Chloride Binding Behavior and Pore Structure Characteristics of Low-Calcium High-Strength Cement Pastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sample
2.3. Characterization
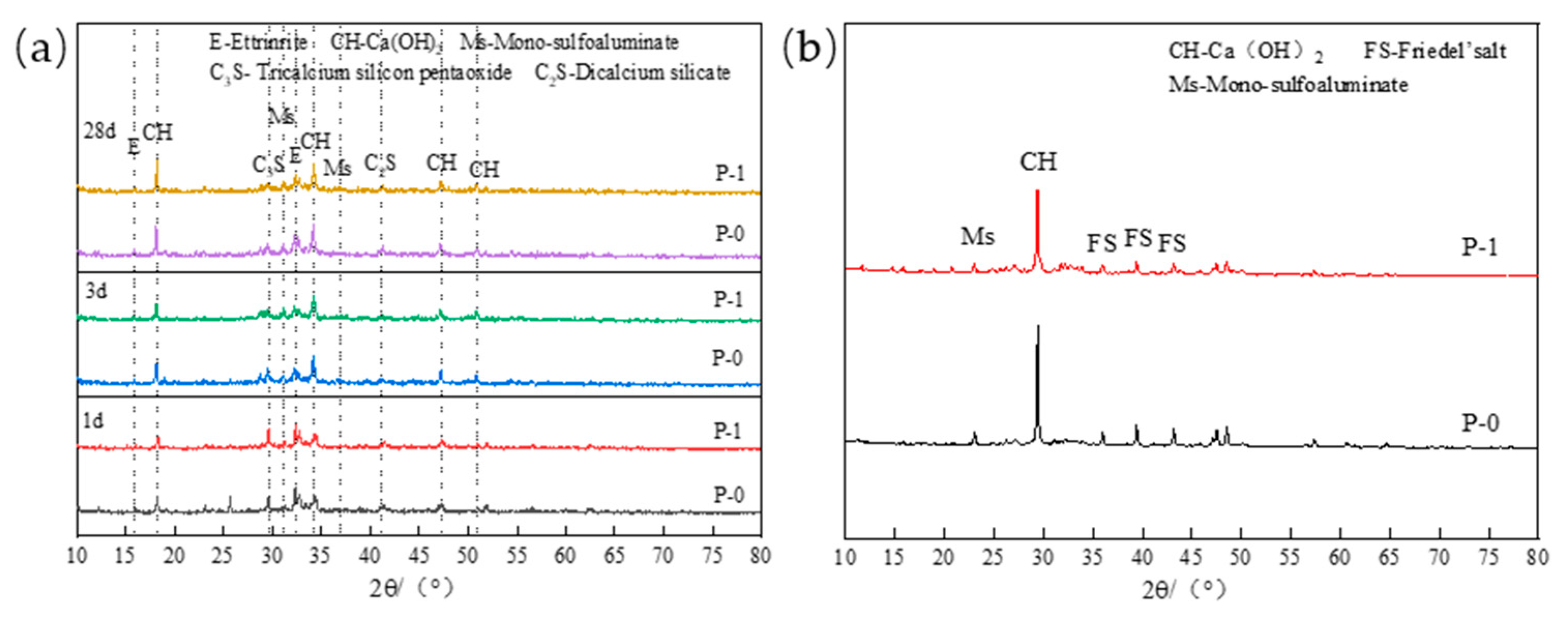
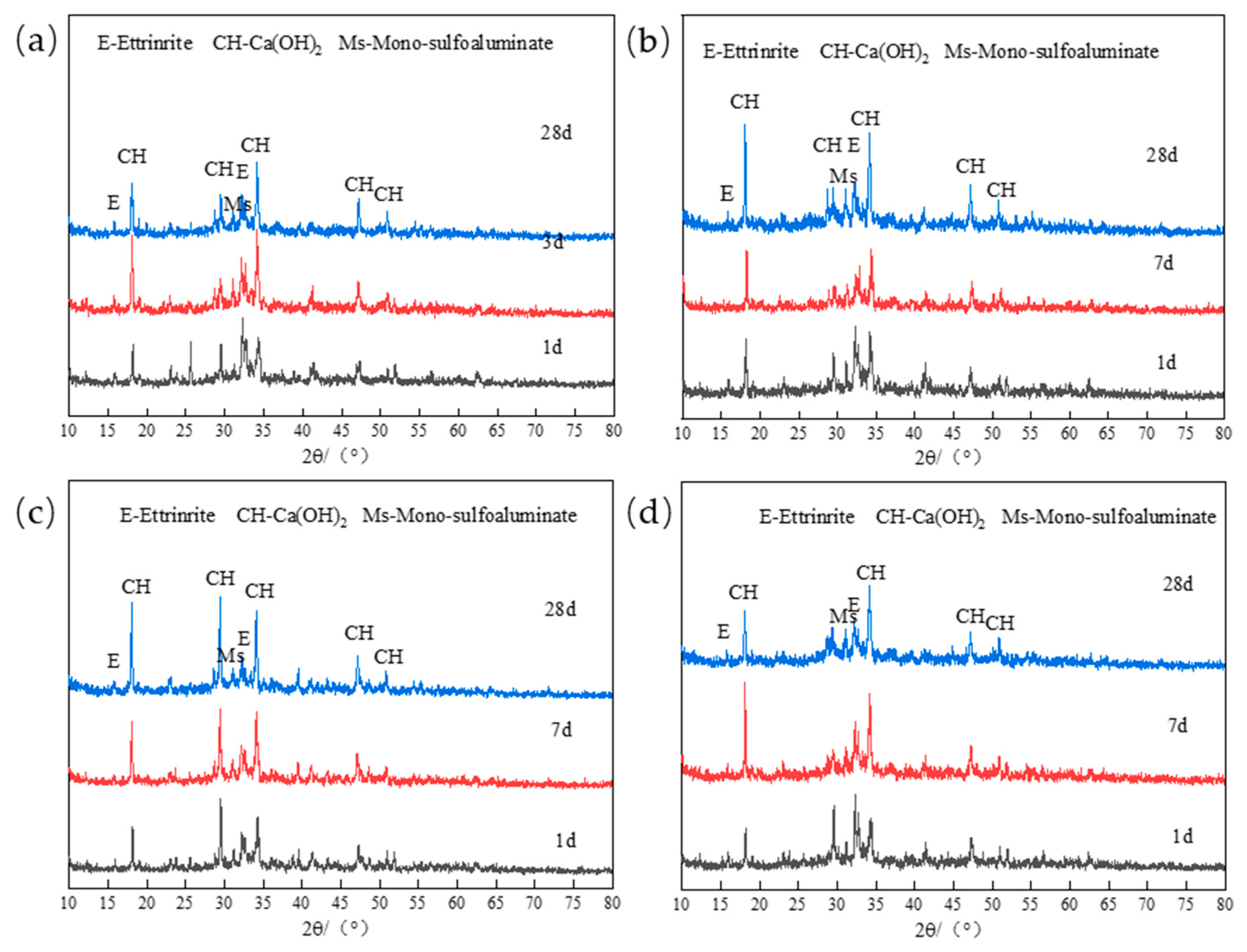
2.3.1. XRD Analysis
2.3.2. Mercury Intrusion Analysis
2.3.3. The Test of Chloride Binding Capacity
3. Results
3.1. Chloride Binding Capacity
3.1.1. Chloride Binding Capacity of Low-Calcium High-Strength Cement Pastes
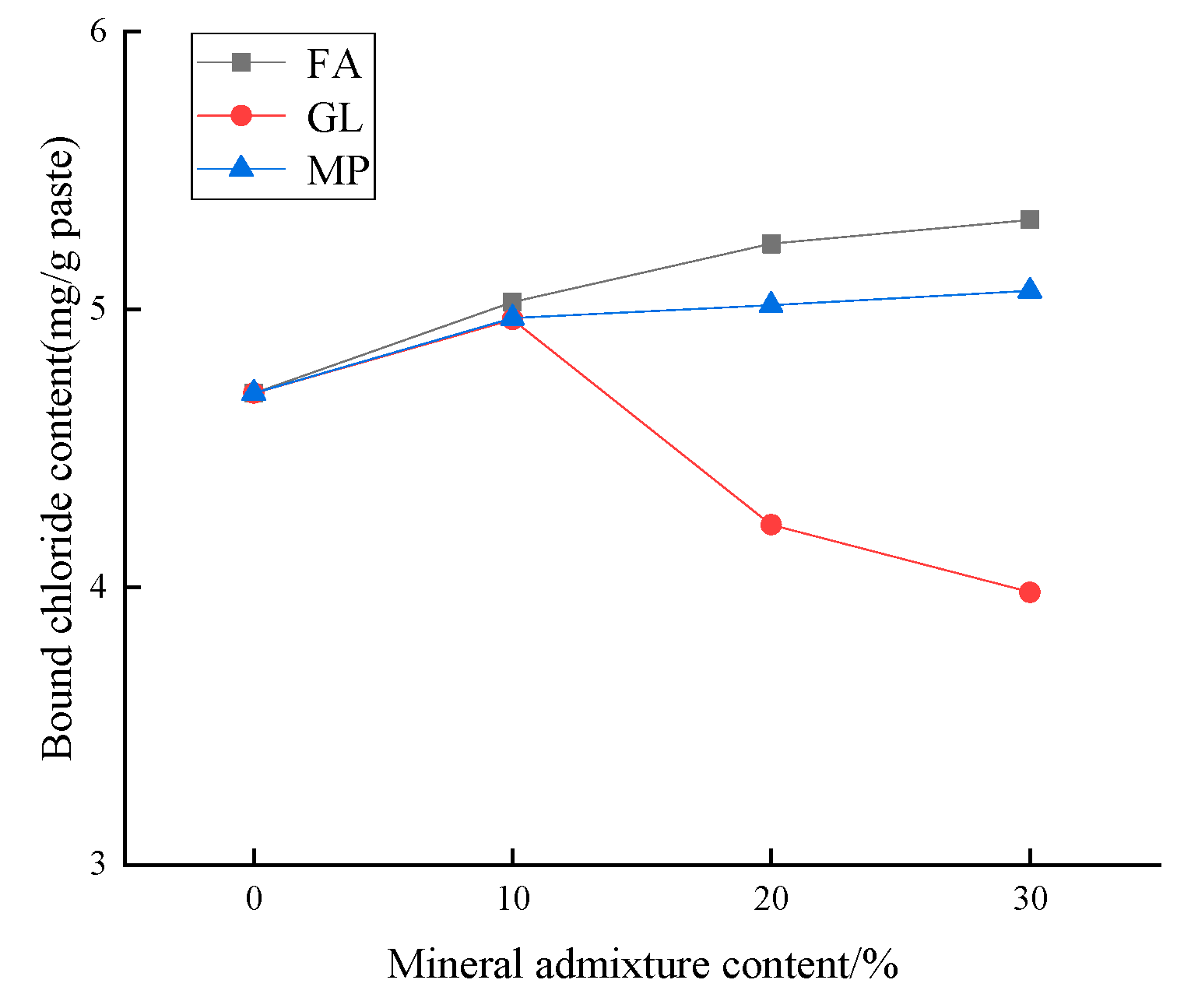
3.1.2. Effect of Different Mineral Admixtures on the Chloride Binding Capacity of Low-Calcium High-Strength Cement Pastes
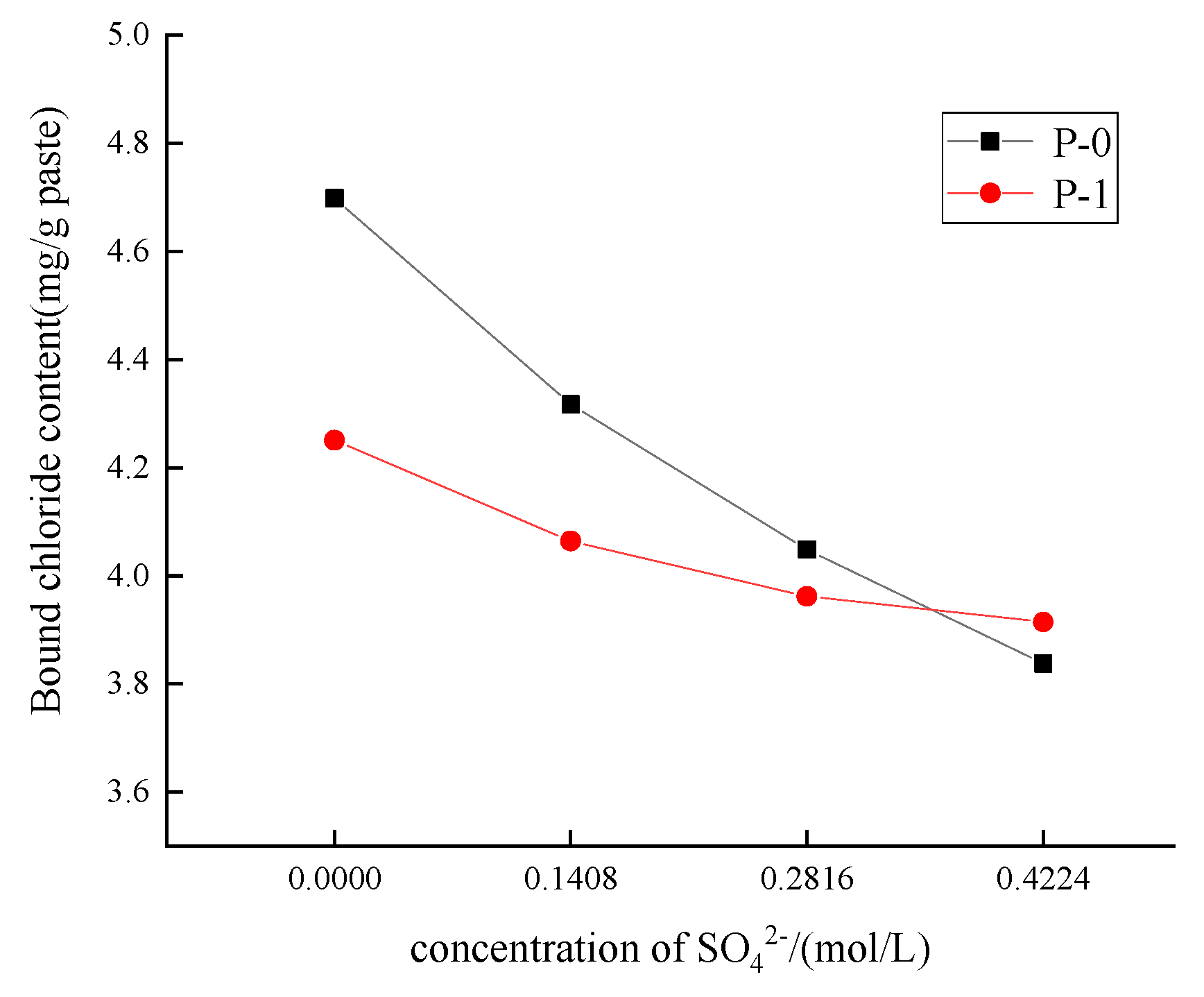
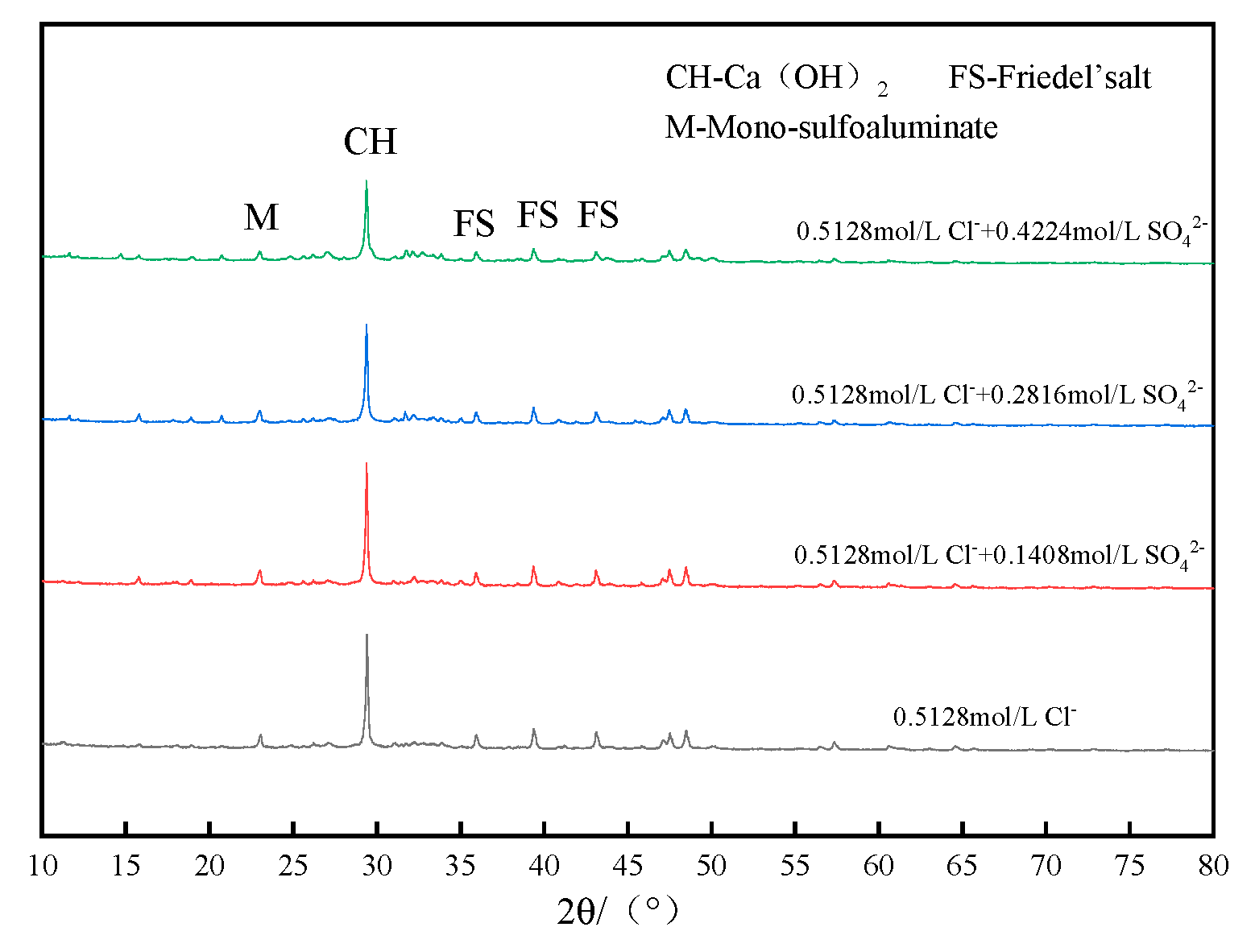
3.1.3. Effect of Different Concentrations of SO42− on the Chloride Binding Capacity of Low-Calcium High-Strength Cement Pastes
3.2. Pore Structure
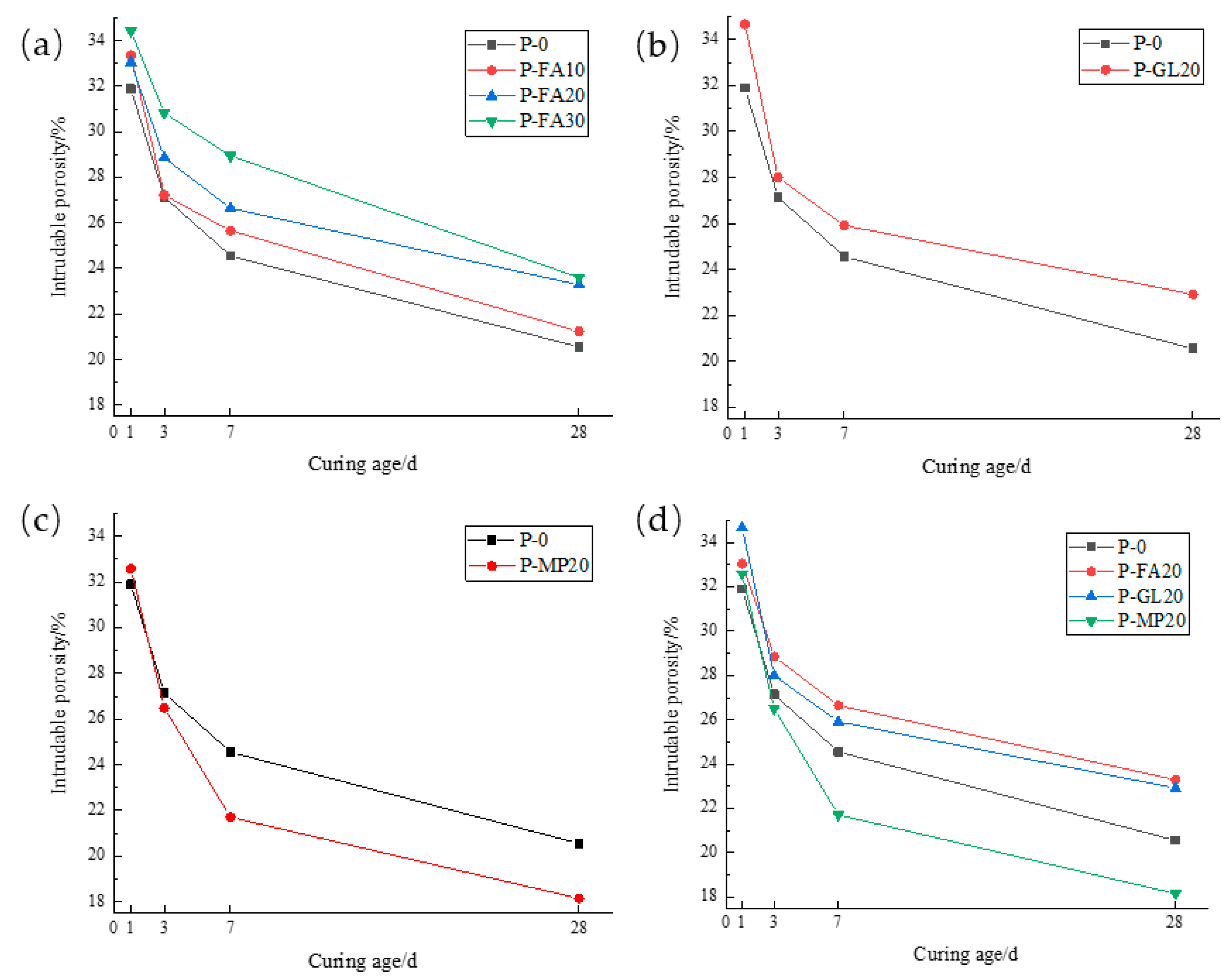
3.2.1. Porosity
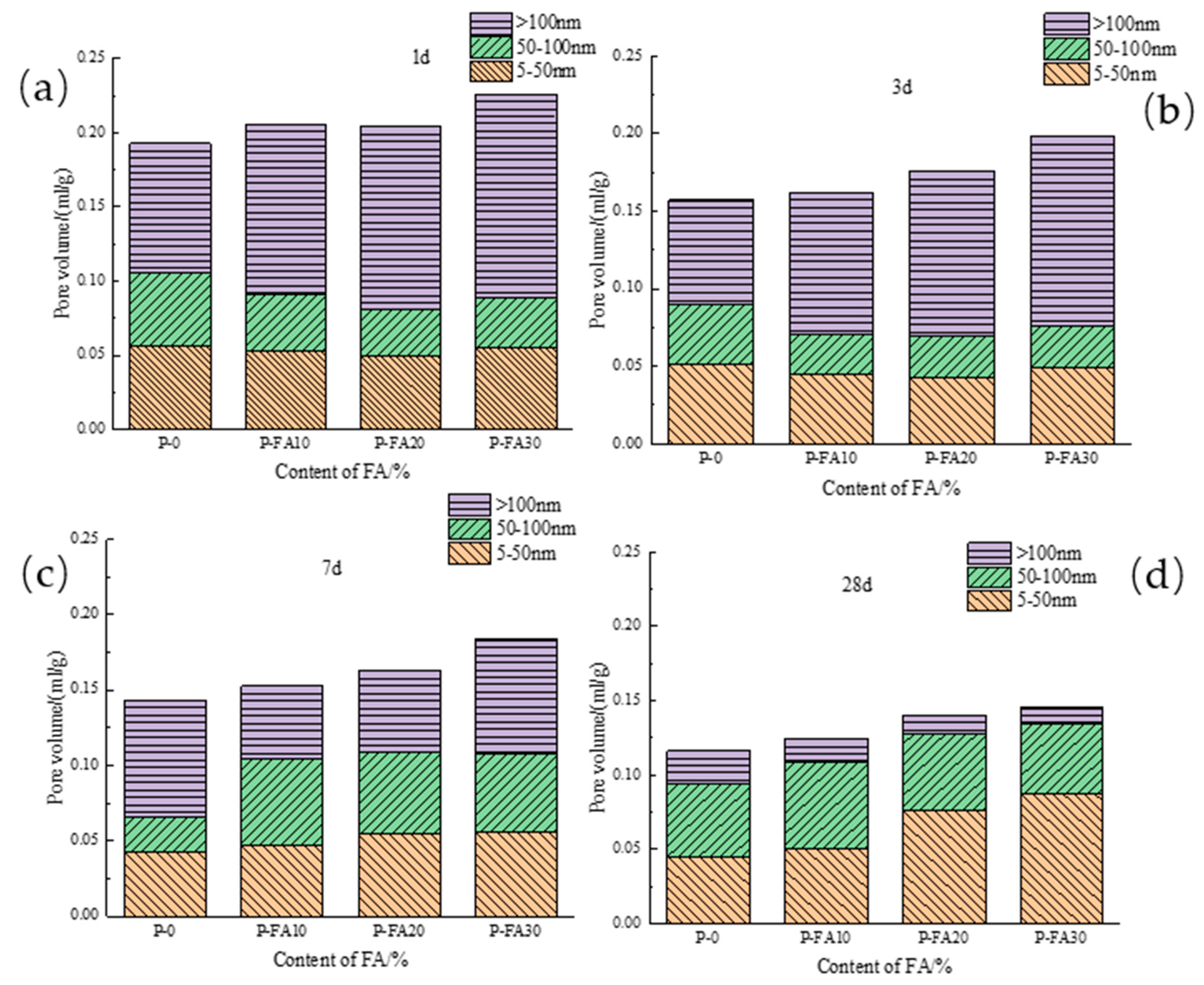
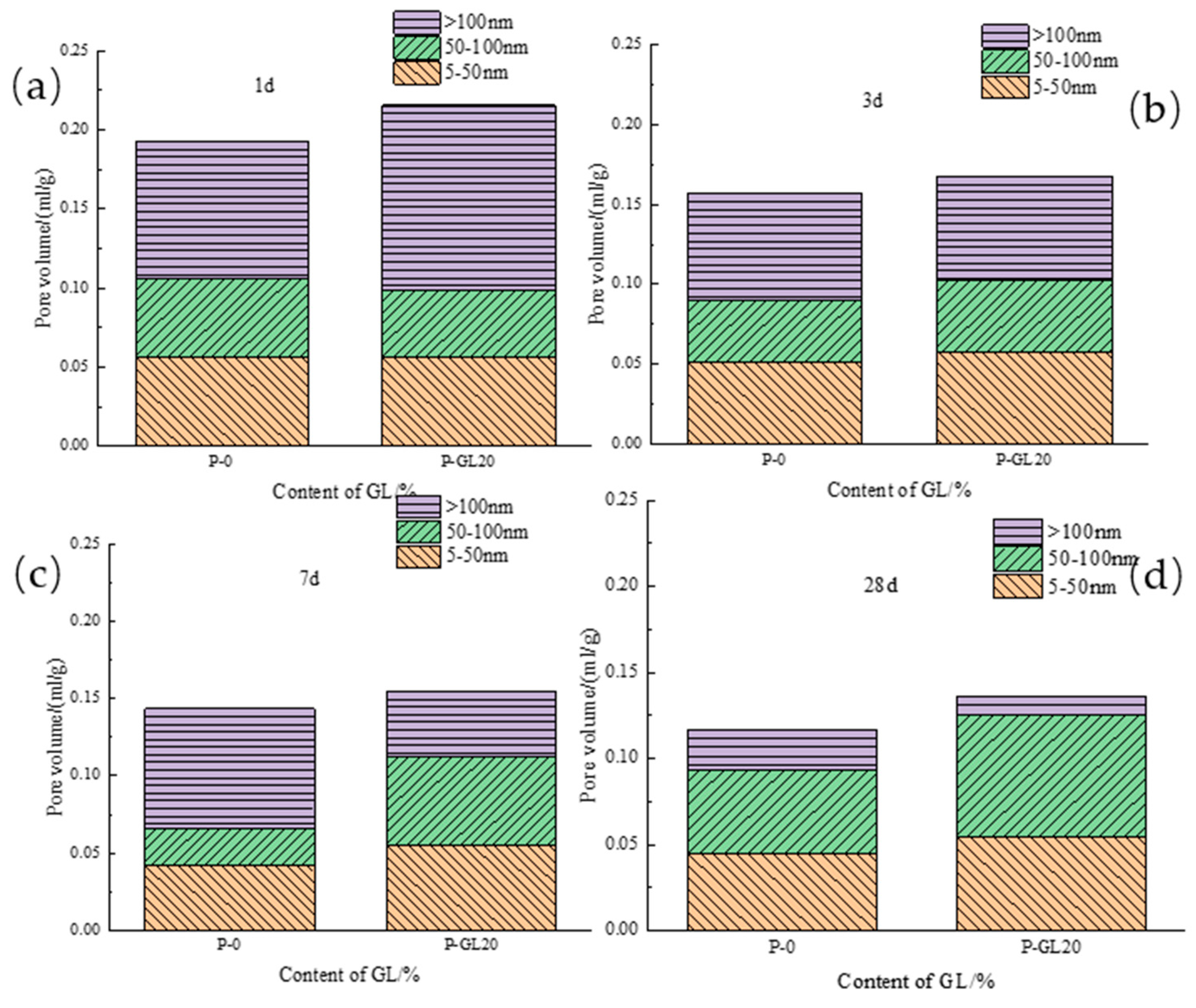
