A Methodology for Shielding-Gas Selection in Wire Arc Additive Manufacturing with Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology
- Keeping the same set values of wire-feed speed (WFSset) and deposition speed (DS) with the three shielding gases, but expecting different mean wire-feed speed (WFSm), mean current (Im), and, consequently, varied amounts of material mass deposited per unit of length (different WFSm/DS ratios);
- Setting different WFSset to reach the same WFSm (actual WFS) for the three shielding gases (resulting in different Im), and, at the same time, adjusting the deposition speed (DS) to maintain the same amount of deposited material mass per length unit (same WFSm/DS ratio);
- Setting different WFSset to reach the same Im (implying different WFSm) and, at the same time, changing disposition speed (DS) to maintain the same amount of deposited material mass per length unit (same WFSm/DS ratio) among the blends.
2.2. Experimental Setup
2.3. Proposed Assessment Parameters
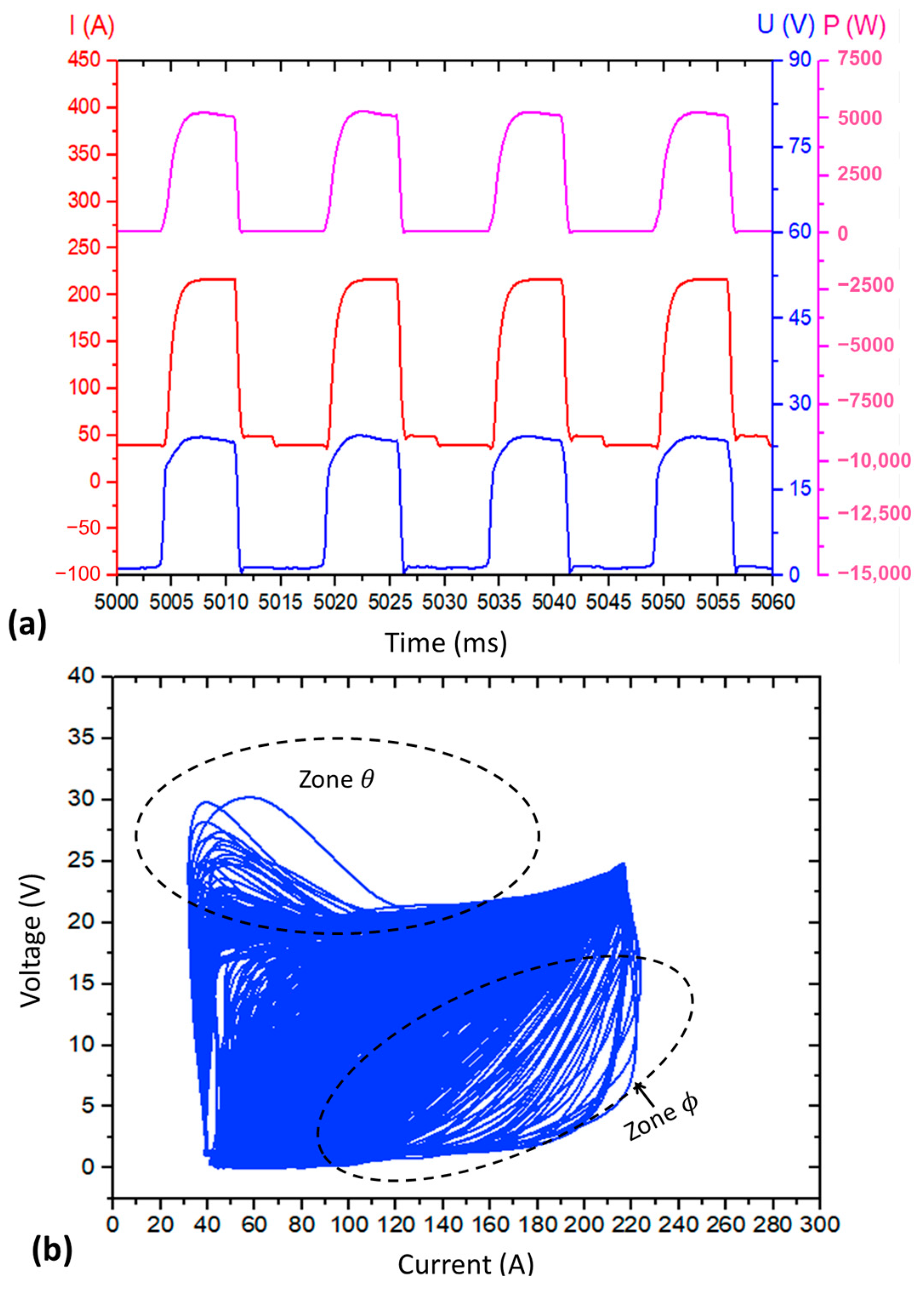
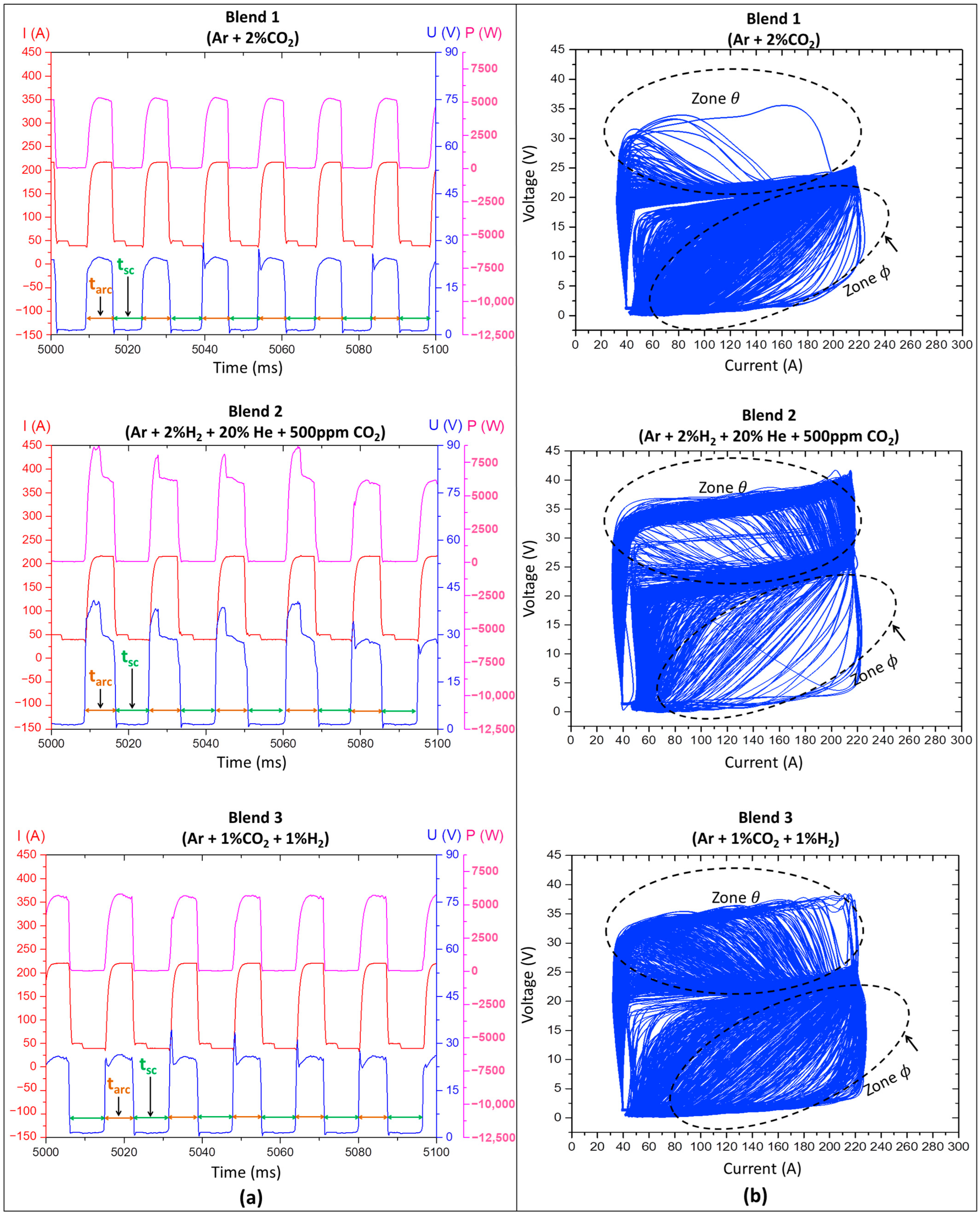
2.3.1. Effect of the Shielding Gas on the Metal Transfer Regularity in Short-Circuiting Mode
- Qualitative analyses were conducted by visually observing the oscillograms (current × time and voltage × time graphs) and cyclograms (voltage × current graphs) resulting from the layer deposition with the three shielding gases. The regularity of the oscillograms can be ascertained when there is maintenance and periodicity of the current and voltage curves as a function of time. The regularity in cyclograms, in turn, will be more significant when the superposition of voltage × current signals is more concentrated;
- Quantitative analyses were carried out by using a metal transfer regularity index, hereafter referred to as IVsc index. For that, mean values and standard deviations of short-circuiting frequency (Fsc), arcing time (tarc), and short-circuiting time (tsc) were calculated (Equation (1)) using voltage traces of 25 layers of each wall, aided by a computer program developed and registered by Vilarinho e Araújo [39]. As described by Jorge et al. [40], the IVsc index is closely linked to the consistency of arcing and short-circuiting durations. In other words, the lower the IVsc value, the more regular the metal transfer.where σtsc and σtarc represent the standard deviation of the monitored short-circuiting time (tsc) and arcing time (tarc), respectively, averaged along the sample duration.
