Effect of Continuous Casting and Heat Treatment Parameters on the Microstructure and Mechanical Properties of Recycled EN AW-2007 Alloy
Abstract
:1. Introduction
- Aerospace components like fittings, fasteners, and brackets;
- Automotive parts, such as engine and transmission components;
- Military applications requiring precision-machined parts;
- Industrial machinery components like gears and shafts.
2. Materials and Methods
Microscopic Examination
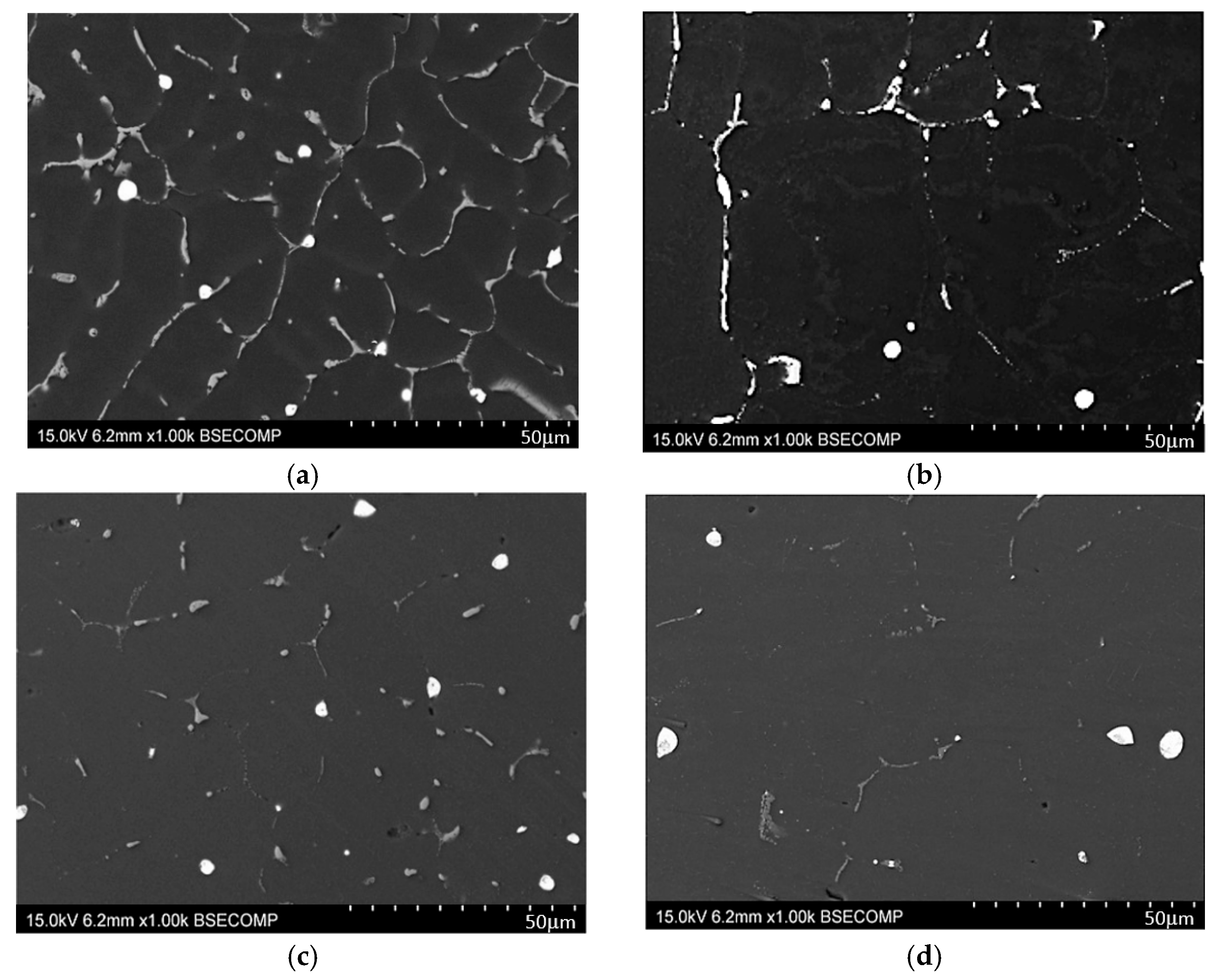
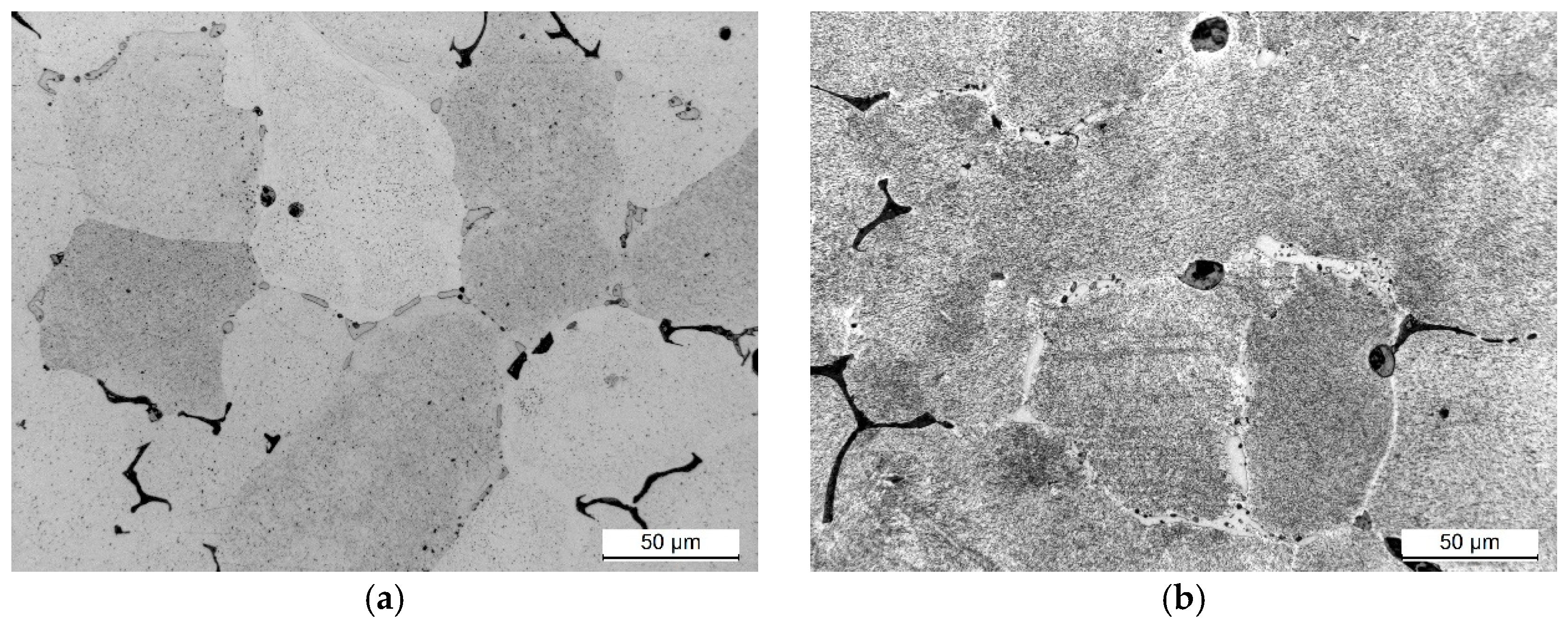
3. Results and Discussion
4. Conclusions
- The findings presented in this article confirm the feasibility of producing EN AW-2007 alloy ingots from scrap via continuous casting. The observed macrostructure and microstructure of the ingots showed no discontinuities or casting defects and were characterized by a uniform and fine-grained structure throughout the cross-section. The ingots displayed good mechanical properties in the as-cast state, with a tensile strength (Rm) of 286.8 MPa, yield strength (R0.2) of 160.0 MPa, hardness of 84 HB, and relative elongation (A5) of 8.2%.