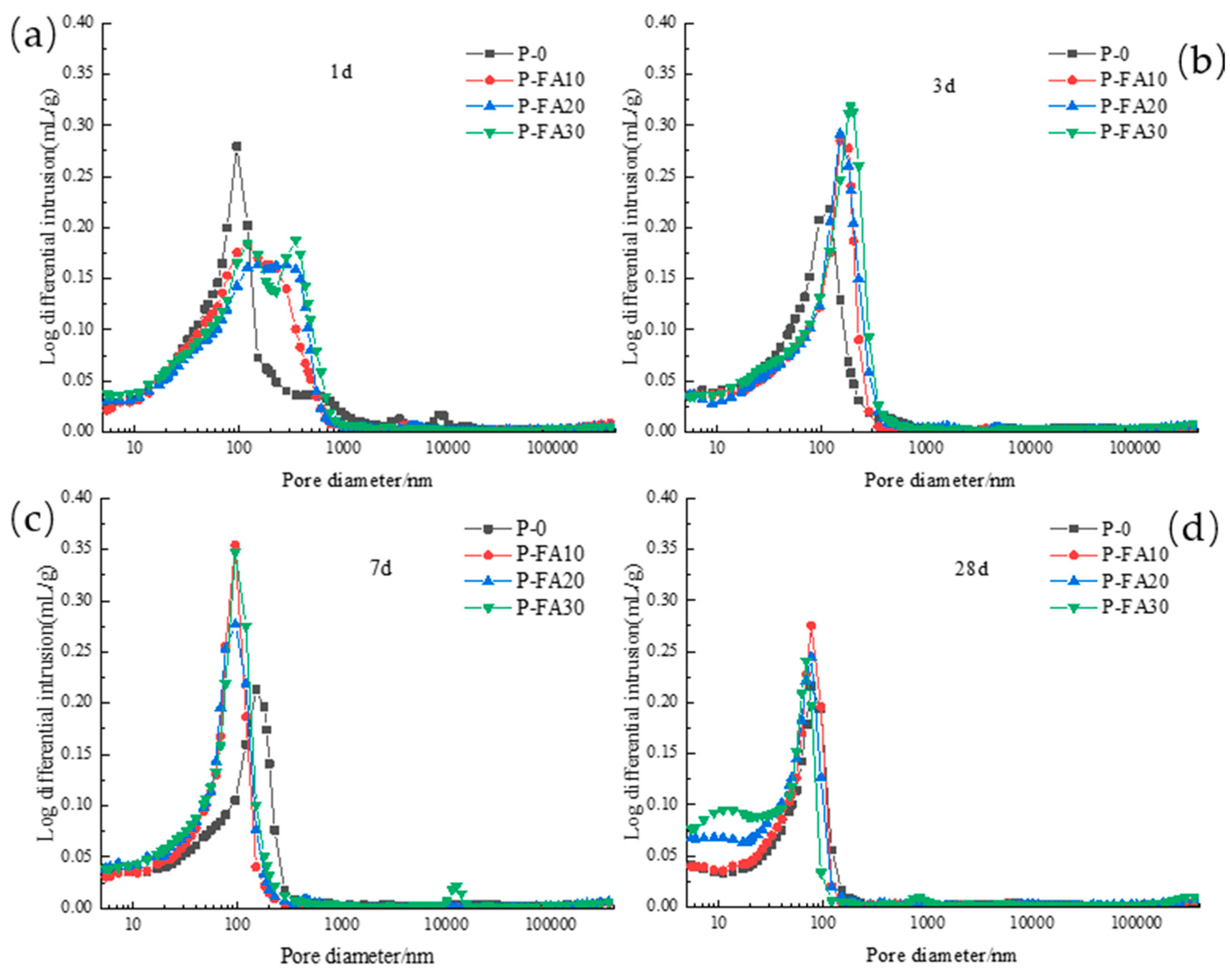
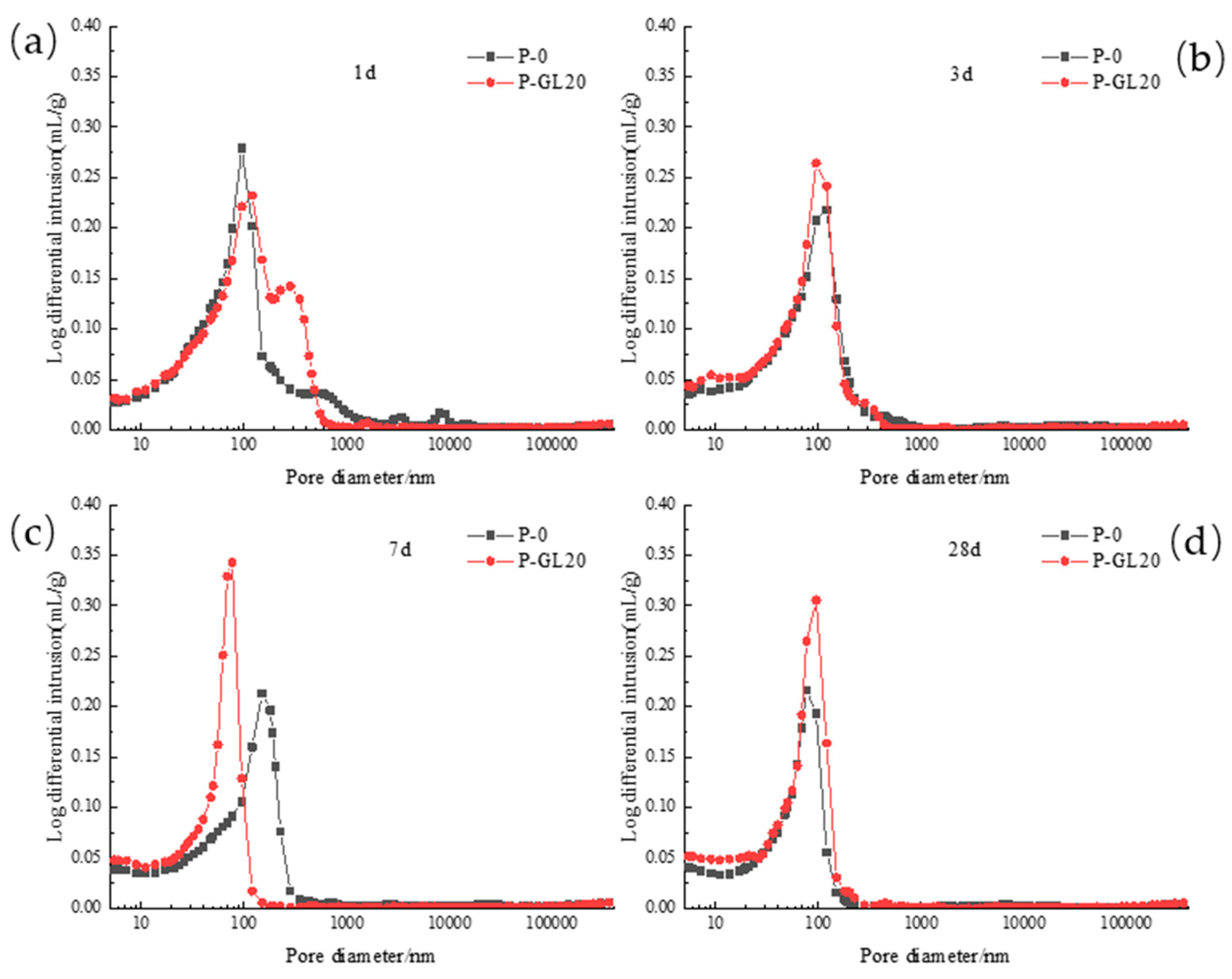
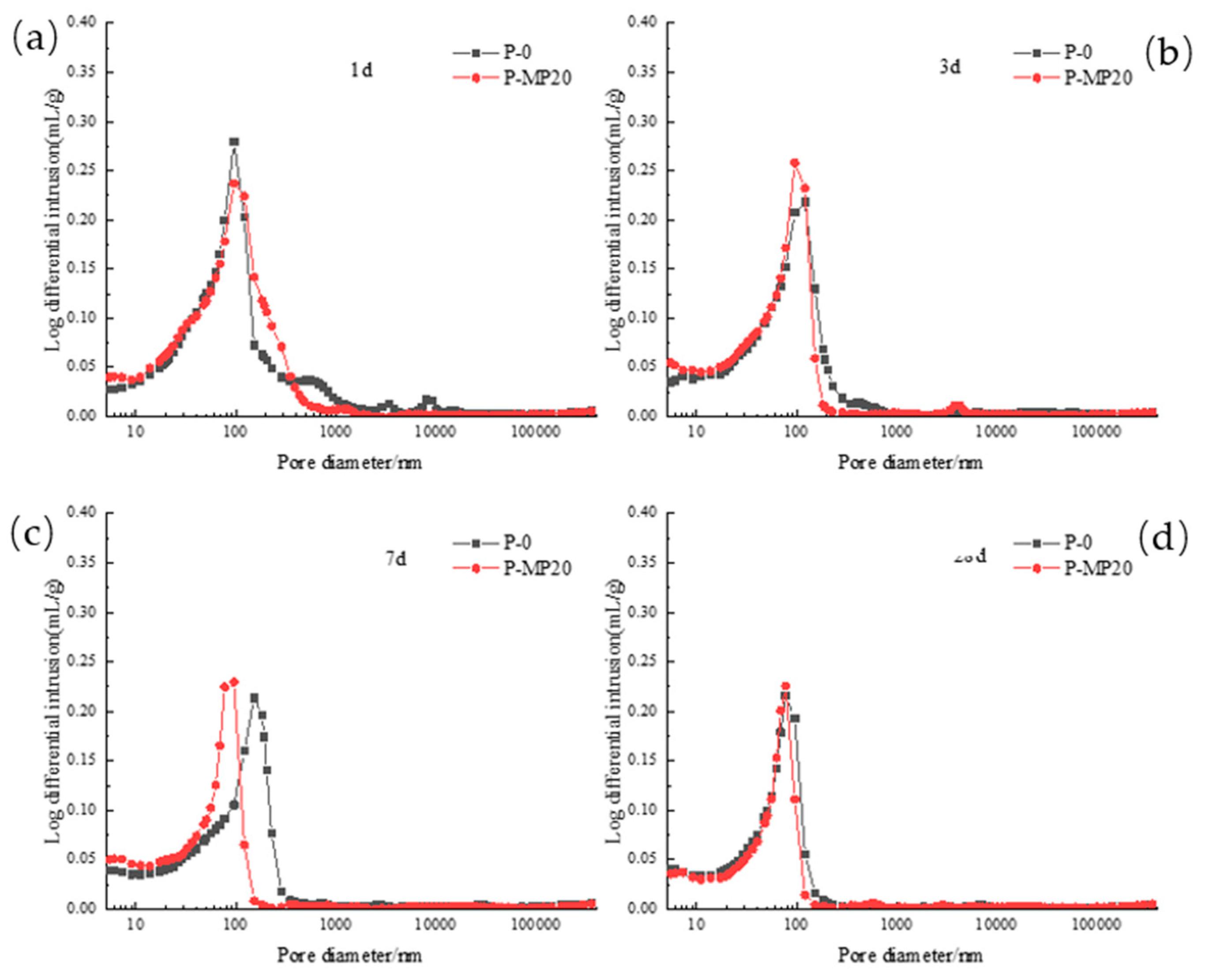
3.2.2. Pore Diameter Distribution
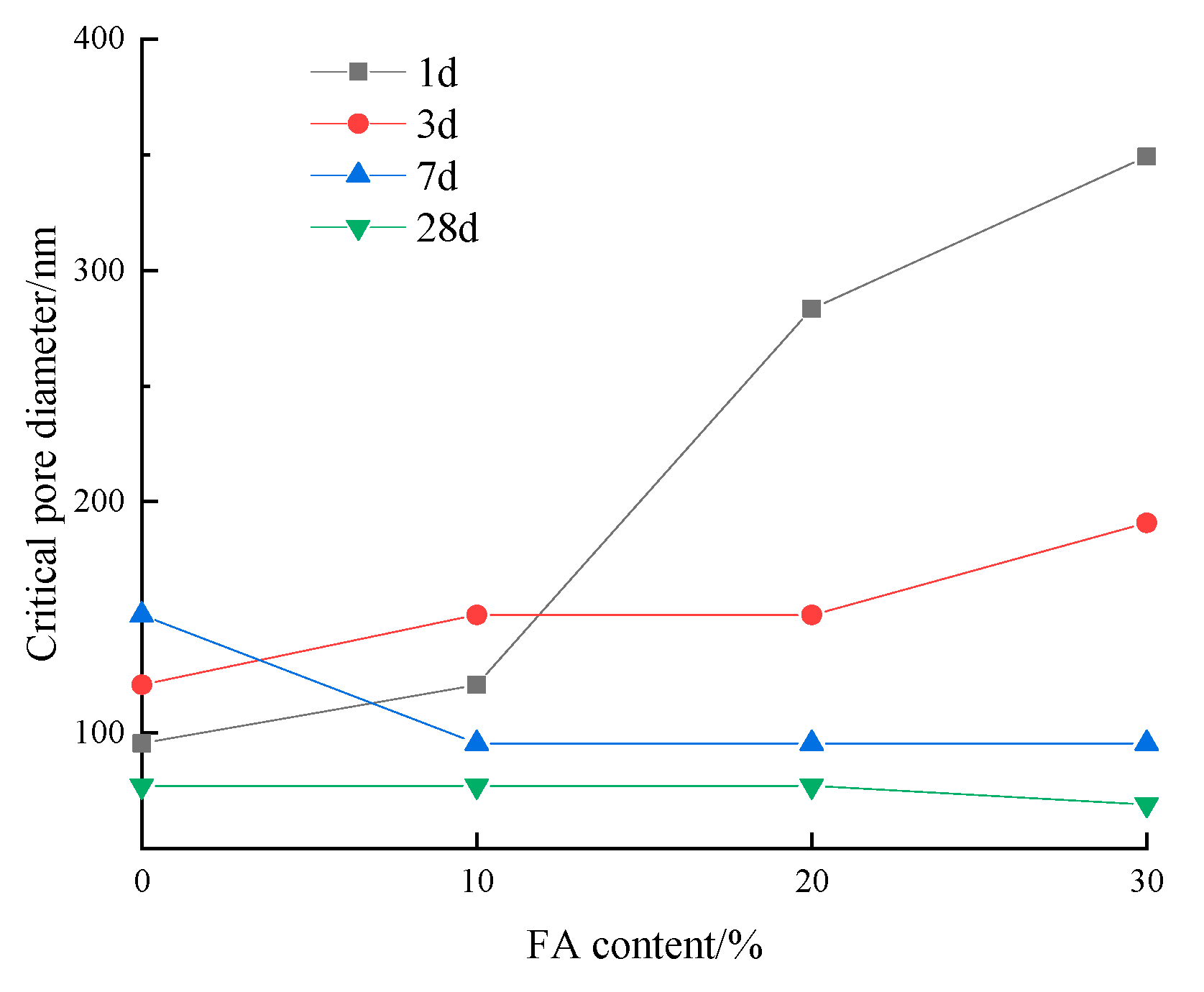
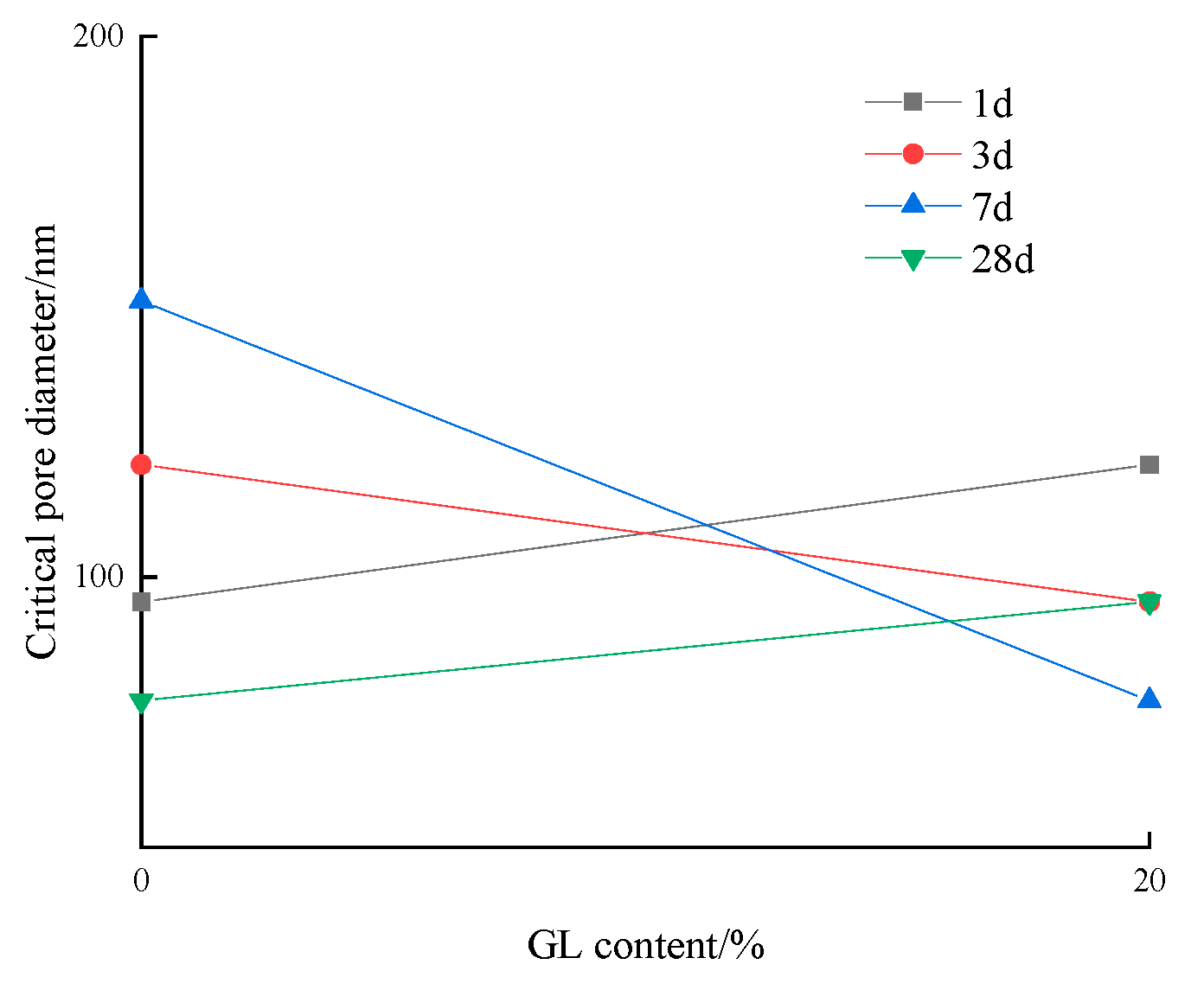
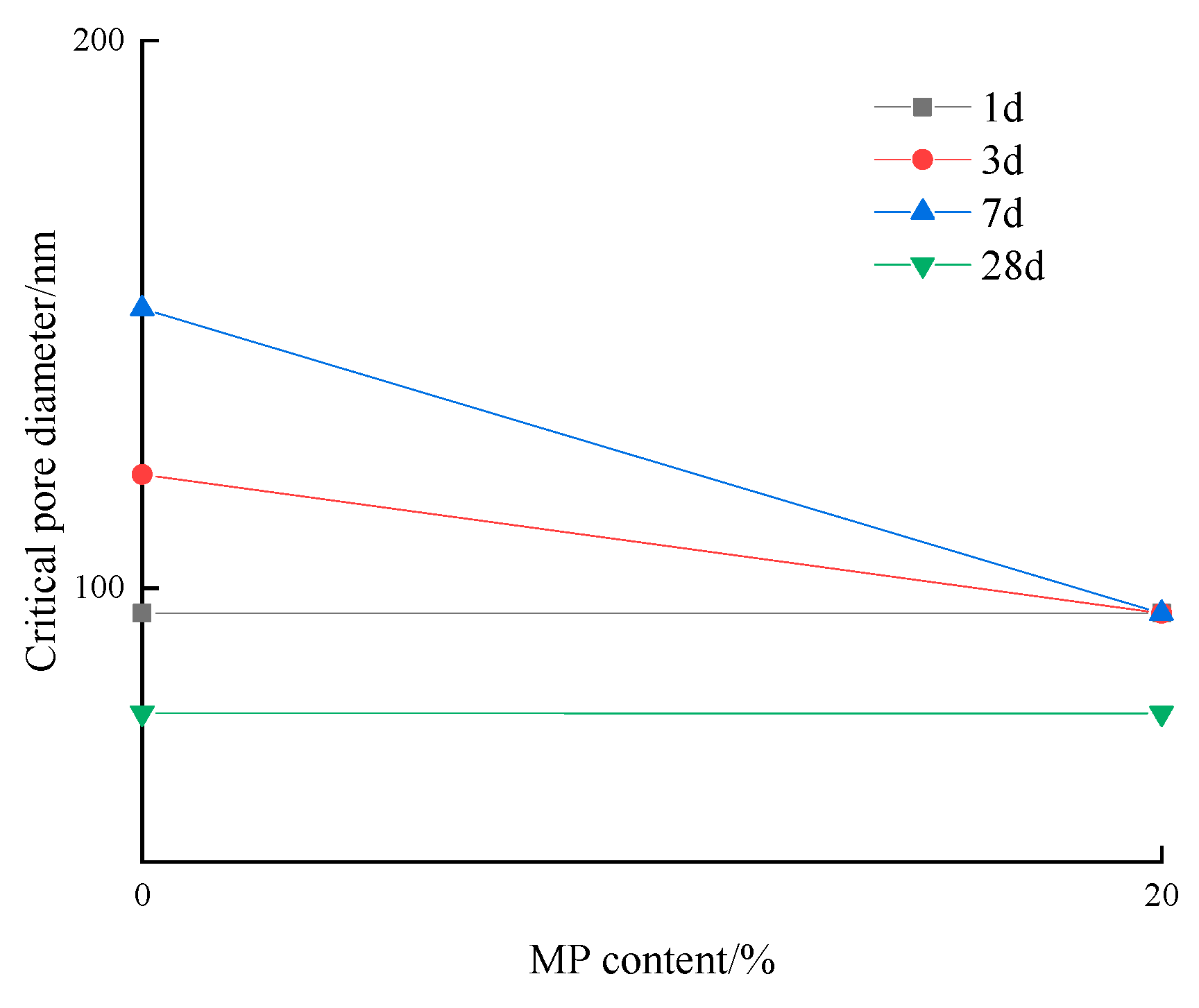
3.2.3. Critical Pore Diameter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, M. Research on the realization path of carbon peak and carbon neutralization in China’s cement industry. Price Theory Amp. 2021, 4–11+53. (In Chinese) [Google Scholar]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Zhou, S.; Zhao, S.; Guan, X.; Wang, L. Research progress of high strength and low calcium Portland cement. Bull. Chin. Silic. Soc. 2014, 33, 5. (In Chinese) [Google Scholar]
- Qu, M.; Chang, H.; Liu, J.; Jin, Z. The influence of different factors on the pure diffusion of chloride ions in concrete. Concrete 2021, 9, 6. (In Chinese) [Google Scholar]
- Wang, L.; Wang, J. Meso-scale numerical simulation of chloride ion diffusion process in concrete. J. Build. Struct. 2008, S1, 5. (In Chinese) [Google Scholar]