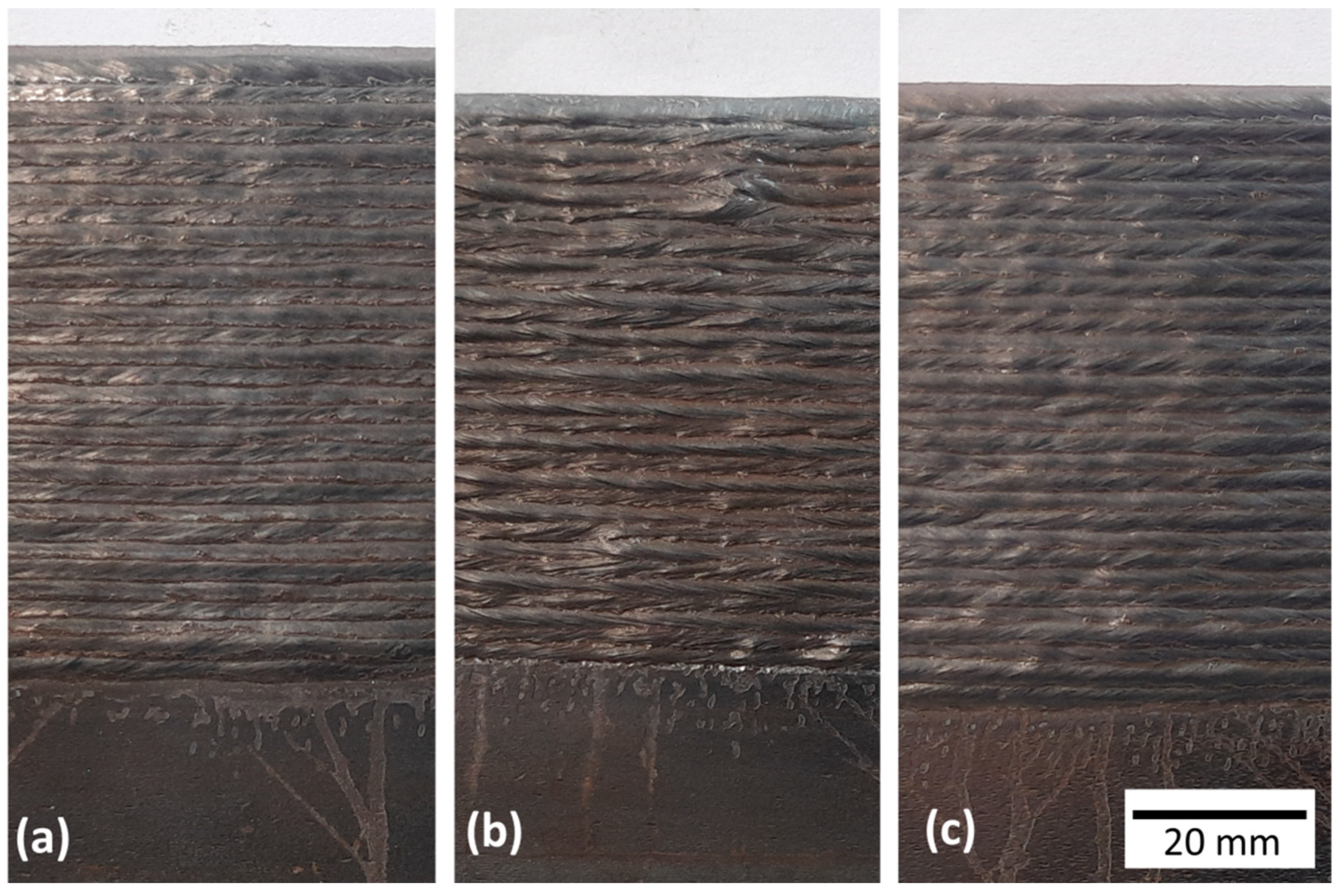
2.3.2. Effect of the Shielding Gas on the Geometrical Features
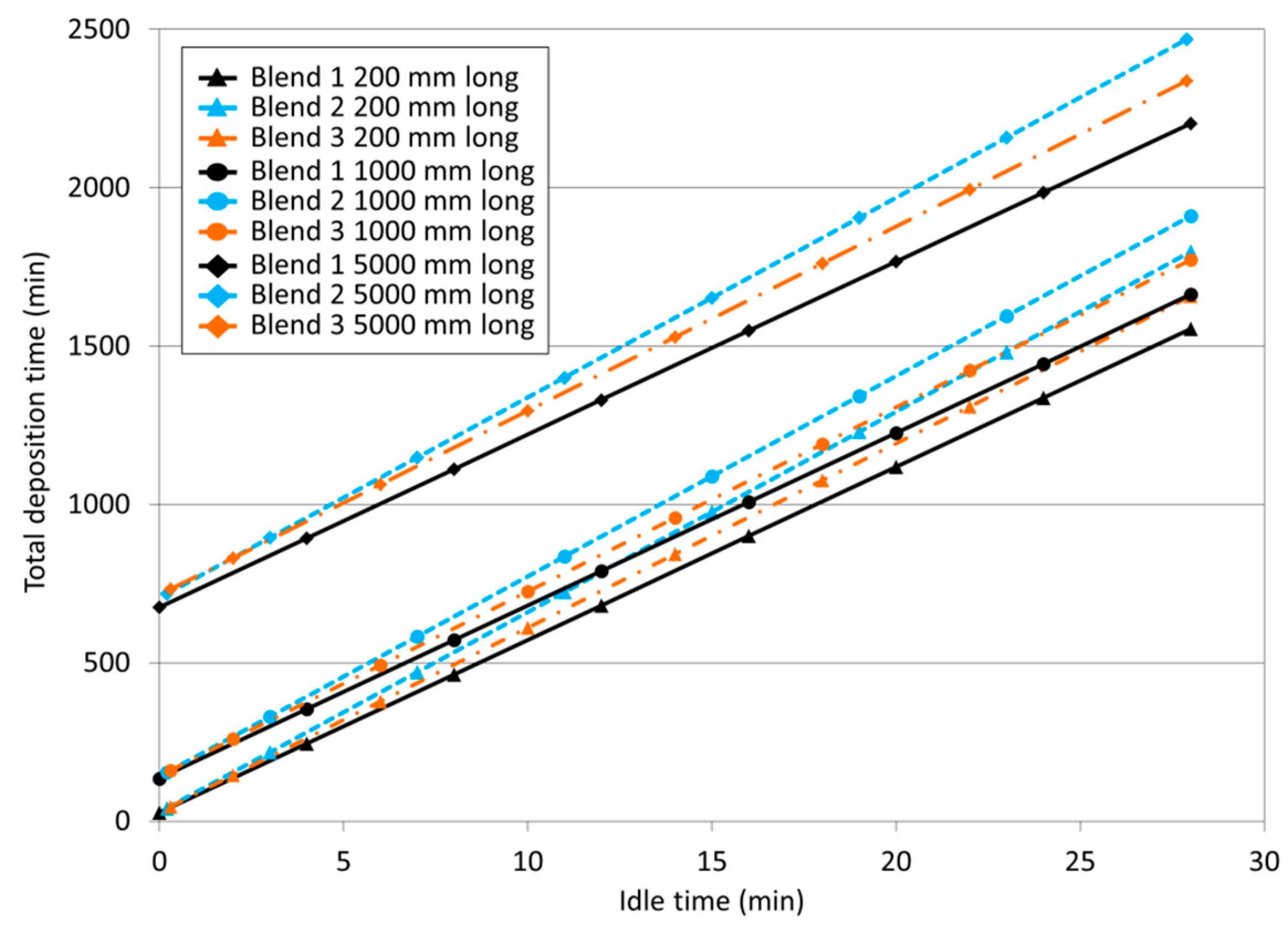
2.3.3. Effect of the Shielding Gas on Total Deposition Time
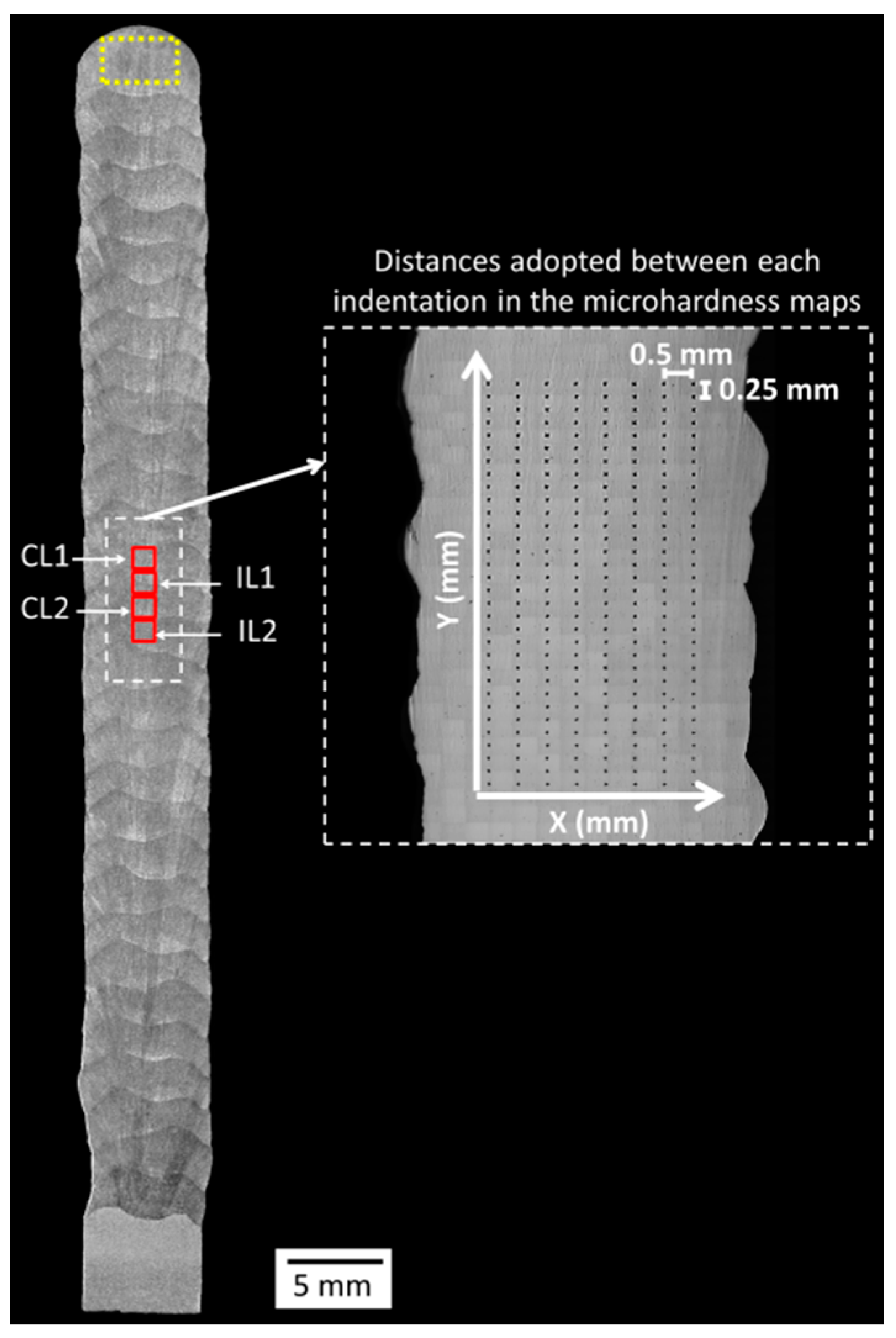
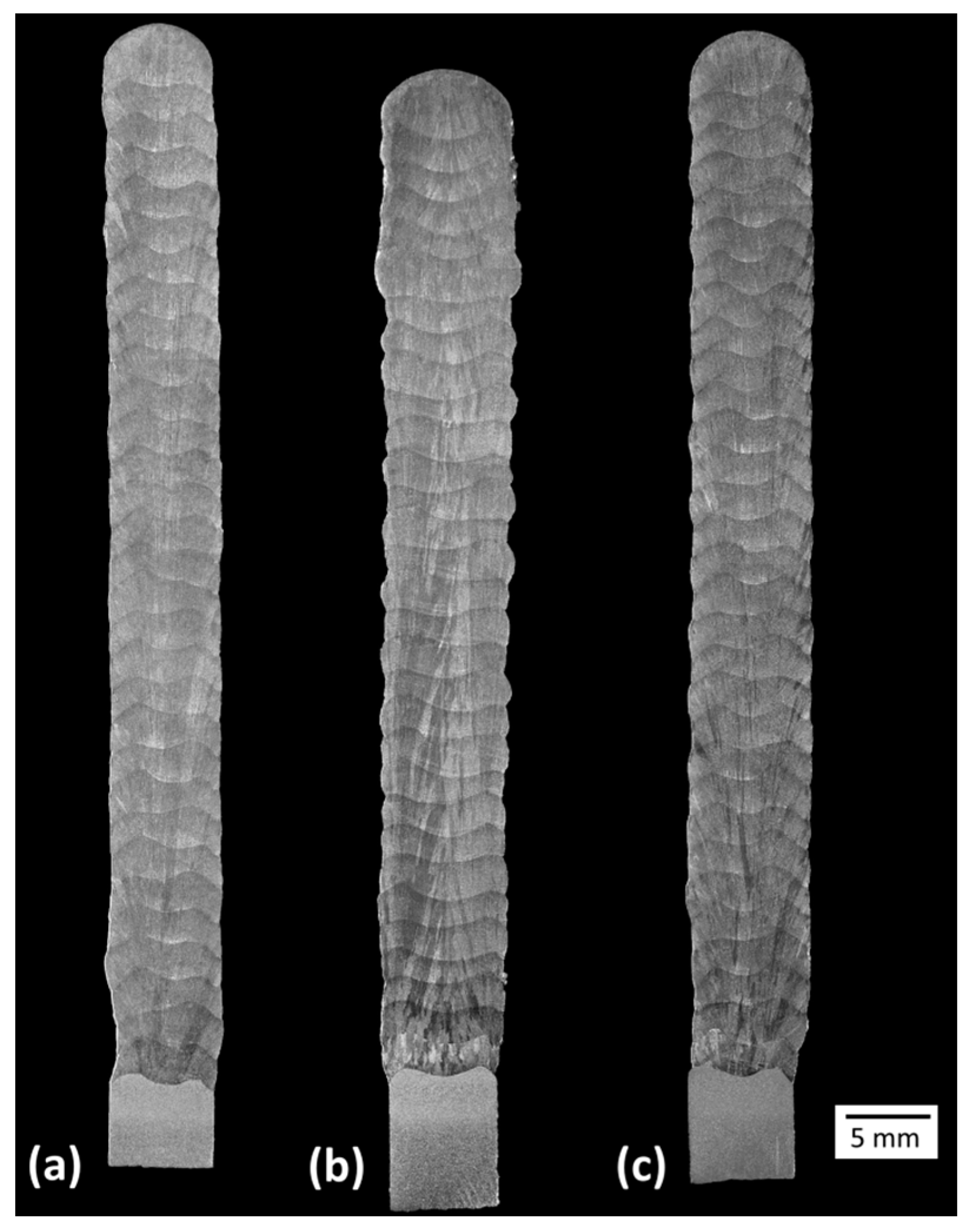
2.3.4. Effect of the Shielding Gas on the Microstructure and Hardness
3. Results and Discussion
3.1. From the Preliminary Experiments
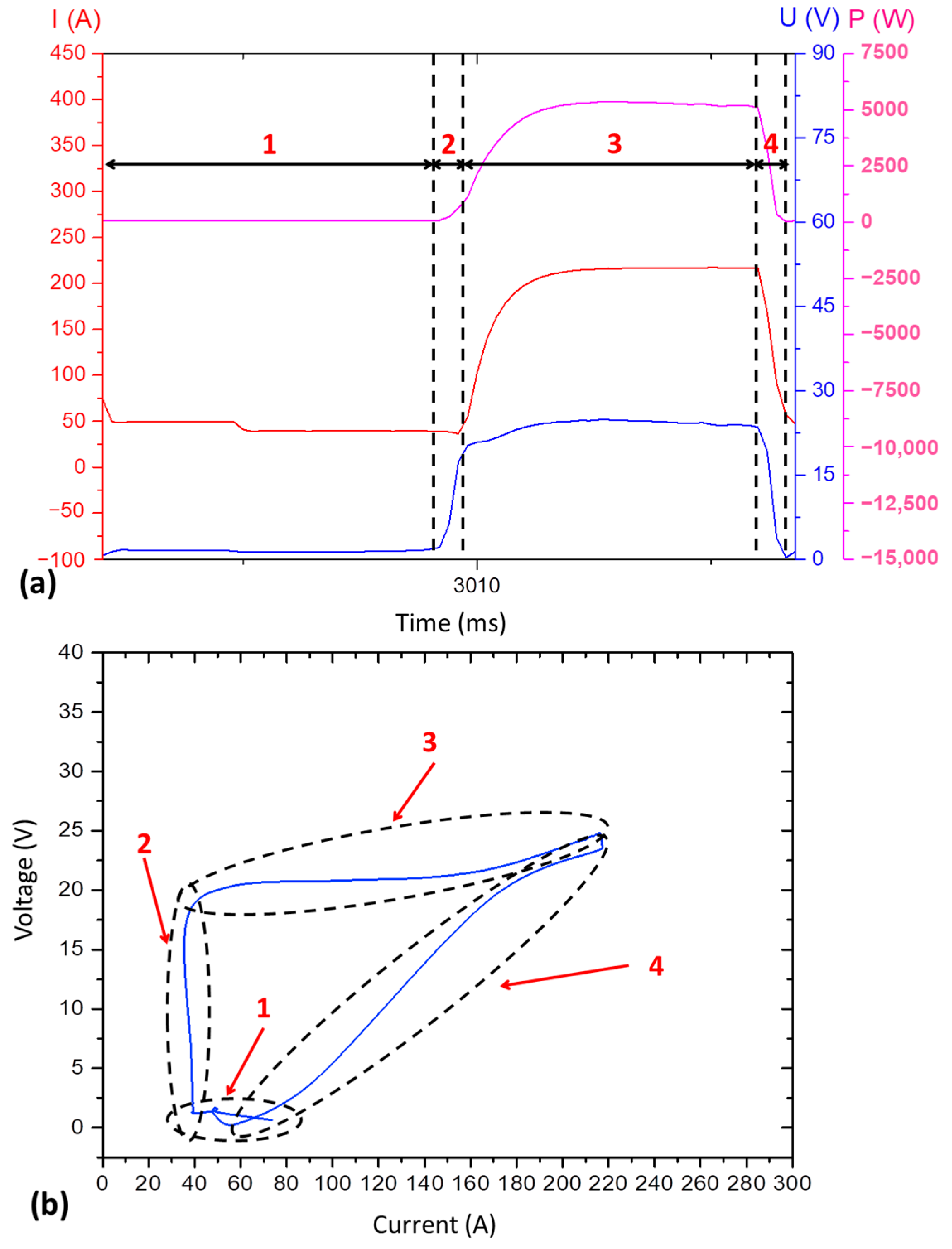
- The type A cyclogram occurs when the current and voltage decrease uniformly (constant slop);
- The type B cyclogram shows an abrupt drop in current at high voltage intensity, followed by an abrupt drop in voltage at low current level;