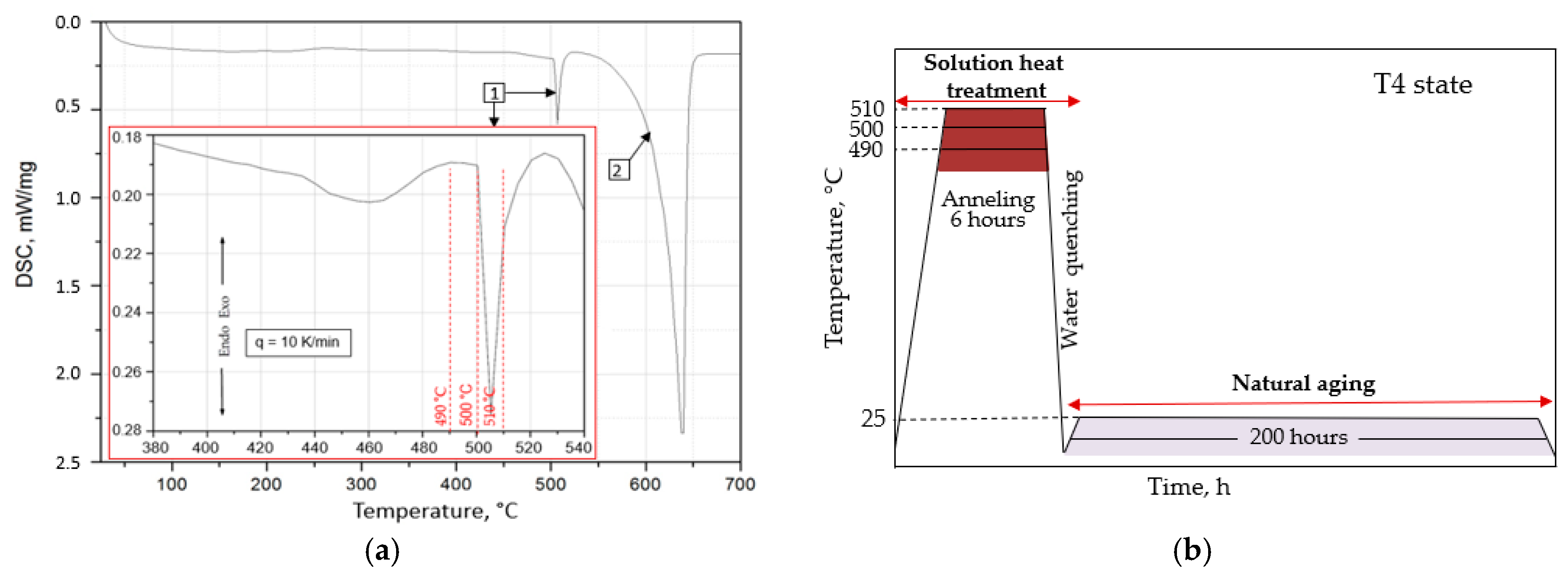
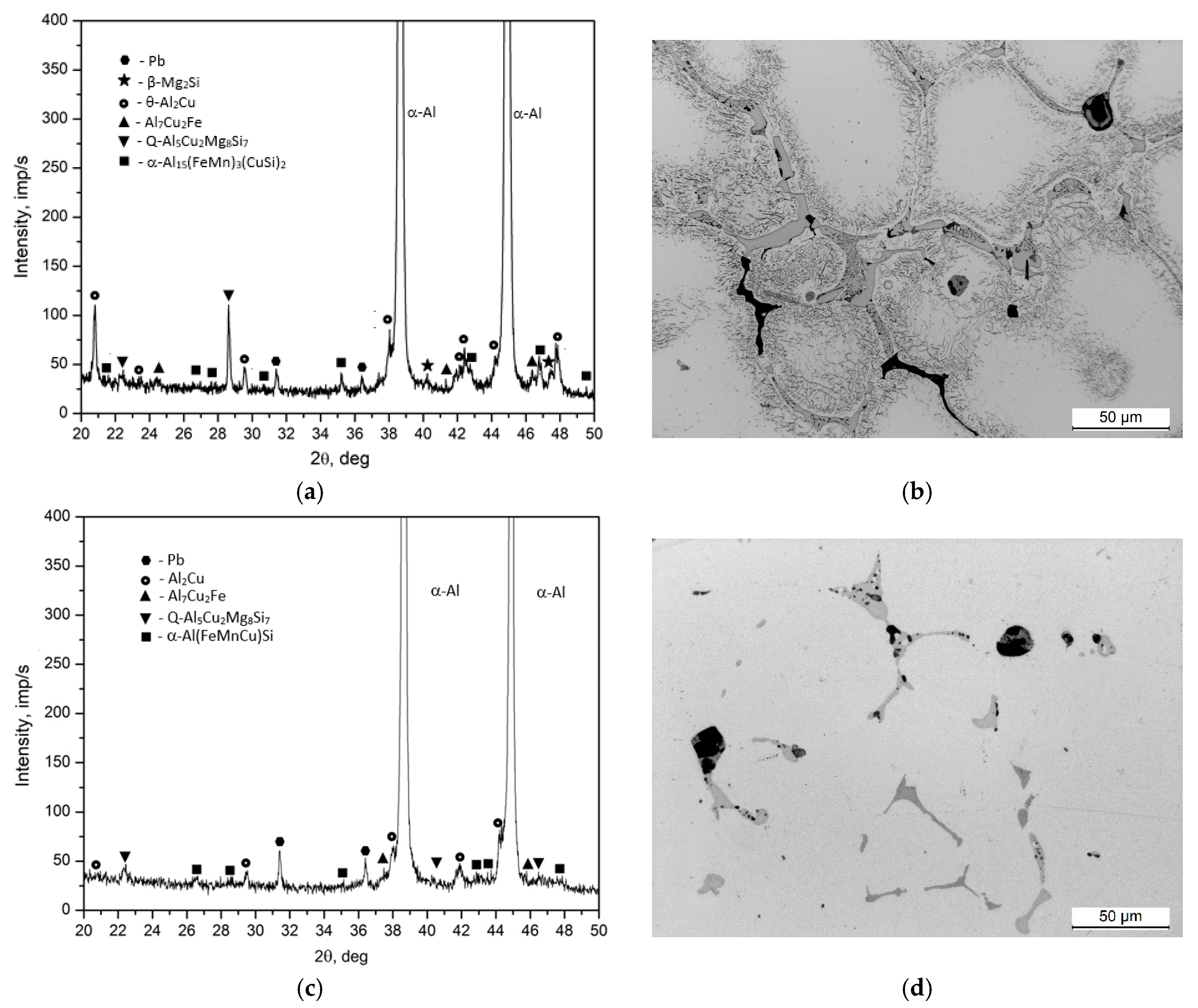
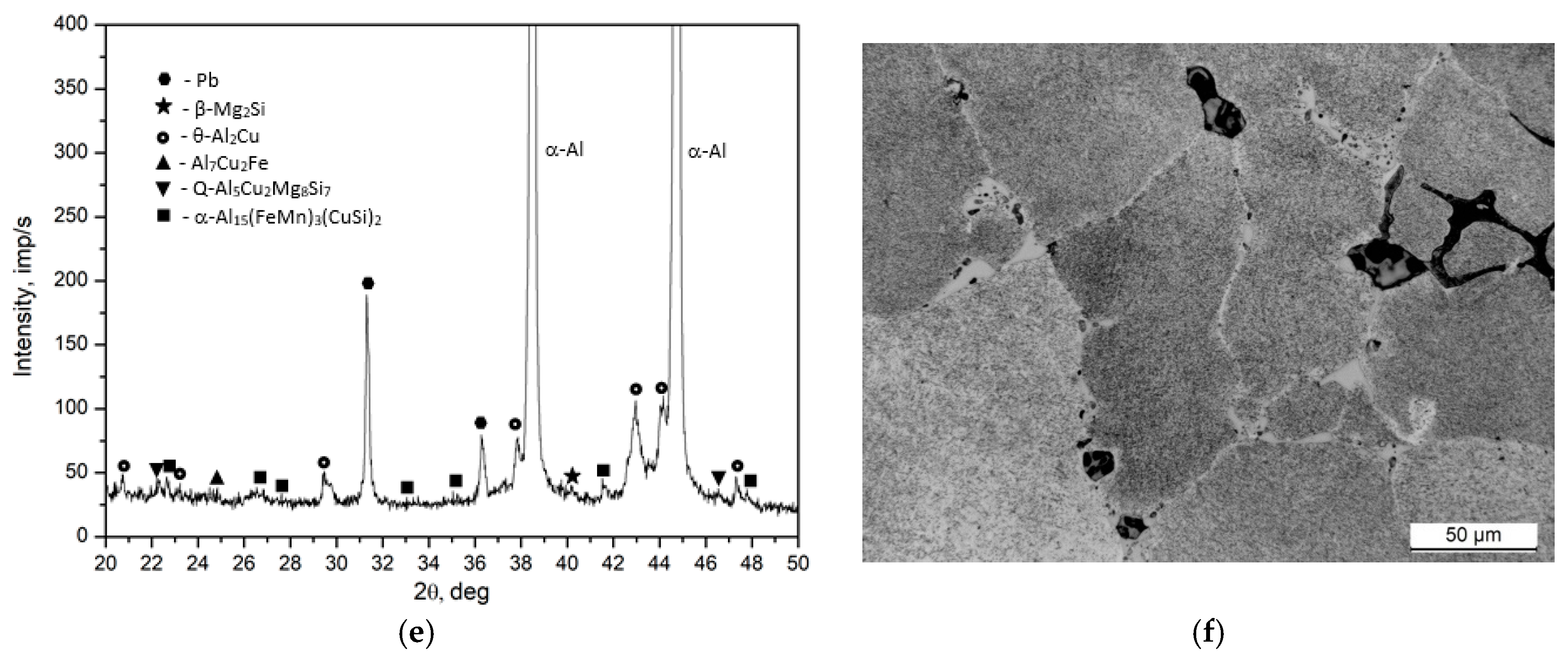
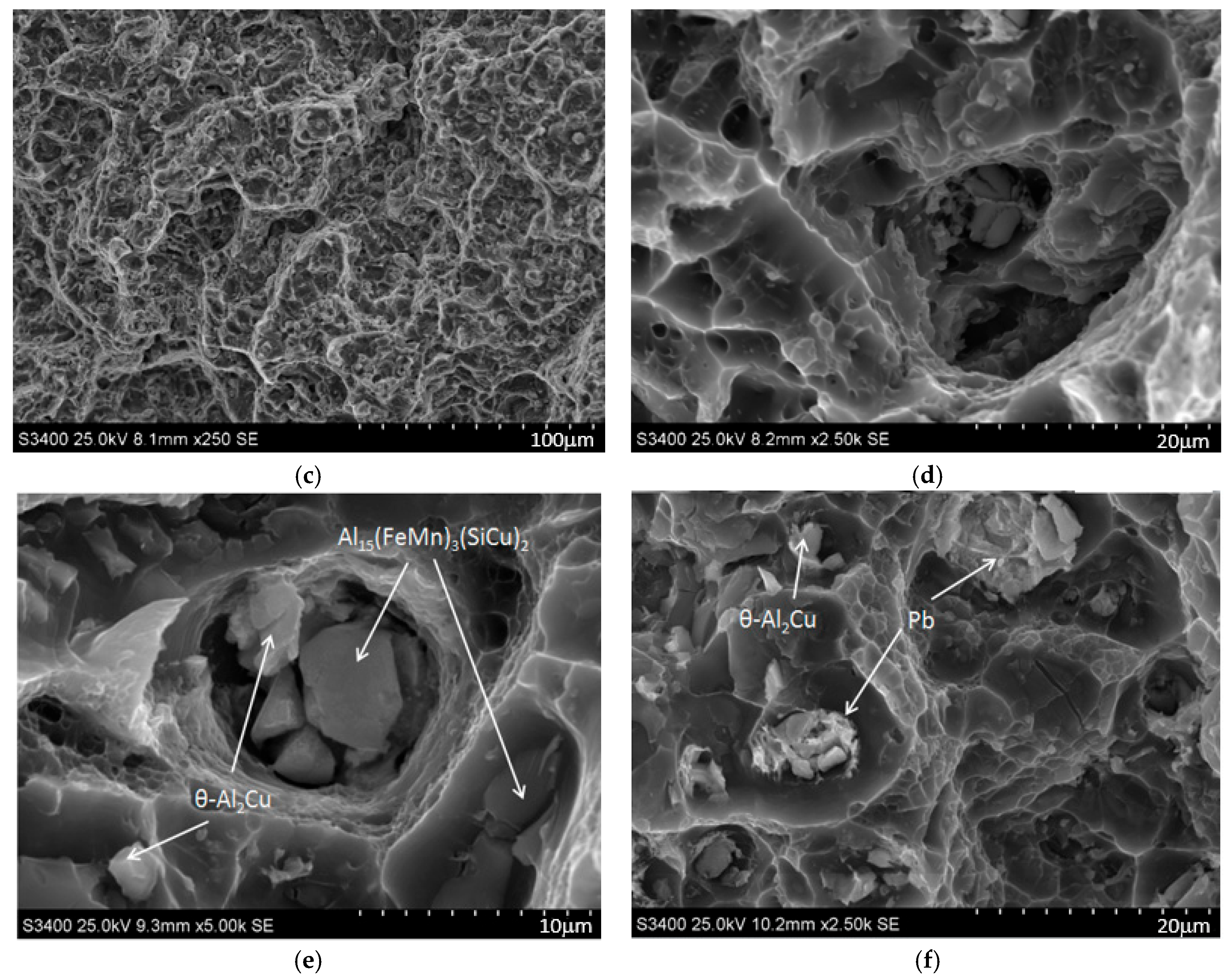
- The microstructure of the EN AW-2007 alloy ingot comprises α-Al solid solution dendrites and precipitates of intermetallic phases, including two-component θ-Al2Cu and β-Mg2Si phases, a three-component Al7Cu2Fe phase, a four-component Q-Al4Cu2Mg8Si7 phase, a five-component α-Al15(FeMn)3(SiCu)2 phase, and Pb particles. During annealing for supersaturation, primary particles of intermetallic phases, particularly θ-Al2Cu, Q-Al4Cu2Mg8Si7, and β-Mg2Si, dissolved into the α-Al solid solution. However, Pb particles remained undissolved, retaining their spheroidal shape as in the cast state.
- The heat treatment parameters that achieved the T4 strengthening state for the EN AW-2007 cast alloy, yielding the highest hardness of 124.8 HB, included annealing at 500 °C for 6 h, supersaturation in cold water, and natural aging for 80 h. Conversely, the highest tensile strength (Rm) of 435 MPa, along with the best plasticity (A5 = 18.1%), were attained after only 9 h of natural aging.
- Fractographic studies revealed that regardless of the heat treatment parameters, decohesion under tensile stress in the studied alloy occurs through the nucleation, growth, and coalescence of voids. Additionally, it was observed that in areas containing primary undissolved Pb particles and θ-Al2Cu phases, the decohesion process initiated at the matrix–particle interface. Particles of primary, hard, and brittle phases that contain Fe, such as α-Al15(FeMn)3(SiCu)2 and Al7Cu2Fe, were found to fragment under tensile loading.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatch, J.E. Aluminium: Properties and Physical Metallurgy; ASM Metals Park: Ohio, OH, USA, 1984. [Google Scholar]
- Singh, G.; Goyal, S.; Sharma, N.; Sharma, P. A comprehensive study on aluminium alloy series—A review. Recent Adv. Mech. Eng. 2017, 1, 11–27. [Google Scholar]
- MacKenzie, S.D.; Totten, G.E. Analytical Characterization of Aluminium, Steel and Superalloys; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- King, F. Aluminium and Its Alloys; John Willey and Sons: New York, NY, USA; Chichester, UK; Brisbane, QLD, Australia; Toronto, BC, Canada, 1987. [Google Scholar]
