Recovery of Metal Ions (Cd2+, Co2+, and Ni2+) from Nitrate and Sulfate on Laser-Induced Graphene Film Using Applied Voltage and Its Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characteristics
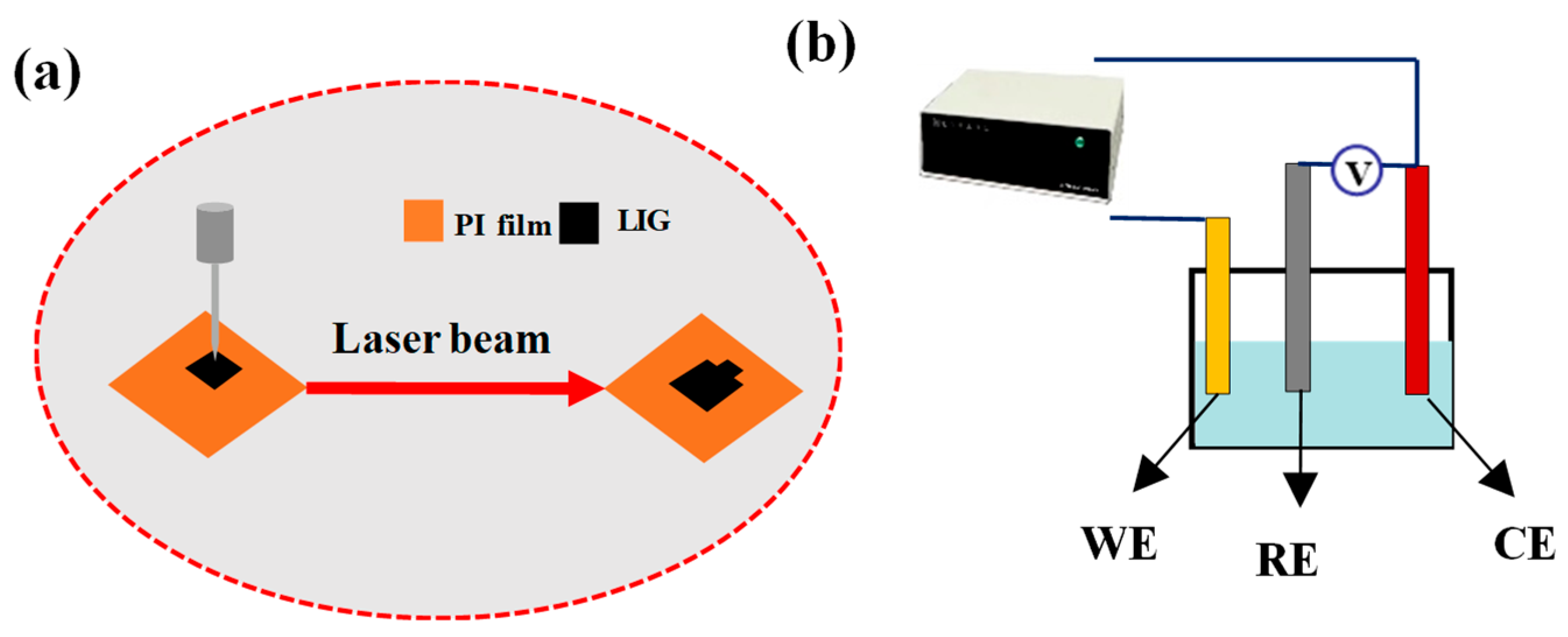
2.2. Preparation of LIG/PI Film Electrodes via Laser Scribing
2.3. Application of Recovered Co(OH)2
3. Results
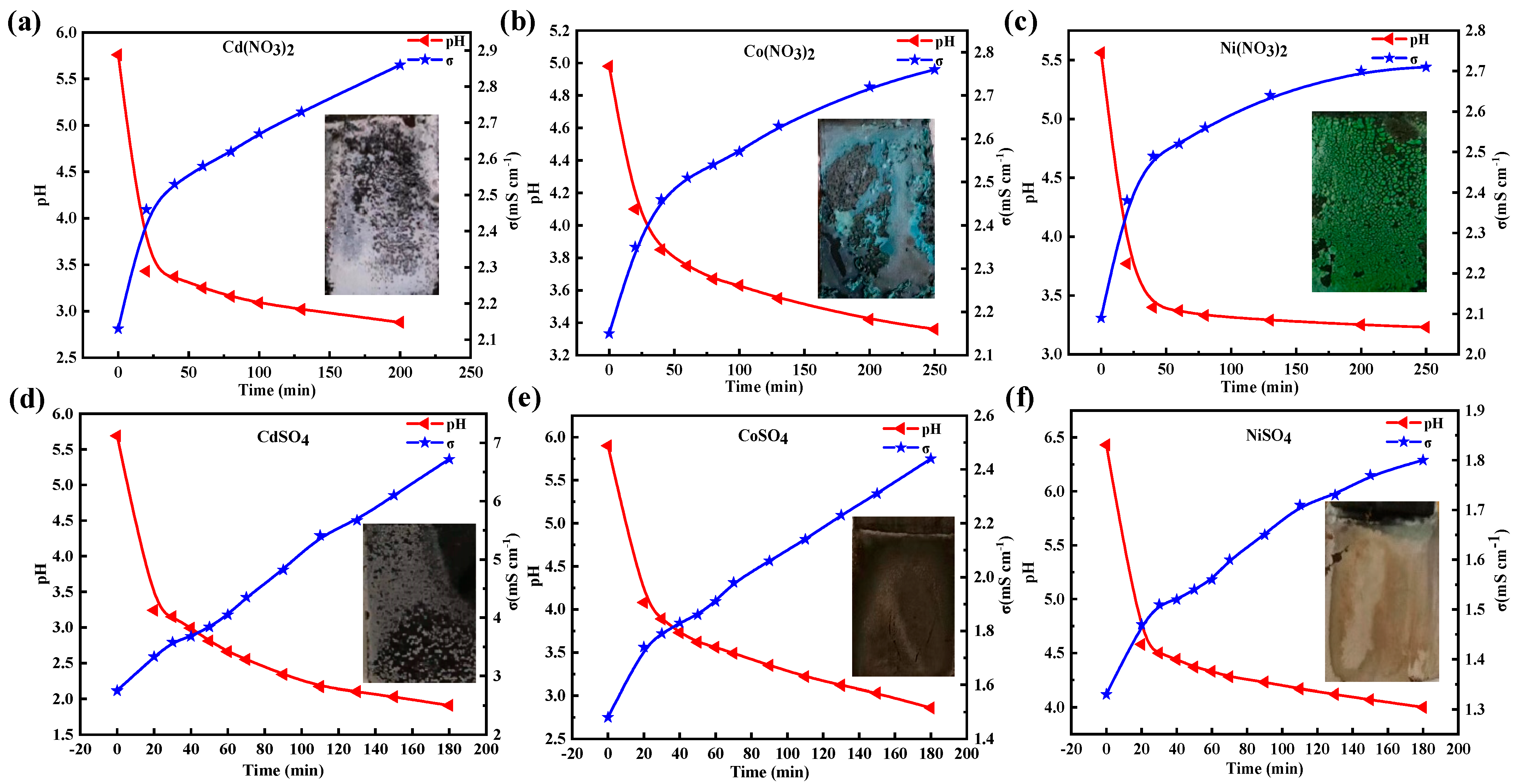
3.1. Change in pH and Conductivity after Applying the External Potential
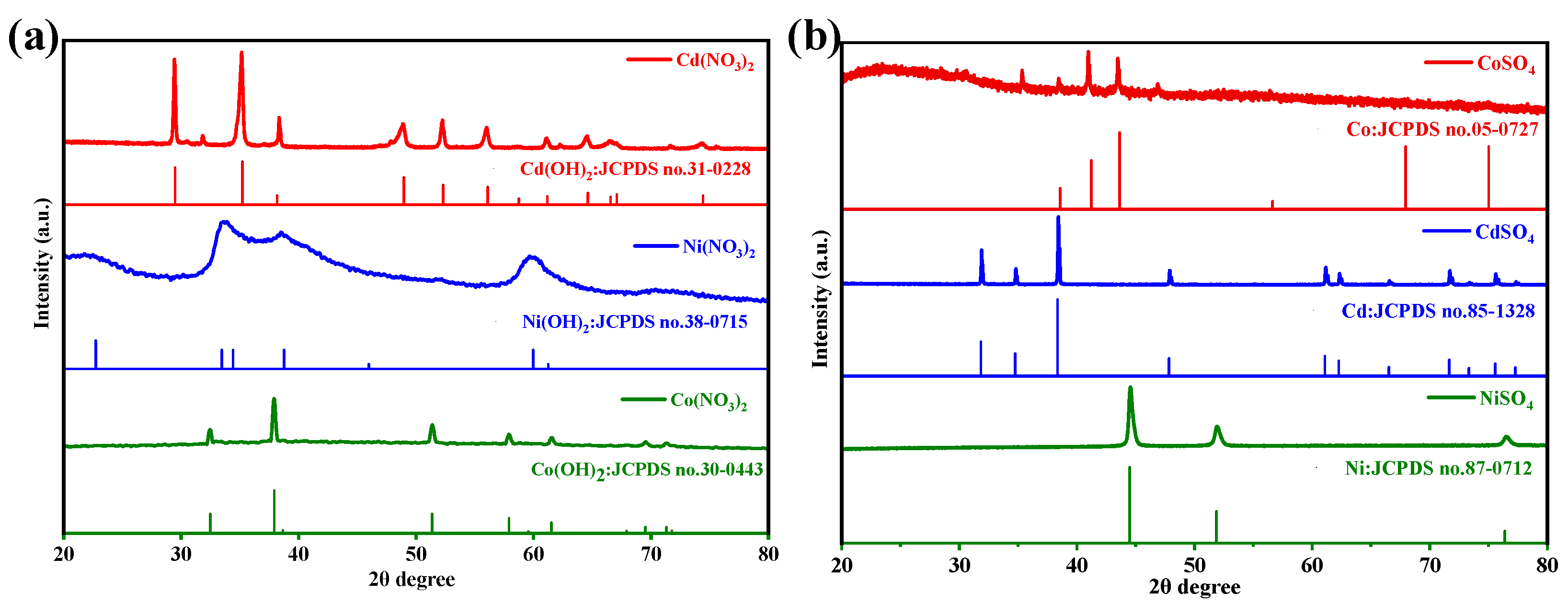
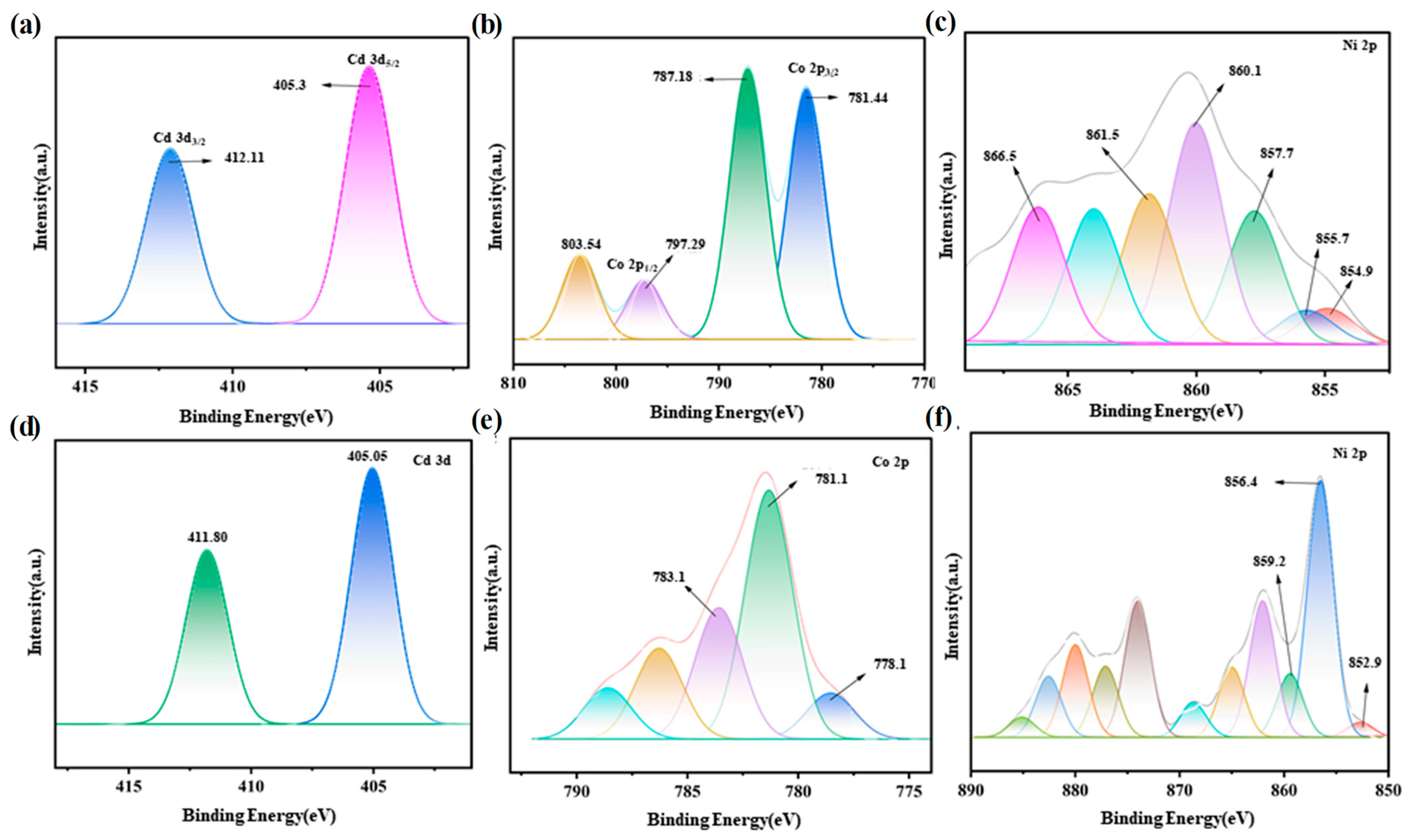
3.2. Analysis of the Adsorption Product
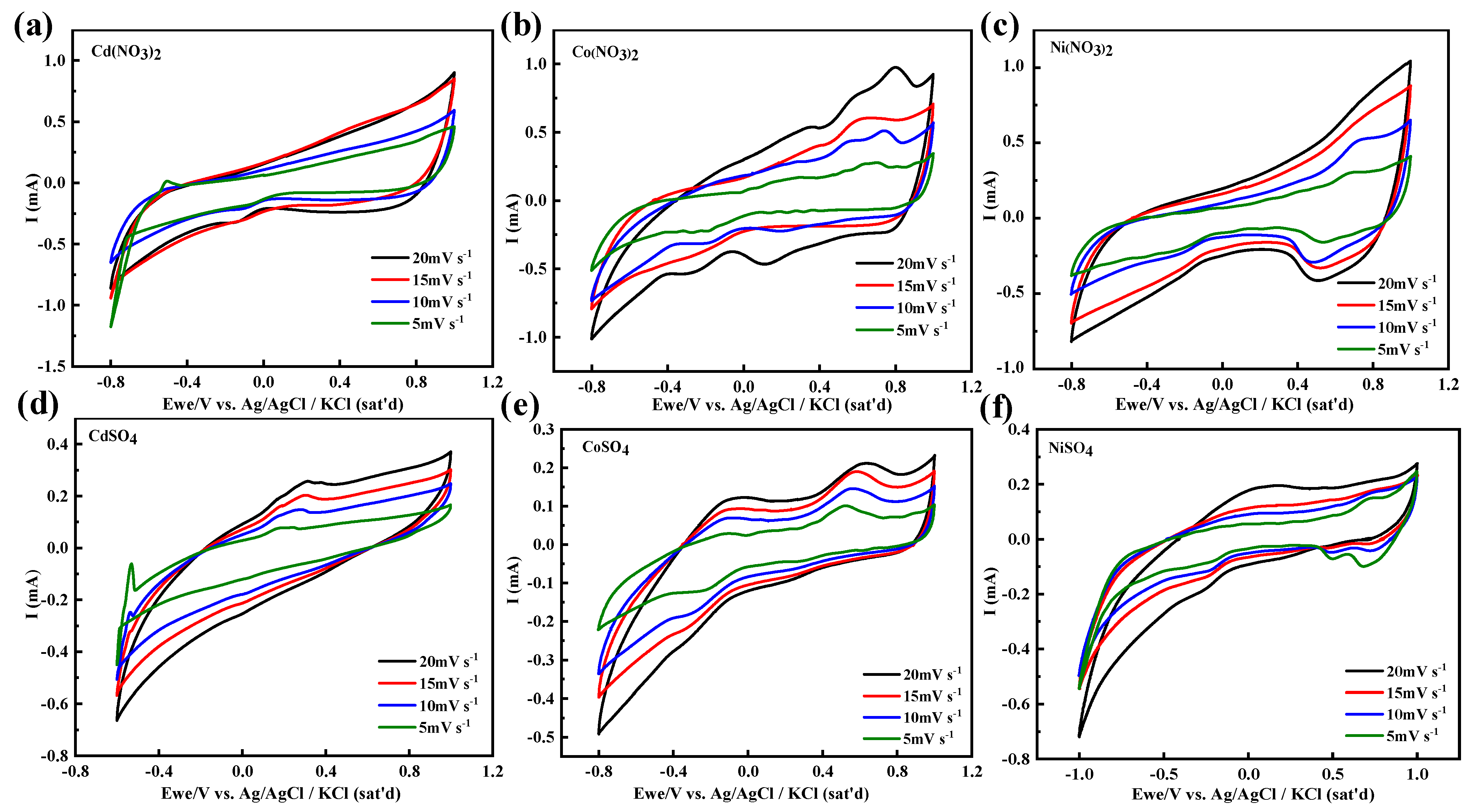
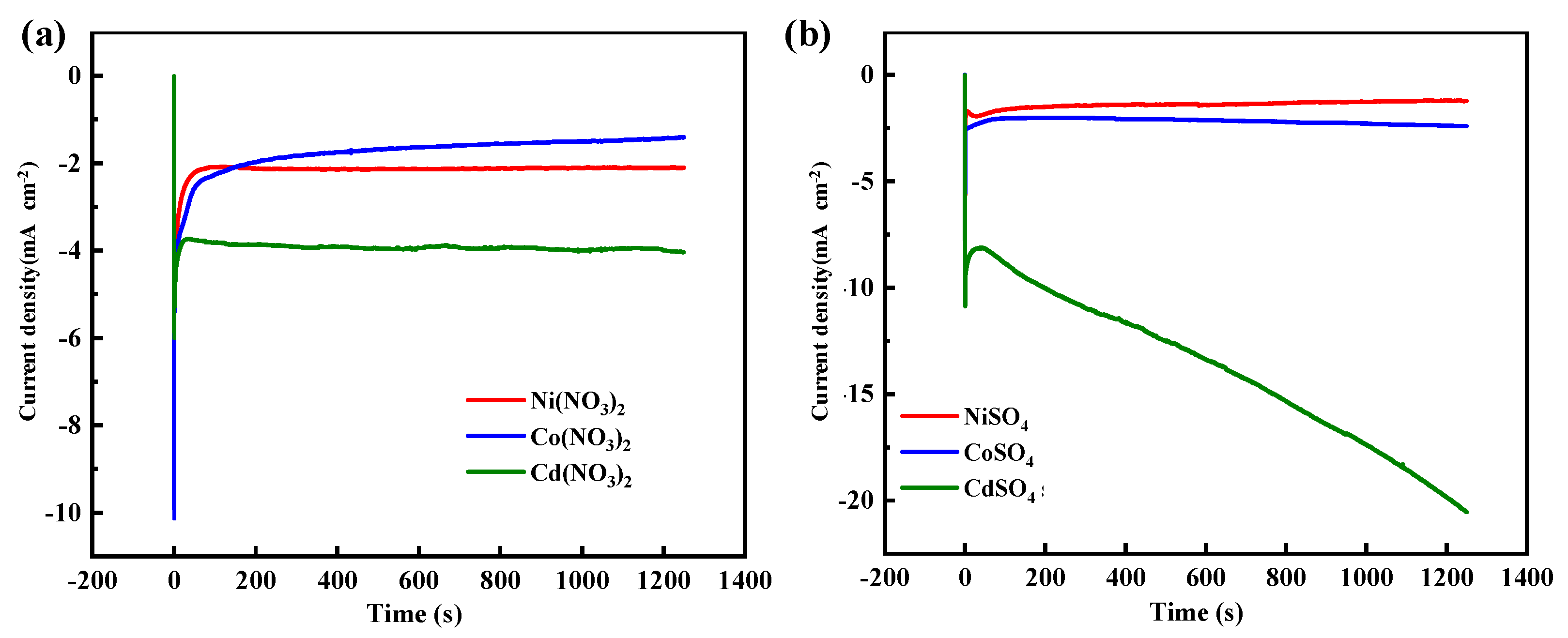
3.3. Electrochemical Properties
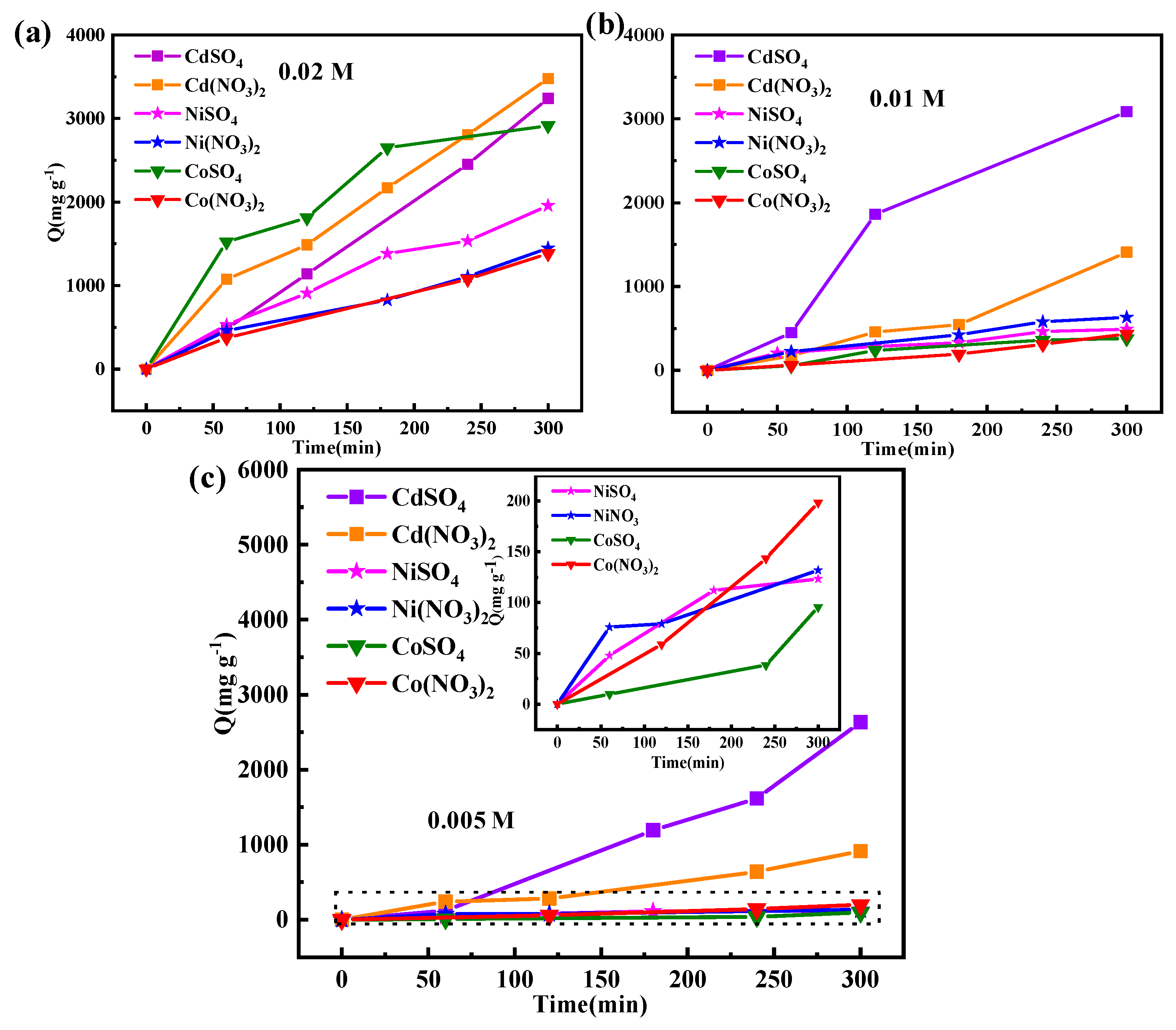
3.4. Adsorption Capacity
3.5. Reaction Mechanism
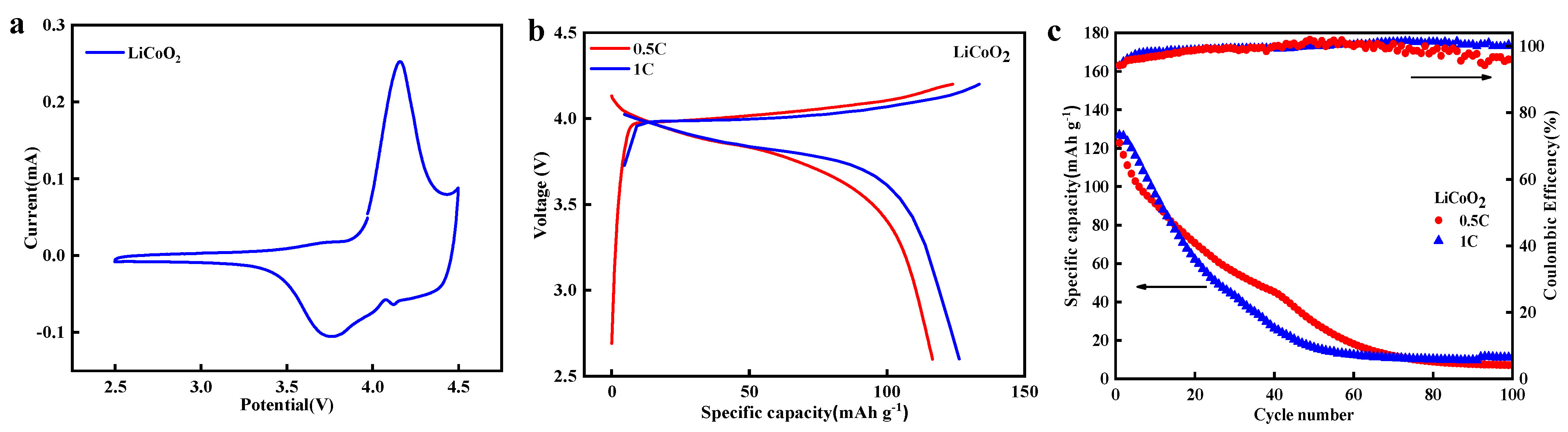
3.6. Application of the Product