- Wang, J.; Zhang, J.; Guo, Y.; Zhou, T. The influence mechanism of different factors on the resistance of concrete to chloride ion permeability. Concrete 2018, 8, 5. (In Chinese) [Google Scholar]
- Long, G.; Chen, S.; Xie, Y. Influencing factors of chloride ion diffusion in mortar. J. Build. Mater. 2008, 11, 6. (In Chinese) [Google Scholar]
- Wang, X.; Cui, S.; Yan, B.; Liu, C.; Ma, G.; Ye, X.; Shi, H. Study on chloride diffusivity of cement-based cementitious materials. Cement 2009, 2, 7. (In Chinese) [Google Scholar]
- Xiao, J.; Guo, M.; Wang, D.; Yin, Z.; Li, Y. Study on chloride ion curing performance of portland cement based on different cation conditions. Bull. Chin. Silic. Soc. 2016, 35, 6. (In Chinese) [Google Scholar]
- Huang, Z.; Li, T. Study on the binding characteristics of chloride ions in ultra-high performance concrete matrix. J. Railw. Sci. Eng. 2016, 13, 7. (In Chinese) [Google Scholar]
- Dhir, R.K.; El-Mohr, M.A.K.; Dyer, T.D. Developing chloride resisting concrete using PFA. Cem. Concr. Res. 1997, 11, 1633–1639. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Yan, X.; Song, Z.; Guo, M.; Cao, C. Role of swelling agent and set-controlling admixtures on chloride binding and diffusion in cement matrix. Constr. Build. Mater. 2020, 230, 117009. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Y.; Geng, J.; Sun, J.; Li, P. Study on chloride ion curing properties of cement-based materials by mineral admixtures and CLDH. J. Xi’an Univ. Archit. Technol. 2019, 51, 6. (In Chinese) [Google Scholar]
- Zhang, S.; Sha, X.; Zong, L. The effect of fly ash and slag on the performance of mortar in chloride environment. Bull. Chin. Silic. Soc. 2017, 36, 893–898. (In Chinese) [Google Scholar]
- Xiao, J.; Wang, J.; Guo, M.; Wang, D.; Zuo, S. Study on chloride ion concentration performance of cement-limestone slurry. Bull. Chin. Silic. Soc. 2017, 36, 7. (In Chinese) [Google Scholar]