- Kaufman, J.G. Aluminum Alloys—Mechanical Engineers’ Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Polmear, I.J. Light Alloys: Metallurgy of Light Metals; Arnold: London, UK; New York, NY, USA; Sydney, NSW, Australia; Auckland, New Zealand, 1995. [Google Scholar]
- Davis, J.R. ASM Specialty Handbook: Aluminium and Aluminium Alloys; International Materials Information Society: Materials Park, OH, USA, 1998. [Google Scholar]
- Mrówka-Nowotnik, G.; Gancarczyk, K.; Nowotnik, A.; Dychtoń, K.; Boczkal, G. Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling. Materials 2021, 14, 89. [Google Scholar]
- Mrówka-Nowotnik, G. Influence of chemical composition variation and heat treatment on microstructure and mechanical properties of 6xxx alloys. Arch. Mater. Sci. Eng. 2010, 46, 98–107. [Google Scholar]
- Schlesinger, M.E. Aluminium Recycling; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2014. [Google Scholar]
- Jirang, C.; Roven, H.J. Recycling of automotive aluminum. Trans. Nonferrous Met. Soc. China 2010, 20, 2057–2063. [Google Scholar]
- Hurtalová, L.; Tillová, E.; Chalupová, M. Effect of heat treatment on fracture surfaces in recycled aluminium cast alloy. Transp. Eng. 2013, 41, 117–122. [Google Scholar] [CrossRef]
- Chino, Y.; Mabuchi, M.; Otsuka, S.; Shimojima, K.; Hosokawa, H.; Yamada, Y.; Wen, C.; Iwasaki, H. Corrosion and Mechanical Properties of Recycled 5083 Aluminum Alloy by Solid State Recycling. Mater. Trans. 2003, 44, 1284–1289. [Google Scholar] [CrossRef]
- Nur, K.Y.; Mohd, A.L.; Azlan, A. Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity. Materials 2017, 10, 902. [Google Scholar] [CrossRef]
- Tenorio, J.A.S.; Espinosa, D.C. Encyclopedia of Aluminum and Its Alloys. Recycling of Aluminium; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; Volume 2, pp. 2341–2360. [Google Scholar]
- Gronostajski, J.; Marciniak, H.; Matuszak, A. New methods of aluminium and aluminium-alloy chips recycling. J. Mater. Process. Technol. 2000, 106, 34–39. [Google Scholar] [CrossRef]