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Kang, Y.; Fan, M.; Li, T.; Wozny, J.; Zhou, Y.; Wang, X.; Chueh, Y.-L.; Liang, Z.; Zhou, G.; et al. Precise separation of spent lithium ion cells in water without discharging for recycling. Energy Storage Mater. 2022, 45, 1092–1099. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, D.; Du, T.; Gong, H.; Luo, W. The current process for the recycling of spent lithium ion batteries. Front. Chem. 2021, 8, 578044. [Google Scholar] [CrossRef] [PubMed]
- Windisch-Kern, S.; Li, B.; Li, J.; Xu, Z. Pyrometallurgical technology in the recycling of a spent lithium ion battery: Evolution and the challenge. Processes 2021, 1, 1369–1382. [Google Scholar]
- Du, K.D.; Ang, E.H.; Wu, X.L.; Liu, Y.C. Progresses in sustainable recycling technology of spent lithium-ion batteries. Energy Environ. Mater. 2022, 5, 1012–1036. [Google Scholar] [CrossRef]
- Gong, R.; Li, C.C.; Meng, Q.; Dong, P.; Zhang, Y.J.; Zhang, B.; Yan, J.; Li, Y. A sustainable closed-loop method of selective oxidation leaching and regeneration for lithium iron phosphate cathode materials from spent batteries. J. Environ. Manage. 2022, 319, 115740. [Google Scholar] [CrossRef] [PubMed]