- The type C cyclogram shows an opposite behaviour in relation to type B: an abrupt drop in voltage at high current, followed by an abrupt reduction in current at low voltage.
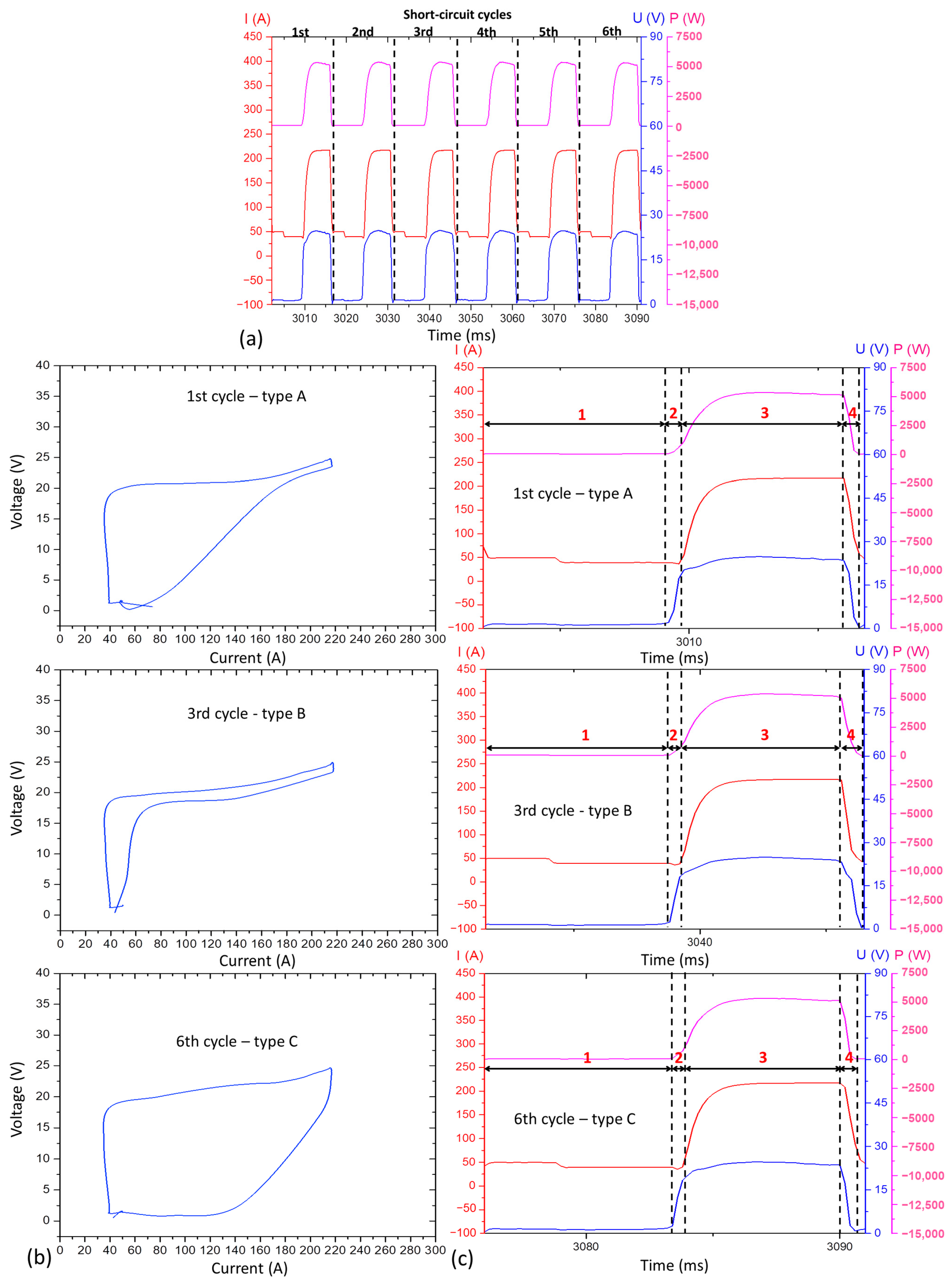
3.2. From the Main Experiments
3.2.1. Effect of the Shielding Gas on the Regularity of the Short-Circuiting Transfer
3.2.2. Effect of the Shielding Gas on the Geometrical Features
3.2.3. Effect of the Shielding Gas on Total Deposition Time
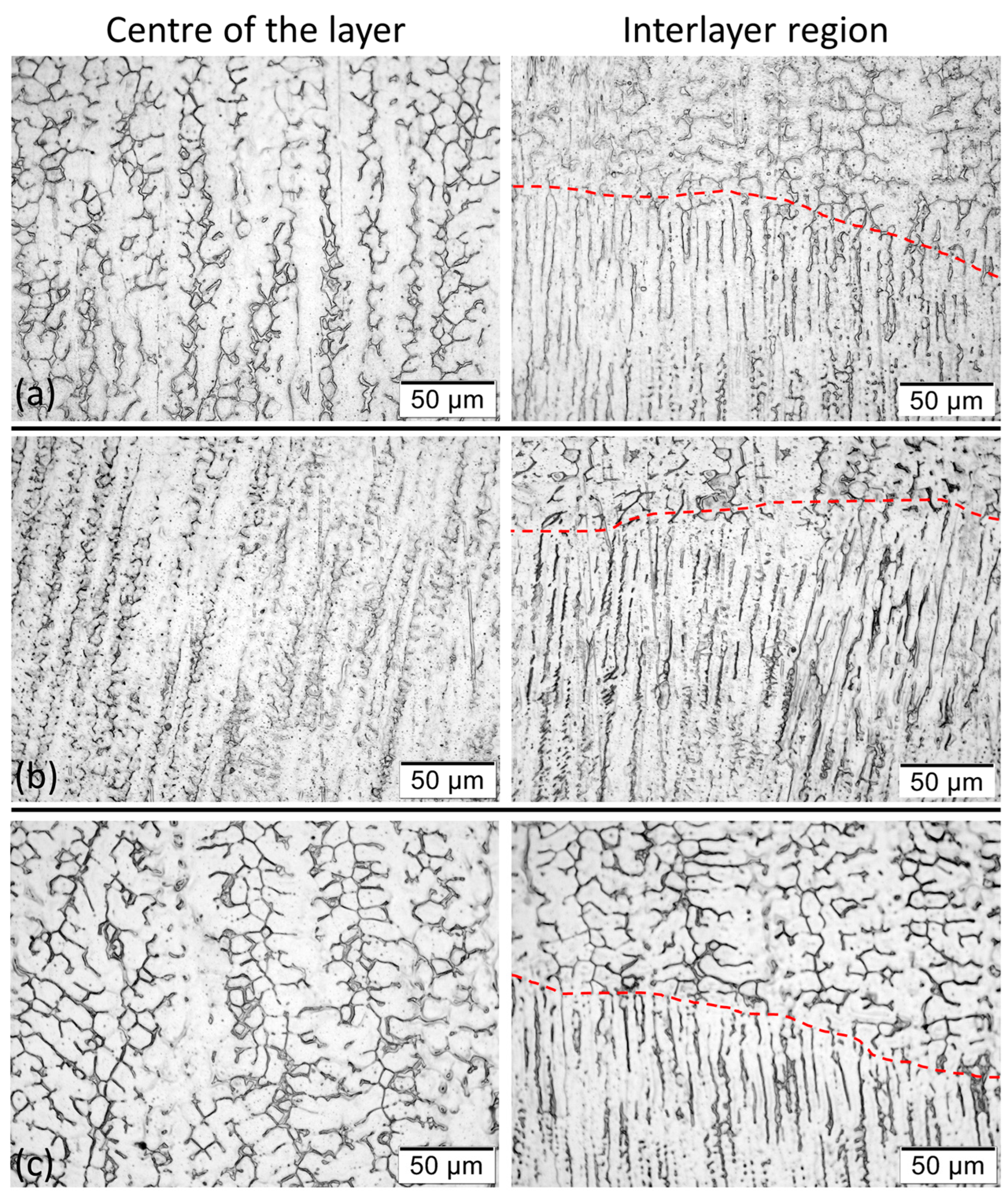
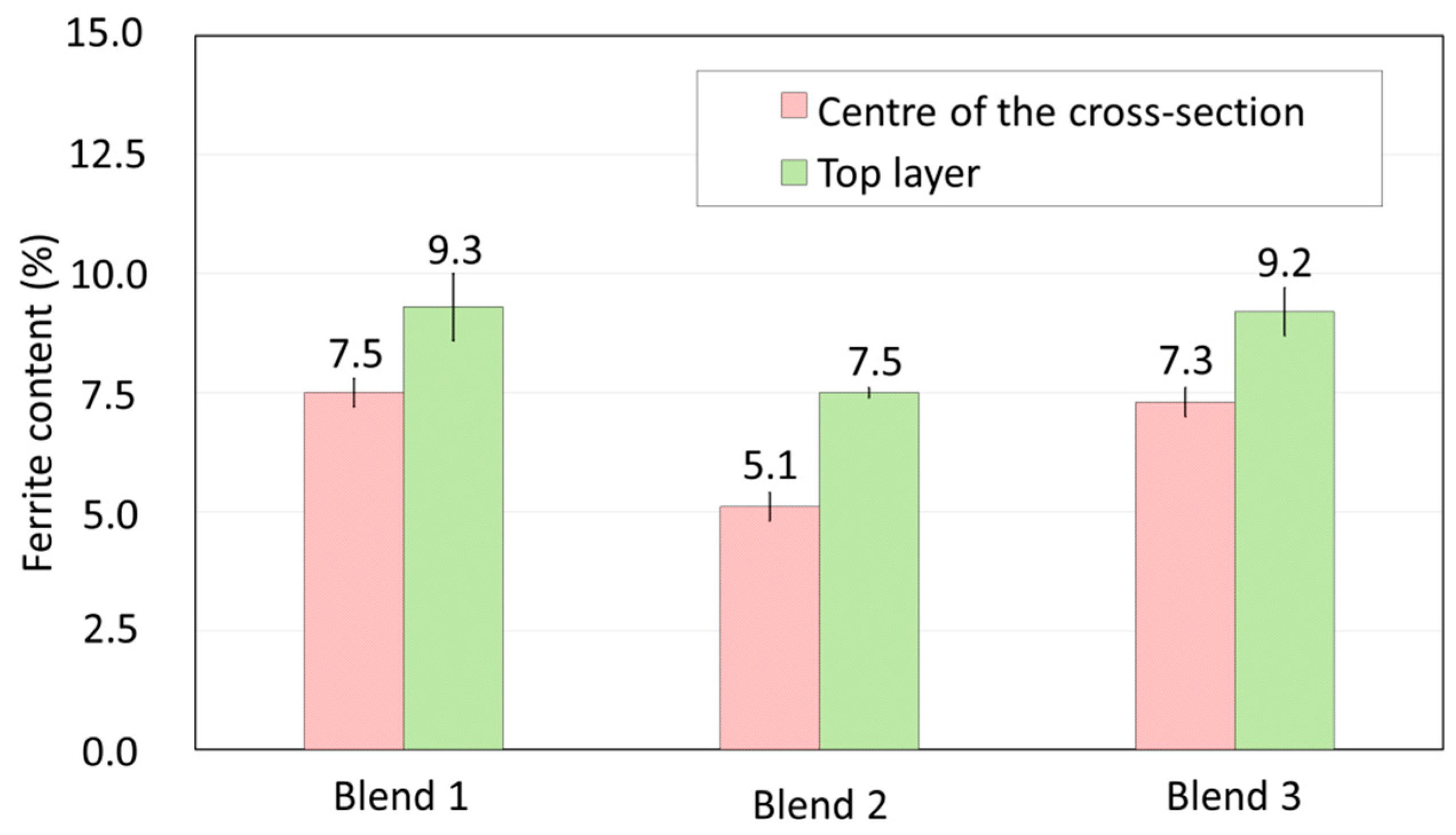
3.2.4. Effect of the Shielding Gas on the Microstructure and Hardness
4. Conclusions
