Diffusion Barrier Performance of Ni-W Layer at Sn/Cu Interfacial Reaction
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Ni-W Diffusion Barrier Layer
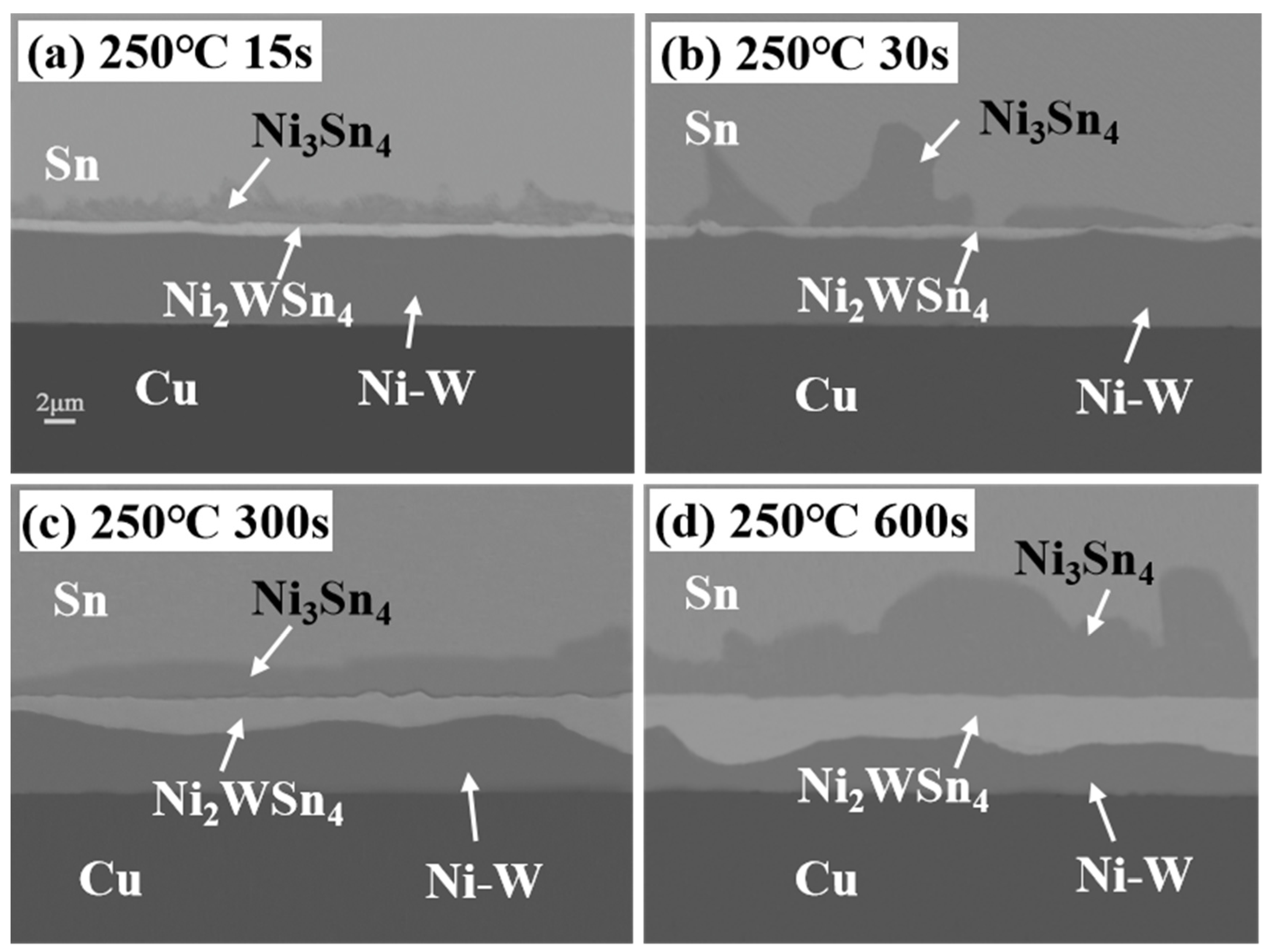
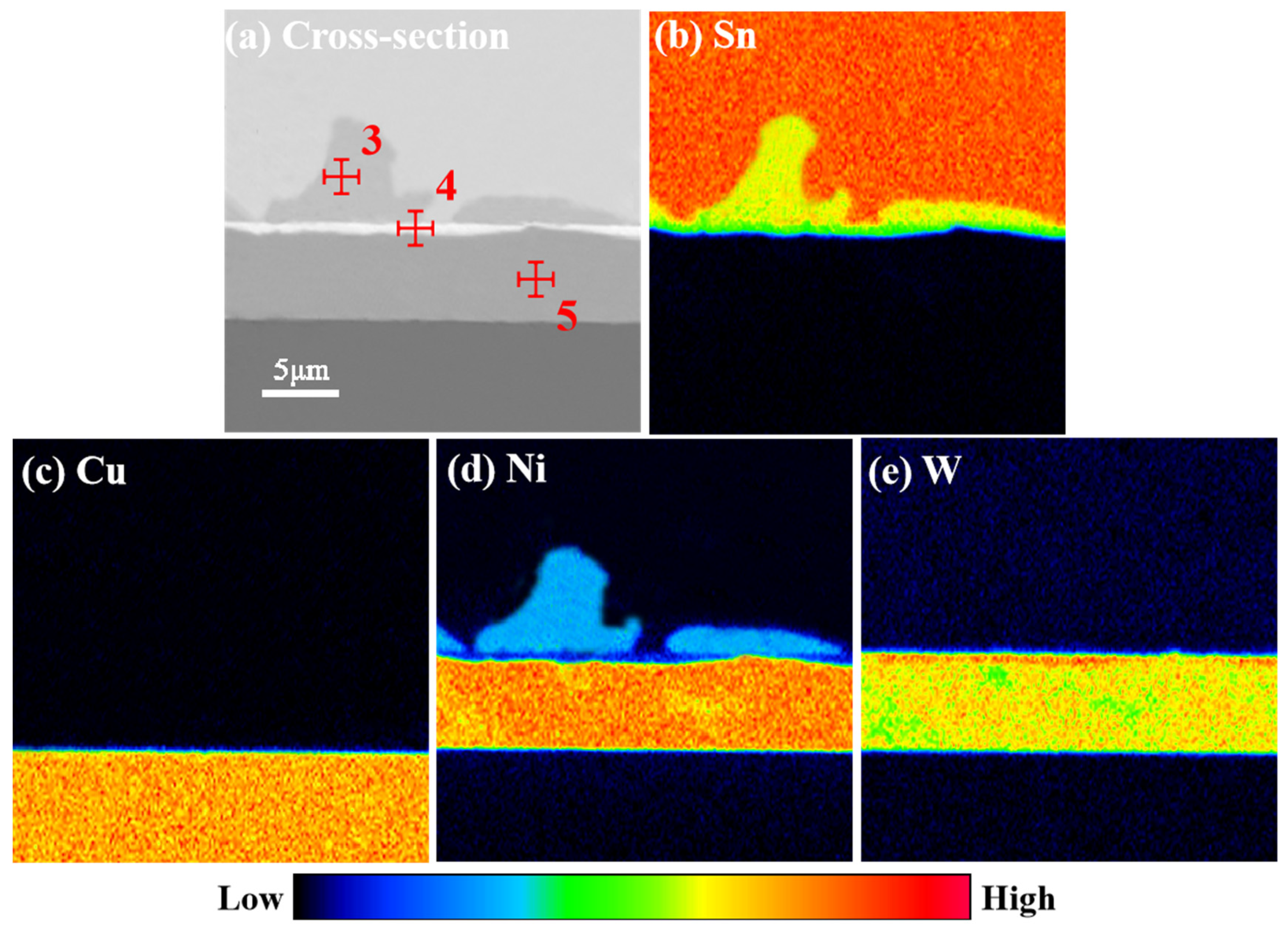
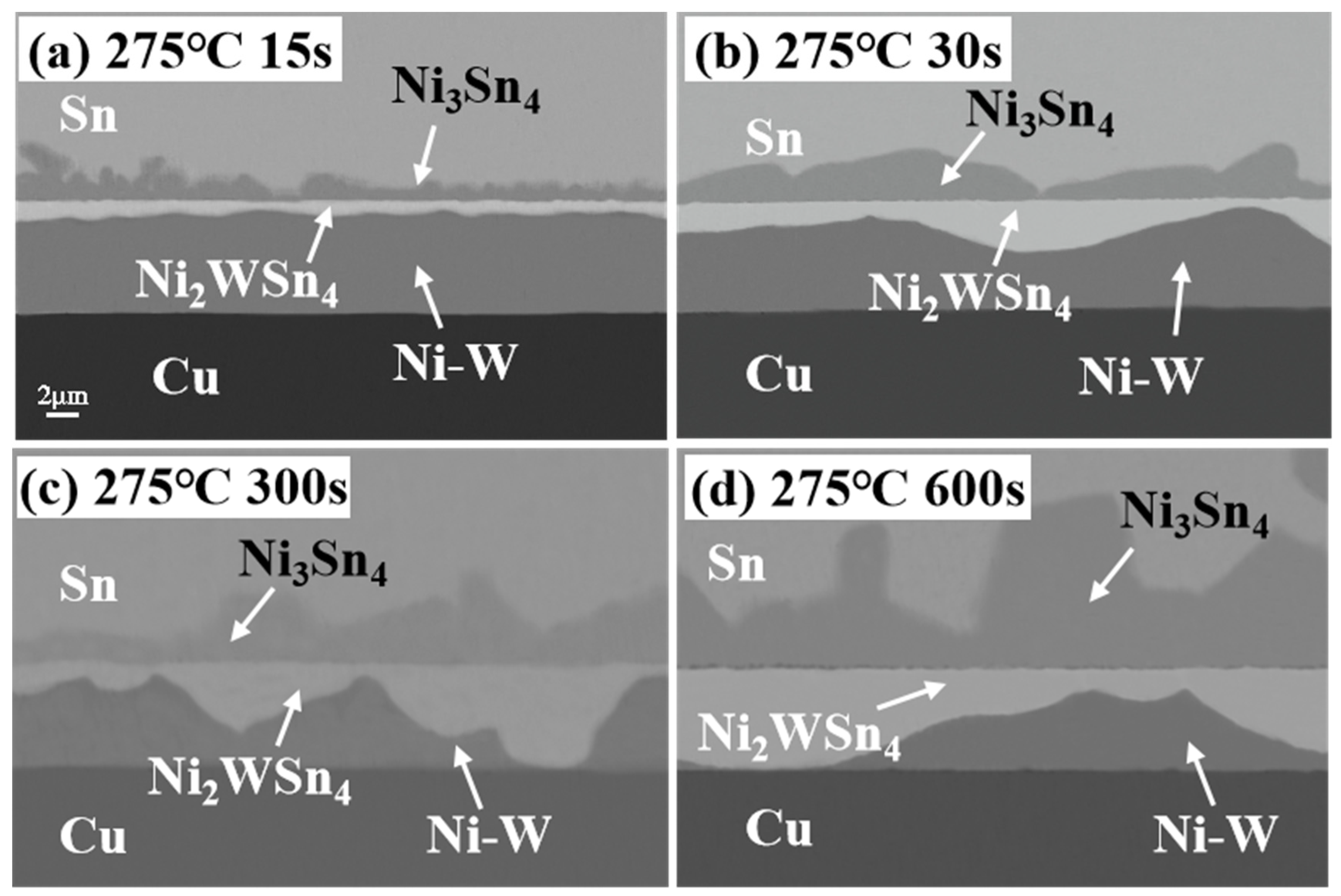
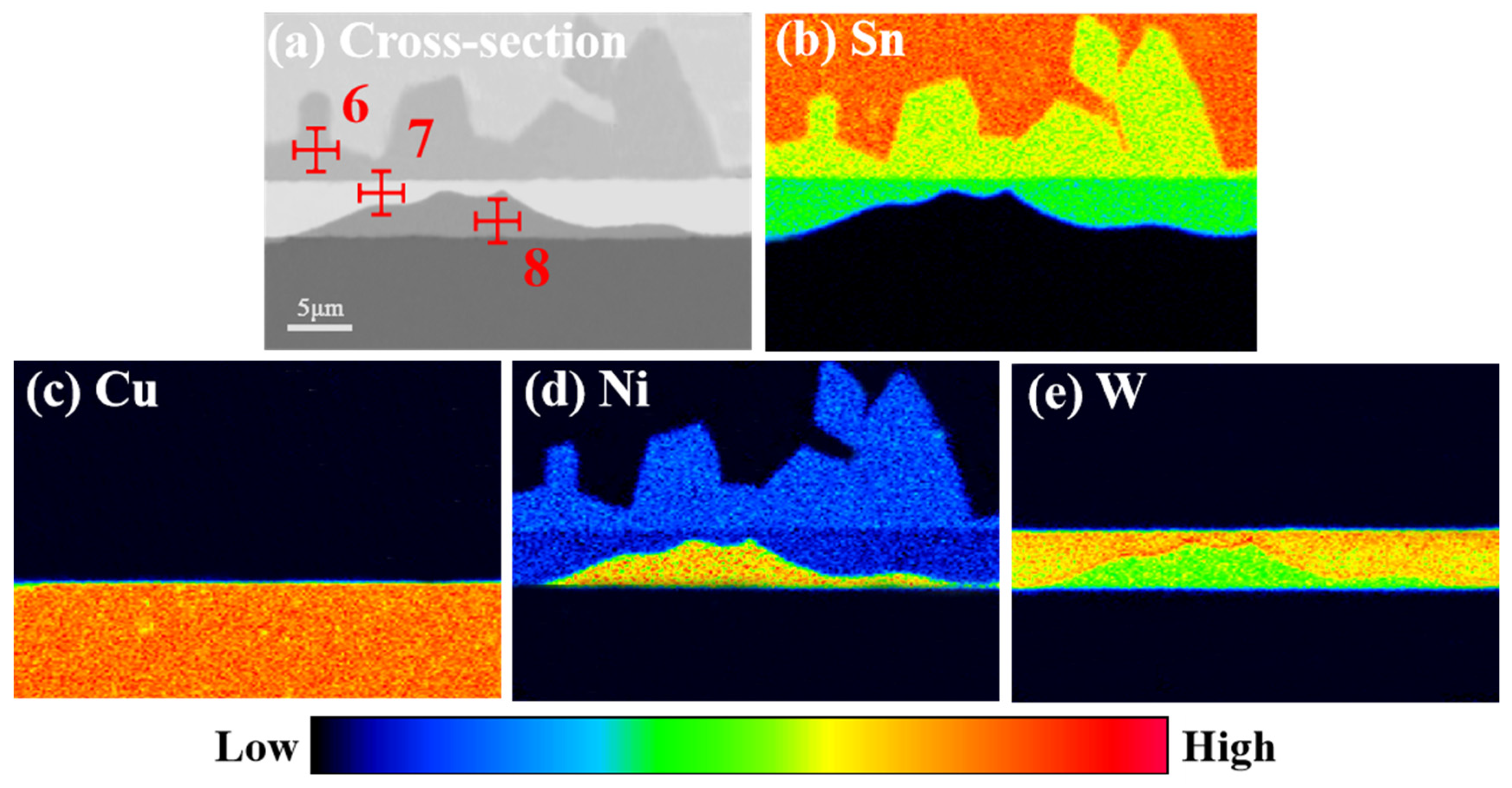
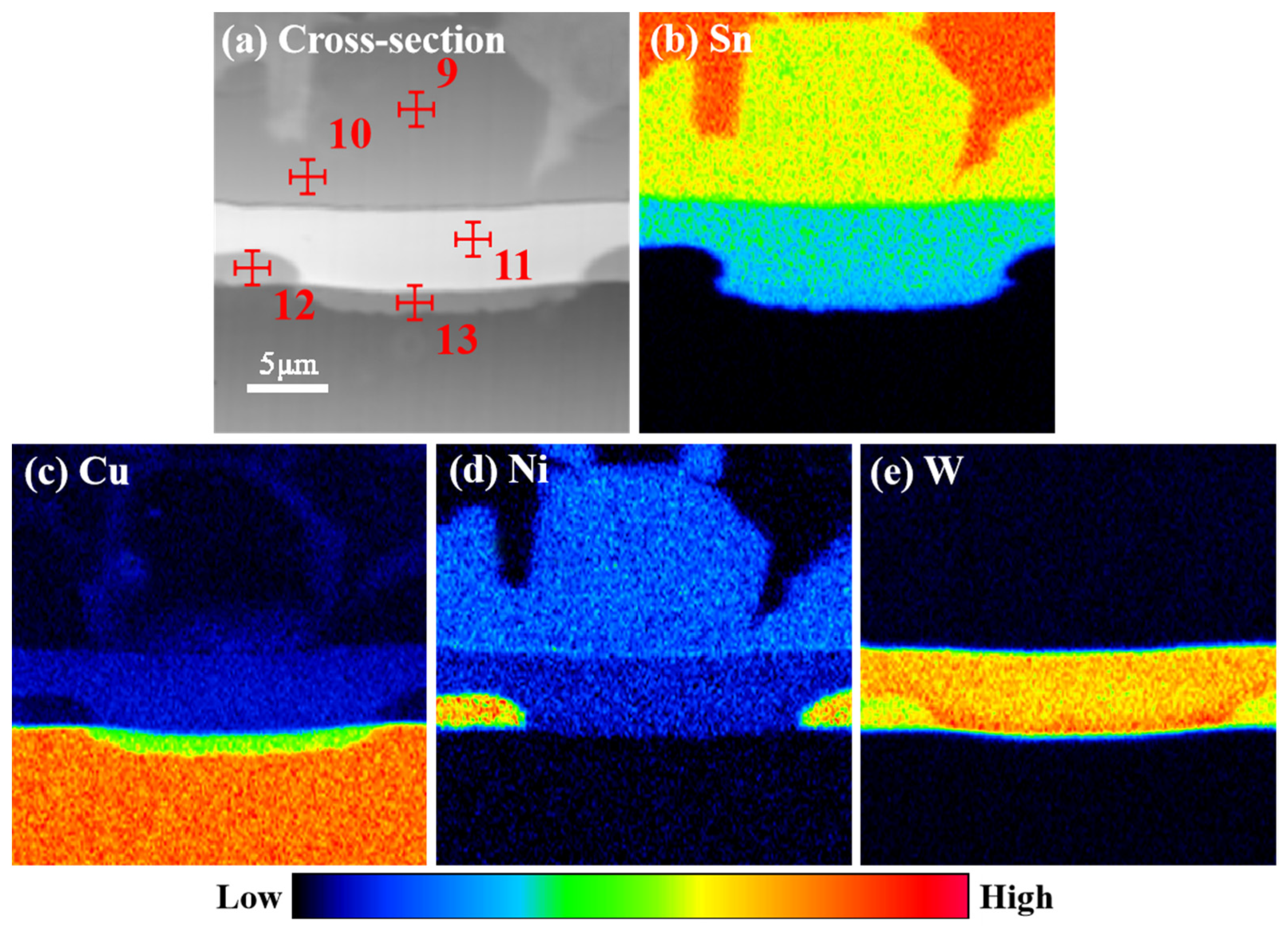
3.2. Microstructure Evolution of IMCs
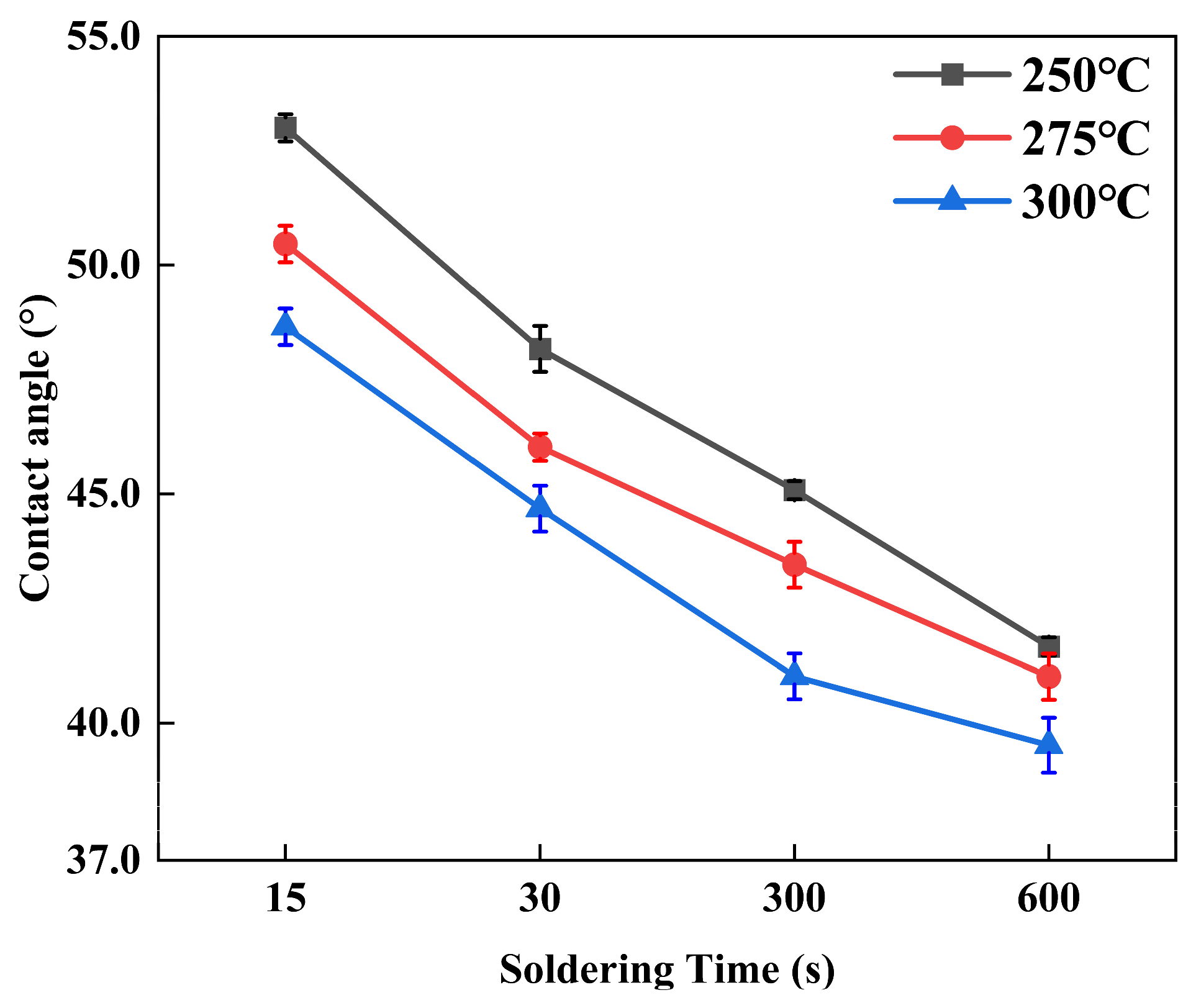
3.3. Growth Kinetics Analysis of Interfacial Reactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, K.; Liu, Y. Recent advances on kinetic analysis of solder joint reactions in 3D IC packaging technology. Mater. Sci. Eng. R Rep. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Jiang, N.; Zhang, L.; Zhong, S. Materials modification of the lead-free solders incorporated with micro/nano-sized particles: A review. Mater. Des. 2021, 197, 109224. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.T.; Ansari, M.; Hanim, M.A.; Oyekanmi, A.A.; Ojo-Kupoluyi, O.J.; Atiqah, A. Recent advances in Sn-based lead free solder interconnects for microelectronics packaging: Materials and technologies. J. Mater. Res. Technol. 2023, 25, 4231–4263. [Google Scholar] [CrossRef]
