The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. NH3/O2 Modified TMS Plasma-Nanocoated Stent
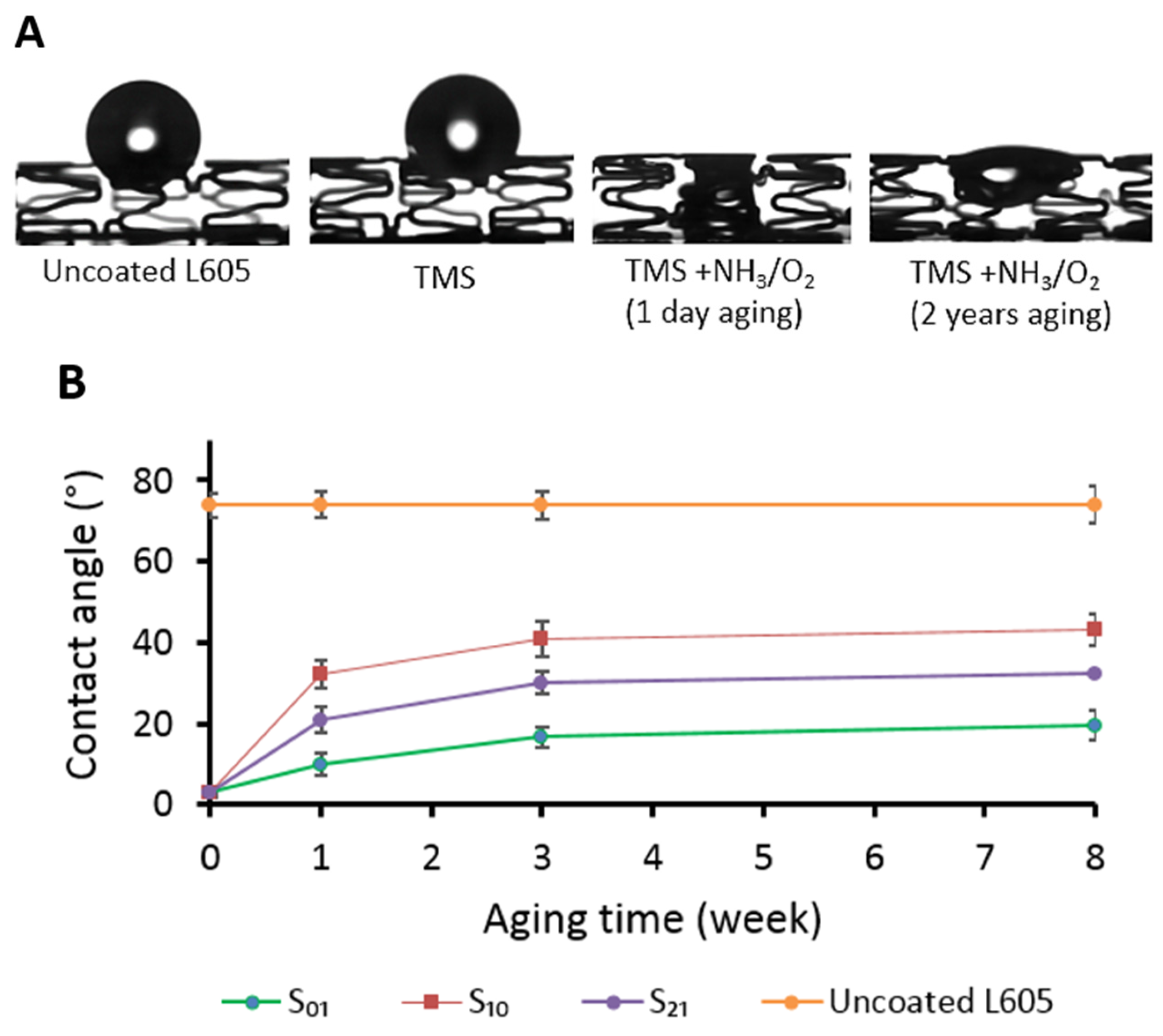
2.3. Qualitative Coating Wettability Assessment
2.4. Stent Dilatation Test with Different Coating Thicknesses
2.5. Immersion Test
2.6. Electrochemical Test
3. Results
3.1. Surface Wettability and Chemistry Assessment of Plasma-Coated Stents
3.2. TMS Plasma Nanocoating Integrity Following Crimping and Expansion
3.3. Corrosion Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Wong, S.C. Intracoronary Stents. A Breakthrough Technology or Just Another Small Step? Circulation 1994, 89, 1323–1327. [Google Scholar] [CrossRef][Green Version]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A Review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Jorge, C.; Dubois, C. Clinical Utility of Platinum Chromium Bare-Metal Stents in Coronary Heart Disease. Med. Devices Evid. Res. 2015, 8, 359–367. [Google Scholar]
- Van der Giessen, W.J.; Serruys, P.W.; van Beusekom, H.M.M.; van Woerkens, L.J.; van Loon, H.; Soei, L.K.; Strauss, B.H.; Beatt, K.J.; Verdouw, P.D. Coronary Stenting with a New, Radiopaque, Balloon-Expandable Endoprosthesis in Pigs. Circulation 1991, 83, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Abbott, J.D. Coronary Stents: History, Design, and Construction. J. Clin. Med. 2018, 7, 126. [Google Scholar] [CrossRef]
- Noad, R.L.; Hanratty, C.G.; Walsh, S.J. Clinical Impact of Stent Design. Interv. Cardiol. Rev. 2014, 9, 89–93. [Google Scholar] [CrossRef]
- Kastrati, A.; Mehilli, J.; Dirschinger, J.; Dotzer, F.; Schühlen, H.; Neumann, F.-J.; Fleckenstein, M.; Pfafferott, C.; Seyfarth, M.; Schömig, A. Intracoronary Stenting and Angiographic Results Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) Trial. Circulation 2001, 103, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Jinnouchi, H.; Torii, S.; Virmani, R.; Finn, A.V. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering 2018, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, R.; Pilgrim, T. The Impact of Thin-Strut, Biodegradable Polymer Stent Designs. Card. Interv. Today 2017, 11, 43–46. [Google Scholar]
- Otsuka, F.; Nakano, M.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathologic Etiologies of Late and Very Late Stent Thrombosis following First-Generation Drug-Eluting Stent Placement. Thrombosis 2012, 2012, 1608593. [Google Scholar] [CrossRef]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; et al. Pathology of Second-Generation Everolimus-Eluting Stents versus First-Generation Sirolimus- and Paclitaxel-Eluting Stents in Humans. Circulation 2013, 129, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Bhatt, D.L.; Gibson, C.M.; James, S.; Kimura, T.; Mehran, R.; Rao, S.V.; Steg, P.G.; Urban, P.; Valgimigli, M.; et al. Bleeding Avoidance Strategies in Percutaneous Coronary Intervention. Nat. Rev. Cardiol. 2022, 19, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Cao, D.; Angiolillo, D.J.; Bangalore, S.; Bhatt, D.L.; Ge, J.; Hermiller, J.; Makkar, R.R.; Neumann, F.-J.; Saito, S.; et al. 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention: A Consensus Document from the Academic Research Consortium for High Bleeding Risk. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-Based or Polymer-Free Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Kirtane, A.J.; Windecker, S.; Latib, A.; Kedhi, E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention with Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients. Circ. Cardiovasc. Interv. 2020, 13, 222–232. [Google Scholar] [CrossRef]
