Structure and Spectral Properties of Er3+-Doped Bismuth Telluride Near-Infrared Laser Glasses
Abstract
:1. Introduction
2. Materials and Methods
3. Results
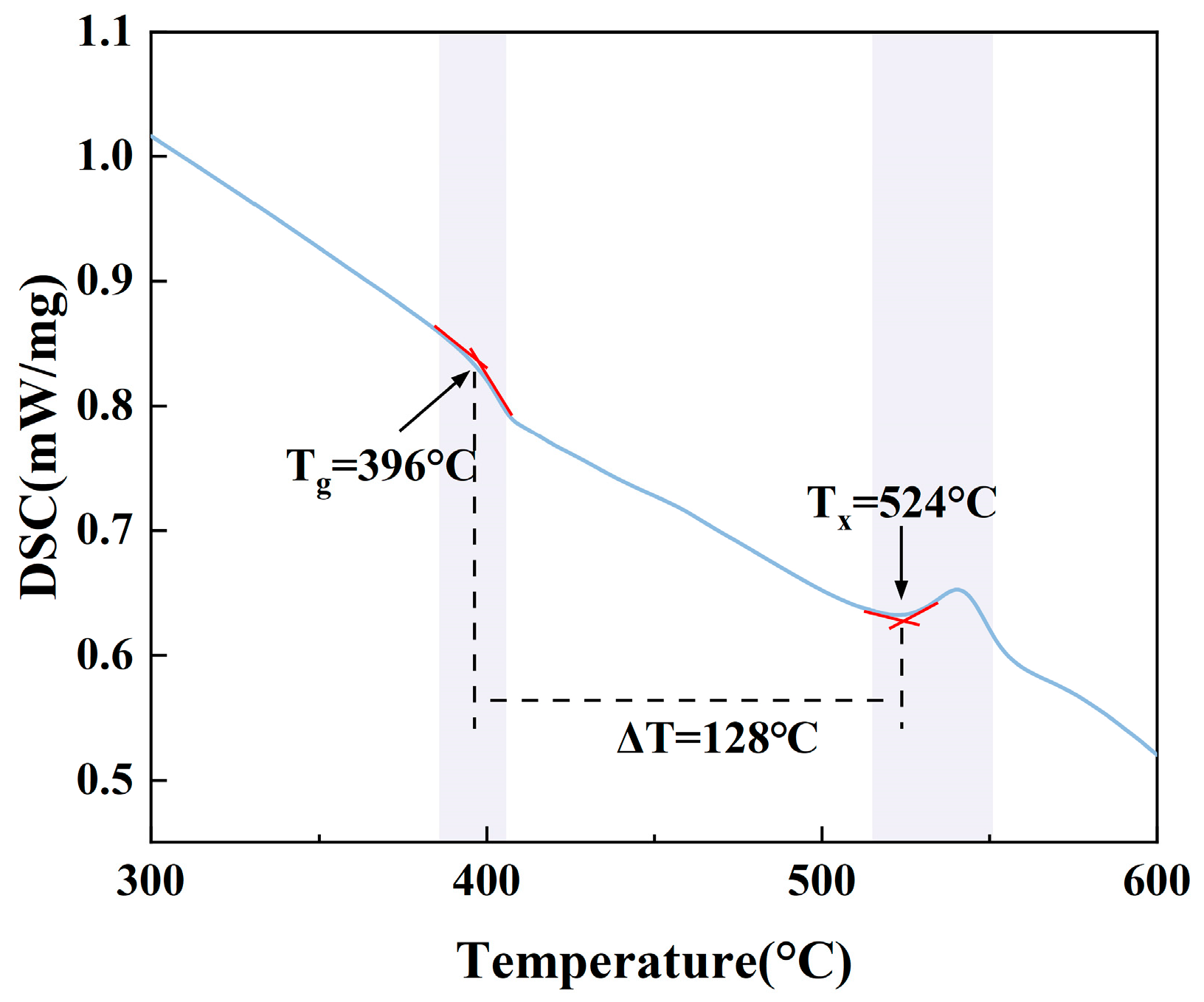
3.1. Thermal Stability
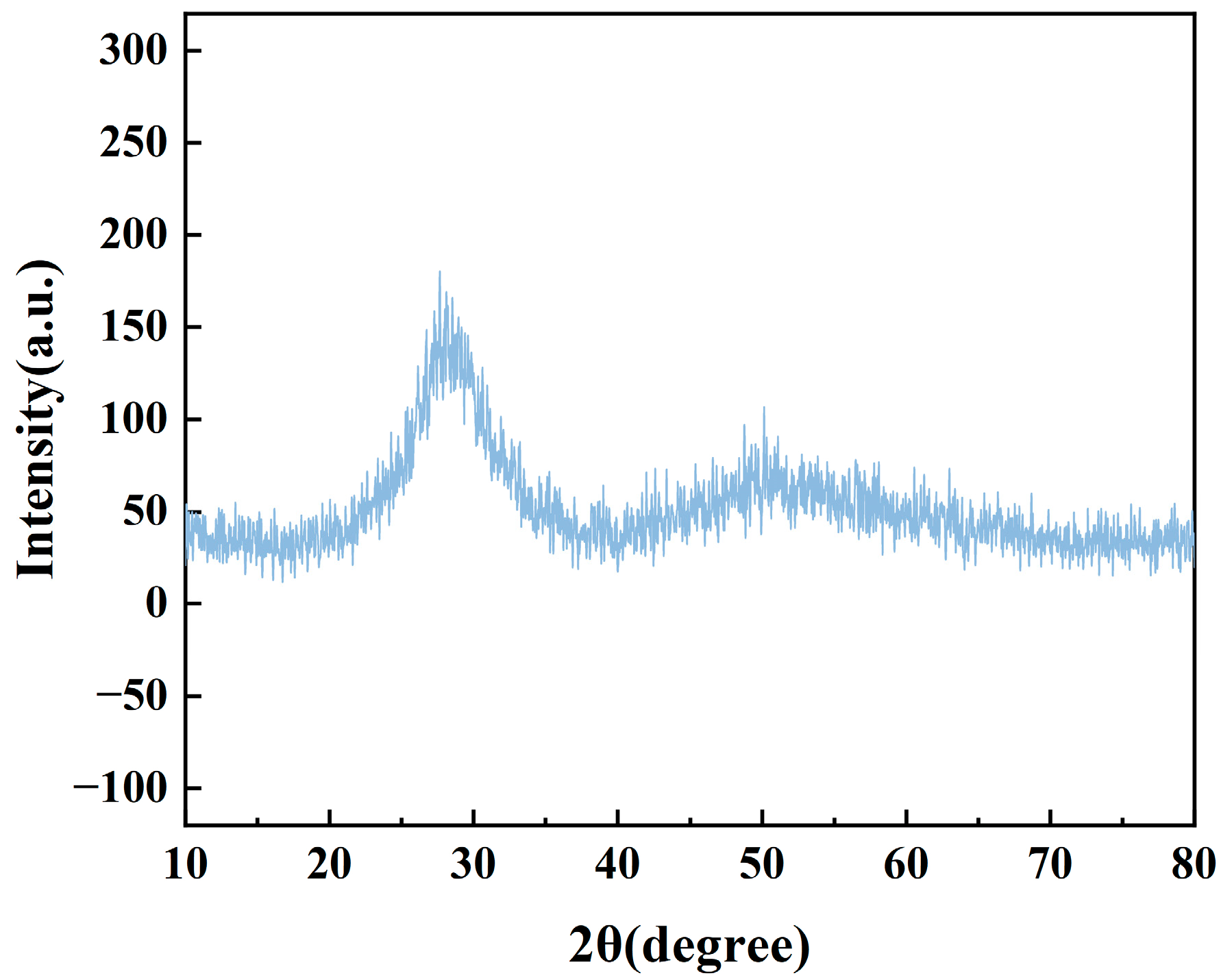
3.2. XRD and Transmission Spectroscopy
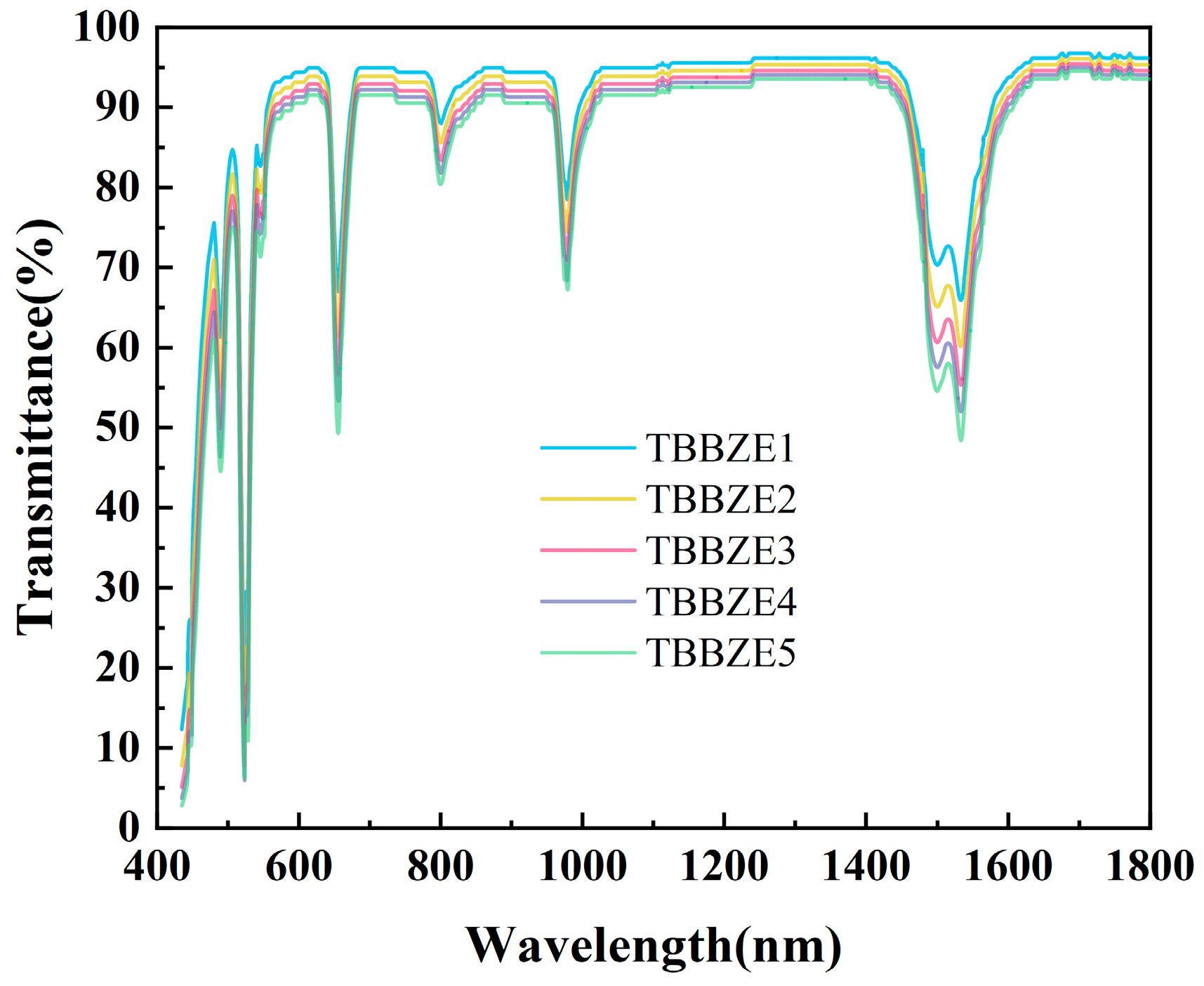
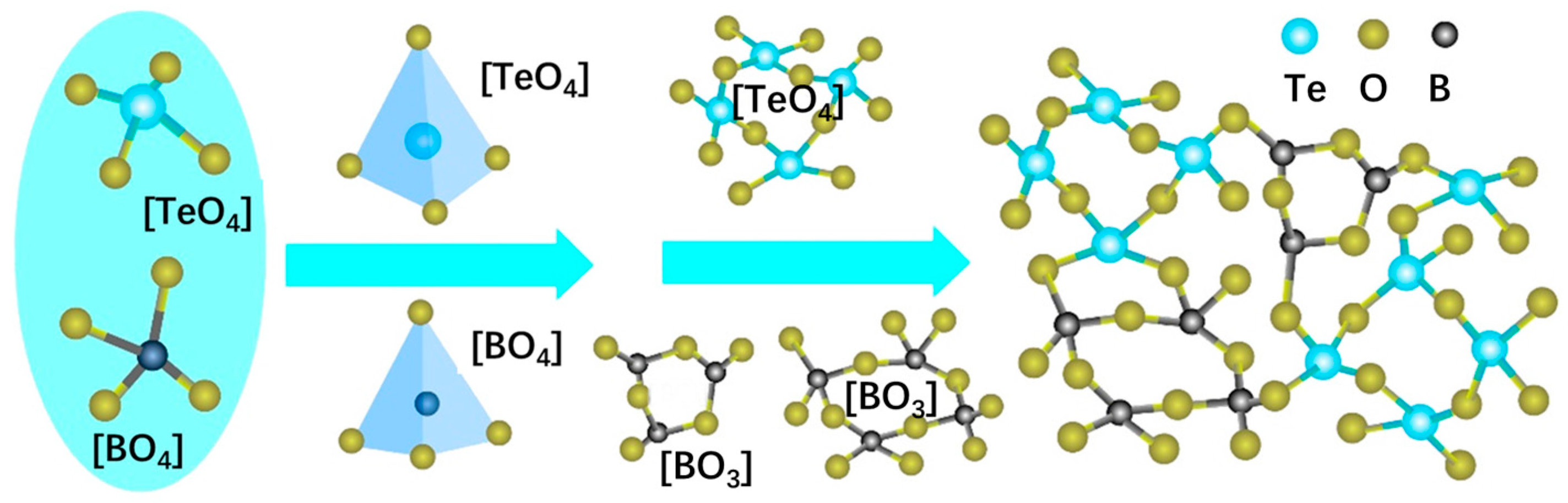
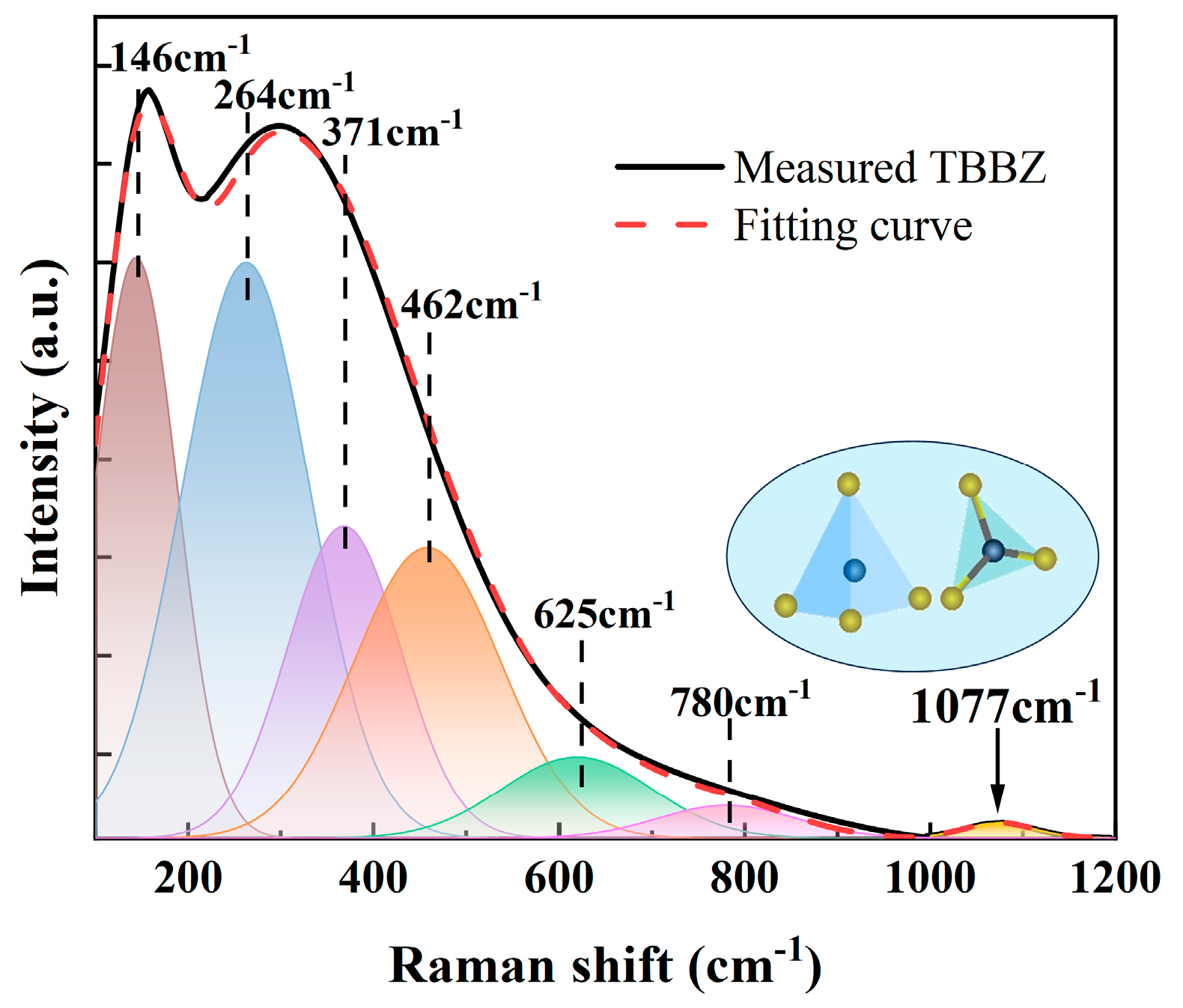
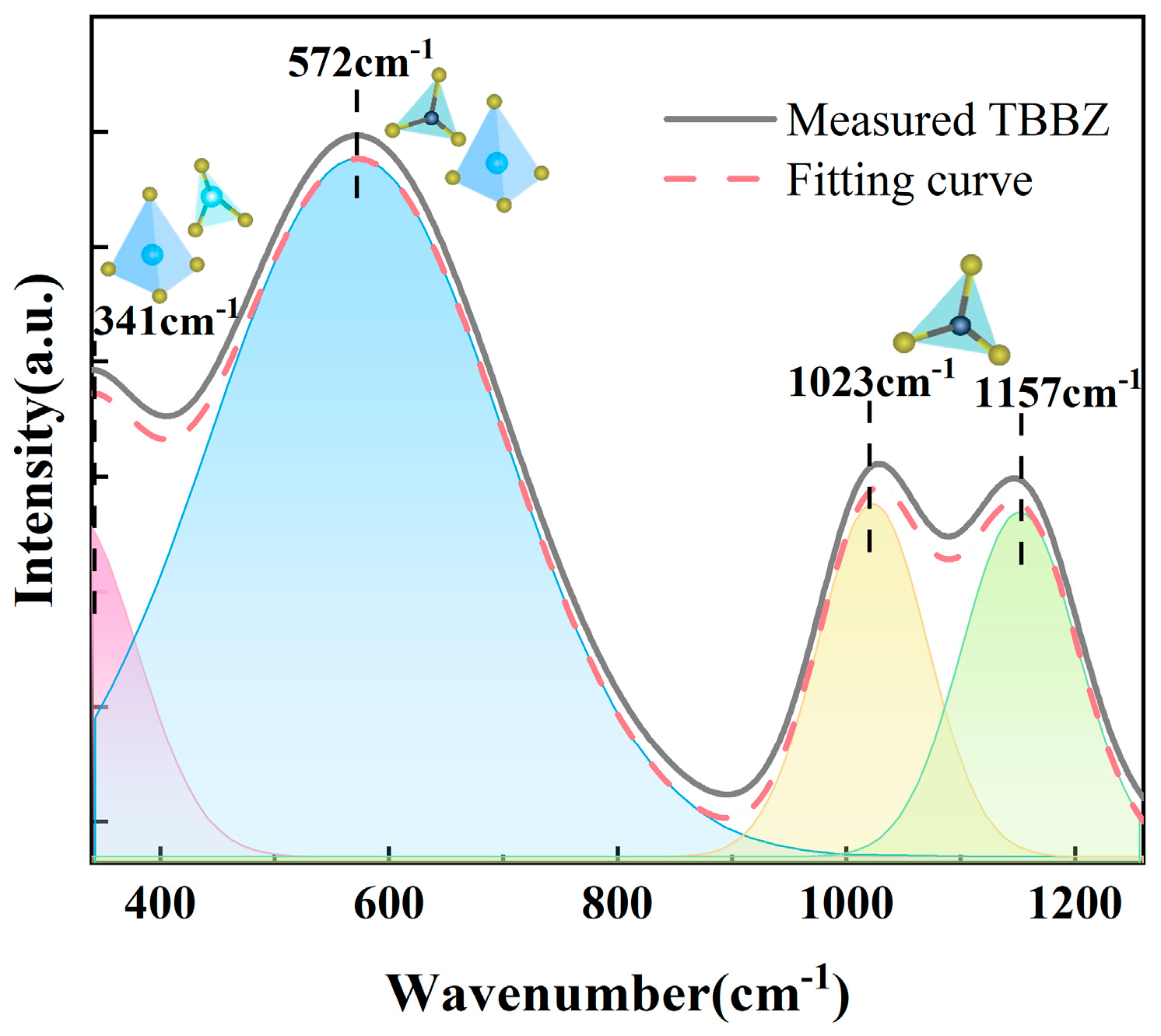
3.3. Raman Spectrum and FT-IR Spectra
3.4. Absorption Spectrum and Judd–Ofelt Analysis
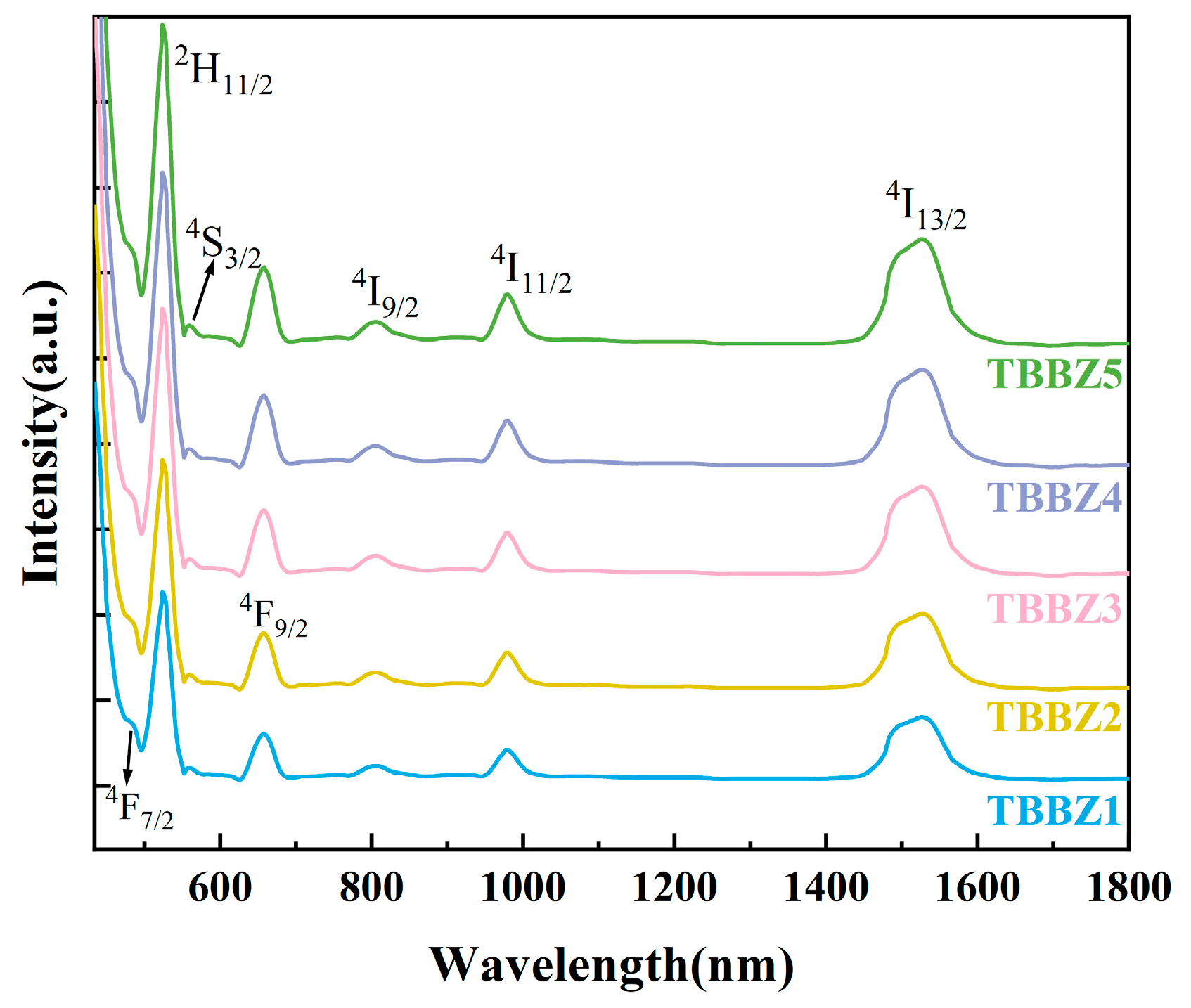
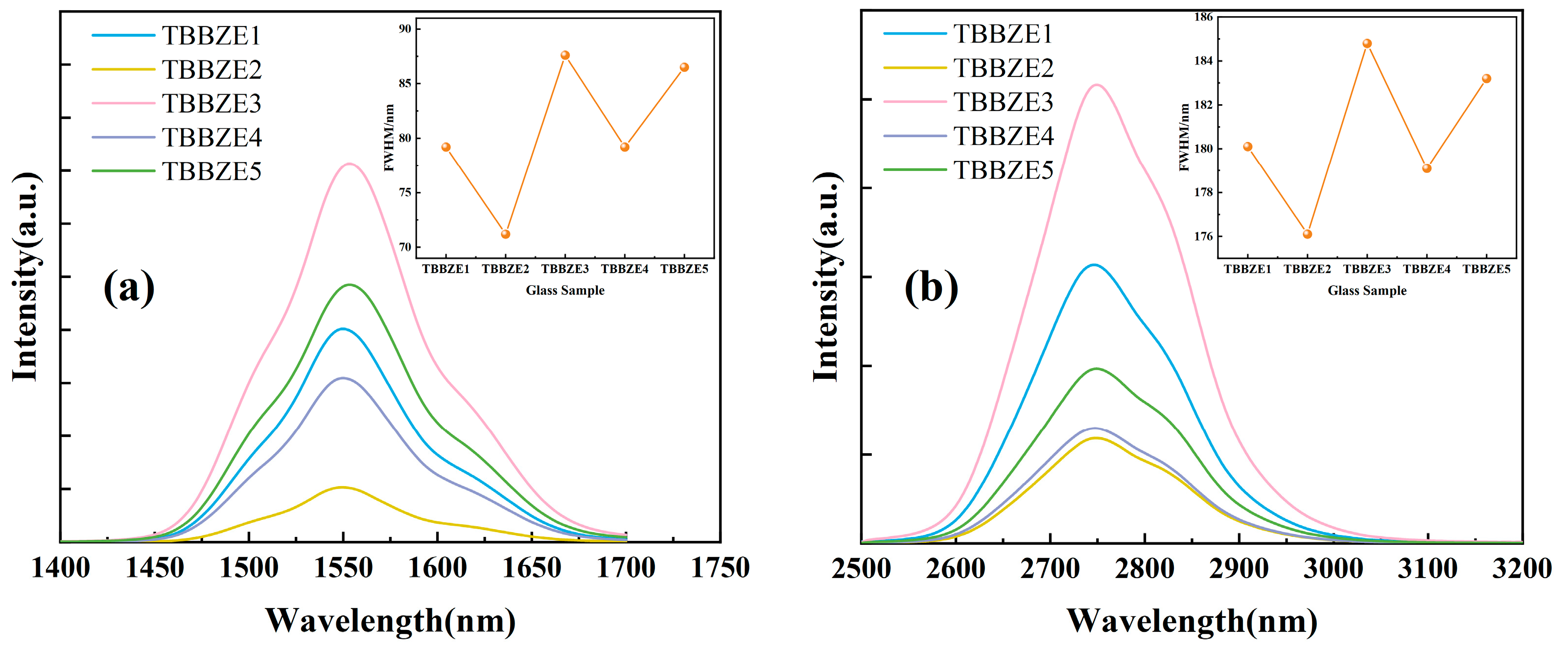
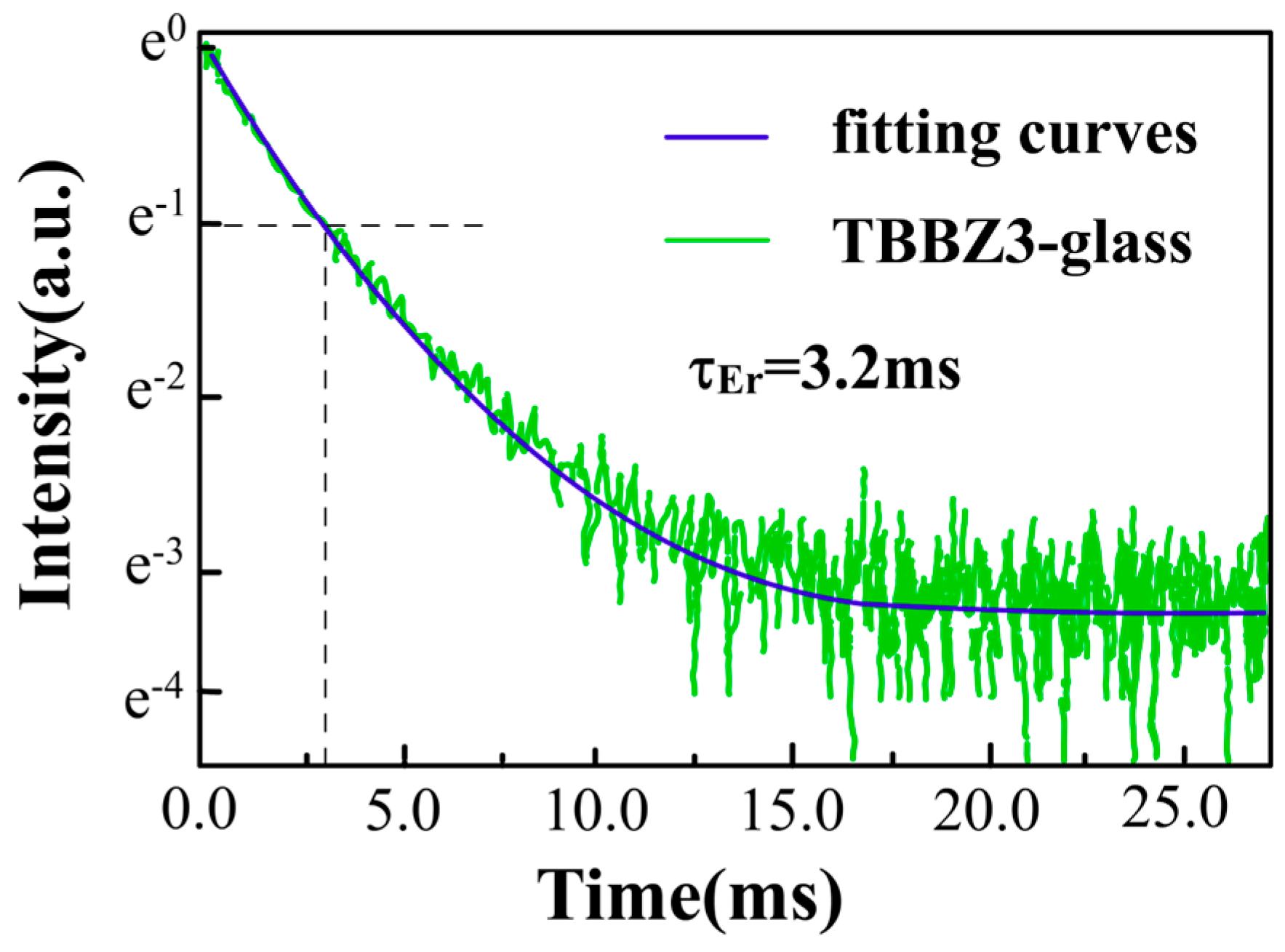
3.5. Fluorescence Spectrum and Lifetime
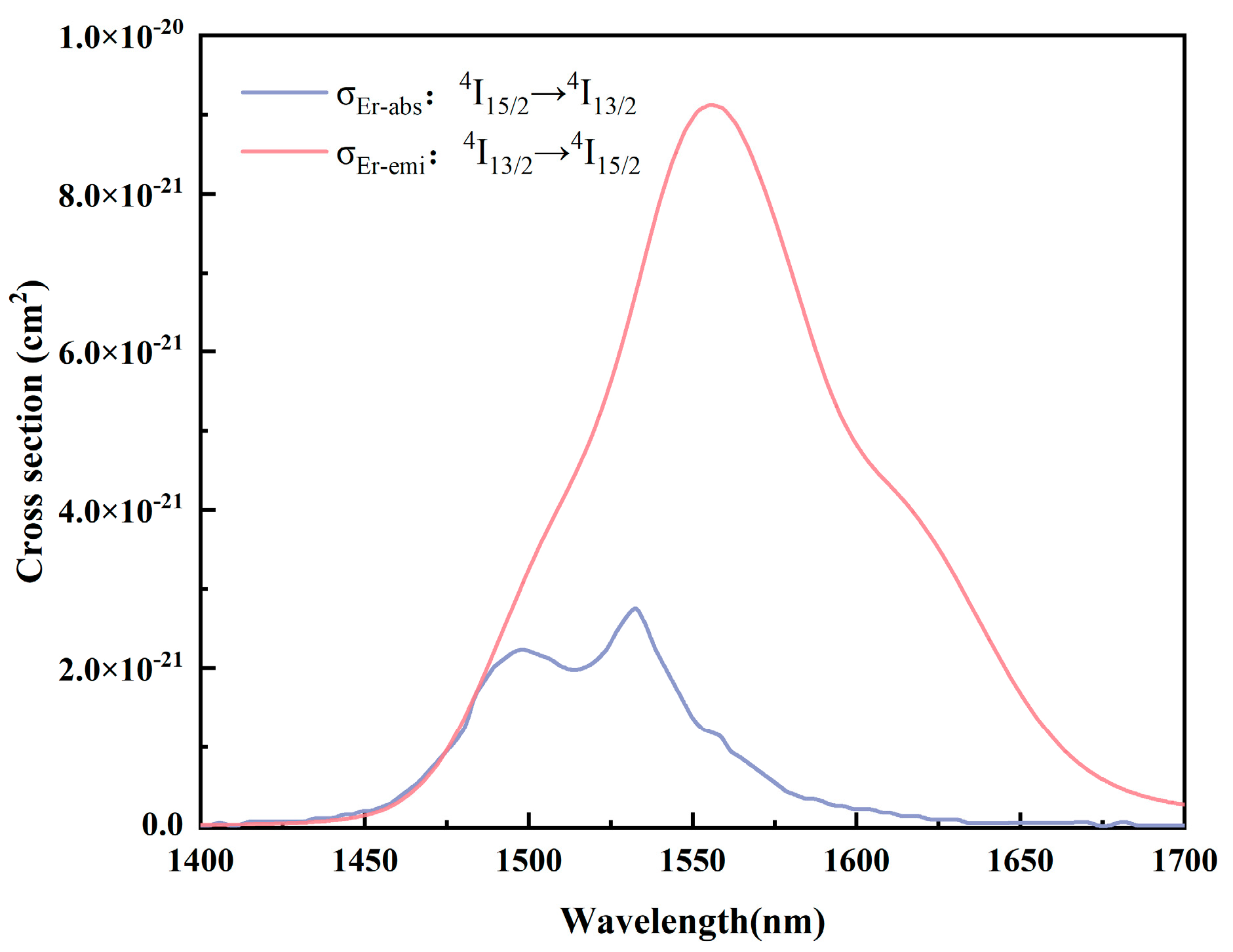
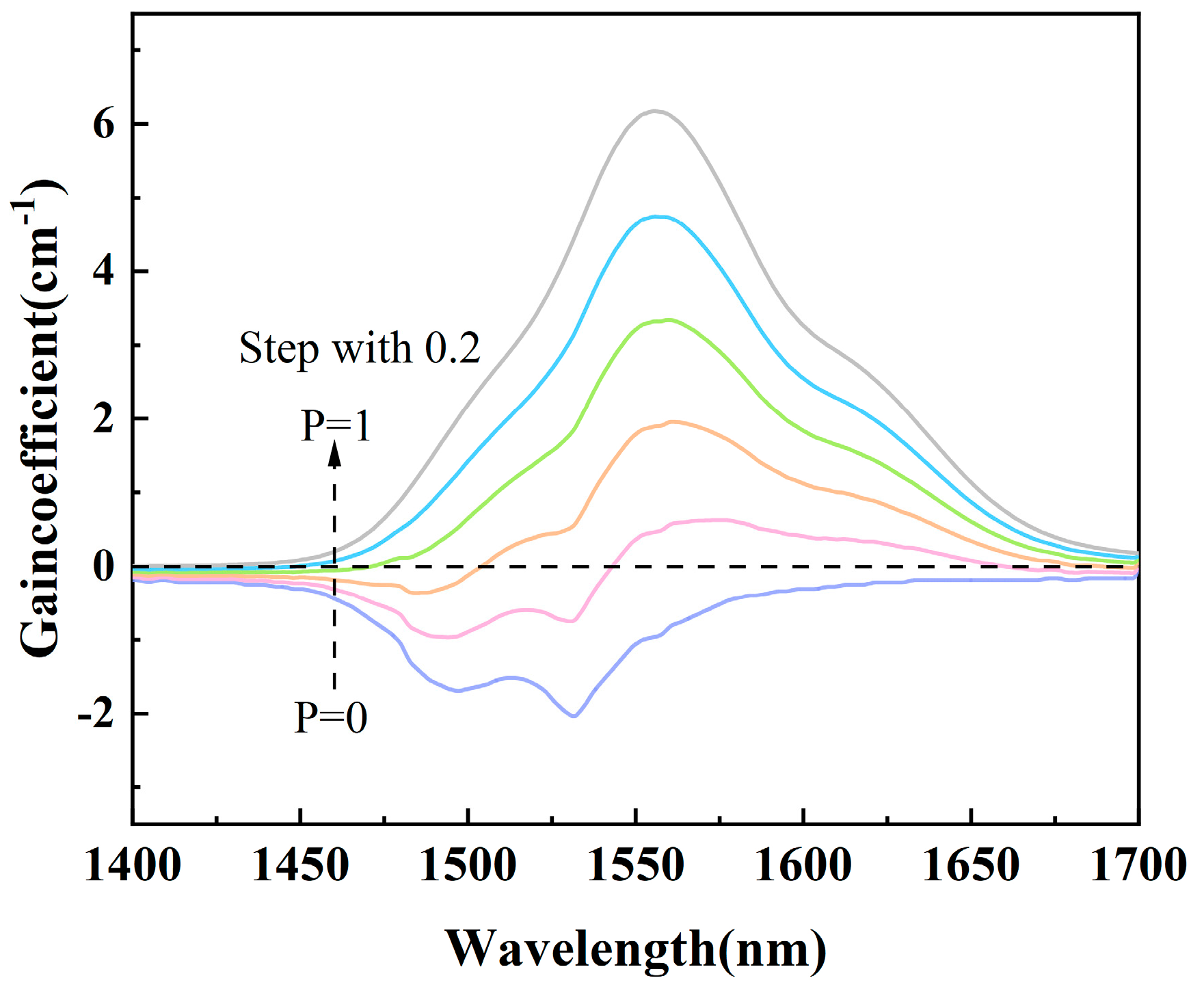
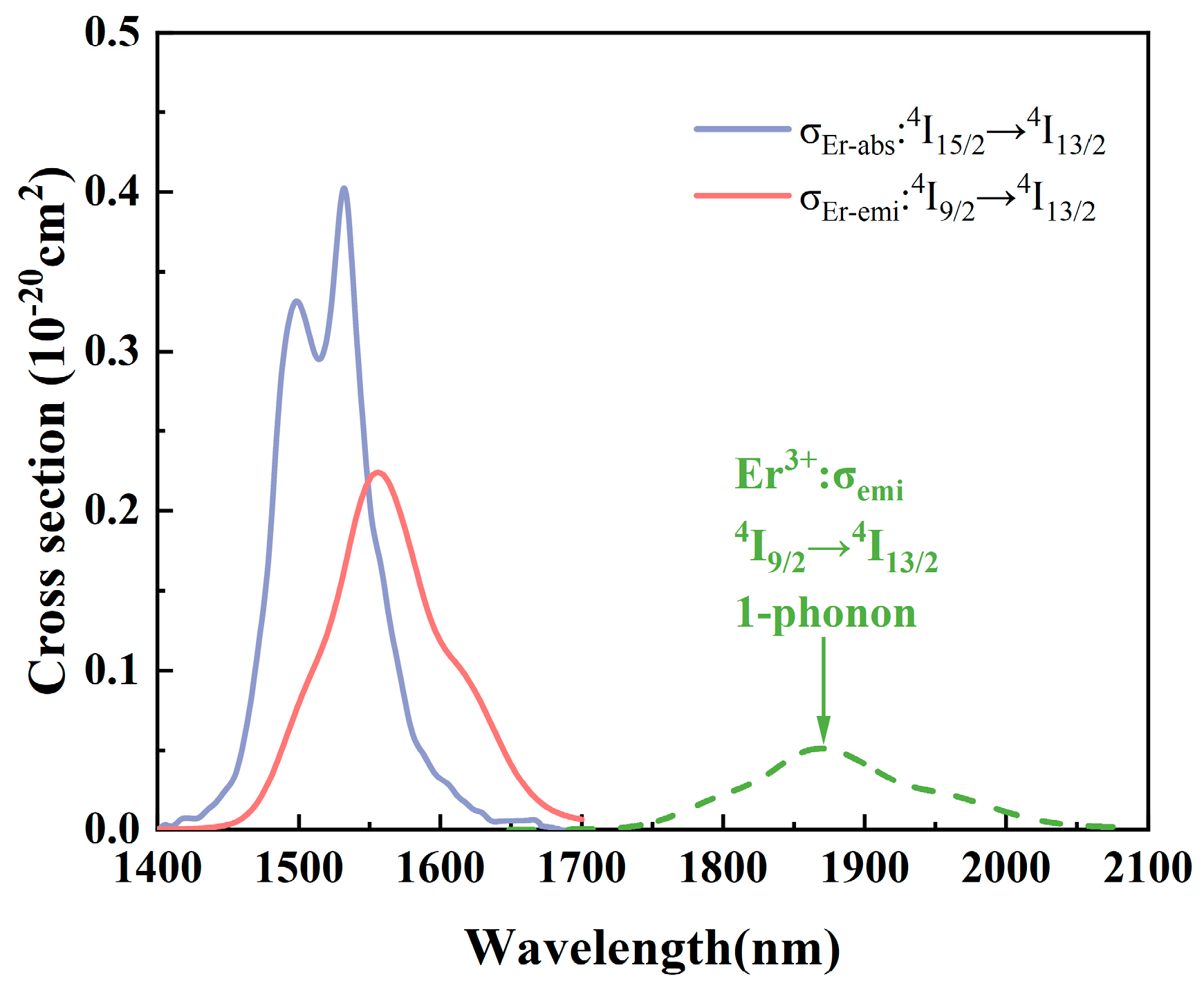
3.6. Absorption and Emission Cross-Sections and Gain Coefficients
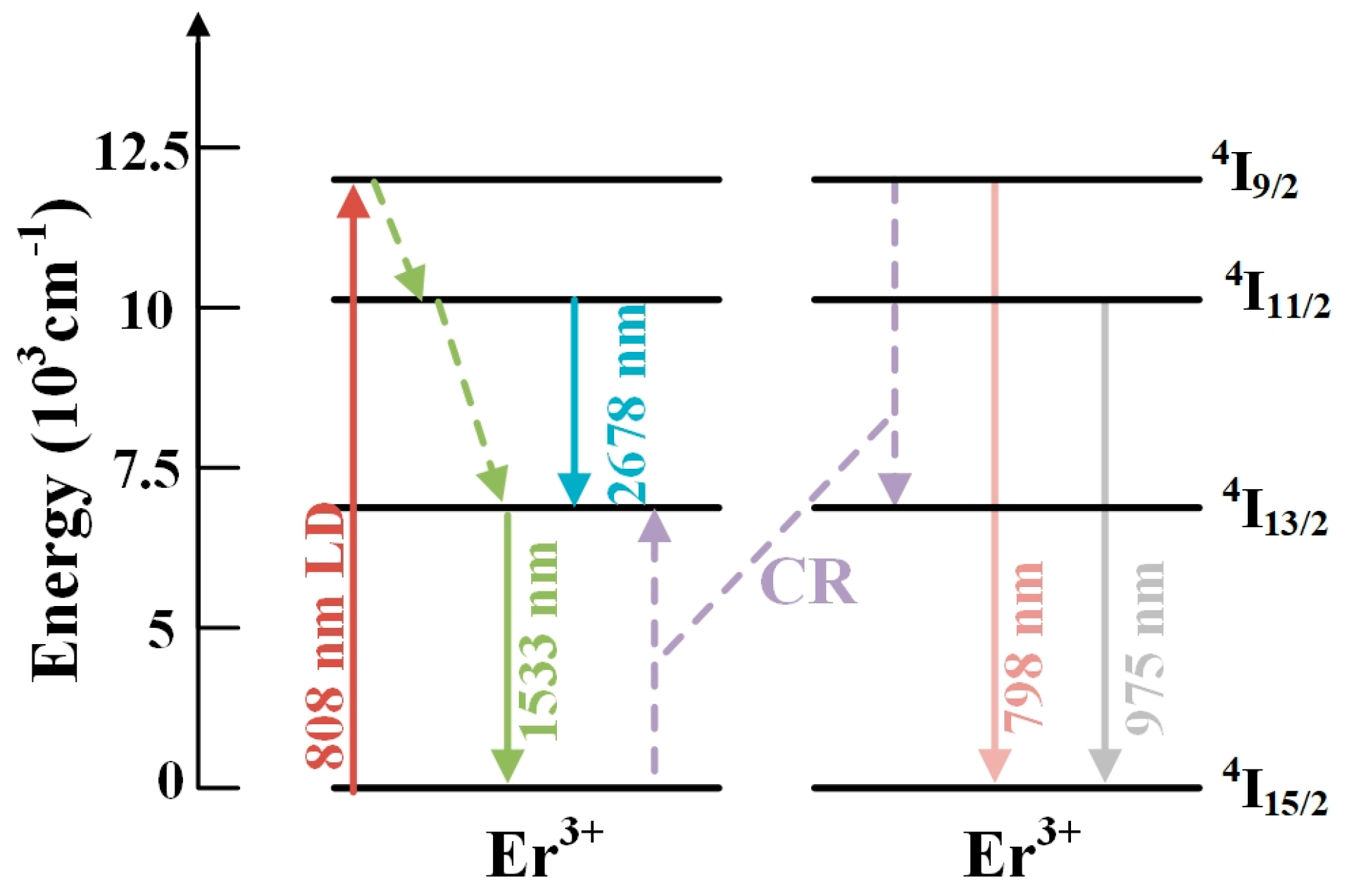
3.7. Energy Transfer Mechanism and Microscopic Parameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhemarajam, J.; Swapna; Babu, M.M.; Prasad, P.S.; Prasad, M. Spectroscopic studies on Er3+ ions incorporated bismuth borolead lithium glasses for solid state lasers and fiber amplifiers. Opt. Mater. 2021, 113, 110818. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Rufaihah, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhu, Y.; Zhou, Y.; Li, J. Broadband and flat near-infrared emission from Er3+/Tm3+ co-doped tellurite glass for amplifier applications. J. Opt. Soc. Am. B 2019, 37, 320–328. [Google Scholar] [CrossRef]