- Sankar, T.K.; Abhilash; Meshram, P. Environmental impact assessment in the entire life cycle of lithium-ioin batteries. Rev. Environ. Contam. Toxicol. 2024, 262, 5. [Google Scholar]
- Liu, Y.; Deng, Y.Y.; Zhang, Q.; Liu, H. Overview of recent developments of resource recovery from wastewater via electrochemistry-based technologies. Sci. Total Environ. 2021, 757, 143901. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Hu, W.B.; Chang, Z.; Liu, T.; Fang, D.F.; Shao, P.H.; Shi, H.; Luo, X.B. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. [Google Scholar] [CrossRef]
- Mousset, E.; Fournier, M.; Su, X. Recent advances of reactive electroseparation systems for water treatment and selective resource recovery. Curr. Opin. Electrochem. 2023, 42, 101384. [Google Scholar] [CrossRef]
- Huang, L.; Sheng, L.; Wan, K.L.; Wang, M.C.; Zhang, H.G.; Yan, J.; Liu, Y.H.; Alhassan, S.I.; Chen, Y.S.; Arulmani, S.R.B. The diversified mechanism of adsorption and electro-adsorption technologies by using Ti3AlC2 for removing fluoride. Desalination 2024, 578, 117449. [Google Scholar] [CrossRef]