- Varenne, O.; Cook, S.; Sideris, G.; Kedev, S.; Cuisset, T.; Carrié, D.; Hovasse, T.; Garot, P.; El Mahmoud, R.; Spaulding, C.; et al. Drug-Eluting Stents in Elderly Patients with Coronary Artery Disease (SENIOR): A Randomised Single-Blind Trial. Lancet 2018, 391, 41–50. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Nammas, W. Excellent Very Early Neointimal Coverage of Bioactive Stents by Optical Coherence Tomography. Scand. Cardiovasc. J. 2015, 49, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Chavarri, M.V.; Bethencourt, A.; Pinar, E.; Gomez, A.; Portales, J.F.; Pomar, F.; Calvo, I.; Minguez, J.R.L.; Valdesuso, R.; Moreu, J.; et al. Titanium-Nitride Oxide-Coated Stents Multicenter Registry in Diabetic Patients: The TIBET Registry. Heart Vessel. 2012, 27, 151–158. [Google Scholar] [CrossRef]
- Windecker, S.; Billinger, M.; Hess, O.M. Stent Coating with Titanium-Nitride-Oxide for Prevention of Restenosis. EuroIntervention 2006, 2, 146–148. [Google Scholar]
- Tonino, P.A.L.; Pijls, N.H.J.; Collet, C.; Nammas, W.; der Heyden, J.V.; Romppanen, H.; Kervinen, K.; Airaksinen, J.K.E.; Sia, J.; Lalmand, J.; et al. Titanium-Nitride-Oxide-Coated Versus Everolimus-Eluting Stents in Acute Coronary Syndrome: The Randomized TIDES-ACS Trial. JACC Cardiovasc. Interv. 2020, 13, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Serruys, P.W. Coronary Stents: Looking Forward. J. Am. Coll. Cardiol. 2010, 56, S43–S78. [Google Scholar] [CrossRef]
- Maillard, L.; Corseaux, D.; Altié, A.; Ung, A.; Courageot, J.; Barakat, M.; Teiger, E.; Belle, E.V. Time Course of Reendothelialization with Polyzene-F Nanocoated Cobra PzF™ Coronary Stent on Rabbit Iliac Arteries. Cardiovasc. Revasc. Med. 2020, 21, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Colleran, R.; Joner, M.; Cutlip, D.; Urban, P.; Maeng, M.; Jauhar, R.; Barakat, M.; Michel, J.M.; Mehran, R.; Kirtane, A.J.; et al. Design and Rationale of a Randomized Trial of COBRA PzF Stenting to REDUCE Duration of Triple Therapy (COBRA-REDUCE). Cardiovasc. Revasc. Med. 2022, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Jauhar, R.; Meraj, P.; Garratt, K.N.; Novack, V.; Novack, L.; Maillard, L.; Erglis, A.; Stoler, R.; Barakat, M.; et al. Five-Year Clinical Outcomes of the COBRA Polyzene F NanoCoated Coronary Stent System. Cardiovasc. Revasc. Med. 2022, 41, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Garratt, K.N.; Novack, V.; Barakat, M.; Meraj, P.; Maillard, L.; Erglis, A.; Jauhar, R.; Popma, J.J.; Stoler, R.; et al. 9-Month Clinical and Angiographic Outcomes of the COBRA Polyzene-F NanoCoated Coronary Stent System. JACC Cardiovasc. Interv. 2017, 10, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Aladin, A.I.; Wermers, J.P.; Morrison, N.; Gray, N.; Waksman, R. Review of Late-Breaking Trials from CRT 2022. Cardiovasc. Revasc. Med. 2022, 40, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.; Vochelet, F.; Peycher, P.; Ayari, A.; Barra, N.; Billé, J.; Joly, P.; Silvestri, M.; Sévilla, J.; Tavildari, A. MAPT (Mono Antiplatelet Therapy) as Regular Regimen after COBRA PzF™ NanoCoated Coronary Stent (NCS) Implantation. Cardiovasc. Revasc. Med. 2020, 21, 785–789. [Google Scholar] [CrossRef]