- The proposed methodology, based on a novel, systematic, and comprehensive quantification of the shielding-gas performance (arc- and wall-related characteristics, such as the metal transfer regularity, deposition time, and geometrical and metallurgical aspects of thin walls deposited by GMA), was shown to reach the global objective, by identifying the influence of different commercial shielding gases on various features of GMA (operational, for the process and parametrisation, and functional, concerning the quality and metallurgy of the printed part). Following this methodology, the decision on the shielding selection can be based on the distinguished arc- and wall-related characteristics of the different shielding gases;
- The composition of the gases affects the operational performance of the shielding gases (consequently, metal transfer and fusion rate are governed by the shielding). For a similar deposition rate per unit of layer length and mean current:
- ○
- Blend 1 (Ar + 2%CO2) and Blend 3 (Ar + 1%CO2 + 1%H2) provided higher metal transfer regularity than Blend 2 (Ar + 2%H2 + 20%He + 500 ppm CO2);
- ○
- Blend 1 (Ar + 2%CO2) leads to shorter total deposition time (), assuming the same idle time between layers, more significantly when the wall length is longer, followed by Blend 3 (Ar + 1%CO2 + 1%H2) and Blend 2 (Ar + 2%H2 + 20%He + 500 ppm CO2) in a row (but idle time can be managed and shortened by the shielding gas itself (leading to lower heat input) or externally (active cooling)).
