High-Emissivity Double-Layer ZrB2-Modified Coating on Flexible Aluminum Silicate Fiber Fabric with Enhanced Oxidation Resistance and Tensile Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
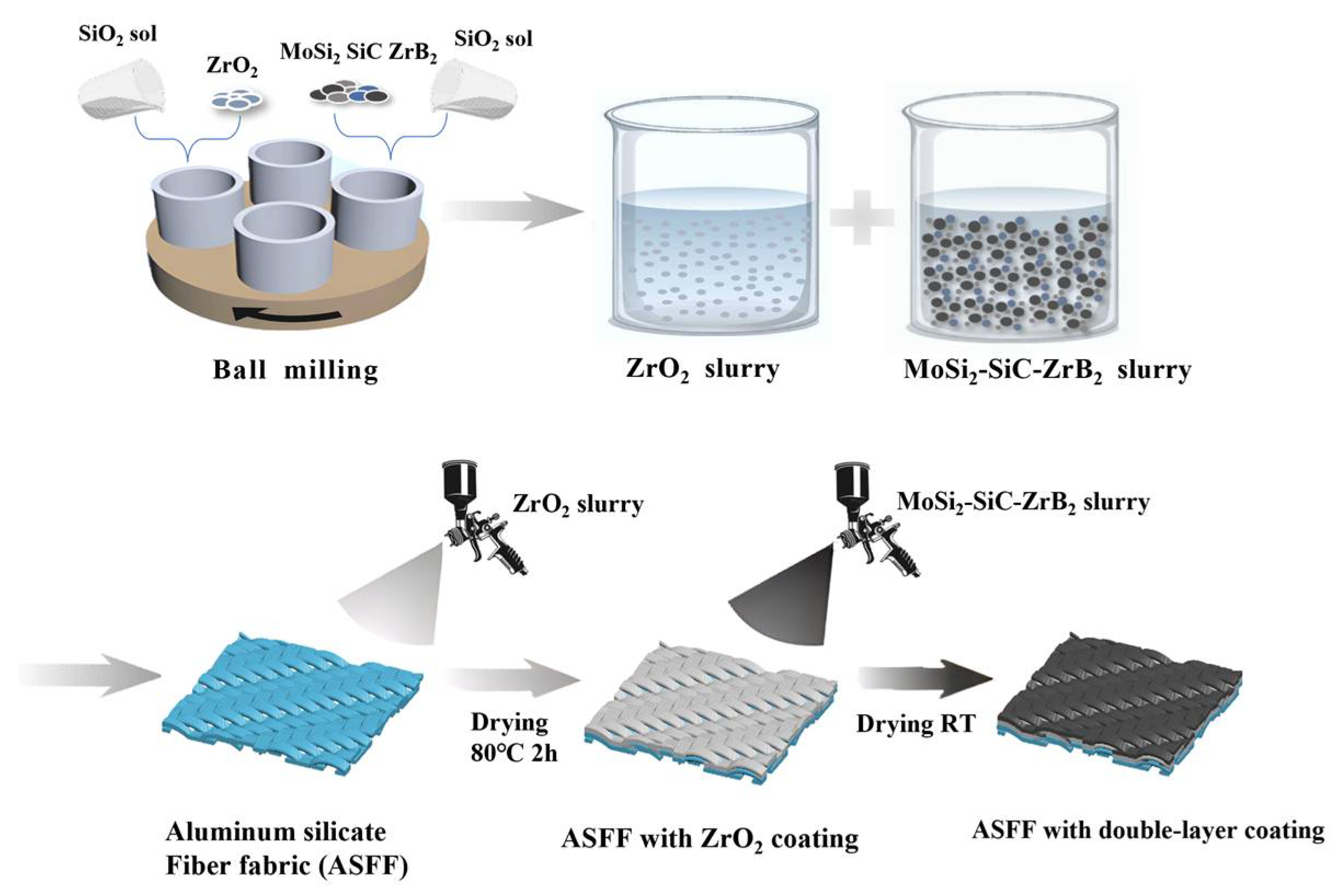
2.2. Preparation of the Double-Layer Coating
2.3. Characterization and Testing
3. Results and Discussion
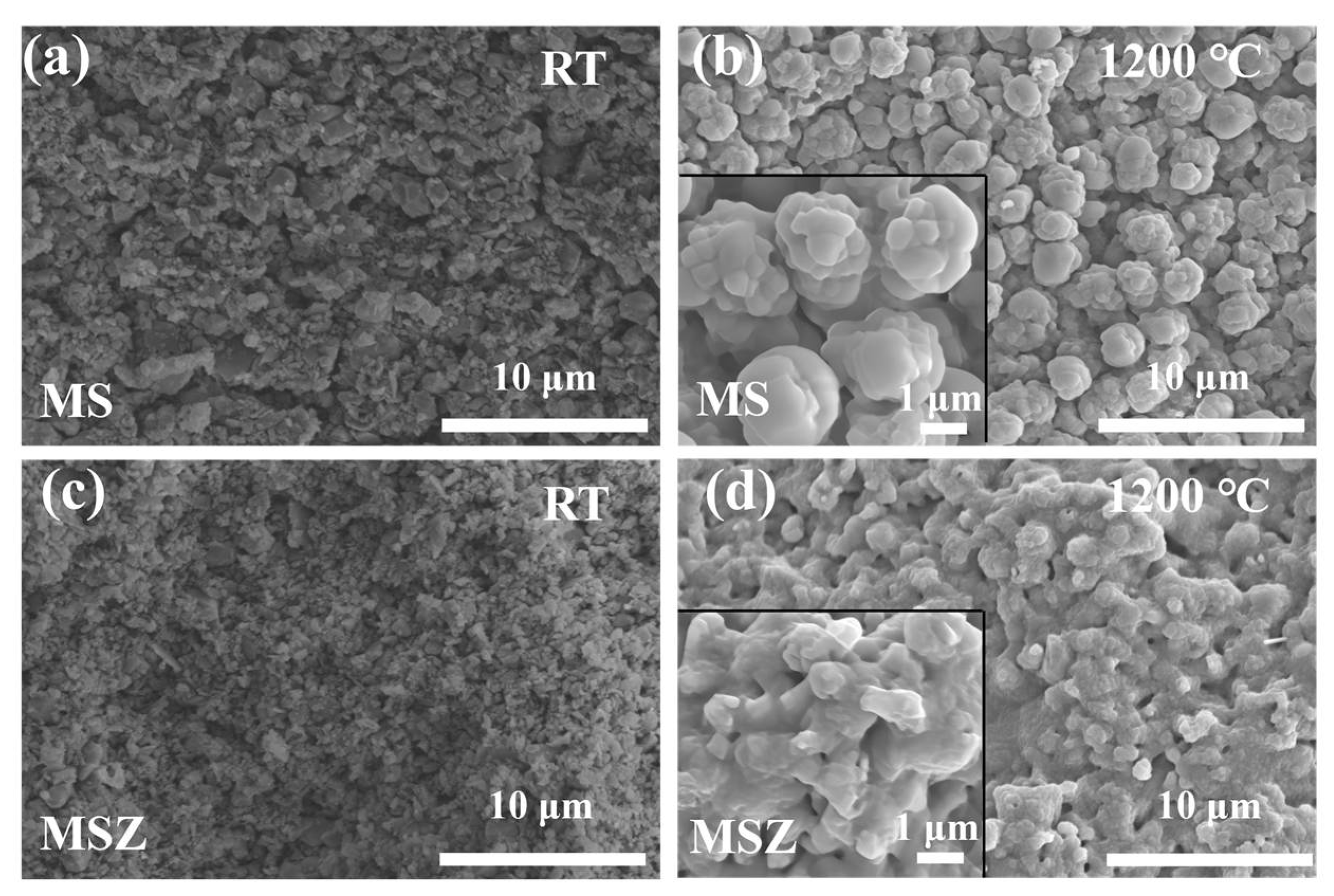
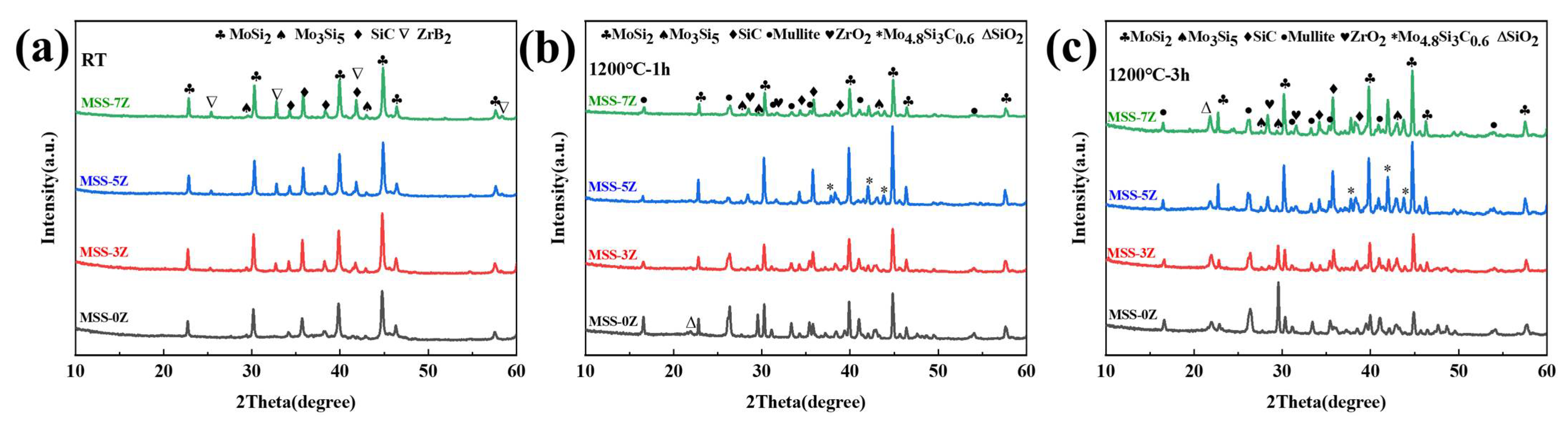
3.1. SEM Images and XRD Spectra of MoSi2–SiC–ZrB2 Powders after High-Temperature Heat Treatment
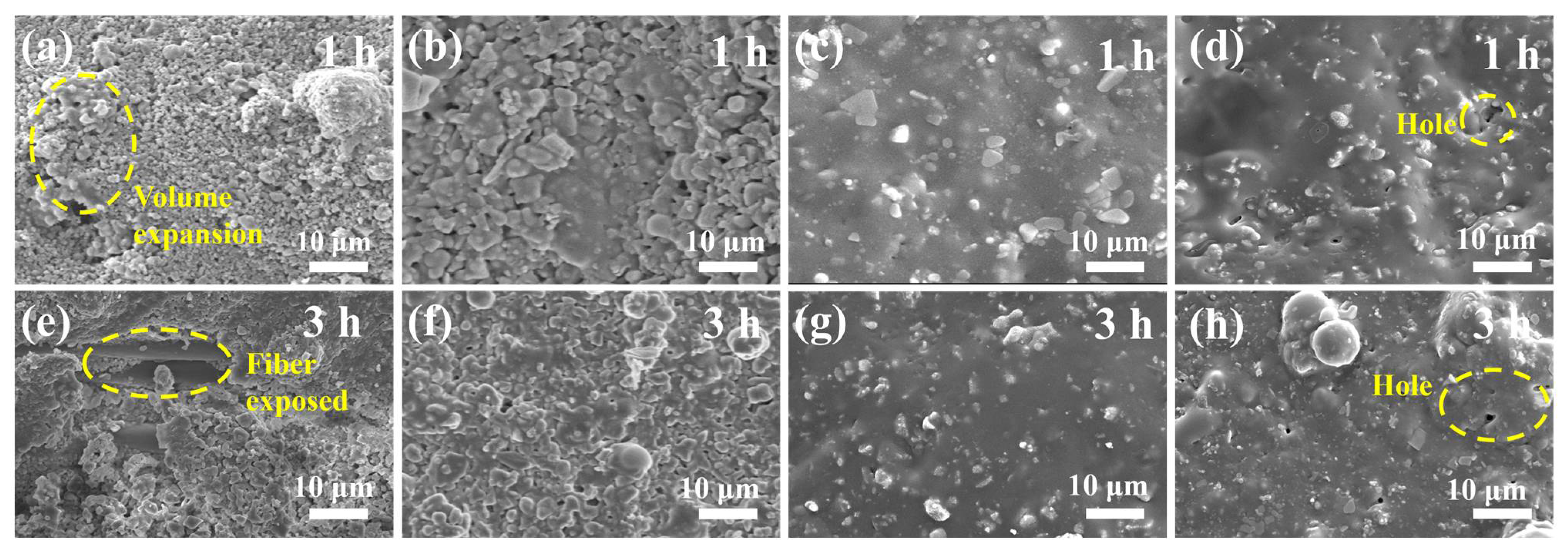
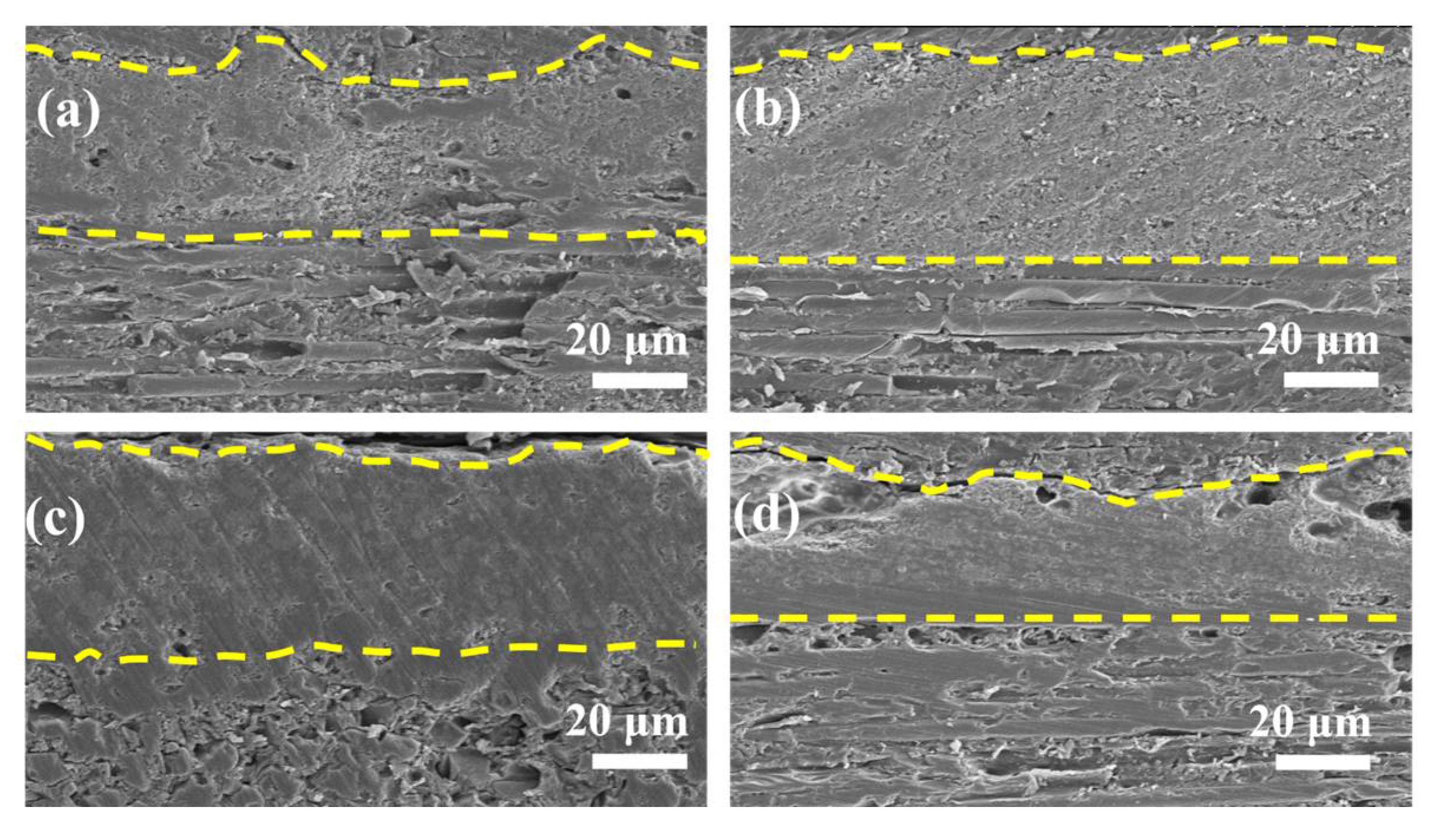
3.2. Morphology and XRD Spectra of Single-Layer MoSi2–SiC–ZrB2 Coating and Double-Layer Coatings after Heat Treatment at 1200 °C for Different Times
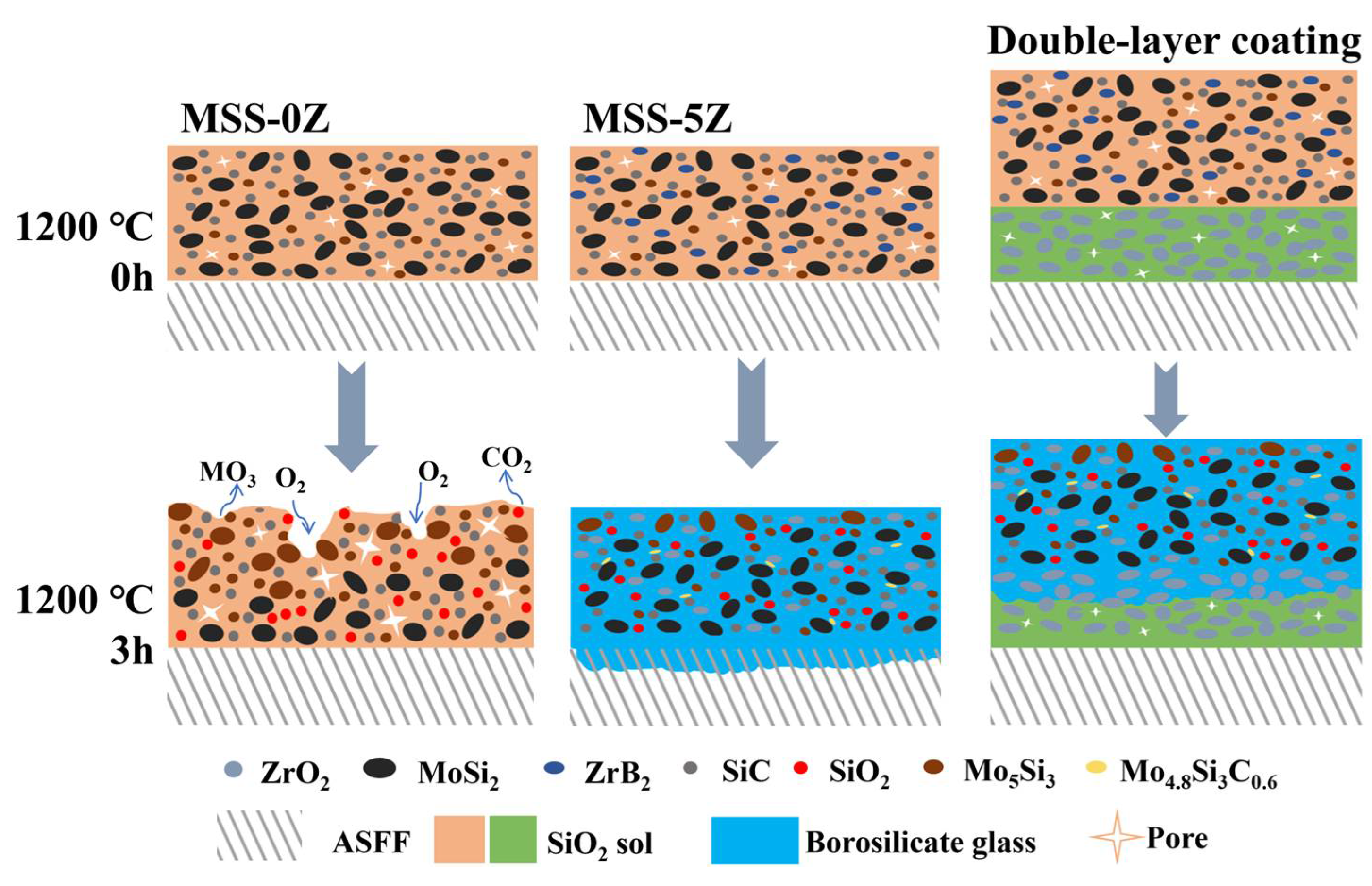
3.3. Oxidation Reaction and Mechanism of Fillers in Coatings at High Temperatures
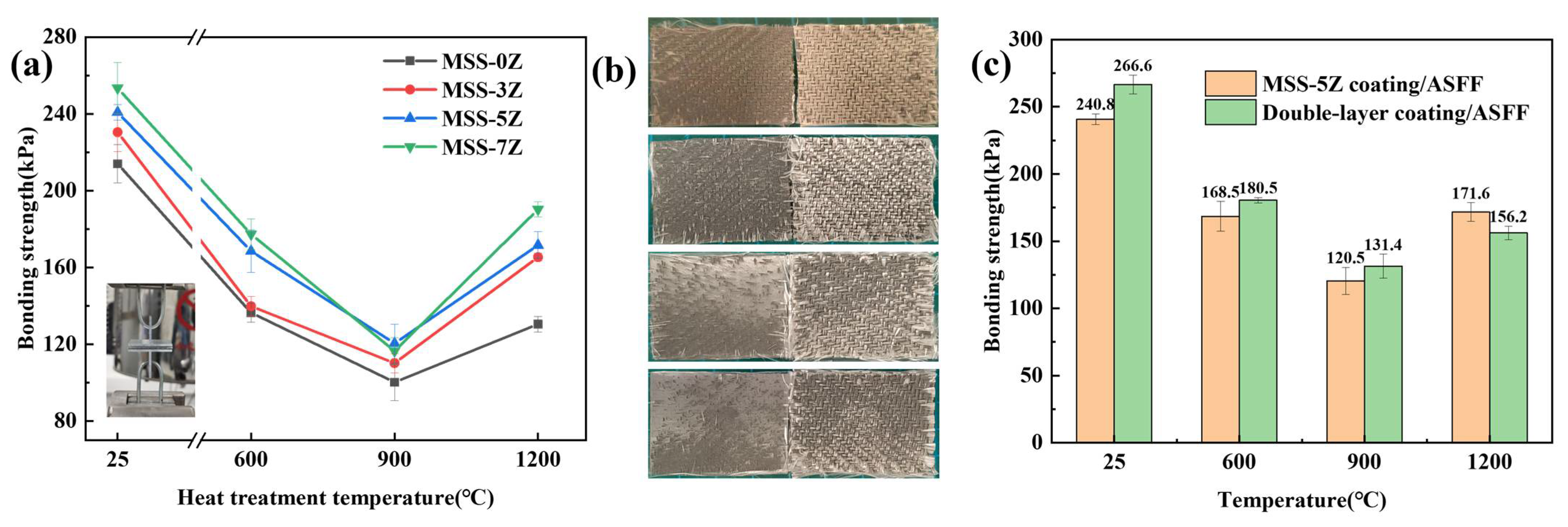
3.4. Analysis of Mechanical Properties of Single-Layer and Double-Layer Coatings after Heat Treatment at Different Temperatures
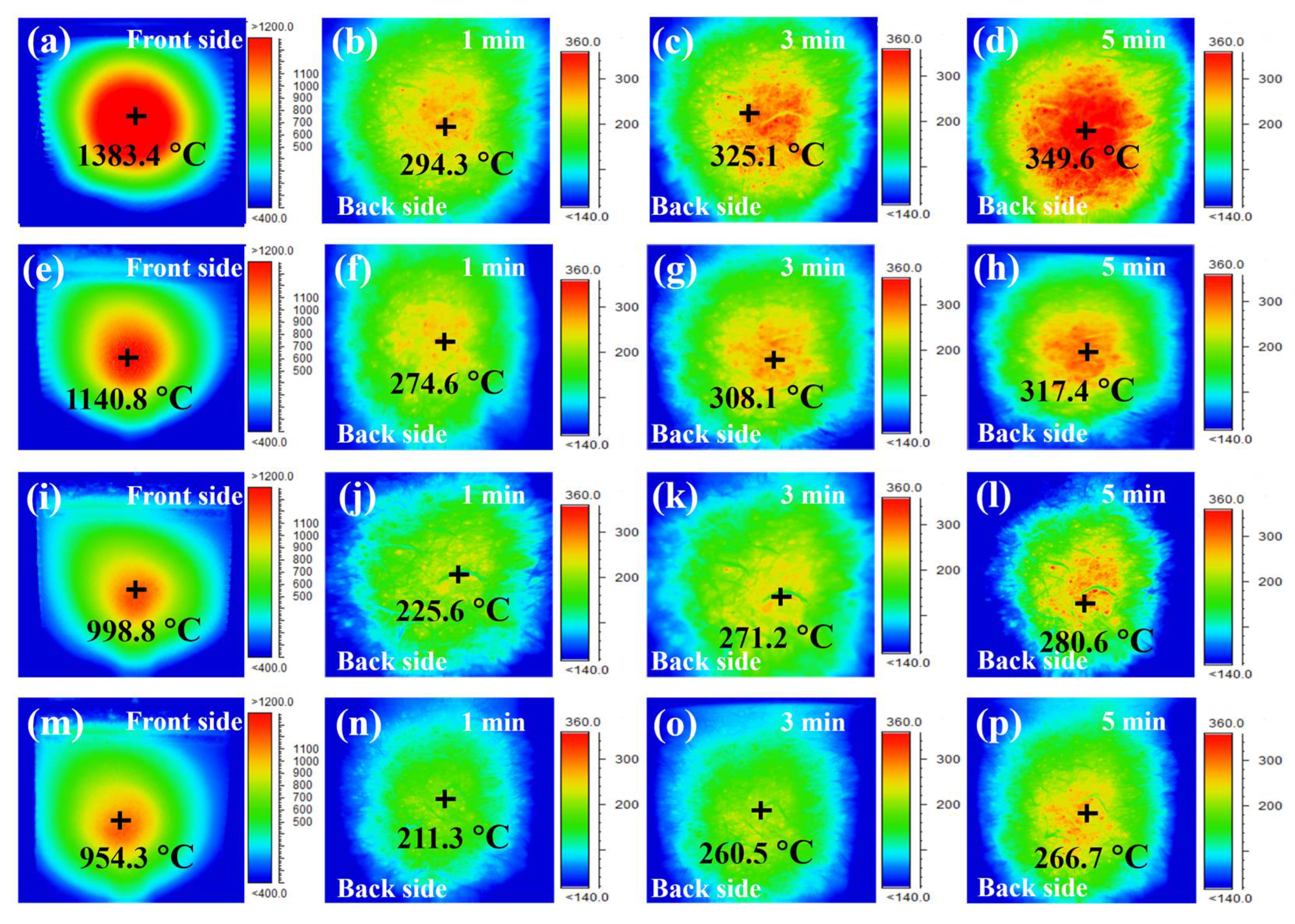
3.5. Comparison of Thermal Insulation Performance between Single-Layer and Double-Layer Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, W.; Wang, T.; Luo, W.; Du, Y.; Ye, L.; Song, R.; Cui, H.; Zhao, T.; Yang, W.; Dai, Z.; et al. Synthesis and Characterization of High-Purity, High-Entropy Diboride Ceramic Powders by a Liquid Phase Method. Materials 2023, 16, 7431. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, Z.; Zhai, C.; Zhang, J.; Li, Z.; Zhang, H.; Xu, X. Low-temperature in-situ grown mullite whiskers toughened heat-resistant inorganic adhesive. J. Alloys Compd. 2020, 836, 155349. [Google Scholar] [CrossRef]