- Rahim, A.S.N.; Laji, M.A.; Ariffin, S. Review on Recycling Aluminum Chips by Hot Extrusion Process. Procedia CIRP 2015, 26, 761–766. [Google Scholar] [CrossRef]
- Samuel, M. A new technique for recycling aluminium scrap. J. Mater. Process. Technol. 2003, 135, 117–124. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Ruiz, N.E.M.; Simón, M.A.; Martinez, M.A. Recycling of aluminium alloy and aluminium matrix composite chips by pressing and hot extrusion. J. Mater. Process. Technol. 2003, 143, 792–795. [Google Scholar] [CrossRef]
- Gronostajski, J.Z.; Marciniak, H.; Matuszak, A. Production of composites on the base of AlCu4 alloy chips. J. Mater. Process. Technol. 1996, 60, 719–722. [Google Scholar] [CrossRef]
- Pawłowska, B.; Śliwa, R.E. Recycling aluminum chips by KoBo method. Met. Form. 2017, 28, 301–316. [Google Scholar]
- Das, S.K.; Kaufman, J.G. Recycling aluminum aerospace alloys. Ligth Met. 2007, 166, 1161–1165. [Google Scholar]
- Korbel, A.; Bochniak, W.; Śliwa, R.E.; Ostachowski, P.; Łagoda, M.; Kusion, Z.; Trzebuniak, B. Low-temperature consolidation of machining chips from hardly-deformable aluminum alloys. Met. Form. 2016, 27, 33–152. [Google Scholar]
- Fawad, T.; Nausheen, N.; Rasheed, A.; Baloch, F. Characterization of Material Properties of 2xxx Series Al-Alloys by Non Destructive Testing Techniques. J. Nondestruct. Eval. 2012, 3, 117–133. [Google Scholar]
- Xia, Q.K.; Liu, Z.Y.; Li, Y.T. Microstructure and properties of Al-Cu-Mg-Ag alloy exposed at 200 °C with and without stress. Trans. Nonferrous Met. Soc. China Engl. Ed. 2008, 18, 789–794. [Google Scholar] [CrossRef]
- Mrówka, N.G.; Sieniawski, J.; Nowotnik, A.; Gradzik, A. Analysis of precipitation strengthening process in 2xxx aluminium alloys. Inżynieria Mater. 2016, 37, 104–108. [Google Scholar]
- Xu, R.; Lin, B.; Li, H.; Xiao, H.; Zhao, Y.; Zhang, W. Microstructure evolution and mechanical properties of Al−6.5 Cu−0.6 Mn−0.5 Fe alloys with different Si additions. Trans. Nonferrous Met. Soc. China 2019, 29, 1583–1591. [Google Scholar] [CrossRef]