- Shin, J.; Lee, G.; Choi, M.; Jang, H.; Lim, Y.; Kim, G.S.; Nam, S.H.; Baek, S.H.; Song, H.C.; Kim, J.; et al. Atomically mixed catalysts on a 3D thin-shell TiO2 for dual-modal chemical detection and neutralization. J. Mater. Chem. A 2023, 11, 18195–18206. [Google Scholar] [CrossRef]
- Wang, X.M.; Chai, Y.J.; Wang, Z.P.; Yu, J.B.; Chen, X.P. A linear and large-range pressure sensor based on hierarchical structural SnO2@carbon nanotubes/polyurethane sponge. Ceram. Int. 2023, 49, 30579–30585. [Google Scholar] [CrossRef]
- Kwak, D.; Kim, H.; Jang, S.; Kim, B.G.; Cho, D.; Chang, H.; Lee, J.O. Investigation of laser-induced graphene (LIG) on a flexible substrate and its functionalization by metal doping for gas-sensing applications. Int. J. Mol. Sci. 2024, 25, 1172. [Google Scholar] [CrossRef]
- Li, P.J.; Gui, Y.; Blackwood, D.J. Development of a nanostructured α-MnO2/carbon paper composite for removal of Ni2+/Mn2+ ions by electrosorption. ACS Appl. Mater. Interfaces 2018, 10, 19615–19625. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, W.T.; Cao, M.; Gao, J.Z.; Chen, J.Q.; Gui, Y.Y.; Zhu, W.M.; Ma, F.Q. Development of nitric acid-modified activated carbon electrode for removal of Co2+/Mn2+/Ni+ by electrosorption. Environ. Sci. Pollut. Res. 2022, 29, 77536–77552. [Google Scholar] [CrossRef]
- Huynh, L.T.N.; Tran, T.N.; Ho, T.T.N.; Le, X.H.; Le, V.H.; Nguyen, T.H. Enhanced electrosorption of NaCl and nickel(II) in capacitive deionization by CO2 activation coconut-shell activated carbon. Carbon Lett. 2022, 32, 1531–1540. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.W.; Tang, J.; Ye, Z.W.; et al. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Wang, X.M.; Chai, Y.J.; Sun, X.Q. Efficient and sustainable electro-sorption of rare earth by laser-induced graphene film. Sep. Purif. Technol. 2022, 300, 121853. [Google Scholar] [CrossRef]