- The composition of the gases affects the heat generated in the arc and that which is transferred to the previous layer (consequently, the layer dimension, wall finish, and metallurgical features are governed by the shielding). For a similar deposition rate per unit of layer length and mean current:
- ○
- Blend 1 (Ar + 2%CO2) leads to narrower total and effective widths and, consequently, a taller layer height than Blend 2 (Ar + 2%H2 + 20%He + 500 ppm CO2) and Blend 3 (Ar + 1%CO2 + 1%H2);
- ○
- Blend 1 (Ar + 2%CO2) and Blend 3 (Ar + 1%CO2 + 1%H2) provide similar surface waviness, which was slightly worse when Blend 2 (Ar + 2%H2 + 20%He + 500 ppm CO2) was shielding the deposition;
- ○
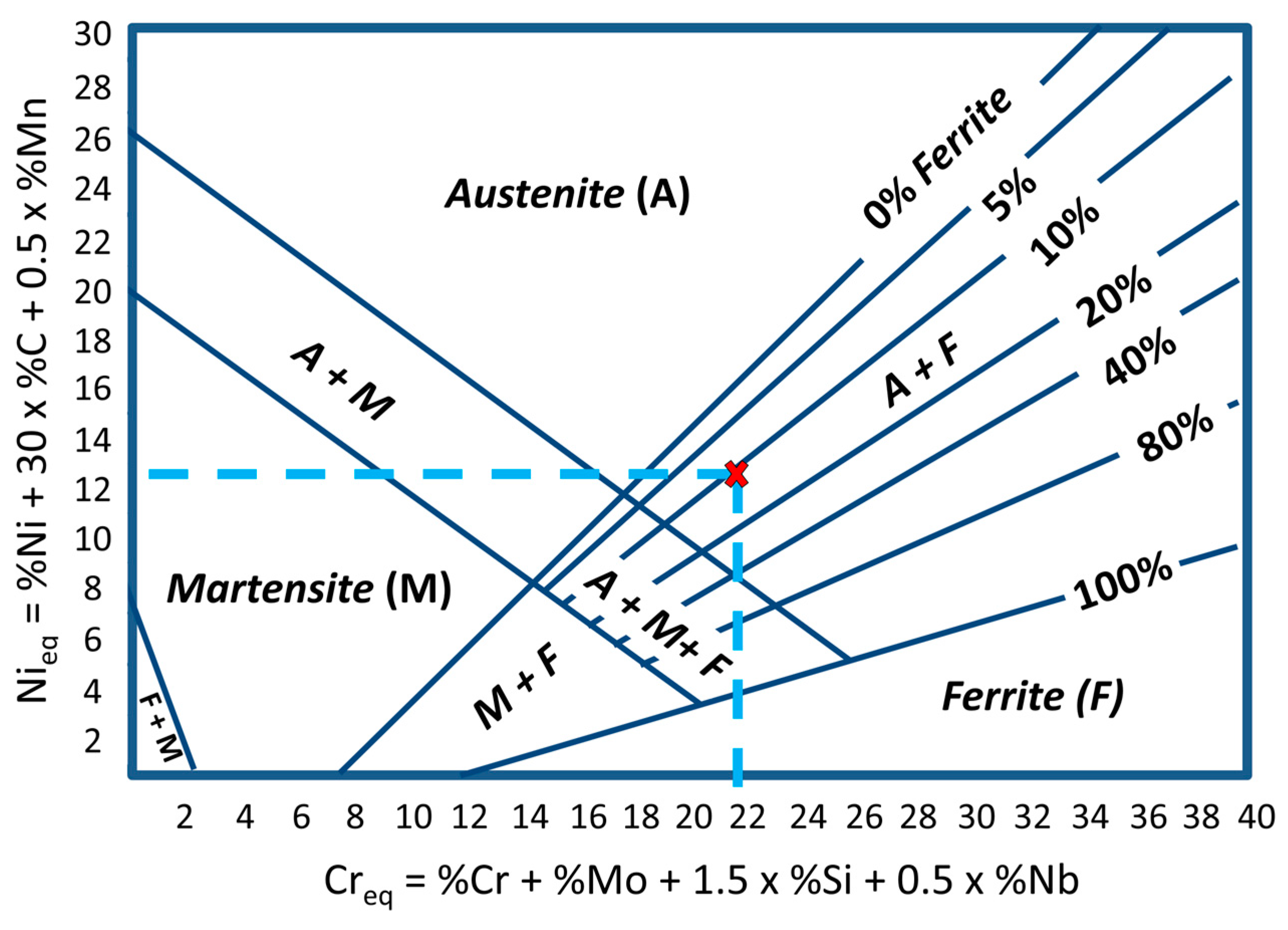
- Blend 2 (Ar + 2%H2 + 20%He + 500 ppm CO2) produces layers with a lower amount of delta ferrite than the other blends. There is no considerable influence of the shielding gas on the microhardness of the build.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norrish, J. Advanced Welding Processes—Technologies and Process Control, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 58–73. [Google Scholar]
- Nobrega, G.; Souza, M.S.; Rodríguez-Martín, M.; Rodríguez-Gonzálvez, P.; Ribeiro, J. Parametric Optimization of the GMAW Welding Process in Thin Thickness of Austenitic Stainless Steel by Taguchi Method. Appl. Sci. 2021, 11, 8742. [Google Scholar] [CrossRef]
- Tasalloti, H.; Kah, P.; Martikainen, J. Effects of welding wire and torch weaving on GMAW of S355MC and AISI 304L dissimilar welds. Int. J. Adv. Manuf. Technol. 2014, 71, 197–205. [Google Scholar] [CrossRef]
- Scotti, A. Mapping transfer modes for stainless steel gas metal arc welding. Sci. Technol. Weld. Join. 2000, 5, 227–234. [Google Scholar] [CrossRef]
- Hertel, M.; Niese, J.; Rose, S.; Häßler, M.; Füssel, U.; Uhrlandt, D. Experimental and numerical investigations into the influence of the shielding gas composition on the GMA spray arc process. Weld. Cut. 2015, 14, 234–241. [Google Scholar]
- Ley, F.H.; Campbell, S.W.; Galloway, A.M.; McPherson, N.A. Effect of shielding gas parameters on weld metal thermal properties in gas metal arc welding. Int. J. Adv. Manuf. Technol. 2015, 80, 1213–1221. [Google Scholar] [CrossRef]
- Murphy, A.B.; Tanaka, M.; Yamamoto, K.; Tashiro, S.; Sato, T.; Lowke, J.J. Modelling of thermal plasmas for arc welding: The role of the shielding gas properties and of metal vapour. J. Phys. D Appl. Phys. 2009, 42, 194006. [Google Scholar] [CrossRef]
- Rao, Z.H.; Hu, J.; Liao, S.M.; Tsai, H.L. Modeling of the transport phenomena in GMAW using argon–helium mixtures. Part I—The arc. Int. J. Heat Mass Transf. 2010, 53, 5707–5721. [Google Scholar] [CrossRef]
- Ferreira Filho, D.F.; Ferraresi, V.A.; Scotti, A. Shielding gas influence on the ferritic stainless steel weldability. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2010, 224, 951–961. [Google Scholar] [CrossRef]
- Liao, M.T.; Chen, W.J. The effect of shielding-gas compositions on the microstructure and mechanical properties of stainless steel weldments. Mater. Chem. Phys. 1998, 55, 145–155. [Google Scholar] [CrossRef]
- Araújo, H.R.; Torres, E.A.; Apolinário, L.H.R.; Vicente, A.A.; Silva, D.C., Jr.; Santos, T.F.A. Evaluation of mechanical performance and microstructural aspects of AISI 304 stainless steel welded joints produced by controlled short circuit GMAW and GTAW. Weld. World 2022, 66, 2443–2459. [Google Scholar] [CrossRef]
- Kannan, A.R.; Shanmugam, N.S.; Vendan, S.A. Effect of cold metal transfer process parameters on microstructural evolution and mechanical properties of AISI 316L tailor welded blanks. Int. J. Adv. Manuf. Technol. 2019, 103, 4265–4282. [Google Scholar] [CrossRef]
- Vishnukumar, M.; Muthupandi, V.; Jerome, S. Effect of post-heat treatment on the mechanical and corrosion behaviour of SS316L fabricated by Directed Energy Deposition. Mater. Lett. 2022, 307, 131015. [Google Scholar] [CrossRef]
- Laghi, V.; Palermo, M.; Gasparini, G.; Veljkovic, M.; Trombetti, T. Assessment of design mechanical parameters and partial safety factors for Wire-and-Arc Additive Manufactured stainless steel. Eng. Struct. 2020, 225, 111314. [Google Scholar] [CrossRef]
- Mvola, B.; Kah, P. Effects of shielding gas control: Welded joint properties in GMAW process optimisation. Int. J. Adv. Manuf. Technol. 2017, 88, 2369–2387. [Google Scholar] [CrossRef]
- Cai, X.; Fan, C.; Lin, S.; Ji, X.; Yang, C. Optimisation of shielding gas composition in narrow gap GMA welding based on response surface methodology. Int. J. Adv. Manuf. Technol. 2018, 95, 2405–2412. [Google Scholar] [CrossRef]
