ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Samples
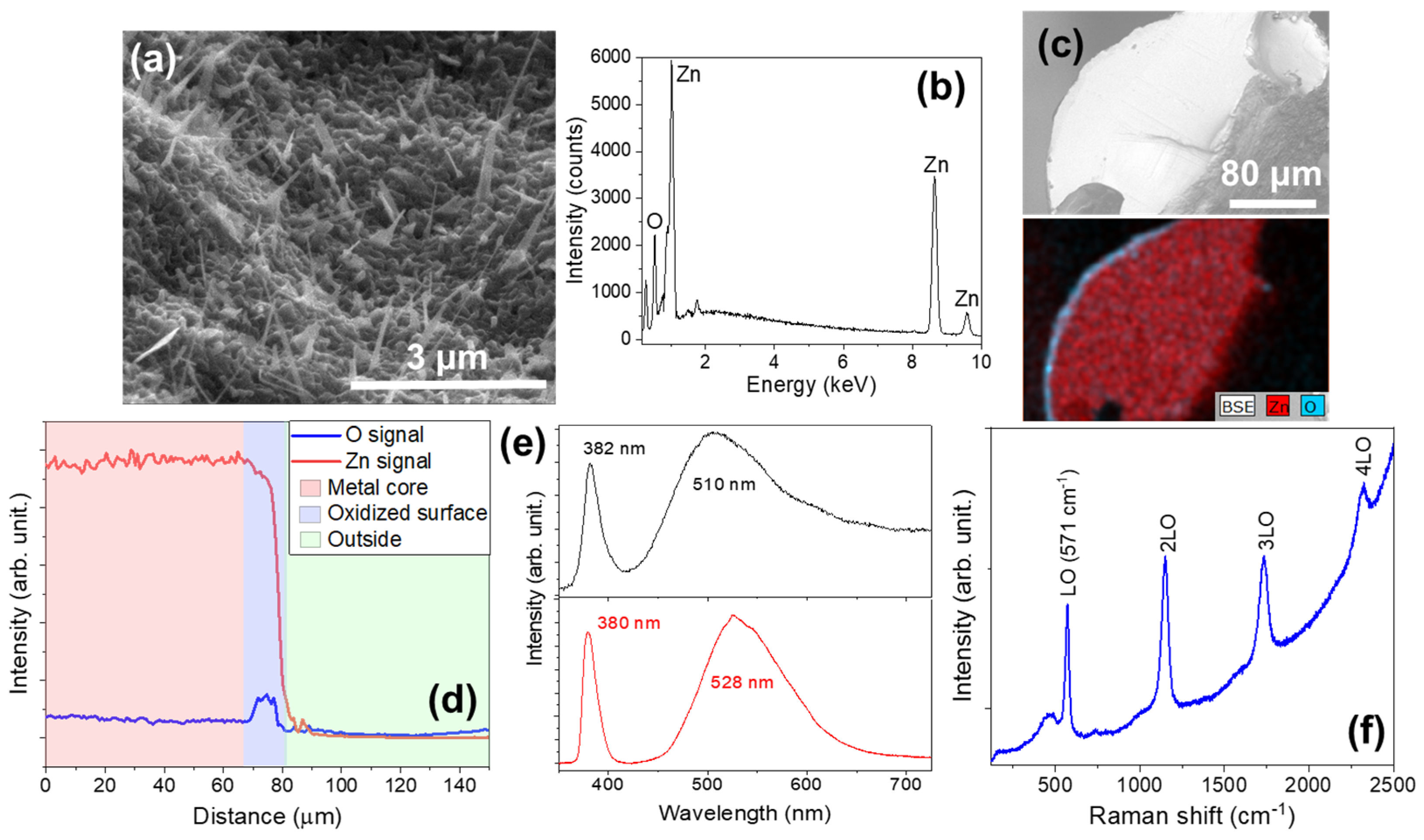
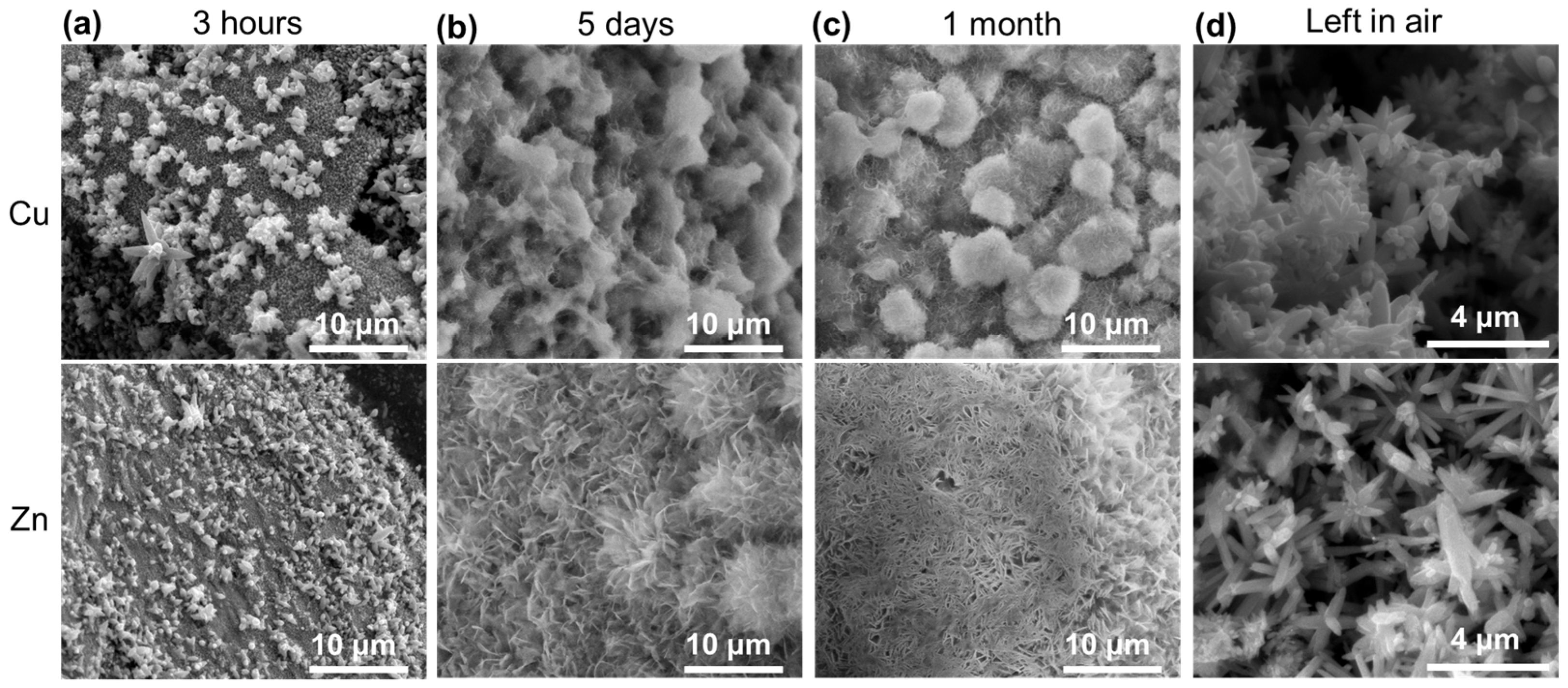
3.1.1. Series SW (Single Wire)
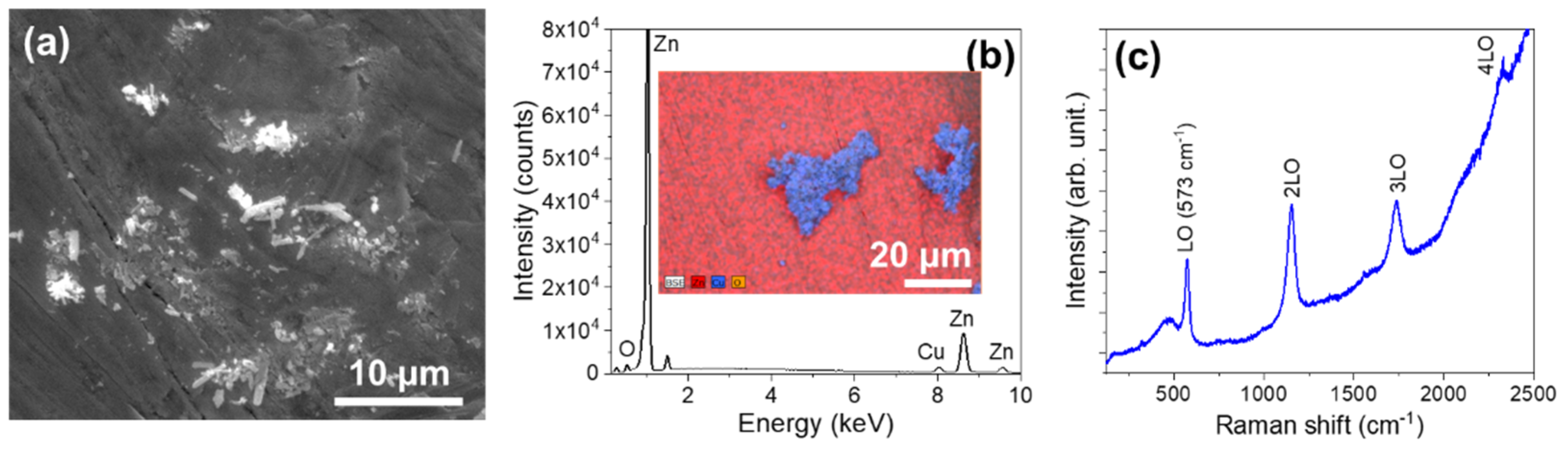
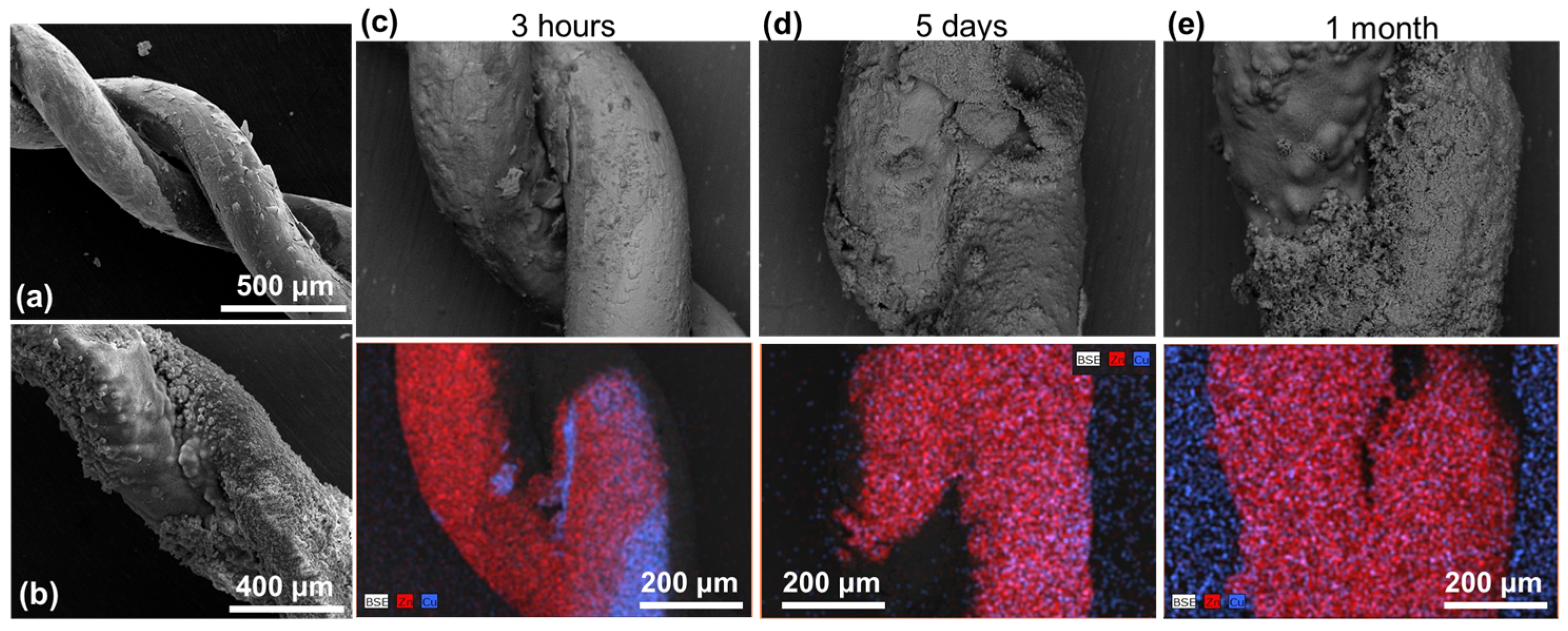
3.1.2. Series IW (Immersed Wires)
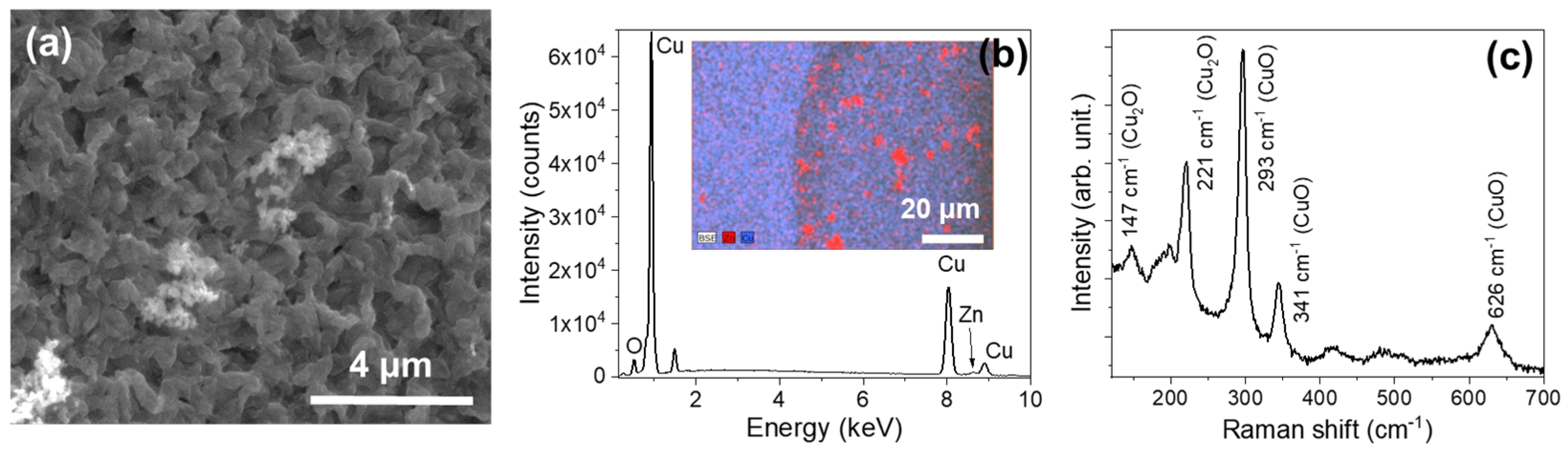
3.1.3. Series BWs (Braided Wires)
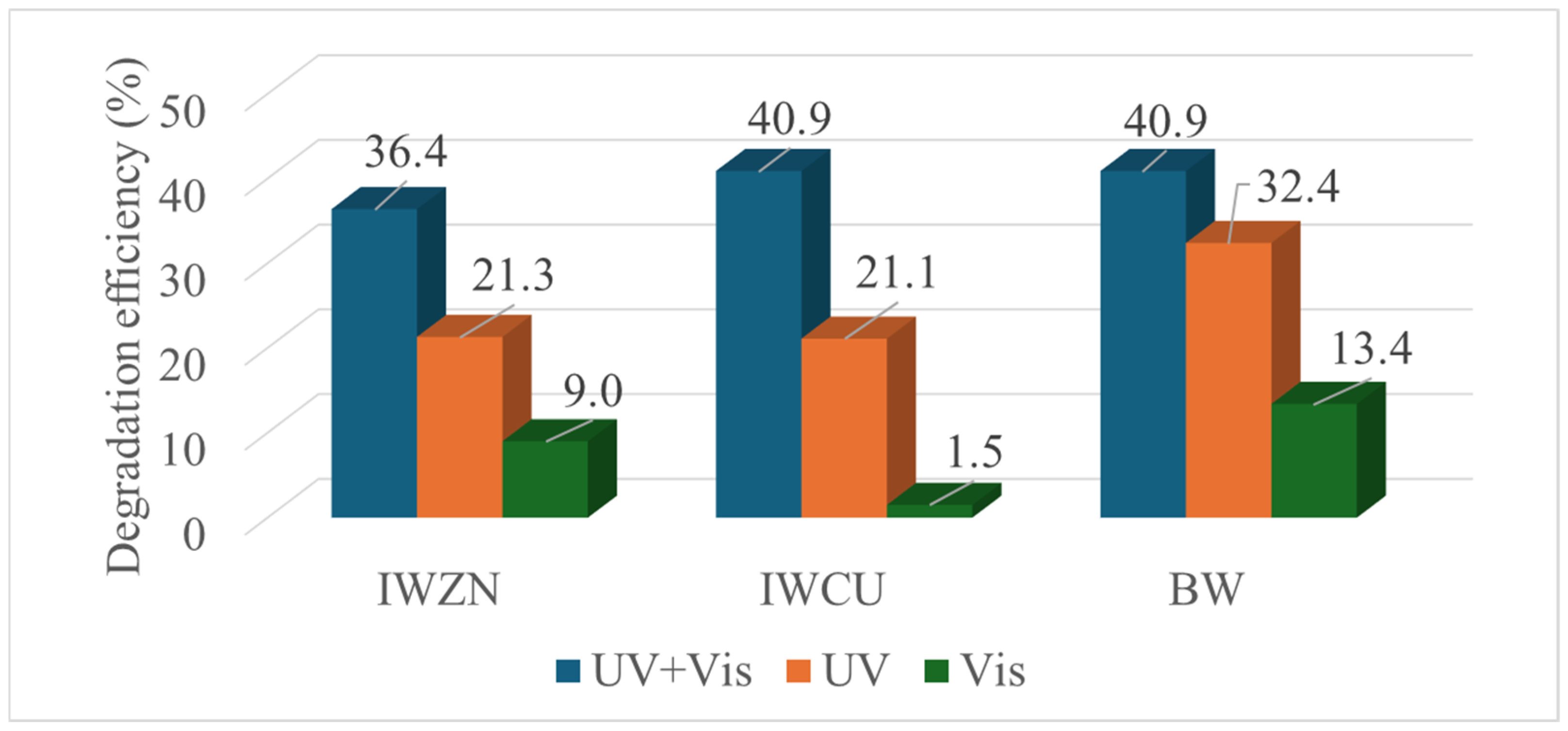
3.2. Photocatalysis Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. “Metal Oxide -Based Heterostructures for Gas Sensors”—A Review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Ponzoni, A.; Zappa, D.; Sberveglieri, G. Metal Oxide Nanowire Chemical and Biochemical Sensors. J. Mater. Res. 2013, 28, 2911–2931. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Jin, W. Earth-Abundant Transition Metal and Metal Oxide Nanomaterials: Synthesis and Electrochemical Applications. Prog. Mater. Sci. 2019, 106, 100574. [Google Scholar] [CrossRef]
- Ke, G.L.; Jia, B.; He, H.C.; Zhou, Y.; Zhou, M. State-of-the-Art Advancements of Transition Metal Oxides as Photoelectrode Materials for Solar Water Splitting. Rare Met. 2022, 41, 2370–2386. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen Vacancies in Metal Oxides: Recent Progress towards Advanced Catalyst Design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal Oxides and Metal Organic Frameworks for the Photocatalytic Degradation: A Review. J. Env. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal Oxides for Optoelectronic Applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A Study on the Mechanism for the Interaction of Light with Noble Metal-Metal Oxide Semiconductor Nanostructures for Various Photophysical Applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Pan, L.; Huang, W. Recent Progress in the ZnO Nanostructure-Based Sensors. Mater. Sci. Eng. B 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials; Springer: Singapore, 2020; Volume 12, ISBN 0123456789. [Google Scholar]
- Chen, H.; Yu, H.; Cui, S.; Xu, J.; Zhang, Y.; Liu, C. Synthesis of Ce:ZnO Nanocomposites: Facile Synthesis and Fast Acetone Gas Sensing Response Properties. Phys. B Condens. Matter 2017, 516, 36–40. [Google Scholar] [CrossRef]
- Liu, S.; Regulacio, M.D.; Tee, S.Y.; Khin, Y.W.; Teng, C.P.; Koh, L.D.; Guan, G.; Han, M.Y. Preparation, Functionality, and Application of Metal Oxide-Coated Noble Metal Nanoparticles. Chem. Rec. 2016, 16, 1965–1990. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Urbieta, A.; Escalante, G.; Hidalgo, P.; Espinós, J.P.; Fernández, P. Study of the Influence of the Precursors on the Sensing Properties of ZnO:Cu System. Ceram. Int. 2020, 46, 8358–8367. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Wang, Q.; Zhang, X. Recent Progress on Transition Metal Oxides as Advanced Materials for Energy Conversion and Storage. Energy Storage Mater. 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Jang, E.S. Recent Progress in Synthesis of Plate-like ZnO and Its Applications: A Review. J. Korean Ceram. Soc. 2017, 54, 167–183. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M. Design and Tailoring of One—Dimensional ZnO Nanomaterials for Photocatalytic Degradation of Organic Dyes: A Review; Springer: Dordrecht, The Netherlands, 2019; Volume 45, ISBN 0123456789. [Google Scholar]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid. Film 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Urbieta, A.; Sánchez, V.; Fernández, P.; Piqueras, J. Fast Growth of Undoped and Sn and Tb Doped ZnO Nanowires by Joule Heating of Zn. CrystEngComm 2018, 20, 4449–4454. [Google Scholar] [CrossRef]
- Piqueras, J.; Hidalgo, P. Growth of Metal Oxide Nanostructures by Thermal Oxidation of Metals Under Influence of External Electric Fields and Electric Current Flow. Phys. Status Solidi A Appl. Mater. Sci. 2021, 218, 2100323. [Google Scholar] [CrossRef]
- Filippo, E.; Tepore, M.; Siciliano, T.; Chirizzi, D.; Malitesta, C.; Guascito, M.R. Room Temperature Facile Synthesis of CuO Nanostructures by Resistive Heating. Phys. E Low-Dimens. Syst. Nanostruct 2014, 60, 59–64. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ching Ng, A.M. Strategies for Improving the Efficiency of Semiconductor Metal Oxide Photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent Developments of Metal Oxide Semiconductors as Photocatalysts in Advanced Oxidation Processes (AOPs) for Treatment of Dye Waste-Water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Achouri, F.; Corbel, S.; Balan, L.; Mozet, K.; Girot, E.; Medjahdi, G.; Said, M.B.; Ghrabi, A.; Schneider, R.; Ben, M.; et al. Porous Mn-Doped ZnO Nanoparticles for Enhanced Solar and Visible Light Photocatalysis. Mater. Des. 2016, 101, 309–316. [Google Scholar] [CrossRef]
- Tabib, A.; Bouslama, W.; Sieber, B.; Addad, A.; Elhouichet, H.; Férid, M.; Boukherroub, R. Structural and Optical Properties of Na Doped ZnO Nanocrystals: Application to Solar Photocatalysis. Appl. Surf. Sci. 2017, 396, 1528–1538. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the Improvement of the Photocatalytic and Antibacterial Activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Mao, T.; Liu, M.; Lin, L.; Cheng, Y.; Fang, C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers 2022, 14, 4484. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-Light Photocatalysts: Prospects and Challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the Criteria Anticipated for the Fabrication of Highly Efficient