- Zhou, D.; Qi, S.; Liu, T.; Gong, H.; Zhou, A.; Pei, H. Diffusion properties of chloride ions in mortar under multi-ion solution immersion environment. J. Chin. Ceram. Soc. 2020, 48, 1817–1823. (In Chinese) [Google Scholar]
- Geng, Y.; Sun, C.; Sun, M.; Zhang, Y.; Duan, J. Review on chloride binding mechanism and influencing factors of cement-based materials. Bull. Chin. Silic. Soc. 2022, 41, 2604–2617. (In Chinese) [Google Scholar]
- Du, J.; Tang, Z.; Li, G.; Yang, H.; Li, L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Li, P.; Su, D.; Wang, S.; Fan, Z. Influence of binder composition and concrete pore structure on chloride diffusion coefficient in concrete. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 160–164. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 3, 247–253. [Google Scholar] [CrossRef]
- Marinescu, M.; Brouwers, J. Chloride Binding Related to Hydration Products Part I: Ordinary Portland Cement; RILEM Book Series; RILEM: Champs-sur-Marne, France, 2012; Volume 3, pp. 125–131. [Google Scholar]
- Zhang, W.; Zhang, J.; Ye, J.; Qian, J.; Shen, W.; Wang, Z. The structure and activity of dicalcium silicate. J. Chin. Ceram. Soc. 2019, 47, 1663–1669. (In Chinese) [Google Scholar]
- Yang, Z.; Gao, Y.; Mu, S.; Chang, H.; Sun, W.; Jiang, J. Improving the chloride binding capacity of cement paste by adding nano-Al2O3. Constr. Build. Mater. 2019, 195, 415–422. [Google Scholar] [CrossRef]
- Hao, J.; Song, Y.; Wang, Z.; Wang, B. Effect of AFm anion type on stability of ettringite in sulphoaluminate cement hydration products. J. Chin. Ceram. Soc. 2019, 47, 1554–1558. (In Chinese) [Google Scholar]
- Maes, M.; De Belie, N. Resistance of concrete and mortar against combined attack of chloride and sodium sulphate. Cem. Concr. Compos. 2014, 53, 59–72. [Google Scholar] [CrossRef]



| Chemicals | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Loss |
|---|---|---|---|---|---|---|---|
| LC | 19.10 | 8.13 | 3.82 | 63.66 | 1.18 | 2.64 | 1.47 |
| C | 21.91 | 5.53 | 4.41 | 65.77 | 0.81 | 0.15 | 1.42 |
| FA | 49.4 | 39.81 | 1.86 | 4.17 | 0.78 | 0.65 | 3.33 |