- Shackleton, D.N.; Lucas, W. Shielding Gas Mixtures for High Quality Mechanized GMA Welding of Q&T Steel. Weld. J. 1974, 53, 537s–547s. [Google Scholar]
- Lozano, J.; Moreda, P.; Lorente, C.L.; Bilmes, P.D. Fusion characteristics of austenitic stainless steel GMAW welds. Lat. Am. Appl. Res. 2003, 33, 27–31. [Google Scholar]
- Costanza, G.; Sili, A.; Tata, M.E. Weldability of austenitic stainless steel by metal arc welding with different shielding gas. Procedia Struct. Integr. 2016, 2, 3508–3514. [Google Scholar] [CrossRef]
- Liskevych, O.; Scotti, A. Influence of the CO2 content on operational performance of short-circuit GMAW. Weld. World 2015, 59, 217–224. [Google Scholar] [CrossRef]
- Souza, D.; Rossi, M.L.; Keocheguerians, F.; Nascimento, V.C.; Vilarinho, L.O.; Scotti, A. Influence of the welding parameter setting on the stability of the MIG/MAG process operating in short circuit. Soldag. Insp. 2011, 16, 22–32. [Google Scholar] [CrossRef]
- Ferreira Filho, D.F.; Ferraresi, V.A. The influence of gas shielding composition and contact tip to work distance in short circuit metal transfer of ferritic stainless steel. Weld. Int. 2010, 24, 206–213. [Google Scholar] [CrossRef]
- Jonsson, P.G.; Murphy, A.B.; Szekely, J. Oxygen additions on argon-shielded gas metal arc welding processes. Weld. J. 1995, 74, 48s–58s. [Google Scholar]
- Cai, X.; Lin, S.; Murphy, A.B.; Dong, B.; Fan, C.; Yang, C. Influence of helium content on a ternary-gas-shielded GMAW process. Weld. World 2018, 62, 973–984. [Google Scholar] [CrossRef]
- Ebrahimnia, M.; Goodarzi, M.; Nouri, M.; Sheikhi, M. Study of the effect of shielding gas composition on the mechanical weld properties of steel ST 37-2 in gas metal arc welding. Mater. Des. 2009, 30, 3891–3895. [Google Scholar] [CrossRef]
- Açar, İ.; Çevik, B.; Gülenç, B. The effect of shielding gas on weldability of the AISI 420 martensitic stainless steel. Pract. Metallogr. 2023, 60, 108–128. [Google Scholar] [CrossRef]
- Gülenç, B.; Develi, K.; Kahraman, N.; Durgutlu, A. Experimental study of the effect of hydrogen in argon as a shielding gas in MIG welding of austenitic stainless steel. Int. J. Hydrogen Energy 2005, 30, 1475–1481. [Google Scholar] [CrossRef]
- Silwal, B.; Nycz, A.; Masuo, C.J.; Noakes, M.W.; Marsh, D.; Vaughan, D. An experimental investigation of the effectiveness of Ar-CO2 shielding gas mixture for the wire arc additive process. Int. J. Adv. Manuf. Technol. 2020, 108, 1285–1296. [Google Scholar] [CrossRef]
- Arana, M.; Ukar, E.; Rodriguez, I.; Iturrioz, A.; Alvarez, P. Strategies to Reduce Porosity in Al-Mg WAAM Parts and Their Impact on Mechanical Properties. Metals 2021, 11, 524. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Ning, J.; Wang, X.; Zhang, G.; Zhang, J.; Na, S.; Fatemeh, B. Comparative study on the microstructures and properties of wire+arc additively manufactured 5356 aluminium alloy with argon and nitrogen as the shielding gas. Addit. Manuf. 2020, 34, 101206. [Google Scholar] [CrossRef]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire + Arc Additive Manufacture of 17-4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2019, 268, 54–62. [Google Scholar] [CrossRef]
- Roy, S.; Silwal, B.; Nycz, A.; Noakes, M.; Cakmak, E.; Nandwana, P.; Yamamoto, Y. Investigating the effect of different shielding gas mixtures on microstructure and mechanical properties of 410 stainless steel fabricated via large scale additive manufacturing. Addit. Manuf. 2021, 38, 101821. [Google Scholar] [CrossRef]
- Scotti, A.; Ponomarev, V. MIG/MAG Welding—Better Understanding, Better Performance, 2nd ed.; Artliber: São Paulo, Brazil, 2008; pp. 96–107. (In Portuguese) [Google Scholar]
- Jorge, V.L.; Teixeira, F.R.; Scotti, A.; Scotti, F.M.; Siewert, E. The significance of supplementary shielding in WAAM of aluminium thin walls. J. Manuf. Process. 2023, 106, 520–536. [Google Scholar] [CrossRef]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 141–225. [Google Scholar]
- Yin, H.; Song, M.; Deng, P.; Li, L.; Prorok, B.C.; Lou, X. Thermal stability and microstructural evolution of additively manufactured 316L stainless steel by laser powder bed fusion at 500–800 °C. Addit. Manuf. 2021, 41, 101981. [Google Scholar] [CrossRef]
- Jorge, V.L.; Teixeira, F.R.; Scotti, A. Pyrometrical interlayer temperature measurement in WAAM of thin wall: Strategies, limitations and functionality. Metals 2022, 12, 765. [Google Scholar] [CrossRef]
- Scotti, A.; Batista, M.A.; Eshagh, M. Inaccuracy in arc power calculation through a product of voltage and current averages. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 11. [Google Scholar] [CrossRef]
- Vilarinho, L.O.; Araújo, D.B. Curtoweld, Computer Program Log. BR Patent Nº BR512019002019-7, 2019. [Google Scholar]