- Haouari, M.; Maaoui, A.; Saad, N.; Bulou, A. Optical temperature sensing using green emissions of Er3+ doped fluoro-tellurite glass. Sens. Actuators A 2017, 261, 235–242. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Maheswari, T.; Depuru, S.R.; Jayasankar, C.K. Sensitizing effect of Yb3+ ions on photoluminescence properties of Er3+ ions in lead phosphate glasses: Optical fiber amplifiers. Opt. Mater. 2018, 86, 256–269. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Zhang, G.; Bai, G.; Fan, S.; Zhang, J.; Hu, L. Highly biocompatible multi-walled carbon nanotube-chitosan nano-particle hybrids as protein carriers. J. Alloys Compd. 2011, 509, 3070–3073. [Google Scholar] [CrossRef]
- Rachkovskaya, G.E.; Zakharevich, G.B. Formation of Low-Temperature Seals and Coatings Based on Low-Melting Lead-Tellurite Glasses. Glass Ceram. 2005, 62, 192–194. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Manzani, D.; Falcao-Filho, E.L.; Messaddeq, Y.; Boudebs, G.; Fedus, K.; de Araújo, C.B. Near-infrared nonlinearity of a multicomponent tellurium oxide glass at 800 and 1064 nm. Appl. Phys. B 2014, 116, 1–5. [Google Scholar] [CrossRef]
- Tan, F.; Xu, P.; Zhou, D.; Wang, L.; Yang, Q.; Song, X.; Han, K. 1.53 μm luminescent properties and energy transfer processes of Er3+/Yb3+ co-doped bismuth germanate glass laser material. J. Lumin. 2021, 239, 118300. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Zheng, S.; Yin, D.; Wang, X. Luminescence properties and energy transfer mechanism of Er3+/Tm3+ co-doped tellurite glasses. J. Alloys Compd. 2013, 556, 221–227. [Google Scholar] [CrossRef]