| GL | 0.81 | 0.14 | 0.03 | 98.22 | 0.47 | 0.01 | 0.32 |
| MP | 32.20 | 17.84 | 0.30 | 37.94 | 7.81 | 1.56 | 2.35 |
| Minerals | C3S | C2S | C3A | C4AF | C4A3$ | C$ | C12A7 | C2AS | C$H2 |
|---|---|---|---|---|---|---|---|---|---|
| LC | 41.58 | 24.14 | 6.05 | 10.62 | 4.83 | 9.00 | 0.22 | 0.23 | |
| C | 50.1 | 26.55 | 7.29 | 12.05 | 4 |
| Sample | LC | C | FA | GL | MP | W/C |
|---|---|---|---|---|---|---|
| P-0 | 100 | 0 | 0 | 0 | 0 | 0.4 |
| P-1 | 0 | 100 | 0 | 0 | 0 | 0.4 |
| P-FA10 | 90 | 0 | 10 | 0 | 0 | 0.4 |
| P-FA20 | 80 | 0 | 20 | 0 | 0 | 0.4 |
| P-FA30 | 70 | 0 | 30 | 0 | 0 | 0.4 |
| P-GL10 | 90 | 0 | 0 | 10 | 0 | 0.4 |
| P-GL20 | 80 | 0 | 0 | 20 | 0 | 0.4 |
| P-GL30 | 70 | 0 | 0 | 30 | 0 | 0.4 |
| P-MP10 | 90 | 0 | 0 | 0 | 10 | 0.4 |
| P-MP20 | 80 | 0 | 0 | 0 | 20 | 0.4 |
| P-MP30 | 70 | 0 | 0 | 0 | 30 | 0.4 |
| mol/L | ||
|---|---|---|
| No. | NaCl | NaSO4 |
| 1 | 0.5128 | 0 |
| 2 | 0.5128 | 0.1408 |
| 3 | 0.5128 | 0.2816 |
| 4 | 0.5128 | 0.4224 |
| No. | Cb(mg/g) |
|---|---|
| P-0 | 4.70 |
