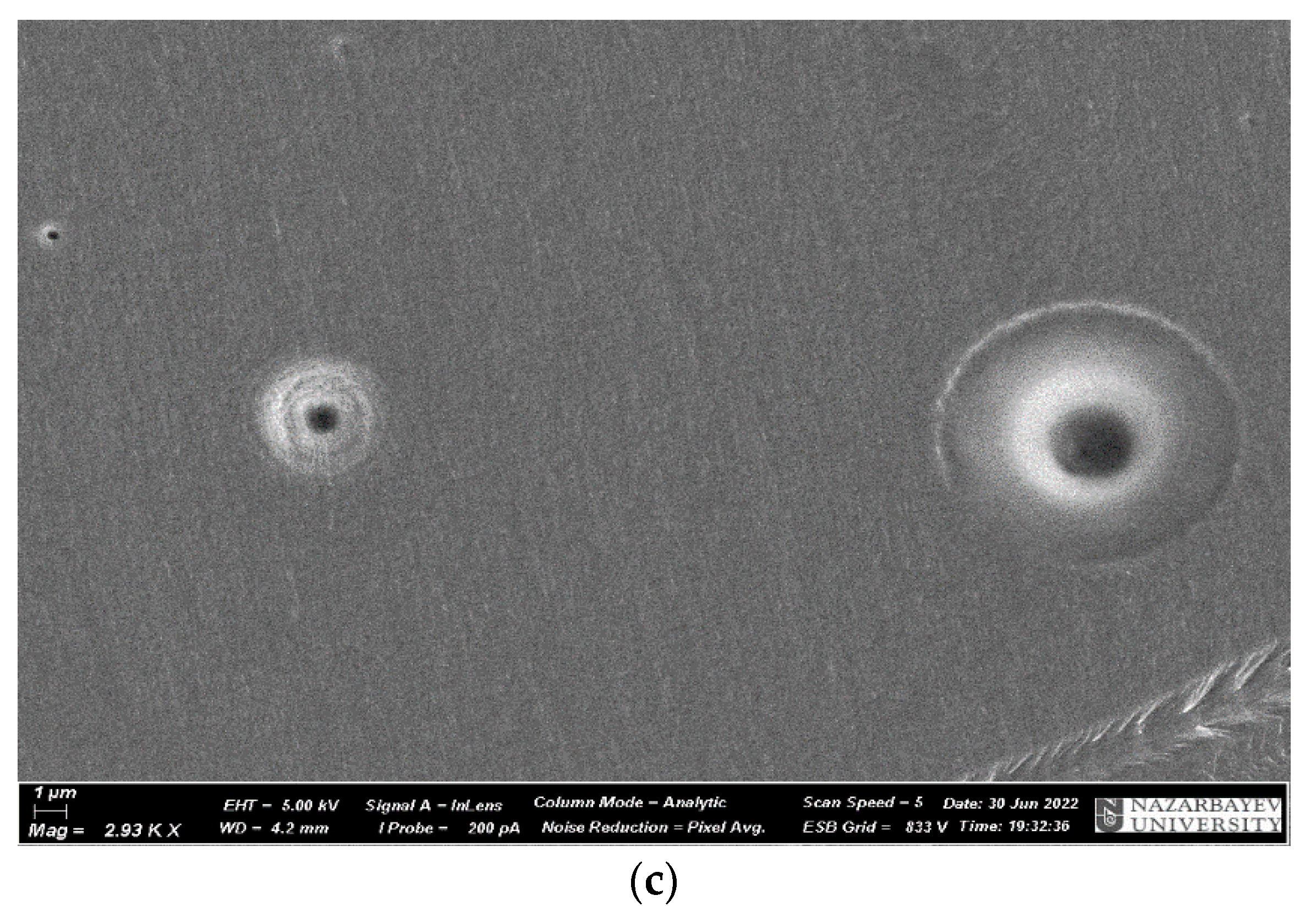



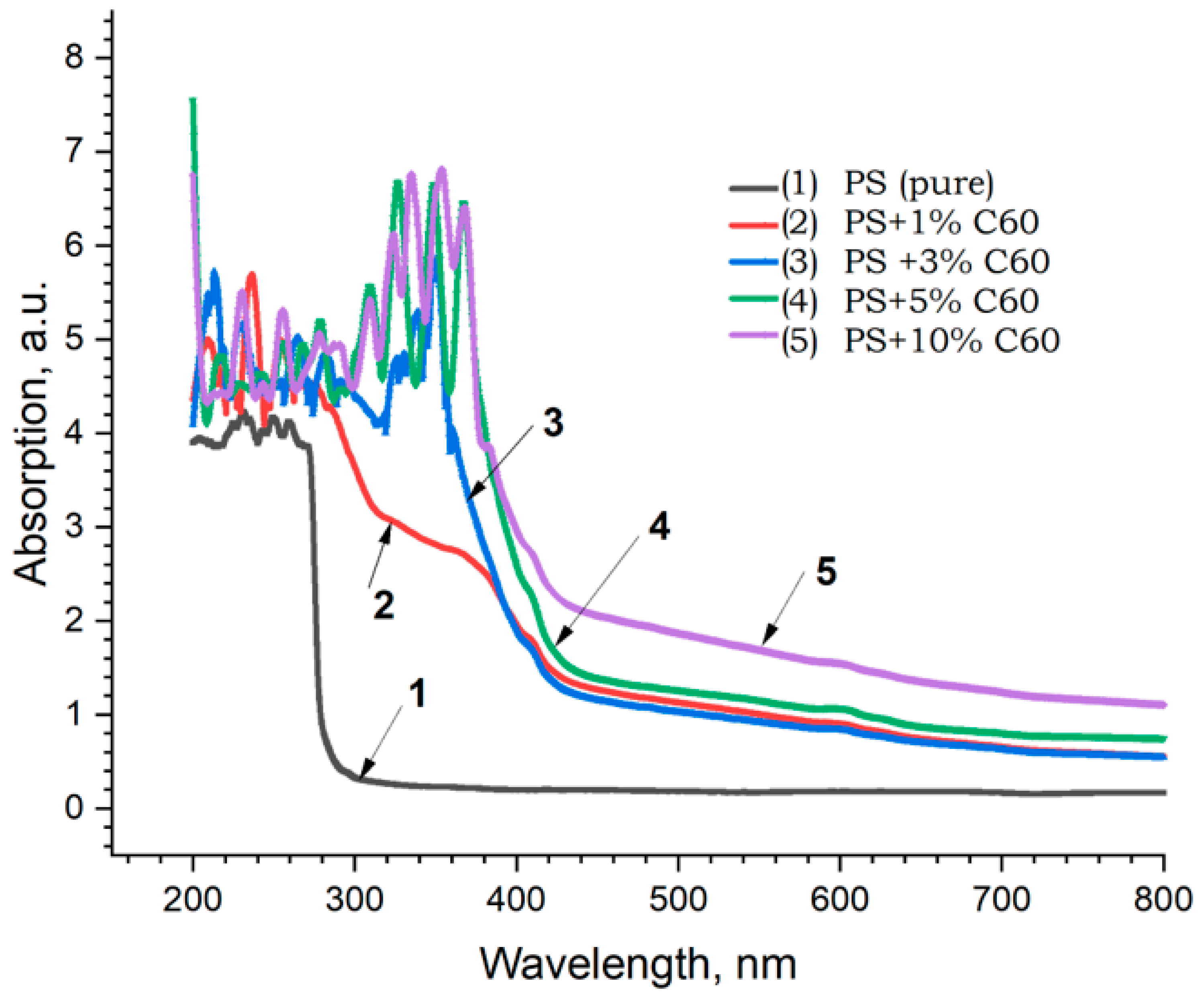
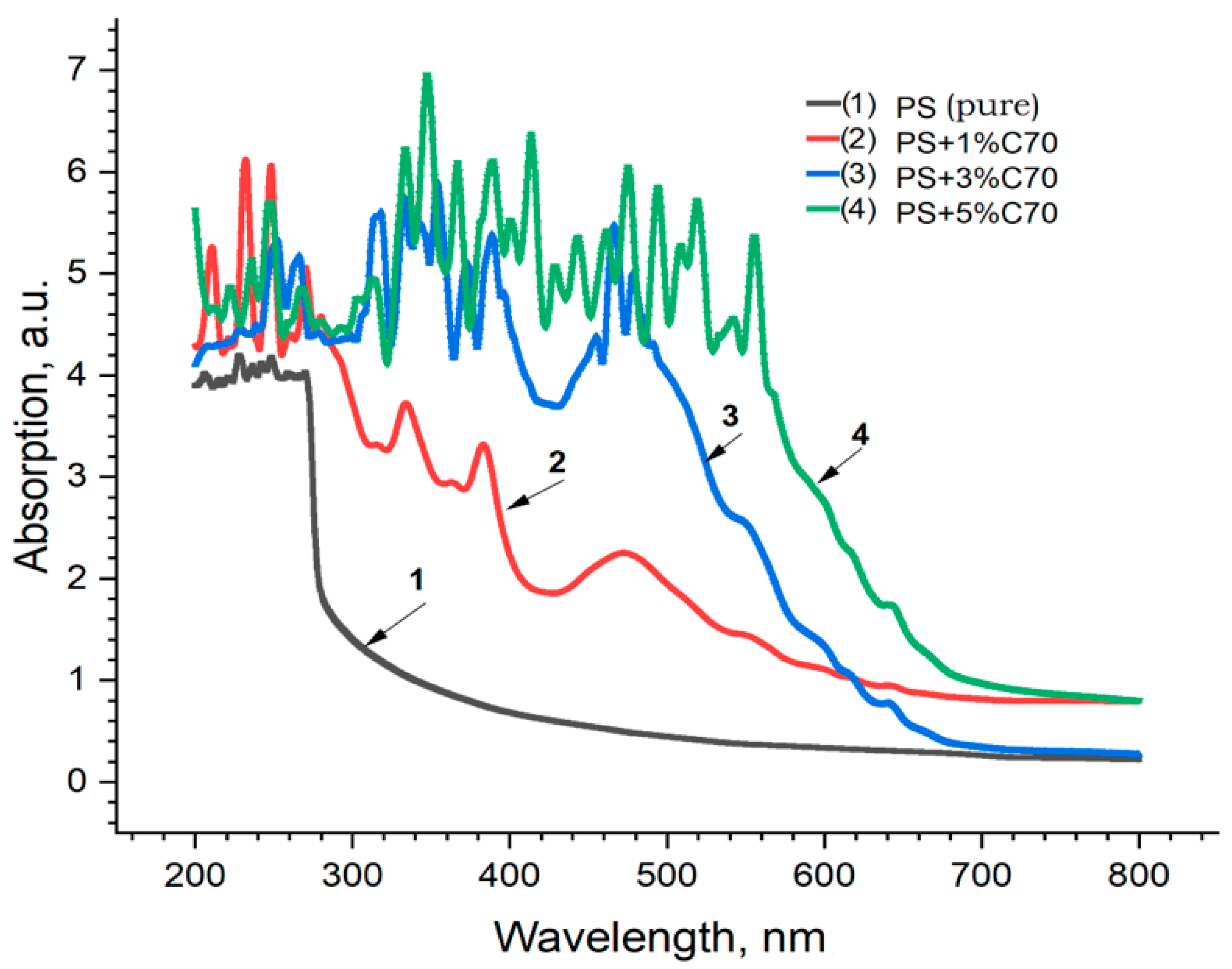
| No. | Samples | Mass Concentration, wt% | Sample Thickness, µm |
|---|---|---|---|
| 1 | Polystyrene (pure) | 0 | 30 |
| 2 | Polystyrene + MWCNT | 0.1 | 85 |
| 0.5 | 95 | ||
| 3 | Polystyrene + Fullerene C60 | 1 | 50 |
| 3 | 45 | ||
| 5 | 105 | ||
| 10 | 110 | ||
| 4 | Polystyrene + Fullerene C70 | 1 | 50 |
| 3 | 65 | ||
| 5 | 90 |
| Chemical Element | Microsphere, % | Microsilica, % |
|---|---|---|
| Na | 0.133 | 0.098 |
| Al | 11.458 | 15.239 |
| Si | 25.312 | 21.485 |
| P | 0.117 | 0.235 |
| K | 0.690 | 0.425 |
| Ca | 1.798 | 2.265 |
| Ti | 0.815 | 0.752 |
| V | 2.200 | 1.780 |
| Mn | 3.450 | 2.990 |
| Fe | 3.270 | 4.110 |
| Sr | 6.260 | 5.120 |
| Zr | 3.780 | 2.220 |