- Yang, N.; Cai, G.; Wan, Y.; Zhang, R.; Li, J.; Zhang, J.; Zhang, H.; Liu, H.; Yu, X.; Wang, M. High anti-ablative epoxy resin-based flame retardant and thermal insulation coating based on spontaneous Ceramization and vitrification. Ceram. Int. 2024, 50, 24233–24251. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Yan, H.; Li, J.; Yin, X.; Sun, J.; Fu, Q.; Riedel, R. Microstructure and evolution of hafnium carbide whiskers via polymer-derived ceramics: A novel formation mechanism. J. Adv. Ceram. 2023, 12, 578–586. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Guo, J.; Meng, X.; Zhang, P.; Fan, F.; Qu, Y.; Gao, F. High wave transmittance and low thermal conductivity Y–α-SiAlON porous ceramics for high-temperature radome applications. J. Adv. Ceram. 2023, 12, 1273–1287. [Google Scholar] [CrossRef]
- Cai, G.; Wan, Y.; Liu, J.; Yang, N.; Guo, J.; Li, J.; Zhou, Y.; Zhang, J.; Zhang, H.; Wang, M. Preparation and performance analysis of methyl-silicone resin-modified epoxy resin-based intumescent flame retardant thermal insulation coating. J. Micromech. Mol. Phys. 2023, 08, 61–82. [Google Scholar] [CrossRef]
- Wang, M.; Bu, F.; Zhou, C.; Zhou, Q.; Wei, T.; Liu, J.; Zhai, W. Bonding performance and mechanism of a heat-resistant composite precursor adhesive (RT-1000°C) for TC4 titanium alloy. J. Micromech. Mol. Phys. 2020, 5, 2050016. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, M.; Lu, R.; Xu, W.; Zhang, T.; Wei, T.; Zhang, J.; Liao, Y. A composite structural high-temperature-resistant adhesive based on in-situ grown mullite whiskers. Mater. Today Commun. 2020, 23, 100944. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J. Preparation and Elasticity & Thermal Insulation Properties of Flexible Fiber Blankets. Mater. Rev. 2023, 37, 21030042. [Google Scholar] [CrossRef]
- Piacquadio, S.; Pridöhl, D.; Henkel, N.; Bergström, R.; Zamprotta, A.; Dafnis, A.; Schröder, K.U. Comprehensive Comparison of Different Integrated Thermal Protection Systems with Ablative Materials for Load-Bearing Components of Reusable Launch Vehicles. Aerospace 2023, 10, 319. [Google Scholar] [CrossRef]