- Phan, T.; Jones, J.E.; Chen, M.; Bowles, D.K.; Fay, W.P.; Yu, Q. A Biocompatibility Study of Plasma Nanocoatings onto Cobalt Chromium L605 Alloy for Cardiovascular Stent Applications. Materials 2022, 15, 5968. [Google Scholar] [CrossRef]
- Phan, T.; Jones, J.E.; Chen, M.; Strawn, T.L.; Khoukaz, H.B.; Ji, Y.; Kumar, A.; Bowles, D.K.; Fay, W.P.; Yu, Q. In vitro biological responses of plasma nanocoatings for coronary stent applications. J. Biomed. Mater. Res. Part A 2023, 111, 1768–1780. [Google Scholar] [CrossRef]
- Standard ASTM F2394-07; Standard Guide for Measuring Securement of Balloon-Expandable Vascular Stent Mounted on Delivery System. ASTM International: West Conshohocken, PA, USA, 2022.
- Kokubo, T.; Takadama, H. How Useful is SBF in Predicting in vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Standard ASTM F2129-15; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices. ASTM International: West Conshohocken, PA, USA, 2015.
- Lewis, F.; Horny, P.; Hale, P.; Turgeon, S.; Tatoulian, M.; Mantovani, D. Study of the Adhesion of Thin Plasma Fluorocarbon Coatings Resisting Plastic Deformation for Stent Applications. J. Phys. D Appl. Phys. 2008, 41, 045310. [Google Scholar] [CrossRef]
- Menown, I.B.A.; Noad, R.; Garcia, E.J.; Meredith, I. The Platinum Chromium Element Stent Platform: From Alloy, to Design, to Clinical Practice. Adv. Ther. 2010, 27, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo Corrosion and Corrosion Protection of Magnesium Alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Mukhamadiyarov, R.A.; Bogdanov, L.A.; Mishinov, S.V.; Kutikhin, A.G. A Novel Technique for Preparation, Staining, and Visualization of Tissue with Metal Implants and Extraskeletal Calcification Areas. Mod. Technol. Med. 2020, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kwok SC, H.; Ha PC, T.; McKenzie, D.R.; Bilek MM, M.; Chu, P.K. Biocompatibility of Calcium and Phosphorus Doped Diamond-Like Carbon Thin Films Synthesized by Plasma Immersion Ion Implantation and Deposition. Diam. Relat. Mater. 2006, 15, 893–897. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, S.-J.; Chung, K.-H.; Chen, Y.-L.; Su, Y.-Y. Increased Corrosion Resistance of Stent Materials by Converting Current Surface Film of Polycrystalline Oxide into Amorphous Oxide. J. Biomed. Mater. Res. 2000, 52, 323–332. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Kinlay, S.; Young, M.M.; Sherrod, R.; Gagnon, D.R. Long-Term Outcomes and Duration of Dual Antiplatelet Therapy after Coronary Intervention with Second-Generation Drug-Eluting Stents: The Veterans Affairs Extended DAPT Study. J. Am. Heart Assoc. 2023, 12, e027055. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; Onuma, Y.; et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, J.S.; Hong, S.J.; Lim, D.S.; Lee, S.Y.; Yun, K.H.; Park, J.K.; Kang, W.C.; Kim, Y.H.; Yoon, H.J.; et al. 1-Month Dual-Antiplatelet Therapy Followed by Aspirin Monotherapy after Polymer-Free Drug-Coated Stent Implantation. J. Am. Coll. Cardiovasc. Interv. 2021, 14, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Gotman, I. Characteristics of metals Used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, R.; Haider, W.; Maffi, S.K. Hemocompatibility Improvement of Chromium-Bearing Bare-Metal Stent Platform after Magnetoelectropolishing. J. Mater. Eng. Perform. 2015, 24, 345–352. [Google Scholar] [CrossRef]