| Composition | Tensile Strength, MPa | Tensile Modulus of Elasticity, MPa |
|---|---|---|
| ED-20 + PEPA | 19.0 | 1598 |
| Microsphere 1% | 19.1 | 1683 |
| Microsphere 2% | 19.2 | 1867 |
| Microsphere 5% | 23.9 | 1954 |
| Microsphere 10% | 15.8 | 1932 |
| Microsphere 15% | 16.9 | 2011 |
| Microsphere 20% | 19.7 | 2101 |
| Microsilica 1% | 22.4 | 1805 |
| Microsilica 2% | 27.6 | 1702 |
| Microsilica 5% | 18.7 | 1321 |
| Microsilica 10% | 12.9 | 989 |
| Microsilica 15% | 13.7 | 1402 |
| Microsilica 20% | 12.1 | 1305 |
| Composition | Impact Energy, J | Impact Strength, J/cm2 |
|---|---|---|
| ED-20 + PEPA | 0.191 | 0.444 |
| ED + Microsphere 1% | 0.242 | 0.452 |
| ED + Microsphere 2% | 0.225 | 0.678 |
| ED + Microsphere 5% | 0.206 | 0.652 |
| ED + Microsphere 10% | 5.325 | 16.398 |
| ED + Microsphere 15% | 5.201 | 16.207 |