- Li, J.F.; Luo, Z.P. Fabrication and performances of preceramic polymer-based high-temperature High emissivity coating. J. Eur. Ceram. Soc. 2020, 40, 5217–5225. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Z.; Yan, S.; Tao, X.; Zou, Y.; Li, J.; Zhou, X.; Zhang, H. The preparation and property analysis of B4C modified inorganic amorphous aluminum phosphates-based intumescent flame retardant coating. Constr. Build. Mater. 2022, 359, 129480. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Ran, N.; Zheng, J.Q.; Huang, Z.R.; Liu, X.J.; Chen, Z.M. High infrared emissivity of SiC-AlN ceramics at room temperature. J. Eur. Ceram. Soc. 2020, 40, 3528–3534. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Li, J.; Liu, J.; Wang, M.; Tao, X. High Emissivity MoSi2-SiC-Al2O3 Coating on Rigid Insulation Tiles with Enhanced Thermal Protection Performance. Materials 2024, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Astapov, A.N.; Potanin, A.Y.; Loginov, P.A.; Shvyndina, N.V.; Eganova, E.M.; Tarasova, A.N.; Levashov, E.A. The effect of Ta on the kinetics and mechanisms of high-temperature oxidation of the (Hf,Ta)B2-SiC ceramics. Corros. Sci. 2024, 227, 111721. [Google Scholar] [CrossRef]
- Astapov, A.N.; Matulyak, A.I. Reaction Synthesis for Producing MoSi2-Based Coatings. Russ. Metall. 2022, 2022, 1569–1577. [Google Scholar] [CrossRef]
- Sajdak, M.; Kornaus, K.; Zientara, D.; Moskala, N.; Komarek, S.; Momot, K.; Golis, E.; Zych, L.; Gubernat, A. Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics. Crystals 2024, 14, 212. [Google Scholar] [CrossRef]
- Guo, L.L.; Hu, X.X.; Tao, X.; Du, H.Y.; Guo, A.R.; Liu, J.C. Low-temperature oxidation resistance of the silica-coated MoSi2 powders prepared by sol-gel preoxidation method. Ceram. Int. 2020, 46, 23471–23478. [Google Scholar] [CrossRef]