- Nagai, A.; Tsutsumi, Y.; Suzuki, Y.; Katayama, K.; Hanawa, T.; Yamashita, K. Characterization of air-formed surface oxide film on a Co–Ni–Cr–Mo alloy (MP35N) and its change in Hanks’ solution. Appl. Surf. Sci. 2012, 258, 5490–5498. [Google Scholar] [CrossRef]
- Halwani, D.O.; Anderson, P.G.; Lemons, J.E.; Jordan, W.D.; Anayiotos, A.S.; Brott, B.C. In-vivo corrosion and local release of metallic ions from vascular stents into surrounding tissue. J. Invasive Cardiol. 2010, 22, 528–535. [Google Scholar] [PubMed]
- Høl, P.J.; Gjerdet, N.R.; Jonung, T. Corrosion and metal release from overlapping arterial stents under mechanical and electrochemical stress—An experimental study. J. Mech. Behav. Biomed. Mater. 2019, 93, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.K.; Brooks, R.P.; Ehrensberger, M.T. Effects of simulated inflammation on the corrosion of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kapnisis, K.; Stylianou, A.; Kokkinidou, D.; Martin, A.; Wang, D.; Anderson, P.G.; Prokopi, M.; Papastefanou, C.; Brott, B.C.; Lemons, J.E.; et al. Multilevel Assessment of Stent-Induced Inflammation in the Adjacent Vascular Tissue. ACS Biomater. Sci. Eng. 2023, 9, 4747–4760. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Leng, Y.; Wan, G.; Huang, N. Corrosion susceptibility investigation of Ti–O film modified cobalt-chromium alloy (L-605) vascular stents by cyclic potentiodynamic polarization measurement. Surf. Coat. Technol. 2011, 206, 893–896. [Google Scholar] [CrossRef]
- Bettini, E.; Eriksson, T.; Boström, M.; Leygraf, C.; Pan, J. Inuence of metal carbides on dissolution behavior of biomedical CoCrMo alloy: SEM, TEM and AFM studies. Electrochim. Acta 2011, 56, 9413–9419. [Google Scholar] [CrossRef]
- Chen, M.; Zamora, P.O.; Som, P.; Peña, L.A.; Osaki, S. Cell attachment and biocompatibility of polytetrafluoroethylene (PTFE) treated with glow-discharge plasma of mixed ammonia and oxygen. J. Biomater. Sci. Polym. Ed. 2003, 14, 917–935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, M.; Zamora, P.O.; Peña, L.; Som, P.; Osaki, S. NH3/O2 mixed gas plasmas alter the interaction of blood components with stainless steel. J. Biomed. Mater. Res. 2003, 67A, 994–1000. [Google Scholar] [CrossRef] [PubMed]



| Elements | TMS+NH3/O2 Plasma-Nanocoated Stents (at%) | Uncoated L605 Stents (at%) |
|---|---|---|
| O | 2.10 ± 0.3 | 35.82 ± 2.32 |
| Ca | 0.01 ± 0 | 11.6 ± 2.01 |
| P | 0.07 ± 0.01 | 8.82 ± 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.; Jones, J.E.; Liao, Y.; Yu, Q.; Chen, M. The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents. Materials 2024, 17, 3699. https://doi.org/10.3390/ma17153699
Phan T, Jones JE, Liao Y, Yu Q, Chen M. The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents. Materials. 2024; 17(15):3699. https://doi.org/10.3390/ma17153699
Chicago/Turabian StylePhan, ThiThuHa, John E. Jones, Yixuan Liao, Qingsong Yu, and Meng Chen. 2024. "The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents" Materials 17, no. 15: 3699. https://doi.org/10.3390/ma17153699
APA StylePhan, T., Jones, J. E., Liao, Y., Yu, Q., & Chen, M. (2024). The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents. Materials, 17(15), 3699. https://doi.org/10.3390/ma17153699






