3.1. XRD Analysis
X-ray diffraction analysis was carried out to study the structure of as-melted glasses. In the diffraction pattern (
Figure 1a) of the SCMS:Pr
3+ glass samples, only two very weak and diffuse peaks with wide halos can be identified (in the range of 2θ = 23–35° and 39–47°), confirming the amorphous nature of the sample. This, in turn, makes their interpretation very difficult. Furthermore, the presence of nanocrystalline (several nm in size) inclusions of silicate phases, which may have characteristic peaks in the regions 2θ = 23–35° and 39–47°, cannot be completely excluded. The most pronounced peak around 2θ = 28.8° is most likely an amorphous halo, which is characteristic of amorphous or highly disordered SiO
2-based structures.
An intense peak at 28.81°, as well as less intense peaks in the region 2θ = 25.99–26.12° and 28.80–29.18°, are characteristic of the SiO2 crystal structure (ICDS code 96-153-2514). The position of the halo with a maximum near 28.8° is also close to the positions of the peaks of the SiO2 structure (coesite, ICDS code 96-900-0805), however, high pressure is required for the formation of this structure. Silicates also have peaks in the region of 30–47°.
The structure of CaMgSi2O6 (ICSD code 30522) has peaks in the range of 26–31° and in the range of 40–44°. However, CaMgSi2O6 does not have the characteristic intense peak near 28.8°, which is characteristic of our glasses and the SiO2 structures.
The significant blurring of the peak (
Figure 1a) with a maximum near 28.8° indicates that the formation of silicate structures (if they occur) is insignificant. Thus, we came to the conclusion that our materials are highly disordered structures based on SiO
2.
3.2. Absorption Spectra, Band Gap, and Refractive Index
Figure 2a shows the absorption spectra of the SCMS glasses doped with Pr
3+ (0.5, 1.0, and 2.5% Pr
3+) in the spectral range from 380 nm to 2200 nm. All observed absorption bands are due to electronic transitions from the
3H
4 ground state. The concentration dependence of the absorption coefficient of the
3H
4→
3P
0 transition of Pr
3+ ions is linear and is presented in the inset of
Figure 2a. As shown in [
16], aluminosilicate glasses containing oxides of strontium, calcium, and barium, have no absorption peaks in the wavelength range from 400 nm to 2400 nm. Therefore, all the well-defined peaks observed in
Figure 2a are due to the presence of Pr
3+ ions in the glass samples.
After doping the glasses with Pr
3+ ions, as shown in
Figure 2a,b, absorption peaks are located at 441 and 447 nm (
3Н
4→
3Р
2 transition), 463 (
3Н
4→
1I
6), 471 nm (
3Н
4→
3Р
1), 482 nm (
3Н
4→
3Р
0), 586 nm (
3Н
4→
1D
2), 980 nm (
3Н
4→
1G
4), 1397 nm (
3Н
4→
3F
4), 1508 nm (
3Н
4→
3F
3), 1904 nm (
3H
4→
3F
2) and near 2200 nm (
3Н
4→
3H
6). These energy transitions are shown in the energy level diagram of the SMCS:2.5%Pr
3+ glass shown in
Figure 3.
In this case, it is worth noticing that the absorption band maxima corresponding to the transitions from the ground state
3H
4 to the excited states
3P
j were close to those recorded in [
16]. On the other hand, the absorption transitions terminated on
1D
2,
3F
4,
3F
3, and
3H
6 Pr
3+ excited states are slightly shifted to the bands described in [
16]. It is the result of the different compositions of the compared silicate glass materials. The relevant aspect of this paper is related to UVC up-converted luminescence, which is excited at 445 nm. Since the used semiconductor excitation source provides quite a high-power density to the glass sample, the impact of higher temperatures on the involved praseodymium absorption bands is worth investigating. In relation, the praseodymium absorption bands attributed to
3Н
4→
3Р
J and
3Н
4→
1D
2 transitions for the SCMS:2.5% Pr
3+ glass were examined as a function of temperature between 300 K and 675 K, i.e., from 26.85 °C to 401.85 °C. Our findings indicate that the examined absorption bands are rather ineffectively broadened at elevated temperatures. Furthermore, the effect of temperature on the
3Н
4→
3Р
2 absorption band is moderate up to T = 675 K, and consequently, the excitation of up-converted praseodymium emission at 445 nm should be efficient even at high powers of the laser diode. The thermal-broadening effect and slight decrease in the absorption coefficient may be perceived for the
3Н
4→
1D
2 absorption band at 586 nm as well.
