A Study of MgZnO Thin Film for Hydrogen Sensing Application
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
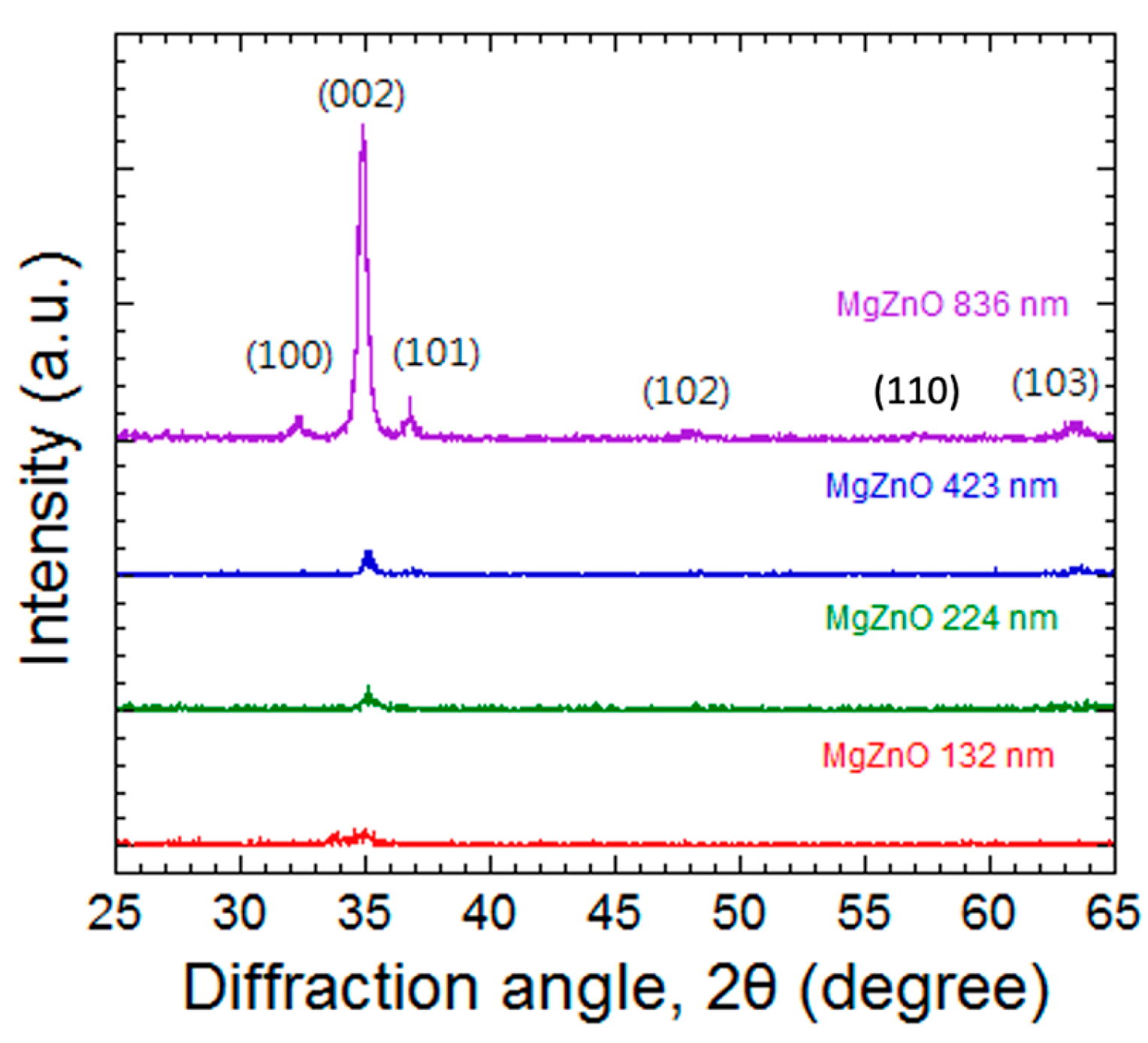
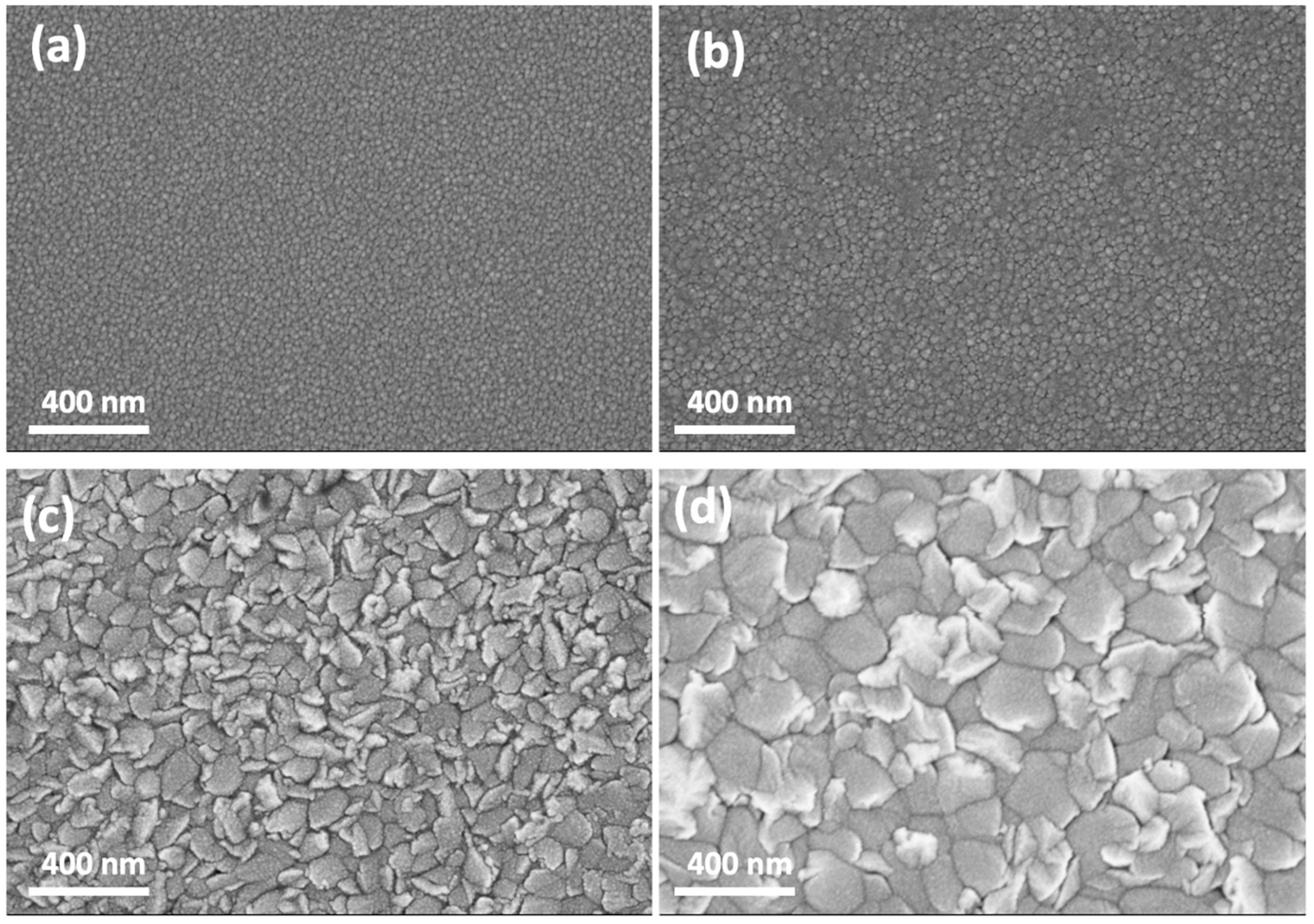