- Lau, J.H. Recent advances and trends in advanced packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 228–252. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Zhou, H.; Li, R.; Wang, S.; Paik, K.; He, P. Challenges and recent prospectives of 3D heterogeneous integration. E-Prime-Adv. Electr. Eng. Electron. Energy 2022, 2, 100052. [Google Scholar] [CrossRef]
- Lau, J.H. Advanced Packaging Trends. In Semiconductor Advanced Packaging; Springer: Singapore, 2021; pp. 465–489. [Google Scholar]
- Li, Y.; Fu, G.; Wan, B.; Wu, Z.; Yan, X.; Zhang, W. A study on the effects of electrical and thermal stresses on void formation and migration lifetime of Sn3.0Ag0.5Cu solder joints. Solder. Surf. Mt. Technol. 2022, 34, 162–173. [Google Scholar] [CrossRef]
- Li, Y.; Fu, G.; Wan, B.; Yan, X.; Zhang, W.; Li, W. Phase-field modelling of lead-free solder joint void growth under thermal-electrical coupled stress. J. Electron. Mater. 2022, 51, 259–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Wang, J.; Han, Y.; Yu, Z.; Wang, Q.; Zhang, P.; Yang, S. Effects of solder thickness on interface behavior and nanoindentation characteristics in Cu/Sn/Cu microbumps. Weld. World 2022, 66, 973–983. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Huo, L.; Li, H.; Tian, W.; Ji, H.; Chen, S. Research on the Reliability of Advanced Packaging under Multi-Field Coupling: A Review. Micromachines 2024, 15, 422. [Google Scholar] [CrossRef]