The optical band gap of SCMS:Pr
3+ glass was calculated employing Equation (1), as it was presented in [
19,
20,
21]:
where A—is a constant, h—is Planck’s constant, and
ν—is the optical frequency. The optical band gap of 4.41 eV was obtained for the SCMS:0.5%Pr
3+ glass. The refractive index (n) was calculated using Fresnel equations [
22]. The optical band gap calculated for the SCMS:0.5%Pr
3+ glass is close to the values of 4.44–4.50 eV obtained in [
22] for TiO
2-SiO
2 mixed thin films.
Figure 4 shows the measured refractive index (n) of SCMS:2.5% Pr
3+ glass as a function of the incident wavelengths. The dispersive qualities of our silicate glass were examined at wide wavelengths ranging from near-infrared to UV. Concerning that, the value of the refractive index is lowered with the wavelength increasing to 1.56 at 2400 nm. For comparison, the comparable findings were described in [
23] for the borosilicate glasses.
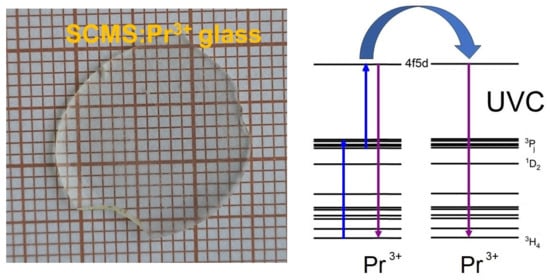
3.3. Up-Conversion Phenomena
We obtained up-convection luminescent radiation in the ultraviolet region in the SCMS:Pr
3+ glass upon excitations with lower-frequency radiation. As shown in
Figure 5, the up-converted luminescence spectrum of Pr
3+-doped SCMS glass contains a broad band in the 230–330 UV spectral region. The maximum of the up-converted luminescence of the SCMS:Pr
3+ glass under the 445 nm excitation is located at 275 nm.
To obtain more insight into the excitation mechanism of the praseodymium UV up-converted emission in the studied silicate glasses, the impact of the incident laser beam power on the integrated anti-Stokes emission was investigated. The result presented in
Figure 6 shows the dependence of the up-converted ultraviolet emission on the 445 nm laser diode excitation power. The plot can very well be approximated by a straight line, and it shows that the excitation power is too low to induce the saturation effect. The slopes of the line indicate that the two-photon excitation process is responsible for up-converted emission in the SCMS:Pr
3+ silicate glass.
The accomplishment of the ultraviolet anti-Stokes praseodymium emission in the studied silicate glasses was confirmed by employing an alternative experimental setup based on an FLS1000 spectrofluorometer.
In contrast to previously described spectra, the currently used detector allows for measuring the up-converted Pr
3+ emission at longer wavelengths up to 410 nm. On the other hand, its sensitivity is significantly reduced at a higher-energy spectral range. For that, additional broad-band up-converted emission was observed within the 320–410 nm region, and these are presented in
Figure 7. The intense emission band is peaked at 392 nm and the weaker UV band is centered at 360 nm. The origin of these bands is explained in the next chapter which describes the excitation and down-converted spectra.
It is recognized that praseodymium ions emit in the UV region of the spectrum in various hosts, including silicate ones. In fact, in [
24], authors display that Pr
3+ ions in LaBSiO
5 powders reveal an intense luminescence band in the 220–280 nm region with a maximum near 231 nm. Xinshun Wang et al. documented up-converted Pr
3+ luminescence in the UV-350 nm region in the Y
2SiO
5:Pr
3+ crystal upon excitation by an infrared femtosecond laser at 800 nm [
25]. The YSO:Pr host was investigated by E.L. Cates et al. [
2], and as a result, the up-conversion spectrum was between 265–360 nm with a maximum of 278 nm under 447 nm excitation. Quite recently, for the other silicate crystal Y
2Si
2O
7:Pr
3+ [
26], two ultraviolet broad bands ranging between ~250 nm to ~390 nm with maximum intensity at 278 nm and 308 nm were achieved under excitation at 445 nm. According to the author’s explanation, this resulting anti-Stokes UV praseodymium emission is a consequence of the sequential absorption of two blue photons, as confirmed by the measurements of excitation power dependence.