| P-1 | 4.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guo, M.; Liu, C.; Lv, Z.; Xiang, T.; Zhang, S.; Chen, D. Chloride Binding Behavior and Pore Structure Characteristics of Low-Calcium High-Strength Cement Pastes. Materials 2024, 17, 3129. https://doi.org/10.3390/ma17133129
Wang Z, Guo M, Liu C, Lv Z, Xiang T, Zhang S, Chen D. Chloride Binding Behavior and Pore Structure Characteristics of Low-Calcium High-Strength Cement Pastes. Materials. 2024; 17(13):3129. https://doi.org/10.3390/ma17133129
Chicago/Turabian StyleWang, Ziwei, Minglei Guo, Chunlin Liu, Zhong Lv, Tengfei Xiang, Shunquan Zhang, and Depeng Chen. 2024. "Chloride Binding Behavior and Pore Structure Characteristics of Low-Calcium High-Strength Cement Pastes" Materials 17, no. 13: 3129. https://doi.org/10.3390/ma17133129
APA StyleWang, Z., Guo, M., Liu, C., Lv, Z., Xiang, T., Zhang, S., & Chen, D. (2024). Chloride Binding Behavior and Pore Structure Characteristics of Low-Calcium High-Strength Cement Pastes. Materials, 17(13), 3129. https://doi.org/10.3390/ma17133129