- Yao, J.; Shang, M.; Chen, X.; Xing, J.; Guo, T.; Wang, Y.; Ma, H.; Gao, Z. Thermal stability and diffusion barrier performance of amorphous Ni-P layer at Sn/Ni-P/Cu interface. J. Mater. Sci. Mater. Electron. 2024, 35, 907. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W.; Li, J.; Dong, S.; Lin, X.; Jian, X.; Wu, P. The obstruction effect of Ni layer on the interdiffusion of Cu substrate and Sn solder: A theoretical investigation. J. Electron. Mater. 2020, 49, 6559–6571. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, Y.; Chen, C.; Liu, K.; Wang, Y.; Liu, T. Microstructural evolution of Cu/solder/Cu pillar-type structures with different diffusion barriers. Metall. Mater. Trans. A 2016, 47, 3971–3980. [Google Scholar] [CrossRef]
- Kim, J.; Park, D.; Ahn, B.; Bang, J.; Kim, M.; Park, H.; Sohn, Y.; Ko, Y. Effective (Pd, Ni)Sn4 diffusion barrier to suppress brittle fracture at Sn-58Bi-xAg solder joint with Ni (P)/Pd (P)/Au metallization pad. Microelectron. Reliab. 2022, 129, 114472. [Google Scholar] [CrossRef]