ZnO-Based Visible-Light-Driven Photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Amin, G.; Zainelabdin, A.; Zaman, S. Zinc Oxide and Copper Oxide Nanostructures: Fundamentals and Applications. Mater. Res. Soc. Symp. Proc. 2012, 1406, 3–10. [Google Scholar] [CrossRef]
- Yu, Z.; Moussa, H.; Liu, M.; Schneider, R.; Wang, W.; Moliere, M.; Liao, H. Development of Photocatalytically Active Heterostructured MnO/ZnO and CuO/ZnO Films via Solution Precursor Plasma Spray Process. Surf. Coat. Technol. 2019, 371, 107–116. [Google Scholar] [CrossRef]
- Ramos-justicia, J.F.; Ballester-and, L.; Urbieta, A.; Fern, P. Applied Sciences Growth of Zr/ZrO2 Core—Shell Structures by Fast Thermal Oxidation. Appl. Sci. 2023, 13, 3714. [Google Scholar] [CrossRef]
- Calvo-Villoslada, A.; Fernández, P.; Sotillo, B. Rapid Growth of Niobium Oxide Nanowires by Joule Resistive Heating. Crystals 2024, 14, 245. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.G. Correlations among Size, Defects, and Photoluminescence in ZnO Nanoparticles. J. Appl. Phys. 2007, 101, 024317. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from Zinc Oxide Nanostructures and Polymers and Their Hybrid Devices. Materials 2010, 3, 2643–2667. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Singh, R.K. Progress on Transition Metal-Doped ZnO Nanoparticles and Its Application. Ind. Eng. Chem. Res. 2019, 58, 17130–17163. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.J.; Hark, S.K.; Li, Q.; Tang, D.; Chu, M.W.; Chen, C.H. Local Electronic Structure and Luminescence Properties of Er Doped ZnO Nanowires. Appl. Phys. Lett. 2006, 89, 221917. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature Dependence of Raman Scattering in ZnO. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Raman Spectra of CuO Nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Kaur, J.; Khanna, A.; Kumar, R.; Chandra, R. Growth and Characterization of Cu2O and CuO Thin Films. J. Mater. Sci. Mater. Electron. 2022, 33, 16154–16166. [Google Scholar] [CrossRef]
- Flores-Carrasco, G.; Rodríguez-Peña, M.; Urbieta, A.; Fernández, P.; Rabanal, M.E. ZnO Nanoparticles with Controllable Ce Content for Efficient Photocatalytic Degradation of MB Synthesized by the Polyol Method. Catalysts 2021, 11, 71. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hiraiwa, K.; Jeem, M.; Zhang, L.; Watanabe, S. Galvanic-Submerged Photosynthesis of Crystallites: Fabrication of ZnO Nanorods@ Cu-Surface. Appl. Surf. Sci. 2019, 489, 313–320. [Google Scholar] [CrossRef]
- Zhao, H.; Du, Z.; Liu, D.; Lai, Y.; Yang, T.; Wang, M.; Ning, Y.; Yin, F.; Zhao, B. Facile Construction of Cu/ZnO Heterojunctions for Enhanced Photocatalytic Performance. Nano 2022, 17, 2250011. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, M.; Yang, Z.; Zeng, C.; Wang, L.; Qiu, M.; Zhang, Y.; Zhang, W. A Galvanic Replacement Reaction to Synthesise Metal/ZnO Heterostructured Films on Zinc Substrates for Enhanced Photocatalytic Performance. RSC Adv. 2016, 6, 103594–103600. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. Aerosol Salt Particle Deposition on Metals Exposed to Marine Environments: A Study Related to Marine Atmospheric Corrosion. J. Electrochem. Soc. 2014, 161, C268–C275. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Calzado, A.; Calvo-Villoslada, A.; Fernández, P.; Sotillo, B. ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis. Materials 2024, 17, 3502. https://doi.org/10.3390/ma17143502
Fernández-Calzado A, Calvo-Villoslada A, Fernández P, Sotillo B. ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis. Materials. 2024; 17(14):3502. https://doi.org/10.3390/ma17143502
Chicago/Turabian StyleFernández-Calzado, Adrián, Aarón Calvo-Villoslada, Paloma Fernández, and Belén Sotillo. 2024. "ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis" Materials 17, no. 14: 3502. https://doi.org/10.3390/ma17143502
APA StyleFernández-Calzado, A., Calvo-Villoslada, A., Fernández, P., & Sotillo, B. (2024). ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis. Materials, 17(14), 3502. https://doi.org/10.3390/ma17143502