- Deopa, N.; Sahu, M.K.; Kaur, S.; Prasad, A.; Swapna, K.; Kumar, V.; Punia, R.; Rao, A.S. Enhanced visible green and 1.5 μm radiative emission of Er3+ions in Li2O-PbO-Al2O3-B2O3 glasses for photonic applications. J. Rare Earths 2021, 39, 520–525. [Google Scholar] [CrossRef]
- Sidek, H.; Rosmawati, S.; Azmi, B.Z.; Shaari, A.H. Effect of ZnO on the Thermal Properties of Tellurite Glass. Adv. Condens. Matter Phys. 2013, 2013, 709–712. [Google Scholar] [CrossRef]
- Silva, L.; Torquato, A.; Assis, I.; Dousti, M.R. Spectroscopic study of Er3+-doped zinc-tellurite glass and opaque glass-ceramic. Solid State Sci. 2020, 112, 106444. [Google Scholar] [CrossRef]
- Song, X.; Han, K.; Zhou, D.; Xu, P.; Xue, X.; Zhang, P. 2 μm emission properties and energy transfer processes in Tm3+ doped Bi2O3-GeO2-Na2O glass laser material. J. Lumin. 2020, 224, 117314. [Google Scholar] [CrossRef]
- Yin, D.; Zhou, Y.; Zheng, S.; Xu, X.; Wang, X.; Dai, S. Near-Infrared Spectroscopic Properties and Energy Transfer Mechanism of Er3+/Tm3+-Codoped TeO2-ZnO2-Na2O Glasses. Chin. E J. Lasers 2013, 40, 0906001. [Google Scholar]
- Yang, X.L.; Wang, W.C.; Zhang, Q.Y. BaF2 modified Cr3+/Ho3+ co-doped germanate glass for efficient 2.0 μm fiber lasers. J. Non-Cryst. Solids 2018, 482, 147–153. [Google Scholar] [CrossRef]
- Shi, D.-m.; Zhao, Y.-g. Structure and Spectroscopic Properties of Er3+-doped GeO2-TeO2-Na2O Glass. Chin. J. Lumin. 2018, 1352–1358. [Google Scholar]