- Gao, Q.C.; Yan, L.W.; Mu, Y.; Xue, Y.J.; Xu, X.J.; Guo, A.R.; Du, H.Y.; Dong, S.; Liu, J.C. TaSi2-SiC high-emissivity coating modified by SiB6 on flexible alumina fibre fabric with enhanced oxidation resistance and high interfacial bonding strength. Ceram. Int. 2023, 49, 12643–12652. [Google Scholar] [CrossRef]
- Shao, G.F.; Wu, X.D.; Cui, S.; Shen, X.D.; Kong, Y.; Lu, Y.C.; Jiao, C.R.; Jiao, J. High emissivity MoSi2-ZrO2-borosilicate glass multiphase coating with SiB6 addition for fibrous ZrO2 ceramic. Ceram. Int. 2016, 42, 8140–8150. [Google Scholar] [CrossRef]
- Zhang, G.L.; Xue, Y.J.; Liu, P.S.; Guo, A.R.; Du, H.Y.; Yan, L.W.; Liu, J.C. High emissivity double-layer coating on the flexible aluminum silicate fiber fabric with enhanced interfacial bonding strength and high temperature resistance. J. Eur. Ceram. Soc. 2021, 41, 1452–1458. [Google Scholar] [CrossRef]
- Grigoriev, O.; Neshpor, I.; Vedel, D.; Mosina, T.; Silvestroni, L. Influence of chromium diboride on the oxidation resistance of ZrB2-MoSi2 and ZrB2-SiC ceramics. J. Eur. Ceram. Soc. 2021, 41, 2207–2214. [Google Scholar] [CrossRef]
- Sedighi, A.; Adeli, M.; Soltanieh, M. Investigation of the effect of SiC additions on the high-temperature oxidation behavior of combustion-synthesized MoSi2. J. Mater. Res. Technol. 2024, 30, 187–196. [Google Scholar] [CrossRef]
- Grigoriev, O.N.; Vinokurov, V.B.; Silvestroni, L. ZrB2-MoSi2 ceramics: Kinetics of compaction during hot pressing in CO environment, phase interactions at interfaces and static fatigue. J. Eur. Ceram. Soc. 2024, 44, 2811–2820. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.; Sytchenko, A.; Pogozhev, Y.; Vorotilo, S.; Orekhov, A.; Loginov, P.; Levashov, E. Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target. Materials 2021, 14, 1932. [Google Scholar] [CrossRef] [PubMed]