- Jorge, V.L.; Scotti, F.M.; Reis, R.P.; Scotti, A. Wire feed pulsation as a means of inducing surface tension metal transfer in Gas Metal Arc Welding. J. Manuf. Process. 2021, 62, 655–669. [Google Scholar] [CrossRef]
- Teixeira, F.R.; Scotti, F.M.; Jorge, V.L.; Scotti, A. Combined effect of the interlayer temperature with travel speed on features of thin wall WAAM under two cooling approaches. Int. J. Adv. Manuf. Technol. 2023, 126, 273–289. [Google Scholar] [CrossRef]
- Lancaster, J.F. The Physics of Welding, 2nd ed.; Pergamon Press: Oxford, UK, 1996; pp. 9–40. [Google Scholar]
- Wang, L.; Xue, J.; Wang, Q. Correlation between arc mode, microstructure, and mechanical properties during wire arc additive manufacturing of 316L stainless steel. Mater. Sci. Eng. A 2019, 751, 183–190. [Google Scholar] [CrossRef]
- Feenstra, D.R.; Cruz, V.; Gao, X.; Molotnikov, A.; Birbilis, N. Effect of build height on the properties of large format stainless steel 316L fabricated via directed energy deposition. Addit. Manuf. 2020, 34, 101205. [Google Scholar] [CrossRef]
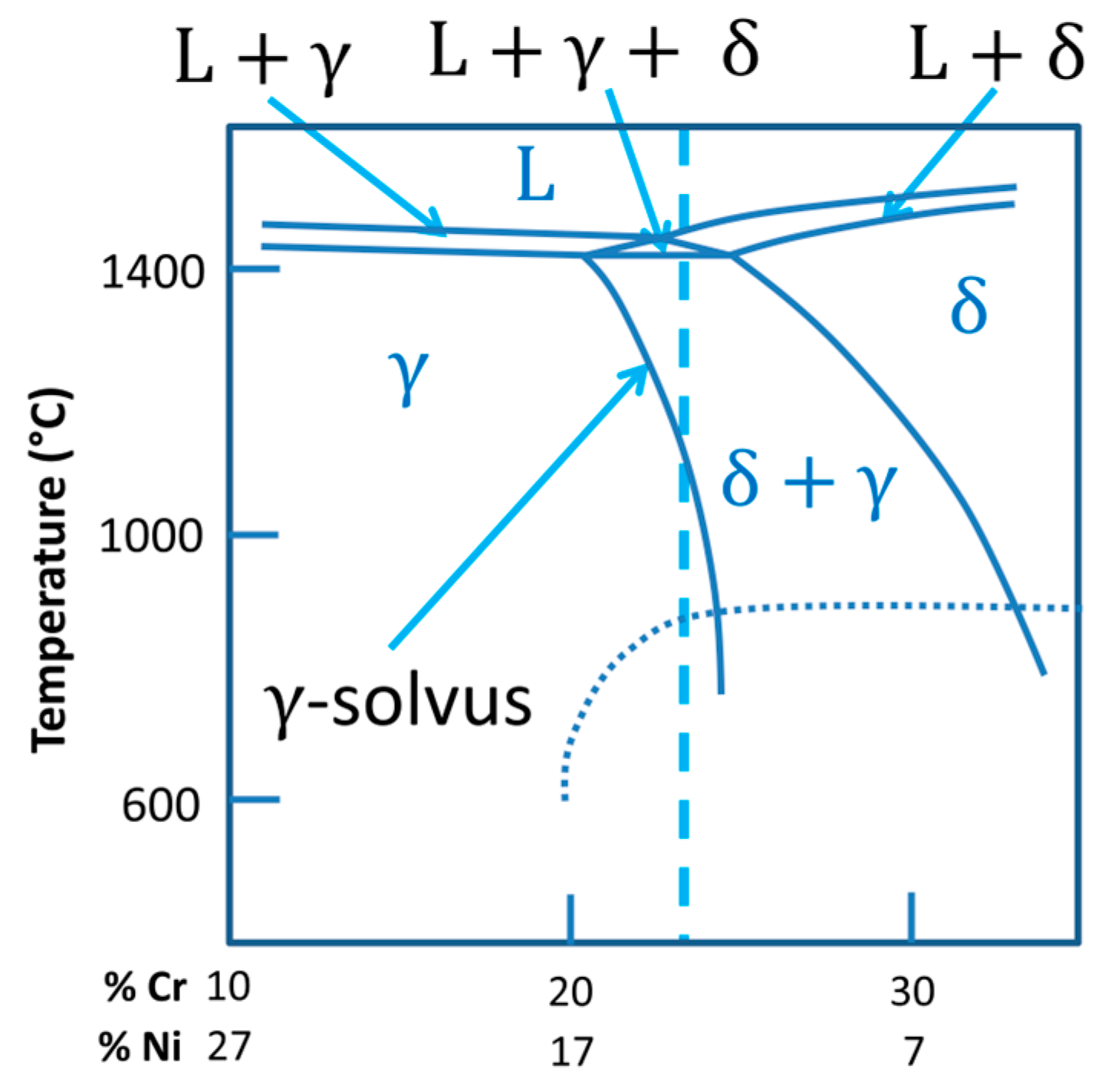
- Kou, S. Welding Metallurgy, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003; pp. 216–241. [Google Scholar]
- Schaeffler, A.L. Constitution diagram for stainless steel weld metal. Met. Prog. 1949, 56, 680. [Google Scholar]
- Elmer, J.W.; Vaja, J.; Carpenter, J.S.; Coughlin, D.R.; Dvornak, M.J.; Hochanadel, P.; Gurung, P.; Johnson, A.; Gibbs, G. Wire-based additive manufacturing of stainless steel components. Weld. J. 2020, 99, 8s–24s. [Google Scholar] [CrossRef]
- David, S.A. Ferrite Morphology and Variations in Ferrite Content in Austenitic Stainless Steel Welds. Weld. J. 1981, 60, 63s–71s. [Google Scholar]
- Le, V.T.; Mai, D.S.; Bui, M.C.; Wasmer, K.; Nguyen, V.A.; Dinh, D.M.; Nguyen, V.C.; Vu, D. Influences of the process parameter and thermal cycles on the quality of 308L stainless steel walls produced by additive manufacturing utilising an arc welding source. Weld. World 2022, 66, 1565–1580. [Google Scholar] [CrossRef]
- Chen, M.H.; Chou, C.P. Effect of thermal cycles on ferrite content of austenitic stainless steel. Sci. Technol. Weld. Join. 1999, 4, 58–62. [Google Scholar] [CrossRef]
- Lundin, C.D.; Chou, C.P.D. Fissuring in the “Hazard HAZ” region of austenitic stainless steel welds. Weld. J. 1985, 64, 113s–118s. [Google Scholar]
- Kou, S.; Le, Y. The effect of quenching on the solidification structure and transformation behavior of stainless steel welds. Metall. Trans. A 1982, 13, 1141–1152. [Google Scholar] [CrossRef]
- Zappa, S.; Svoboda, H.G.; Surian, E.S. Supermartensitic Stainless Steel Deposits: Effects of Shielding Gas and Postweld Heat Treatment. Weld. J. 2013, 92, 297s–303s. [Google Scholar]



| Gas | WFSset (m/min) | DS (cm/min) | AL | DC | Im (A) | Um (V) | WFSm (m/min) | Em (J/mm) | WFSm/ DS |
|---|---|---|---|---|---|---|---|---|---|
| Blend 1 | 4.9 | 35.0 | −30 | −4 | 112.7 ± 0.8 | 11.6 ± 0.2 | 3.8 ± 0.1 | 367.5 ± 3.7 | 10.9 ± 0.2 |
| Blend 2 | 5.7 | 36.8 | −30 | −5 | 110.9 ± 1.4 | 15.0 ± 0.3 | 4.0 ± 0.1 | 442.5 ± 8.7 | 10.9 ± 0.3 |
| Blend 3 | 5.2 | 33.4 | −30 | −4 | 111.8 ± 1.1 | 12.1 ± 0.2 | 3.8 ± 0.1 | 401.7 ± 3.6 | 11.2 ± 0.2 |
| Gas | WFSm (m/min) | DS (cm/min) | WFSm/ DS | Fcc (Hz) | IVsc | tarc (ms) | tsc (ms) | tarc/tsc |
|---|---|---|---|---|---|---|---|---|
| Blend 1 | 3.8 ± 0.1 | 35.0 | 10.9 ± 0.2 | 66.9 ± 0.7 | 0.05 ± 0.02 | 7.1 ± 0.1 | 7.8 ± 0.1 | 0.9 ± 0.0 |
| Blend 2 | 4.0 ± 0.1 | 36.8 | 10.9 ± 0.3 | 57.8 ± 1.1 | 0.11 ± 0.03 | 8.3 ± 0.2 | 9.0 ± 0.2 | 0.9 ± 0.0 |
| Blend 3 | 3.8 ± 0.1 | 33.4 | 11.2 ± 0.2 | 60.8 ± 0.7 | 0.06 ± 0.02 | 7.5 ± 0.1 | 9.0 ± 0.2 | 0.8 ± 0.0 |
| C | Mn | Si | S | P | Ni | Cr | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.018 | 1.770 | 0.880 | 0.006 | 0.024 | 11.230 | 18.380 | 2.080 | 0.100 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, F.R.; Jorge, V.L.; Scotti, F.M.; Siewert, E.; Scotti, A. A Methodology for Shielding-Gas Selection in Wire Arc Additive Manufacturing with Stainless Steel. Materials 2024, 17, 3328. https://doi.org/10.3390/ma17133328
Teixeira FR, Jorge VL, Scotti FM, Siewert E, Scotti A. A Methodology for Shielding-Gas Selection in Wire Arc Additive Manufacturing with Stainless Steel. Materials. 2024; 17(13):3328. https://doi.org/10.3390/ma17133328
Chicago/Turabian StyleTeixeira, Felipe Ribeiro, Vinicius Lemes Jorge, Fernando Matos Scotti, Erwan Siewert, and Americo Scotti. 2024. "A Methodology for Shielding-Gas Selection in Wire Arc Additive Manufacturing with Stainless Steel" Materials 17, no. 13: 3328. https://doi.org/10.3390/ma17133328