- Song, C.; Xu, P.; Zhou, D.; Zhang, K.; Ma, B.; Cong, Y.; Bai, Y.; Cao, J.; Han, K. Effect of phonon energy on 2 μm emission in Tm3+/Yb3+ co-doped bismuth-tellurite glasses based on WO3 or B2O3 adjustment. J. Lumin. Interdiscip. J. Res. Excit. State Process. Condens. Matter 2023, 255, 119610. [Google Scholar] [CrossRef]
- Liu, Z.; She, J.; Peng, B. Spectroscopic properties of Er3+-doped fluoroindate glasses. J. Rare Earths 2022, 40, 1037–1042. [Google Scholar] [CrossRef]
- Chen, L.; Lai, Y. Thermal, structural, and metallization properties of 0.95[50B2O3-30BaO-(20−x)ZnO-xBi2O3]+0.05V2O5 glasses. J. Non-Cryst. Solids 2020, 556, 120552. [Google Scholar] [CrossRef]
- Jose, R.; Arai, Y.; Ohishi, Y. Raman scattering characteristics of the TBSN-based tellurite glass system as a new Raman gain medium. J. Opt. Soc. Am. B 2007, 24, 1517–1526. [Google Scholar] [CrossRef]
- Berwal, N.; Dhankhar, S.; Sharma, P.; Kundu, R.S.; Punia, R.; Kishore, N. Physical, structural and optical characterization of silicate modified bismuth-borate-tellurite glasses. J. Mol. Struct. 2017, 1127, 636–644. [Google Scholar] [CrossRef]
- Kotkova, K.; Ticha, H.; Tichy, L. Raman studies and optical properties of some (PbO)x(Bi2O3)0.2(B2O3)0.8x glasses. J. Raman Spectrosc. 2008, 39, 1219–1226. [Google Scholar] [CrossRef]
- Rada, S. Structure of TeO2·B2O3 glasses inferred from infrared spectroscopy and DFT calculations. J. Non-Cryst. Solids 2008, 354, 5491–5495. [Google Scholar] [CrossRef]