| ED + Microsphere 20% | 5.403 | 16.302 |
| ED + Microsilica 1% | 0.298 | 1.085 |
| ED + Microsilica 2% | 0.375 | 1.224 |
| ED + Microsilica 5% | 0.219 | 0.696 |
| ED + Microsilica 10% | 0.258 | 0.819 |
| ED + Microsilica 15% | 0.201 | 0.621 |
| ED + Microsilica 20% | 0.198 | 0.602 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifov, D.; Niyazbekova, R.; Mirzo, A.; Shansharova, L.; Serekpayeva, M.; Aldabergenova, S.; Ibzhanova, A.; Machnik, R.; Bembenek, M. The Study of Composite Materials Properties Based on Polymers and Nano-Additives from Industrial Wastes from Kazakhstan. Materials 2024, 17, 2959. https://doi.org/10.3390/ma17122959
Sharifov D, Niyazbekova R, Mirzo A, Shansharova L, Serekpayeva M, Aldabergenova S, Ibzhanova A, Machnik R, Bembenek M. The Study of Composite Materials Properties Based on Polymers and Nano-Additives from Industrial Wastes from Kazakhstan. Materials. 2024; 17(12):2959. https://doi.org/10.3390/ma17122959
Chicago/Turabian StyleSharifov, Dzhumakhon, Rimma Niyazbekova, Avazi Mirzo, Lazzat Shansharova, Mira Serekpayeva, Saule Aldabergenova, Ainur Ibzhanova, Ryszard Machnik, and Michał Bembenek. 2024. "The Study of Composite Materials Properties Based on Polymers and Nano-Additives from Industrial Wastes from Kazakhstan" Materials 17, no. 12: 2959. https://doi.org/10.3390/ma17122959
APA StyleSharifov, D., Niyazbekova, R., Mirzo, A., Shansharova, L., Serekpayeva, M., Aldabergenova, S., Ibzhanova, A., Machnik, R., & Bembenek, M. (2024). The Study of Composite Materials Properties Based on Polymers and Nano-Additives from Industrial Wastes from Kazakhstan. Materials, 17(12), 2959. https://doi.org/10.3390/ma17122959