- Park, J.M.; Hyeon, D.Y.; Ma, H.; Kim, S.; Kim, S.; Nguyen, Y.N.; Son, I.; Yi, S.; Kim, K.T.; Park, K. Enhanced output power of thermoelectric modules with reduced contact resistance by adopting the optimized Ni diffusion barrier layer. J. Alloys Compd. 2021, 884, 161119. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Chen, P.; Liu, J.; Hu, A.; Li, M. Effect of Co-W and Co-Fe-W Diffusion Barriers on the Reliability of the Solder/Cu Interface during Reflow Conditions. Electron. Mater. Lett. 2024, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.; Chen, P.; Cao, L.; Li, M.; Hang, T. Interface reliability and diffusion barrier property of Co-W barrier layer with modulated structure. Mater. Lett. 2023, 331, 133501. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent advances in barrier layer of cu interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef]
- Sohn, Y. Effect of Morphological Change of Ni3Sn4 Intermetallic Compounds on the Growth Kinetics in Electroless Ni-P/Sn-3.5 Ag Solder Joint. Metall. Mater. Trans. A 2020, 51, 2905–2914. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Li, J.; Chen, Z. Comparison of interfacial reactions and isothermal aging of cone Ni-P and flat Ni-P with Sn3.5Ag solders. Appl. Surf. Sci. 2023, 625, 157219. [Google Scholar] [CrossRef]
- Chi, P.; Li, Y.; Pan, H.; Wang, Y.; Chen, N.; Li, M.; Gao, L. Effect of Ni (P) Layer Thickness on Interface Reaction and Reliability of Ultrathin ENEPIG Surface Finish. Materials 2021, 14, 7874. [Google Scholar] [CrossRef]