- Palceka, P.; Porubcana, J.; Blažeka, D.; Trojanováb, Z. Internal Friction in Commercial Aluminium Alloy AW-2007. Procedia Eng. 2011, 10, 1226–1231. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A. Iron in Aluminium Alloys: Impurity and Alloying Element; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Warmuzek, M.; Gazda, A.; Sieniawski, J.; Mrówka, G. Processes of the formation of the Fe(Mn)-bearing intermetallic phases in the Al-Fe(Mn)-Si alloys. Adv. Mater. Sci. 2003, 2, 81–91. [Google Scholar]
- Bäckerud, L.; Król, E.; Tamminen, J. Solidification Characteristic of Aluminium Alloys. Wrought Alloys; Skanaluminium: Oslo, Norway, 1968. [Google Scholar]
- Kent, D.; Schaffer, G.B.; Drennan, J. Age hardening of a sintered Al–Cu–Mg–Si–(Sn) alloy. Mater. Sci. Eng. A 2005, 405, 65–73. [Google Scholar] [CrossRef]
- Ghosh, K.S. Calorimetric studies of 2024 Al–Cu–Mg and 2014 Al–Cu–Mg–Si alloys of various tempers. J. Therm. Anal. Calorim. 2019, 136, 447–459. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J.; Gao, N. Precipitation hardening in Al–Cu–Mg alloys revisited. Scr. Mater. 2006, 54, 287–291. [Google Scholar] [CrossRef]
- Banerjee, S.; Robi, P.S.; Srinivasan, A. Calorimetric study of precipitation kinetics of Al–Cu–Mg and Al–Cu–Mg-0.06 wt. % Sn alloys. Met. Mater. Int. 2010, 16, 523–531. [Google Scholar] [CrossRef]
- Bassani, P.; Gariboldi, E.; Vimercati, G. Calorimetric analysis on aged Al-4.4Cu-0.5 Mg-0.9Si-0.8Mn alloy (AA2014 grade). J. Therm. Anal. Calorim. 2007, 87, 247–253. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. The thermodynamics of and strengthening due to co-clusters: General theory and application to the case of Al–Cu–Mg alloys. Acta Mater. 2009, 57, 2376–2389. [Google Scholar] [CrossRef]
- Eskin, D.G. Decomposition of supersaturated solid solutions in Al–Cu–Mg–Si Alloys. J. Mater. Sci. 2003, 38, 279–290. [Google Scholar] [CrossRef]
- Hutchuinson, C.R.; Ringer, S.P. Precipitation process in Al–Cu–Mg alloys microalloyed with Si. Metall. Mater. Trans. A 2000, 31, 2721–2733. [Google Scholar] [CrossRef]
- Ringer, S.P.; Hono, K.; Sakurai, T.; Polmear, I.J. Cluster hardening in an aged Al–Cu–Mg alloy. Scr. Mater. 1997, 31, 517–521. [Google Scholar] [CrossRef]
- PN EN 573-1; Aluminium i Stopy Aluminium-Skład Chemiczny i Rodzaje Wyrobów Przerobionych Plastycznie-Część 1: System Oznaczeń Numerycznych. Polski Komitet Normalizacyjny: Warsaw, Poland, 2006.