- Cao, R.Y.; Zhang, J.F.; Wang, D.; Sun, F.W.; Li, N.; Li, J.X. Electrodeposition cobalt sulfide nanosheet on laser-induced graphene as capacitive deionization electrodes for uranium adsorption. Chem. Eng. J. 2023, 461, 142080. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sivakumar, M.; Chen, S.M.; Hou, Y.S.; Veeramani, V.; Madhu, R.; Miyamoto, N. A facile synthesis of Cd(OH)2-rGO nanocomposites for the practical electrochemical detection of acetaminophen. Electroanalysis 2017, 29, 280–286. [Google Scholar] [CrossRef]
- Maile, N.C.; Shinde, S.K.; Koli, R.R.; Fulari, A.V.; Kim, D.Y.; Fulari, V.J. Effect of different electrolytes and deposition time on the supercapacitor properties of nanoflake-like Co(OH)2 electrodes. Ultrason. Sonochem. 2019, 51, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.J.; Zheng, L.C.; Su, Y.M.; Meng, P.P.; Zhou, Q.Y.; Zeng, H.; Zhang, T.; Yu, H.J. Competitive adsorption of Cd(II), Pb(II) and Cu(II) ions from acid mine drainage with zero-valent iron/phosphoric titanium dioxide: XPS qualitative analyses and DFT quantitative calculations. Chem. Eng. J. 2022, 445, 136778. [Google Scholar] [CrossRef]