- Yu, H.Y.; Yang, T.H.; Chiu, Y.S.; Kao, C.R. Surface diffusion and the interfacial reaction in Cu/Sn/Ni micro-pillars. J. Electron. Mater. 2020, 49, 88–95. [Google Scholar] [CrossRef]
- Labie, R.; Ruythooren, W.; Van Humbeeck, J. Solid state diffusion in Cu–Sn and Ni–Sn diffusion couples with flip-chip scale dimensions. Intermetallics 2007, 15, 396–403. [Google Scholar] [CrossRef]
- Tsai, Y.; Wu, F.; Chen, Y.; Peng, P.; Duh, J.; Tsai, S. Thermal stability and mechanical properties of Ni–W–P electroless deposits. Surf. Coat. Technol. 2001, 146, 502–507. [Google Scholar] [CrossRef]
- Huang, L.; Dong, J.X.; Yang, F.Z.; Xu, S.K.; Zhou, S.M. Studies on the mechanism, structure and microhardness of Ni-W alloy electrodeposits. Trans. IMF 1999, 77, 185–187. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Sheng, M.; Wang, H.; Guo, R.; Qu, T.; Hu, S. The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating. Coatings 2023, 13, 1651. [Google Scholar] [CrossRef]
- Mizushima, I.; Tang, P.T.; Hansen, H.N.; Somers, M.A. Residual stress in Ni–W electrodeposits. Electrochim. Acta 2006, 51, 6128–6134. [Google Scholar] [CrossRef]