- Azzali, N.; Meucci, M.; Di Rosa, D.; Mercatelli, L.; Silvestroni, L.; Sciti, D.; Sani, E. Spectral emittance of ceramics for high temperature solar receivers. Sol. Energy 2021, 222, 74–83. [Google Scholar] [CrossRef]
- Pellegrini, C.; Balat-Pichelin, M.; Rapaud, O.; Bêche, E. Oxidation resistance of Zr- and Hf-diboride composites containing SiC in air plasma up to 2600 K for aerospace applications. Ceram. Int. 2022, 48, 2177–2190. [Google Scholar] [CrossRef]
- Vedel, D.V.; Grigoriev, O.N.; Mazur, P.V.; Osipov, A.E. Structure, Strength, and Oxidation Resistance of Ultrahigh-Temperature ZrB2-SiC-WC Ceramics. Powder Metall. Met. Ceram. 2021, 60, 60–68. [Google Scholar] [CrossRef]
- Li, X.F.; Feng, J.Z.; Jiang, Y.G.; Lin, H.; Feng, J. Preparation and anti-oxidation performance of Al2O3-containing TaSi2-MoSi2-borosilicate glass coating on porous SiCO ceramic composites for thermal protection. RSC Adv. 2018, 8, 13178–13185. [Google Scholar] [CrossRef]
- Cai, G.; Wu, J.; Guo, J.; Wan, Y.; Zhou, Q.; Zhang, P.; Yu, X.; Wang, M. A Novel Inorganic Aluminum Phosphate-Based Flame Retardant and Thermal Insulation Coating and Performance Analysis. Materials 2023, 16, 4498. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Yang, N.; Hou, Y.; Chen, D.; Liu, J.; Cai, G.; Wang, M. The Effect of Different Diluents and Curing Agents on the Performance of Epoxy Resin-Based Intumescent Flame-Retardant Coatings. Materials 2024, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Meng, G.H.; Chen, L.; Li, G.R.; Liu, M.J.; Zhang, W.X.; Zhao, L.N.; Zhang, Q.; Zhang, X.D.; Wan, C.L.; et al. Progress in ceramic materials and structure design toward advanced thermal barrier coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Wang, T.C.; Zhang, Z.H.; Dai, C.H.; Li, Q.; Li, Y.C.; Kong, J.; Wong, C.P. Amorphous silicon and silicates-stabilized ZrO2 hollow fiber with low thermal conductivity and high phase stability derived from a cogon template. Ceram. Int. 2019, 45, 7120–7126. [Google Scholar] [CrossRef]