It Is worth noticing that values ‘f th’ energy gap Eg = 4.82 eV and 4.78 eV were reported for Y
2SiO
5 and Y
2Si
2O
7 silicates, respectively [
27]. These are slightly higher than Eg = 4.41 eV estimated for our SCMS glass. It can be concluded that during the effective excitation process at 445 nm, the
3P
J multiplets of Pr
3+ ions are populated, after which, due to the UC process, the levels of the 4f
15d
1 electronic configuration are fed, and as a result, UV anti-Stokes 4f
15d
1→
3H
J emission is observed.
3.4. Excitation and Down-Converted Luminescence Spectra
The optical properties of Pr
3+ ions were studied in the UV, visible, and near-IR regions. The luminescence excitation spectra of SCMS glasses monitored at 605 nm and 730 nm are presented in
Figure 8a,b. As can be seen in the 425–500 nm wavelength range, three peaks are noticeable at 441, 471, and 486 nm, caused by
3H
4→
3P
2,
3H
4→
3P
1, and
3H
4→
3P
0 transitions, respectively. These peaks are close to those presented in [
6] for oxyfluoride silicate glasses doped with Pr
3+ ions (~443, 468, and 481 nm—transitions from the ground state
3H
4 to excited states
3P
2,
3P
1, and
3P
0, manifolds, respectively). It can be discerned that the spectral characteristic of the praseodymium excitation band is not affected by the concentration of Pr
3+ ions in the material under study. In addition, as shown in
Figure 8b, the luminescence excitation spectra of glasses contain a broad band in the UV region with a maximum at 273 nm related to
3H
J-4f
15d
1 inter-configurational transitions of praseodymium. This band is especially intense for the SCMS:2.5% Pr
3+ glass with a high concentration of dopant.
An additional peak of the luminescence excitation band at 386 nm was observed for all SCMS glass samples. In [
8], an additional peak with a maximum at 355 nm was also observed for the luminescence excitation spectra of La
2Zr
2O
7:Pr
3+ nanophosphors, and the authors associated the appearance of this peak with the intrinsic defect absorption or the virtual charge transfer of Pr
3+ ions.
The appearance of a band with a maximum of 330 nm is characteristic of the luminescence excitation spectra of our glasses when detected at 385 nm (
Figure 8c). When excited into this band at 335 nm, an intense luminescence band is observed (
Figure 9c) with a maximum near 400 nm. A similar luminescence band was studied in [
28] for the porous SiO
2 matrix. The authors of this work showed that when the pure SiO
2 matrix was excited at 335 nm, a broad luminescence band with a maximum at 380 nm (at room temperature) was observed and suggested that the nature of this band could be due to either isolated silanol groups or OH-related centers. However, during the synthesis of our glass, tetraethoxysilane was not used. We carried out the synthesis at high temperatures, so the more likely appearance of this band is associated with the presence of defects in the examined SCMS glasses.
In [
29], it was stated that silicate glasses obtained in a reducing atmosphere (H
2/He) can have a luminescence band with a maximum at 3.1 eV (400 nm) (strong) and 4.2 eV (295 nm) (very weak) with excitation at 5.17 eV (240 nm). The authors stated that such bands are also characteristic of studies in an oxidizing atmosphere. This band is identified as a Si-related center, which was assigned to Si(II), dissolved in the silica network.
It can be assumed that the presence of a band with a maximum at 400 nm in our glasses can also be due to the formation of chains of two-fold coordinated Si atoms with two oxygen neighbors; moreover, the presence of oxygen vacancies is also possible.
The luminescence spectra of the SCMS:Pr
3+ glasses were measured in the visible and near-infrared regions. Samples of the SCMS glasses doped with Pr
3+ were excited into absorption bands at 237 nm and 447 nm. The luminescence spectra of the doped glasses are presented in
Figure 9a,b. The involved transitions are shown as the solid arrows in the energy level scheme of Pr
3+ ions in the SCMS glasses (
Figure 3).
When praseodymium luminescence is excited at 447 nm (
Figure 9a), the emission bands appear with a maximum at 486 nm (
3P
0→
3H
4), 527 nm (
3P
1→
3H
5), and 552 nm (
3P
0→
3H
5). The most intense band with a maximum at 605 nm (
1D
2→
3H
4) contains weaker components near 631 nm (
3P
0→
3H
6) and at 645 nm (
3P
0→
3F
2). The remaining maxima can be discerned at 692 nm (
3P
1→
3F
4), 708 nm (
3P
0→
3F
3), 730 nm (
3P
0→
3F
4), 880 nm (
3P
1→
1G
4), and at 823 nm (
1D
2→
3H
6).