- Jung, D.; Jung, J. Review of the wettability of solder with a wetting balance test for recent advanced microelectronic packaging. Crit. Rev. Solid State Mater. Sci. 2019, 44, 324–343. [Google Scholar] [CrossRef]
- Hasnine, M.; Tolla, B.; Karasawa, M. Effect of Ge addition on wettability, copper dissolution, microstructural and mechanical behavior of SnCu–Ge solder alloy. J. Mater. Sci. Mater. Electron. 2017, 28, 16106–16119. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Liu, Z.; Sun, L.; Long, W.; He, P.; Xiong, M.; Zhao, M. Reliability issues of lead-free solder joints in electronic devices. Sci. Technol. Adv. Mater. 2019, 20, 876–901. [Google Scholar] [CrossRef]
- Lin, K.; Ling, H.; Hu, A.; Wu, Y.; Gao, L.; Hang, T.; Li, M. Growth behavior and formation mechanism of porous Cu3Sn in Cu/Sn solder system. Mater. Charact. 2021, 178, 111271. [Google Scholar] [CrossRef]
- King-Ning, T.U. Solder Joint Technology, Materials, Properties, and Reliability; Springer Series in Materials Science; Springer: New York, NY, USA, 2007; Volume 117, p. 373. [Google Scholar]

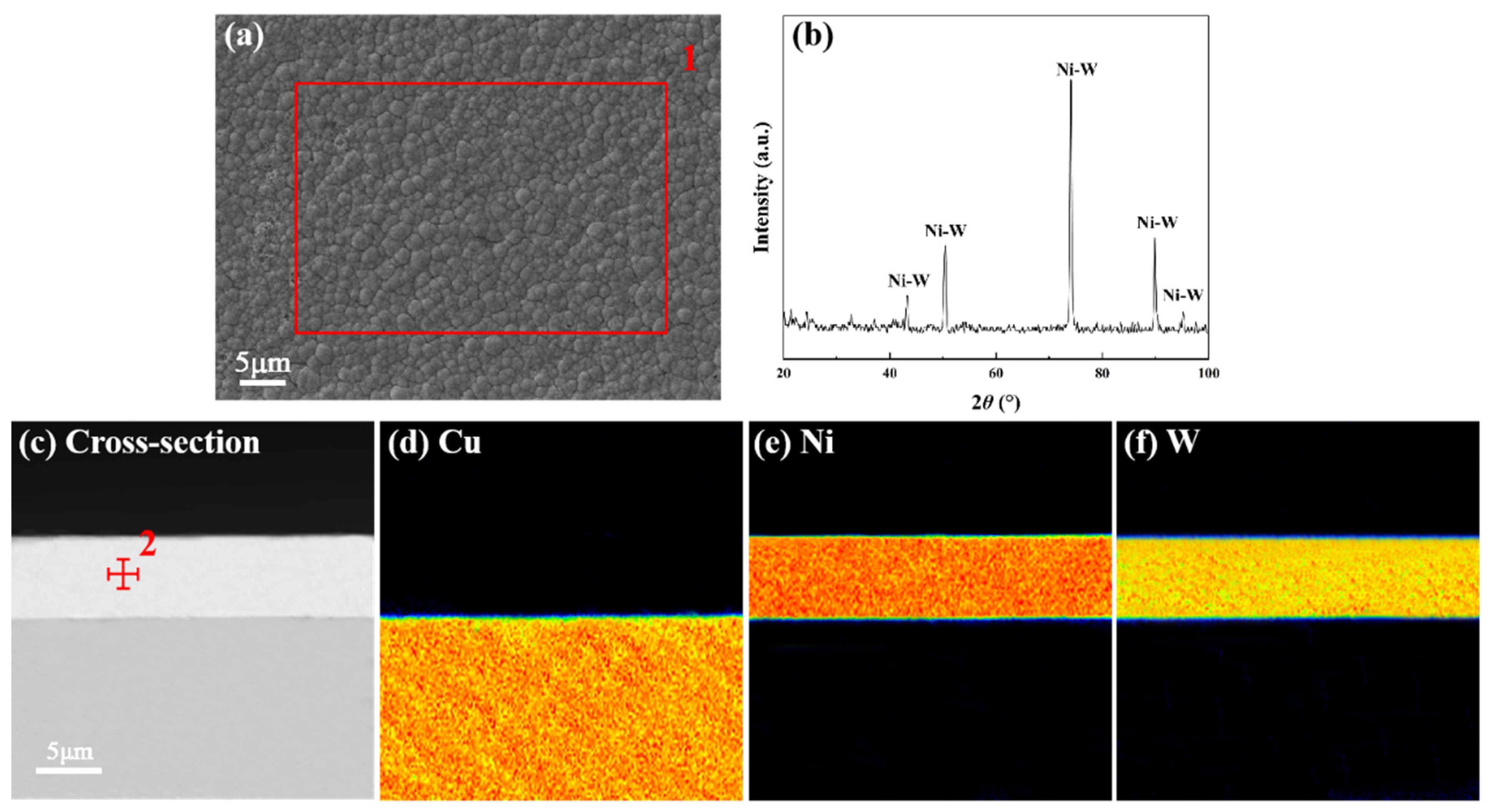


| Material | NiCl2·6H2O | NiSO4·6H2O | Na3C6H5O7·H2O | Na2WO4·2H2O | C6H4SO2NNaCO·2H2O | C12H25SO4Na |
|---|---|---|---|---|---|---|
| Concentration | 15 g/L | 80 g/L | 100 g/L | 80 g/L | 5 g/L | 0.5 g/L |
| Indicated Position | Element | wt.% | at.% |
|---|---|---|---|