- ICDD PDF-4+ Diffraction Data Base; International Centre for Diffraction Data: Newtown Square, PA, USA, 2020.
- PN-EN 10002-1:2004; Metale. Próba Rozciągania. Część 1: Metoda Badania w Temperaturze Otoczenia. Polski Komitet Normalizacyjny: Warsaw, Poland, 2004.
- ASTM Standard E8/E8M; Standard Test Methods of Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2013. Available online: www.astm.org (accessed on 1 June 2024).
- Data and Facts for Application EN AW-2007 Hardenable, Easily Workable Alloy. Available online: https://www.leichtmetall.eu/app/uploads/leichtmetall-data-sheet-EN-AW-2007.pdf (accessed on 1 June 2024).
- 2007-T4 Aluminum. Available online: https://www.makeitfrom.com/material-properties/2007-T4-Aluminum (accessed on 1 June 2024).
- ENAW-AlCu4PbMgMn (ENAW-2007). Available online: https://www.steelnumber.com/en/steel_alloy_composition_eu.php?name_id=1030 (accessed on 1 June 2024).
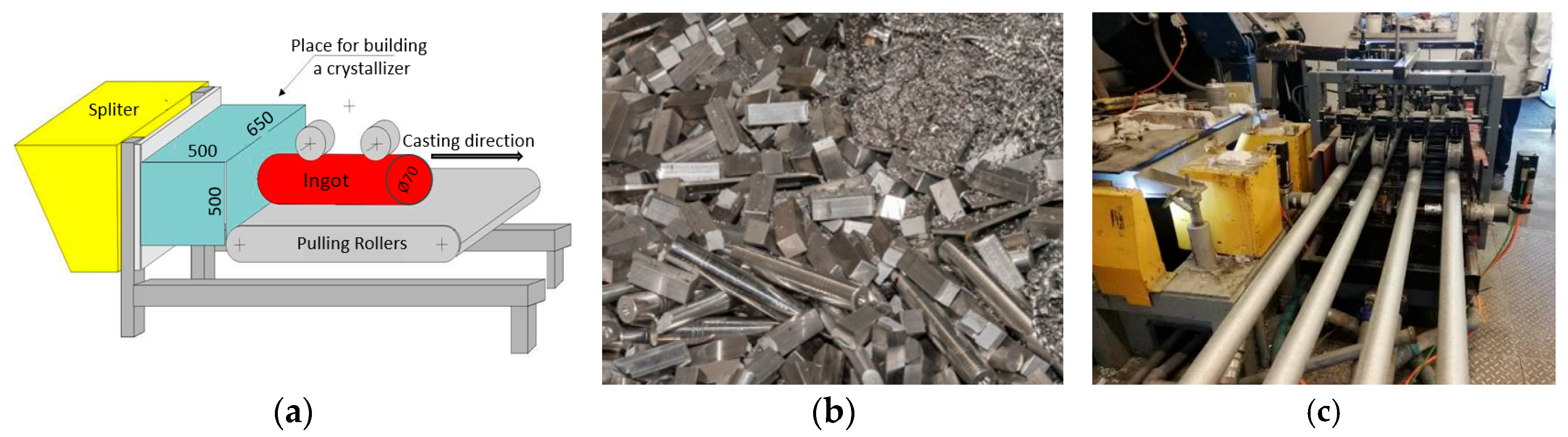
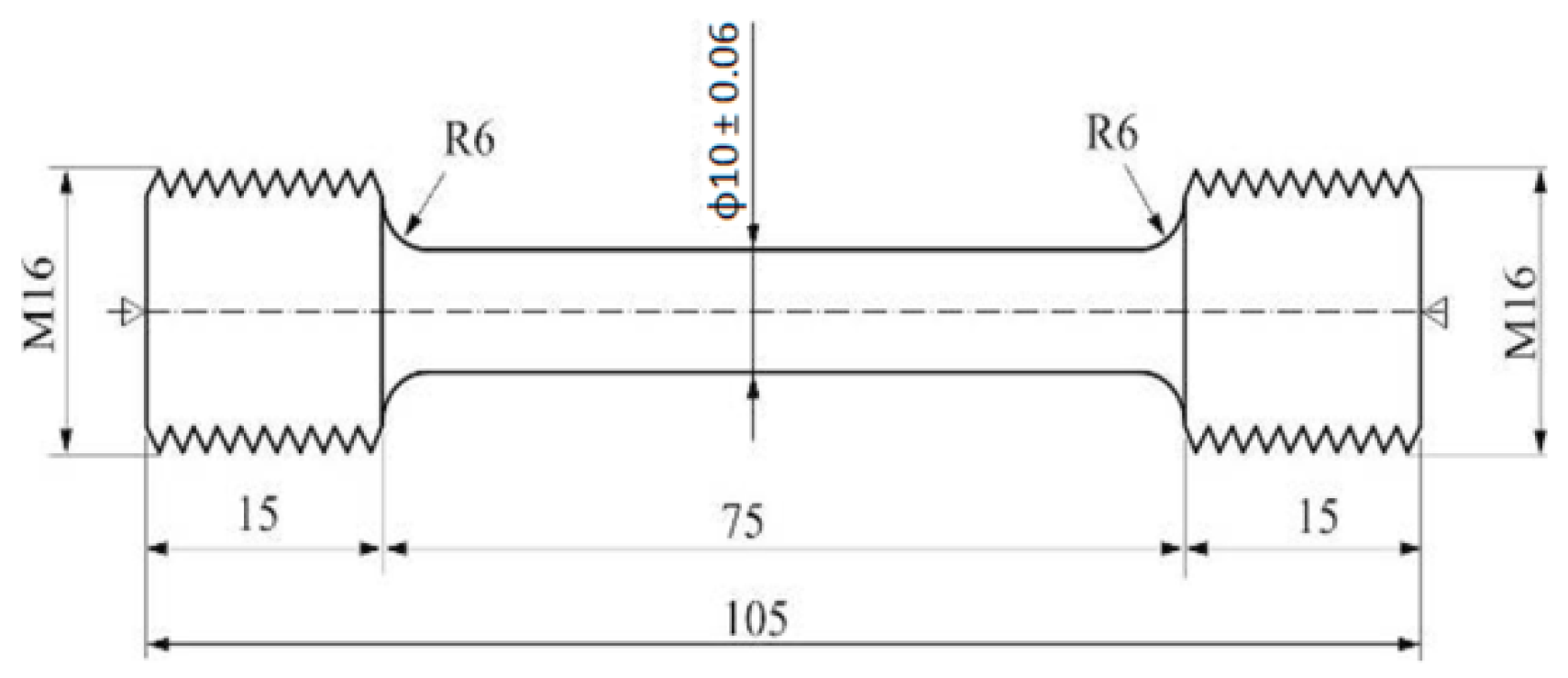
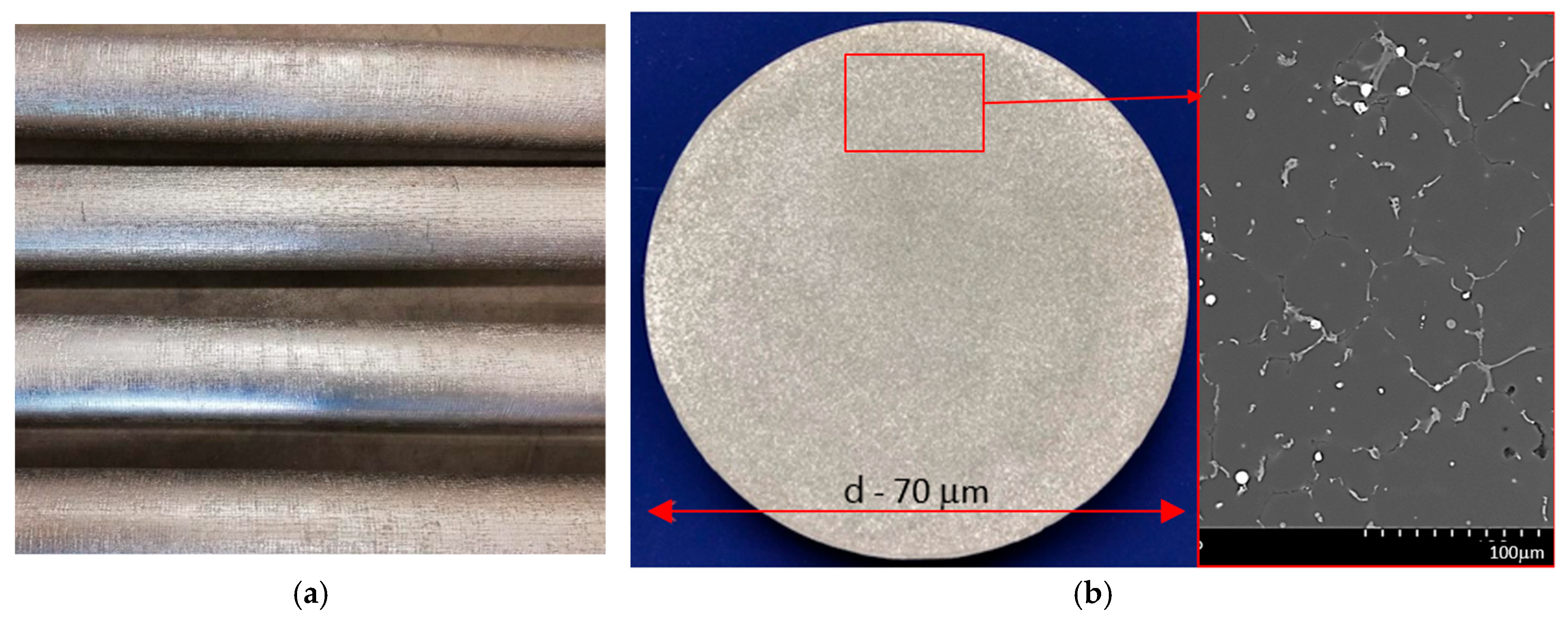


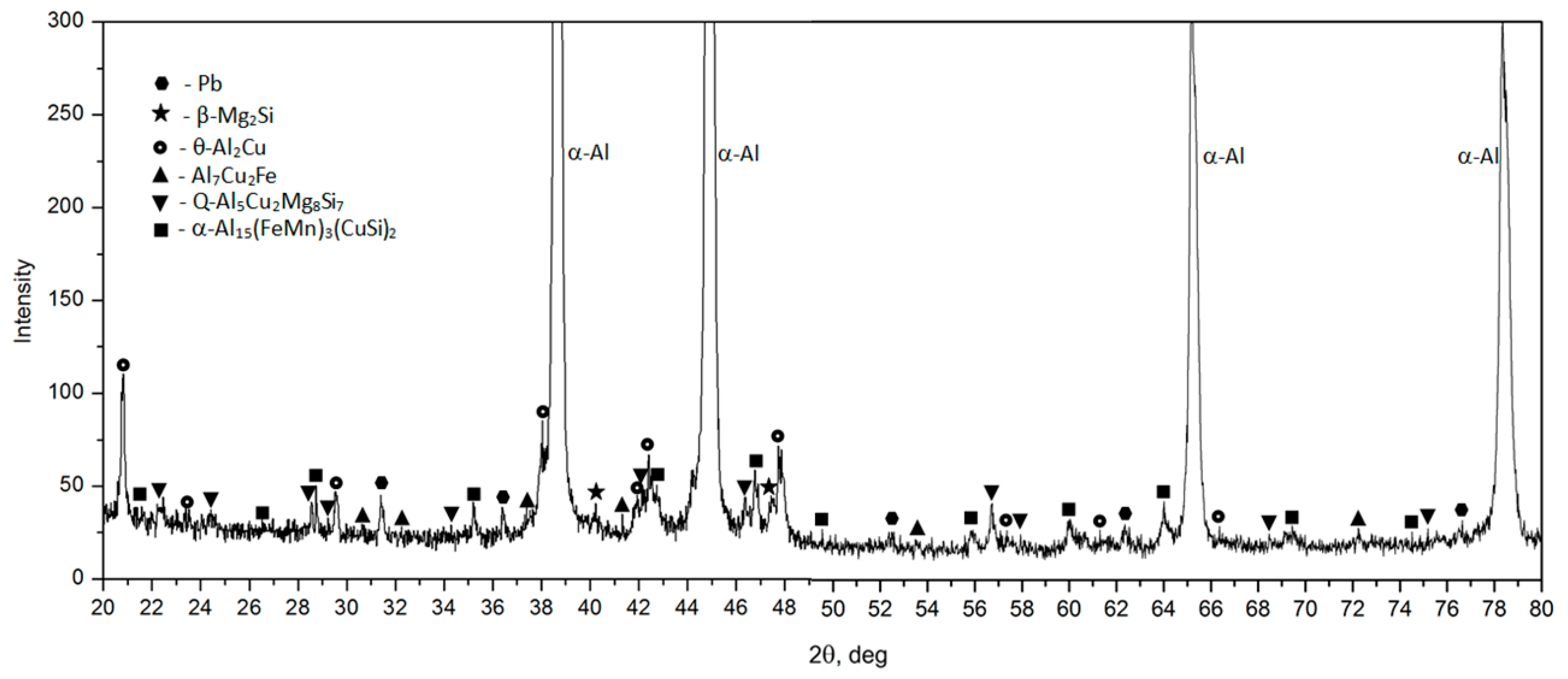




| Parameters | Value |
|---|---|
| Metal temperature in furnace | 730 °C |
| Metal temperature in classifier | 680–700 °C |
| Cooling water volume | 25 L/min |
| Casting speed | 3.5–4.0 mm/s |
| Remarks | Total amount of water for four crystallizers 120 L/m |
| Alloy | Elements Content, wt % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Pb | Ti | |
| EN AW-2007 | 0.76 | 0.45 | 3.43 | 0.64 | 0.70 | 0.023 | 0.008 | 0.20 | 0.53 | 0.027 |