- Wang, M.C.; Liu, J.X.; Chen, Z.L.; Hu, X.X.; Zhai, W.Z.; Tao, X.; Liu, J.C. Joining zirconia with nickel-based superalloys for extreme applications by using a pressure-free high-temperature resistant adhesive. Ceram. Int. 2022, 48, 8025–8030. [Google Scholar] [CrossRef]
- Zhang, T.; Song, X.; Qi, G.; An, B.; Dong, W.; Zhao, Y.; Wang, Z.; Yi, X.; Yuan, Z.; Zhao, Y.; et al. Investigation of High-Temperature Normal Infrared Spectral Emissivity of ZrO2 Thermal Barrier Coating Artefacts by the Modified Integrated Blackbody Method. Materials 2022, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Wu, Y.; Feng, Z.; Li, J.; Feng, Z.; Zhang, Q.; Wang, M. The preparation and properties of an alloying modified ceramic-precursor high-temperature resistant adhesive for joining zirconia ceramics and titanium-based superalloys. Mater. Today Commun. 2022, 33, 104353. [Google Scholar] [CrossRef]
- Aribuga, D.; Karaahmet, O.; Balci-Çagiran, Ö.; Çiçek, B. Effect of Al2O3 and ZrO2 Filler Material on the Microstructural, Thermal and Dielectric Properties of Borosilicate Glass-Ceramics. Micromachines 2023, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.I.; Al Saad, A.; Seren, M.H.; Ko, J.Y.P.; Nygren, K.E.; Trice, R.W.; Sangid, M.D. Residual elastic strain and survivability of stabilized zirconia coated carbon-carbon (C/C) composite. Surf. Coat. Technol. 2023, 470, 129811. [Google Scholar] [CrossRef]
- Al Saad, A.; Martinez, C.; Trice, R.W. Ablation performance of rare earth oxide (REO)-stabilized tetragonal and cubic zirconia coatings as a thermal protection system (TPS) for carbon/carbon composites. J. Eur. Ceram. Soc. 2023, 43, 6449–6460. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, G.H.; Zhao, S.M.; Meng, H.J.; Xie, Z. A fused glass coating for oxidation protection of Mo-W-ZrO2 cermet. Surf. Coat. Technol. 2015, 261, 189–194. [Google Scholar] [CrossRef]