Figure 9b shows the luminescence spectrum excited at 237 nm, and consequently, the emission bands appear with maxima near 491 nm (
3P
0→
3H
4), 533 nm (
3P
1→
3H
5), 556 nm (
3P
0→
3H
5), the most intense 605 nm (
1D
2→
3H
4), with shoulder maxima at 631 nm (
3P
0→
3H
6), and 647 nm (
3P
0→
3F
2), as well as a band with a small maximum at 703 nm and 736 nm (
3P
1→
3F
3,
4). Eventually, it can be perceived that in the visible luminescence spectra of the SCMS:Pr
3+ glasses, red emission is prominent at ~605 nm (
1D
2→
3H
4 transition). The comparable branching ratio of praseodymium luminescence and the location of the examined peaks were documented in [
30] for Pr
3+-doped calcium aluminosilicate glasses. Moreover, the emission peaks of Pr
3+ ions observed in our SCMS glasses coincide with the emission peaks reported in [
6] for SiO
2-Al
2O
3-CaO-CaF
2-TiO
2: Pr
2O
3 glasses excited at 443 nm.
It should be noted that when the glass samples are excited at 237, the maximum luminescence intensity of the 1D2-3H4 transition (605 nm) decreases for the SCMS:1% Pr3+ and SCMS:2.5% Pr3+ samples by a factor of 1.5–2 in relation to a glass with a low concentration of Pr3+ ions.
The decrease in praseodymium luminescence intensity related to the
1D
2→
3H
4 transition near 605 nm with increasing dopant concentration may be due to cross-relaxation phenomena taking place between two neighboring optically active ions. As can be seen from
Figure 9a, the ratio of peak intensities at 484 nm relative to the peak intensity at 605 nm is 0.18, 0.23, and 0.51, with an increase in Pr
3+ ion concentration from 0.5 to 1.0, and 2.5%, respectively. Our findings indicated that visible emission of praseodymium in the SCMS glasses can be efficiently excited employing excitation bands at 441, 471, and 486 nm corresponding to
3H
4→
3P
2,
3H
4→
3P
1, and
3H
4→
3P
0 transitions, and a higher-energy UV excitation is useful as well.
The luminescence of SCMS glass doped with 1% Pr
3+ measured in the near-infrared wavelength range of 800–1700 nm with excitation at 447 nm is displayed in
Figure 10. The NIR emission spectra consist of three bands that may be assigned to
3P
1→
1G
4 (888 nm),
1D
2→
3F
4 (1055 nm), and
1D
2→
1G
4 (1496 nm) transitions. The band centered at 1055 nm is the most intense, and a transition terminated on the
1G
4 level covers a wider 1300–1630 spectral range.
3.5. Impact of Temperature on Excitation and Luminescence Spectra
A study on the effect of 85 K (−188/15 °C)–715 K (441.85 °C) temperature on the luminescence excitation spectra of the SCMS:1% Pr
3+ glass (
3H
4-
3P
j transitions) has been prepared, and the resulting spectra are presented in
Figure 11a. At higher temperatures, the higher-energy crystal field components of praseodymium ground state
3H
4 are more effectively populated, consequently, the consecutive transitions to the involved sublevels of
3P
J multiplets take place. This effect, combined with the inherent thermal line shift and broadening, gives rise to the emission band extension, especially at longer wavelengths. In contrast to that, overall band intensity is reduced with temperature increasing. Particularly, at low temperatures, two high-energy components at 441 and 447 nm are more pronounced and become efficiently depressed at higher temperatures.
The praseodymium luminescence originating In
3P
J and
1D
2 multiplets was measured as a function of temperature 85 K (−188/15 °C)–715 K (441.85 °C) for the SCMS glass doped with 1% Pr
3+, and the adequate spectra are presented in
Figure 11b. The most intense band at 605 nm is effectively broadened within shorter wavelengths, and the peak maximum is blue-shifted as well. It is a consequence of the population of higher-energy crystal field sublevels attributed to the involved praseodymium luminescent excited states.