| Phase | % | Elements | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Si | Mn | Fe | Mg | Cu | Pb | ||
| Pb | wt | 1.3 ÷ 1.8 | - | - | - | - | - | 98.2 ÷ 98.7 |
| at | 9.0 ÷ 4 | - | - | - | - | - | 90.6 ÷ 91.0 | |
| θ-Al2Cu | wt | 46.9 ÷ 47.6 | - | - | - | - | 52.4 ÷ 53.1 | - |
| at | 67.1 ÷ 68.5 | - | - | - | - | 31.9 ÷ 32.6 | - | |
| β-Mg2Si | wt | 2.45 ÷ 3.8 | 52.6 ÷ 61.2 | - | - | 37.9 ÷ 48.2 | - | - |
| at | 2.55 ÷ 3.7 | 47.2 ÷ 48.9 | - | - | 48.5 ÷ 52.9 | - | - | |
| Al7Cu2Fe | wt | 61.4 ÷ 65.1 | - | - | 8.8 ÷ 9.3 | - | 25.1 ÷ 29.0 | |
| at | 75.6 ÷ 78.7 | - | - | 5.4 ÷ 5.7 | - | 13.2 ÷ 14.7 | ||
| Q Al5Cu2Mg8Si6 | wt | 33.7 ÷ 56.4 | 16.5 ÷ 26.9 | - | - | 15.5 ÷ 23.5 | 11.5 ÷ 18.7 | - |
| at | 37.1 ÷ 59.6 | 16.5 ÷ 27.6 | - | 18.5 ÷ 28.1 | 5.2 ÷ 8.7 | - | ||
| α-Al15(FeMn)3(SiCu)2 | wt | 56.7 ÷ 59.91 | 5.7 ÷ 6.8 | 9.6 ÷ 12.3 | 17.4 ÷ 19.6 | - | 4.2 ÷ 7.3 | - |
| at | 71.0 ÷ 72.9 | 7.0 ÷ 8.1 | 5.7 ÷ 7.2 | 10.2 ÷ 11.7 | - | 2.6 ÷ 3.7 | - | |
| Aging Time, h | Solution Temperature, °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 490 | 500 | 510 | |||||||
| R0.2, MPa | Rm, MPa | A5, % | R0.2, MPa | Rm, MPa | A5, % | R0.2, MPa | Rm, MPa | A5, % | |
| 5 | 223.9 ± 2.1 | 387.9 ± 1.7 | 10.5 ± 0.2 | 233.0 ± 1.1 | 413.1 ± 1.1 | 16.3 ± 0.1 | 226.9 ± 1.8 | 416.6 ± 1.8 | 14.5 ± 0.1 |
| 9 | 237.5 ± 2.2 | 396.6 ± 2.1 | 10.9 ± 0.1 | 240.5 ± 2.7 | 435.0 ± 1.3 | 18.1 ± 0.1 | 235.5 ± 2.2 | 420.0 ± 1.3 | 15.8 ± 0.3 |