- Ren, Y.; Qian, Y.H.; Xu, J.J.; Jiang, Y.; Zuo, J.; Li, M.S. Oxidation and cracking/spallation resistance of ZrB2-SiC-TaSi2-Si coating on siliconized graphite at 1500 °C in air. Ceram. Int. 2020, 46, 6254–6261. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol-gel coatings. J. Mater. Sci. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Xiao, B.; Li, J.; Hu, Z.; Zhang, R.; Hu, X.; Liu, J.; Cai, G.; Liu, H.; et al. The preparation and performance analysis of zirconium-modified aluminum phosphate-based high-temperature (RT–1500 °C) resistant adhesive for joining alumina in extreme environment. J. Adv. Ceram. 2024. [Google Scholar] [CrossRef]
- GB/T 1447-2005; Fiber-Reinforced Plastics Composites—Determination of Tensile Properties. China Building Materials Federation: Beijing, China, 2005.
- GB/T 5210-2006; Paints and Varnishes—Pull-Off Test for Adhesion. China Petroleum and Chemical Industry Federation: Beijing, China, 2006.
- Zhu, Q.; Shobu, K.; Tani, E.; Kishi, K.; Umebayashi, S. Oxidation behavior of Mo≤5Si3C≤1 and its composites. J. Mater. Sci. 2000, 35, 863–872. [Google Scholar] [CrossRef]
- Guo, W.M.; Xiao, H.N.; Gao, P.Z.; Xie, W.; Li, Q.; Hu, J.L. Investigation of MoSi2 melt infiltrated RSiC and its oxidation behavior. Ceram. Int. 2012, 38, 111–117. [Google Scholar] [CrossRef]
- Mao, J.W.; Ding, S.Y.; Li, Y.J.; Li, S.H.; Liu, F.S.; Zeng, X.; Cheng, X.D. Preparation and investigation of MoSi2/SiC coating with high infrared emissivity at high temperature. Surf. Coat. Technol. 2019, 358, 873–878. [Google Scholar] [CrossRef]
- Shao, G.F.; Wu, X.D.; Kong, Y.; Shen, X.D.; Cui, S.; Guan, X.; Jiao, C.R.; Jiao, J. Microstructure, radiative property and thermal shock behavior of TaSi2-SiO2-borosilicate glass coating for fibrous ZrO2 ceramic insulation. J. Alloys Compd. 2016, 663, 360–370. [Google Scholar] [CrossRef]