| 1 | Ni | 75.18 | 90.43 |
| W | 24.82 | 9.57 | |
| 2 | Ni | 74.61 | 90.29 |
| W | 25.39 | 9.71 |
| Indicated Position | Element | wt.% | at.% |
|---|---|---|---|
| 3 | Cu | 0.07 | 0.03 |
| Sn | 74.36 | 57.11 | |
| Ni | 25.57 | 42.86 | |
| W | 0.00 | 0.00 | |
| 4 | Cu | 0.00 | 0.00 |
| Sn | 60.63 | 57.40 | |
| Ni | 16.41 | 28.59 | |
| W | 22.96 | 14.01 | |
| 5 | Cu | 0.58 | 0.67 |
| Sn | 1.55 | 0.96 | |
| Ni | 73.29 | 88.79 | |
| W | 24.58 | 9.58 |
| Indicated Position | Element | wt.% | at.% |
|---|---|---|---|
| 6 | Cu | 0.08 | 0.04 |
| Sn | 74.84 | 57.77 | |
| Ni | 23.78 | 42.19 | |
| W | 0.00 | 0.00 | |
| 7 | Cu | 0.00 | 0.00 |
| Sn | 60.91 | 57.86 | |
| Ni | 16.21 | 28.25 | |
| W | 22.88 | 13.89 | |
| 8 | Cu | 0.57 | 0.93 |
| Sn | 1.66 | 1.03 | |
| Ni | 72.87 | 88.18 | |
| W | 24.90 | 9.86 |
| Indicated Position | Element | wt.% | at.% |
|---|---|---|---|
| 9 | Cu | 0.73 | 1.03 |
| Sn | 75.13 | 57.41 | |
| Ni | 24.24 | 41.56 | |
| W | 0.00 | 0.00 | |
| 10 | Cu | 1.82 | 2.56 |
| Sn | 75.02 | 57.03 | |
| Ni | 23.16 | 40.41 | |
| W | 0.00 | 0.00 | |
| 11 | Cu | 0.00 | 0.00 |
| Sn | 60.28 | 57.46 | |
| Ni | 16.28 | 28.31 | |
| W | 23.44 | 14.23 | |
| 12 | Cu | 0.53 | 0.87 |
| Sn | 1.76 | 1.09 | |
| Ni | 72.68 | 88.13 | |
| W | 25.03 | 9.91 | |
| 13 | Cu | 63.50 | 76.78 |