| 30 | 246.0 ± 1.6 | 399.5 ± 1.9 | 11.7 ± 0.2 | 253.6 ± 1.3 | 432.8 ± 1.4 | 16.3 ± 0.1 | 244.0 ± 1.6 | 418.0 ± 0.9 | 15.2 ± 0.4 |
| 45 | 250.0 ± 3.2 | 404.2 ± 2.3 | 13.5 ± 0.1 | 255.1 ± 1.4 | 426.6 ± 1.8 | 15.9 ± 0.1 | 247.0 ± 1.2 | 409.2 ± 2.1 | 14.5 ± 0.2 |
| 80 | 253.7 ± 0.9 | 421.1 ± 2.2 | 11.6 ± 0.3 | 258.3 ± 2.1 | 393.8 ± 2.2 | 11.6 ± 0.1 | 251.7 ± 1.9 | 389.0 ± 2.5 | 12.6 ± 0.7 |
| Alloy Condition | R0.2, MPa | Rm, MPa | A5, % | Reference |
|---|---|---|---|---|
| As-cast | 81 | 184 | 21 | [45] |
| 160 | 286.8 | 8.2 | * | |
| T4 | 220 | 340 | 8 | [45] |
| 240 | 380 | 8 | [46] | |
| 210–250 | 330–370 | 7–8 | [47] | |
| 240.5 | 435 | 18.1 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrówka-Nowotnik, G.; Boczkal, G.; Nowotnik, A. Effect of Continuous Casting and Heat Treatment Parameters on the Microstructure and Mechanical Properties of Recycled EN AW-2007 Alloy. Materials 2024, 17, 3447. https://doi.org/10.3390/ma17143447
Mrówka-Nowotnik G, Boczkal G, Nowotnik A. Effect of Continuous Casting and Heat Treatment Parameters on the Microstructure and Mechanical Properties of Recycled EN AW-2007 Alloy. Materials. 2024; 17(14):3447. https://doi.org/10.3390/ma17143447
Chicago/Turabian StyleMrówka-Nowotnik, Grażyna, Grzegorz Boczkal, and Andrzej Nowotnik. 2024. "Effect of Continuous Casting and Heat Treatment Parameters on the Microstructure and Mechanical Properties of Recycled EN AW-2007 Alloy" Materials 17, no. 14: 3447. https://doi.org/10.3390/ma17143447