We investigated the variation of luminescence intensity ratio (LIR) related to (
3P
0-
3H
4/
3P
1-
3H
5) praseodymium transitions as a function of 85–715 K temperature to evaluate the suitability of the studied materials for applications in optical sensing thermometry. The impact of temperature on the determined luminescence intensity ratio was used to estimate the corresponding absolute (S
A) and relative (S
R) thermal sensitivities for SCMS:1%Pr and SCMS:2.5%Pr glasses. The achieved results are depicted in
Figure 12a–d. The luminescence intensity ratio was fitted according to the relation:
where ∆E is the energy difference between the thermalized
3P
0,1 levels, k
B is the Boltzmann constant, T is the temperature expressed in absolute scale [K] and A and B are constants.
Luminescence intensity ratios LIR (527/486) increase exponentially with temperature elevation, and the maximum absolute temperature sensitivity is reached for T = 223 K and T = 230 K, and amount to 8.71 × 10−4 K−1 and 9.83 × 10−4 K−1 for SCMS:1%Pr3+ and SCMS:2.5%Pr3+ respectively.
The highest values of relative sensitivity, S
R = 0.52% K
−1 at T = 330 K and S
R = 0.48% K
−1 at T = 322 K, were found for SCMS:1%Pr
3+ and SCMS:2.5%Pr
3+ glasses, respectively. Concerning that, our glasses can be considered a potential luminescent optical temperature sensor, applying thermally coupled levels of praseodymium. Quite recently, a maximum relative sensitivity of 1.0% K
−1 has been reported for Pr
3+/Yb
3+ co-doped fluoride phosphate glass [
31]. This estimated sensitivity is higher in relation to SCMS:Pr
3+ glass, regardless of our optical systems can be useful at extended temperature ranges up to 715 K.
3.6. Relaxation Dynamic of Pr3+ Excited States
Luminescence decay curves were measured for
3P
0 and
1D
2 excited states at 495 nm or 605 nm under excitation at 447 nm. As can be seen from
Table 1 and
Figure 13a, the experimental lifetime of the
3P
0 excited state changes slightly with increasing dopant concentration in the studied glass. In fact, an increase in the concentration of Pr
3+ ions from 0.5% to 2.5% leads to an insignificant decrease in average lifetime
τexp. of
3P
0 levels from 2.20 μs to 2.06 μs.
A different effect is recognized for
1D
2 levels of Pr
3+-doped SCMS (
Table 1 and
Figure 13b). An increase in the concentration of Pr
3+ ions leads to a considerable decrease in
τexp. (from 187 μs to 74 μs at a concentration of Pr
3+ ions of 0.5 and 2.5%, respectively). Our findings indicate that the efficiency of Pr-Pr energy transfer can be found to be around 60% in SCMS glasses.
To examine the interaction between optically active ions in SCMS:Pr
3+ glasses, the Inokuti–Hirayama model has been utilized [
32]. When the effectiveness of interionic energy transfer is more significant in relation to the time evolution of praseodymium, luminescence intensity can be described as:
where
A is constant,
Φ(
t) denotes the emission intensity after pulse excitation,
S = 6 for dipole-dipole interactions,
τ0 defines the intrinsic decay probability of the donor involved excited state when the acceptor is absent; furthermore, α is the parameter expressed as:
where
R0 is the critical ion-ion energy transfer distance,
Na is the acceptor concentration Γ = 1.77 (for
S = 6) is Euler’s function. The nonexponential decay curve recorded for
1D
2 luminescence in heavily doped SCMS:2.5%Pr
3+ glass was applied to estimate energy transfer quality. In relation to that, the α parameter was fitted to be 1.9 and the critical energy transfer distance
R0 is equal to 8.9 Å for the studied glass host. Employing the relations C
da =
R06τ0−1 and W
da = C
daR0−6 the energy transfer parameter corresponds to the value of 2.40 × 10
−39 cm
6s
−1 and a donor-acceptor energy transfer rate is estimated to be 5 × 10
6 s
−1. The estimated critical distance R
0 is higher than 5 Å, hence the applied IH model validates the contribution of multipolar Pr-Pr interactions in the material under study [
33]. For the comparison, the energy transfer parameters C
da = 6.39 × 10
−43 cm
8s
−1 and W
da = 13 × 10
6 s
−1 were estimated for Pr-doped multi-component silicate photonic films [
34]. In relation to our glass, this praseodymium highly-doped silicate optical system is characterized by a significant D-A energy transfer rate and especially dipole-quadrupole mechanisms leading to significant quenching phenomena. Contrary, for higher Pr
2O
3 concentration in P
2O
5-Na
2O-Al
2O
3-Gd
2O
3 glass [
35], particularly the reliable fitting is documented for
S = 6 and dipole-dipole interaction between optically active ions takes place.