| Sn | 36.46 | 23.2 | |
| Ni | 0.00 | 0.00 | |
| W | 0.04 | 0.02 |
| Temperature (°C) | k (μm/s1/2) | n | |
|---|---|---|---|
| Ni3Sn4-based IMCs (Growth) | 250 | 0.54 | 0.24 |
| 275 | 0.68 | 0.24 | |
| 300 | 0.80 | 0.33 | |
| Ni2WSn4 (Growth) Ni-W diffusion barrier layer (Consumption) | 250 | 0.30 | 0.37 |
| 275 | 0.48 | 0.30 | |
| 300 | 0.65 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Li, C.; Shang, M.; Chen, X.; Wang, Y.; Ma, H.; Ma, H.; Liu, X. Diffusion Barrier Performance of Ni-W Layer at Sn/Cu Interfacial Reaction. Materials 2024, 17, 3682. https://doi.org/10.3390/ma17153682
Yao J, Li C, Shang M, Chen X, Wang Y, Ma H, Ma H, Liu X. Diffusion Barrier Performance of Ni-W Layer at Sn/Cu Interfacial Reaction. Materials. 2024; 17(15):3682. https://doi.org/10.3390/ma17153682
Chicago/Turabian StyleYao, Jinye, Chenyu Li, Min Shang, Xiangxu Chen, Yunpeng Wang, Haoran Ma, Haitao Ma, and Xiaoying Liu. 2024. "Diffusion Barrier Performance of Ni-W Layer at Sn/Cu Interfacial Reaction" Materials 17, no. 15: 3682. https://doi.org/10.3390/ma17153682
APA StyleYao, J., Li, C., Shang, M., Chen, X., Wang, Y., Ma, H., Ma, H., & Liu, X. (2024). Diffusion Barrier Performance of Ni-W Layer at Sn/Cu Interfacial Reaction. Materials, 17(15), 3682. https://doi.org/10.3390/ma17153682