- Ardelean, I.; Cora, S.; Ciceo-Lucacel, R. Structural Investigations of CuO–B2O3–Bi2O3 Glasses by Means of EPR and FT-IR Spectroscopies. Mod. Phys. Lett. B 2004, 18, 803–810. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Baki, S.O.; Lira, A.; Kityk, I.V.; Mahdi, M.A. Structural, thermal, and optical absorption studies of Er3+, Tm3+, and Pr3+-doped borotellurite glasses. J. Non-Cryst. Solids 2017, 459, 150–159. [Google Scholar] [CrossRef]
- Saritha, D.; Markandeya, Y.; Salagram, M.; Vithal, M.; Singh, A.K.; Bhikshamaiah, G. Effect of Bi2O3 on physical, optical and structural studies of ZnO-Bi2O3-B2O3 glasses. J. Non-Cryst. Solids 2008, 354, 5573–5579. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Huang, F.; Ren, J.; Yuan, S.; Chen, G. Characterization of new tellurite glasses and crystalline phases in the TeO2–PbO–Bi2O3–B2O3 system. J. Non-Cryst. Solids 2014, 386, 90–94. [Google Scholar] [CrossRef]
- Tao, J.; Chen, X.; Lin, W.; Zhou, G.; Zhao, G.; Chen, L. Calculation and analysis on J-O spectral parameters of tellurate luminescent glass doped Er3+. Chin. J. Electron. 2018, 35, 257–263. [Google Scholar]
- Zhang, J.; Xi, Z.; Wang, X.; Long, W.; Cui, M.; Zou, M. Spectrum Properties and Judd-Ofelt Analysis of PMN-PT: Er3+ Crystal. J. Synth. Cryst. 2020, 1023–1029+1056. [Google Scholar]
- Aouaini, F.; Maaoui, A.; Mohamed, N.; Alanazi, M.M.; El Maati, L.A. Visible to infrared down conversion of Er3+ doped tellurite glass for luminescent solar converters. J. Alloys Compd. 2022, 894, 162506. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Ferid, M.; Barthou, C. JuddOfelt analysis and improvement of thermal and optical properties of tellurite glasses by adding P2O5. J. Lumin. 2010, 130, 2394–2401. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Huang, Y.; Wang, D. 2 μm Luminescence Properties of Bismuth Tellurite Glass Co-doped with Ho3+\Yb3+\Tm3+\Ce3+. J. Mater. Sci. Eng. 2020, 6, 917–923. [Google Scholar]