- An, Z.; Chunhua, L.U.; Zhongzi, X.U. Research on Chemical Stability of ZrO2-doped Borosilicate Glass and Analysis of Its Structure. Mater. Rev. 2006, 20, 134–136. [Google Scholar] [CrossRef]
- Manara, J.; Arduini-Schuster, M.; Keller, M. Infrared-optical characteristics of ceramics at elevated temperatures. Infrared Phys. Technol. 2011, 54, 395–402. [Google Scholar] [CrossRef]
- Cheng, X.D.; Min, J.; Zhu, Z.Q.; Ye, W.P. Preparation of high emissivity NiCr2O4 powders with a spinel structure by spray drying. Int. J. Miner. Metall. Mater. 2012, 19, 173–178. [Google Scholar] [CrossRef]






| Sample | Composition (wt.%) | ||||
|---|---|---|---|---|---|
| MoSi2 | SiC | ZrB2 | ZrO2 | SiO2-Sol | |
| MSS-0Z | 25 | 25 | 0 | 0 | 50 |
| MSS-3Z | 25 | 25 | 3 | 0 | 47 |
| MSS-5Z | 25 | 25 | 5 | 0 | 45 |
| MSS-7Z | 25 | 25 | 7 | 0 | 43 |
| ZrO2 coating | 0 | 0 | 0 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, X.; Yan, L.; Guo, A.; Du, H.; Liu, J. High-Emissivity Double-Layer ZrB2-Modified Coating on Flexible Aluminum Silicate Fiber Fabric with Enhanced Oxidation Resistance and Tensile Strength. Materials 2024, 17, 3234. https://doi.org/10.3390/ma17133234
Li W, Zhang X, Yan L, Guo A, Du H, Liu J. High-Emissivity Double-Layer ZrB2-Modified Coating on Flexible Aluminum Silicate Fiber Fabric with Enhanced Oxidation Resistance and Tensile Strength. Materials. 2024; 17(13):3234. https://doi.org/10.3390/ma17133234
Chicago/Turabian StyleLi, Wei, Xueying Zhang, Liwen Yan, Anran Guo, Haiyan Du, and Jiachen Liu. 2024. "High-Emissivity Double-Layer ZrB2-Modified Coating on Flexible Aluminum Silicate Fiber Fabric with Enhanced Oxidation Resistance and Tensile Strength" Materials 17, no. 13: 3234. https://doi.org/10.3390/ma17133234