- Wang, R.F.; Deng, L.G.; Fan, X.J.; Li, K.; Lu, H.Q.; Li, W. Removal of heavy metal ion cobalt (II) from wastewater via adsorption method using microcrystalline cellulose–magnesium hydroxide. Int. J. Biol. Macromol. 2021, 189, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Rohit, R.C.; Jenifer, A.; Jagadale, A.D.; Kumbhar, V.S.; Lee, H.; Lee, K. Facile synthesis of Ce-doped α-cobalt hydroxide nanoflakes battery type electrode with an enhanced capacitive contribution for asymmetric supercapacitors. J. Energy Storage 2020, 28, 101227. [Google Scholar] [CrossRef]
- Mark, C.; Biesinger, B.P.; Payne, A.P.; Grosvenor, L.W.M.; Lau, A.R.; Gerson, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar]
- Altamirano-Garcia, L.; Vazquez-Arenas, J.; Pritzker, M.; Luna-Sánchez, R.; Cabrera-Sierra, R. Effects of saccharin and anions (SO42−, Cl−) on the electrodeposition of Co-Ni alloys. J. Solid State Electrochem. 2015, 19, 423–433. [Google Scholar] [CrossRef]
- Ma, H.L.; Wang, F.; Shen, M.H.; Tong, Y.; Wang, H.X.; Hu, H.L. Advances of LiCoO2 in cathode of aqueous lithium-Ion batteries. Small Methods 2023, 2300820. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-m.; Su, T.; Chai, Y. Recovery of Metal Ions (Cd2+, Co2+, and Ni2+) from Nitrate and Sulfate on Laser-Induced Graphene Film Using Applied Voltage and Its Application. Materials 2024, 17, 2965. https://doi.org/10.3390/ma17122965
Wang X-m, Su T, Chai Y. Recovery of Metal Ions (Cd2+, Co2+, and Ni2+) from Nitrate and Sulfate on Laser-Induced Graphene Film Using Applied Voltage and Its Application. Materials. 2024; 17(12):2965. https://doi.org/10.3390/ma17122965
Chicago/Turabian StyleWang, Xiu-man, Tong Su, and Yujun Chai. 2024. "Recovery of Metal Ions (Cd2+, Co2+, and Ni2+) from Nitrate and Sulfate on Laser-Induced Graphene Film Using Applied Voltage and Its Application" Materials 17, no. 12: 2965. https://doi.org/10.3390/ma17122965