- Pugliese, D.; Boetti, N.G.; Lousteau, J.; Ceci-Ginistrelli, E.; Bertone, E.; Geobaldo, F.; Milanese, D. Concentration quenching in an Er-doped phosphate glass for compact optical lasers and amplifiers. J. Alloys Compd. 2016, 657, 678–683. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, R.; Hu, L.; Zhang, J. Spectroscopic properties and energy transfer process in Er3+ doped ZrF4-based fluoride glass for 2.7 mm laser materials. Opt. Mater.-Amst. 2011, 34, 308–312. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, L.; Hu, L.; Zhang, J. Emission enhancement in Er3+/Pr3+-codoped germanate glasses and their use as a 2.7-mm laser material. Chin. Opt. Lett. 2013, 11, 12160101–12160103. [Google Scholar]
- Huang, F.; Zhang, Y.; Hu, L.; Chen, D. Judd-Ofelt analysis and energy transfer processes of Er3+ and Nd3+ doped fluoroaluminate glasses with low phosphate content. Opt. Mater. 2014, 38, 167–173. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, G.; Xu, J.; Zhang, N.; Ma, Z.; Hu, L. Energy transfer and 1.8 μm emission in Yb3+/Tm3+ co-doped bismuth germanate glass. Ceram. Int. 2014, 40, 6037–6043. [Google Scholar] [CrossRef]
- Miguel, A.; Morea, R.; Gonzalo, J.; Arriandiaga, M.A.; Fernández, J.; Balda, R. Near-infrared emission and upconversion in Er3+-doped TeO2-ZnO-ZnF2 glasses. J. Lumin. 2013, 140, 38–44. [Google Scholar] [CrossRef]
- Ouannes, K.; Soltani, M.; Boulon, G.; Alombert-Goget, G.; Guyot, Y.; Pillonnet, A.; Lebbou, K. Spectroscopic properties of Er3+-doped antimony oxide glass. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid State Chem. Phys. 2014, 603, 132–135. [Google Scholar] [CrossRef]
- Huang, F.; Guo, Y.; Tian, Y.; Xu, S.; Zhang, J. Intense 2.7 μm emission in Er3+ doped zinc fluoride glass. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 42–45. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, Z.; He, J.; Lin, A. Intense 2.7 μm emission of Er3+-doped water-free fluorotellurite glasses. Opt. Lett. 2012, 37, 3408–3410. [Google Scholar] [CrossRef]
- Yang, J.-X.; Zhao, X.; Zhang, J.-H.; Lin, H. 1.2 m Near Infrared Fluorescence Emission of Holmium Ions in Thermal Ion-exchanged Aluminum Germanate Waveguide Glasses. Chin. J. Lumin. 2018, 1519–1526. [Google Scholar]
- Klinkov, V.A.; Tsimerman, E.A.; Rokhmin, A.S.; Andreeva, V.D.; Tagil’tseva, N.O.; Babkina, A.N. 1.53 μm luminescent properties of Er3+-doped fluoroaluminate glasses. Opt. Mater. 2021, 121, 111585. [Google Scholar] [CrossRef]
- Sajna, M.S.; Thomas, S.; Jayakrishnan, C.; Joseph, C.; Biju, P.R.; Unnikrishnan, N.V. NIR emission studies and dielectric properties of Er3+-doped multicomponent tellurite glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 130. [Google Scholar] [CrossRef] [PubMed]
| Glass | Ω2 (×10−20 cm2) | Ω4 (×10−20 cm2) | Ω6 (×10−20 cm2) | Ref. |
|---|---|---|---|---|
| TBBZ1 | 4.31 | 1.44 | 1.40 | This work |
| TBBZ2 | 3.54 | 1.19 | 1.14 | This work |
| TBBZ3 | 3.13 | 1.05 | 1.0 | This work |
| TBBZ4 | 2.80 | 0.94 | 0.9 | This work |
| TBBZ5 | 2.57 | 0.86 | 0.82 | This work |
| HYTC0.6 | 2.84 | 1.56 | 1.82 | [33] |
| Tellurite | 1.88 | 1.98 | 1.39 | [34] |
| Fluoride | 3.08 | 1.46 | 1.69 | [35] |
| Transition | λ (nm) | Arad (s−1) | ∑A (s−1) | β (%) | τrad (ms) |
|---|---|---|---|---|---|
| 4I13/2→4I15/2 | 1533 | 284.41 | 284.41 | 100.00 | 3.52 |
| 4I11/2→4I13/2 | 2678 | 24.61 | 612.25 | 4.02 | 1.63 |
| →4I15/2 | 975 | 587.65 | 95.98 | ||
| 4I9/2→4I11/2 | 4395 | 1.05 | 922.91 | 0.11 | 1.08 |
| →4I13/2 | 1664 | 65.37 | 7.08 | ||
| →4I15/2 | 798 | 856.49 | 92.80 |
| Glass | ET | Phonon Number (N) and Contribution Radio (%) | Transfer Constant (10−40 cm6/s) | |
|---|---|---|---|---|
| TBBZE3 | CR | 0 | 1 | CD-A = 13.8 |
| 99.999 | 0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Xie, G.; Ma, Y.; Zhang, Y.; Gao, B.; Cui, S.; Chen, D.; Ban, Y.; Zhou, D. Structure and Spectral Properties of Er3+-Doped Bismuth Telluride Near-Infrared Laser Glasses. Materials 2024, 17, 3292. https://doi.org/10.3390/ma17133292
Tan F, Xie G, Ma Y, Zhang Y, Gao B, Cui S, Chen D, Ban Y, Zhou D. Structure and Spectral Properties of Er3+-Doped Bismuth Telluride Near-Infrared Laser Glasses. Materials. 2024; 17(13):3292. https://doi.org/10.3390/ma17133292
Chicago/Turabian StyleTan, Fang, Guoxing Xie, Yuqin Ma, Yunlong Zhang, Binhao Gao, Shunfa Cui, Dexiao Chen, Yumeng Ban, and Dechun Zhou. 2024. "Structure and Spectral Properties of Er3+-Doped Bismuth Telluride Near-Infrared Laser Glasses" Materials 17, no. 13: 3292. https://doi.org/10.3390/ma17133292