3.1. Thickness Effect of MgZnO Thin Film
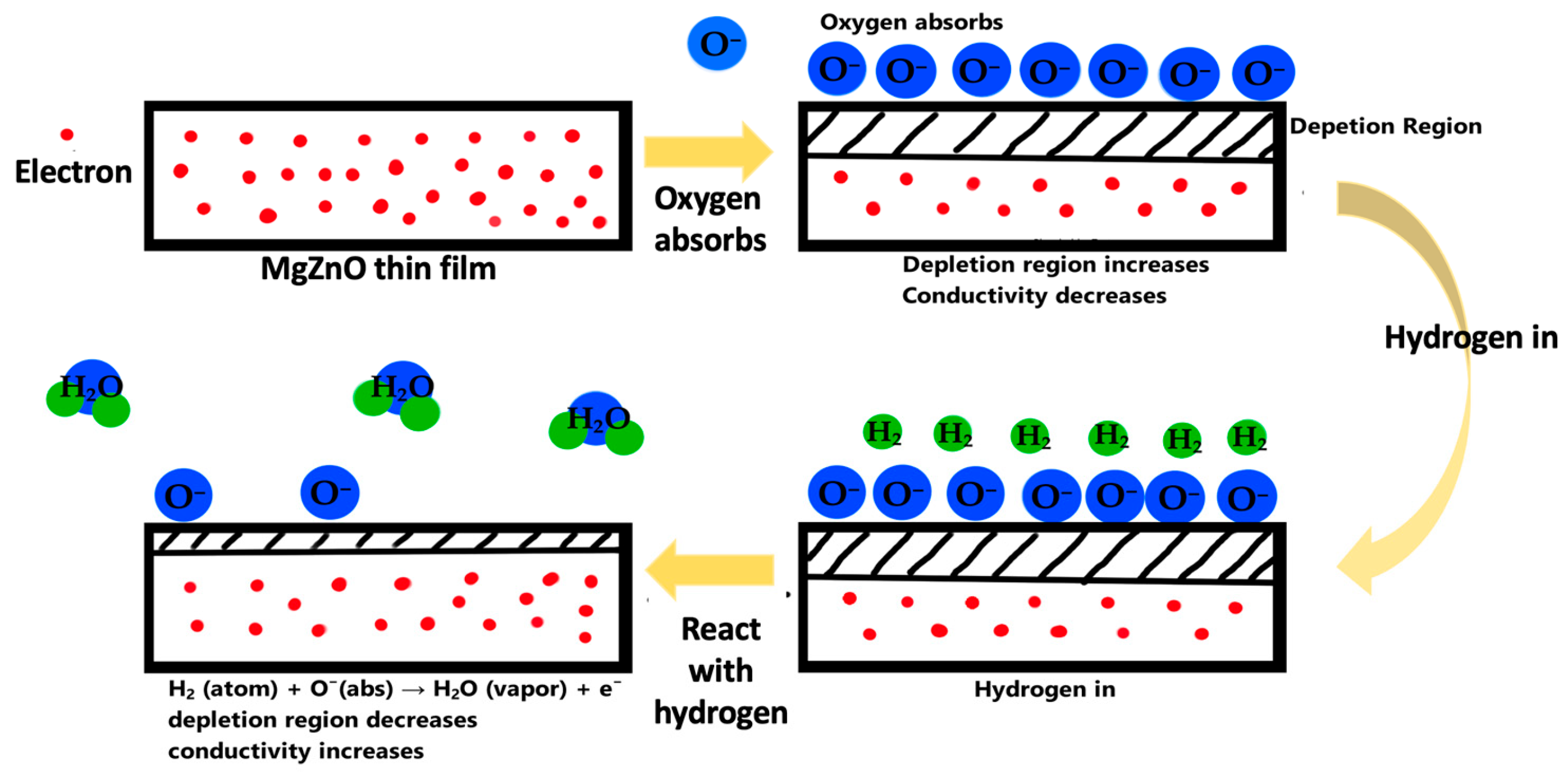
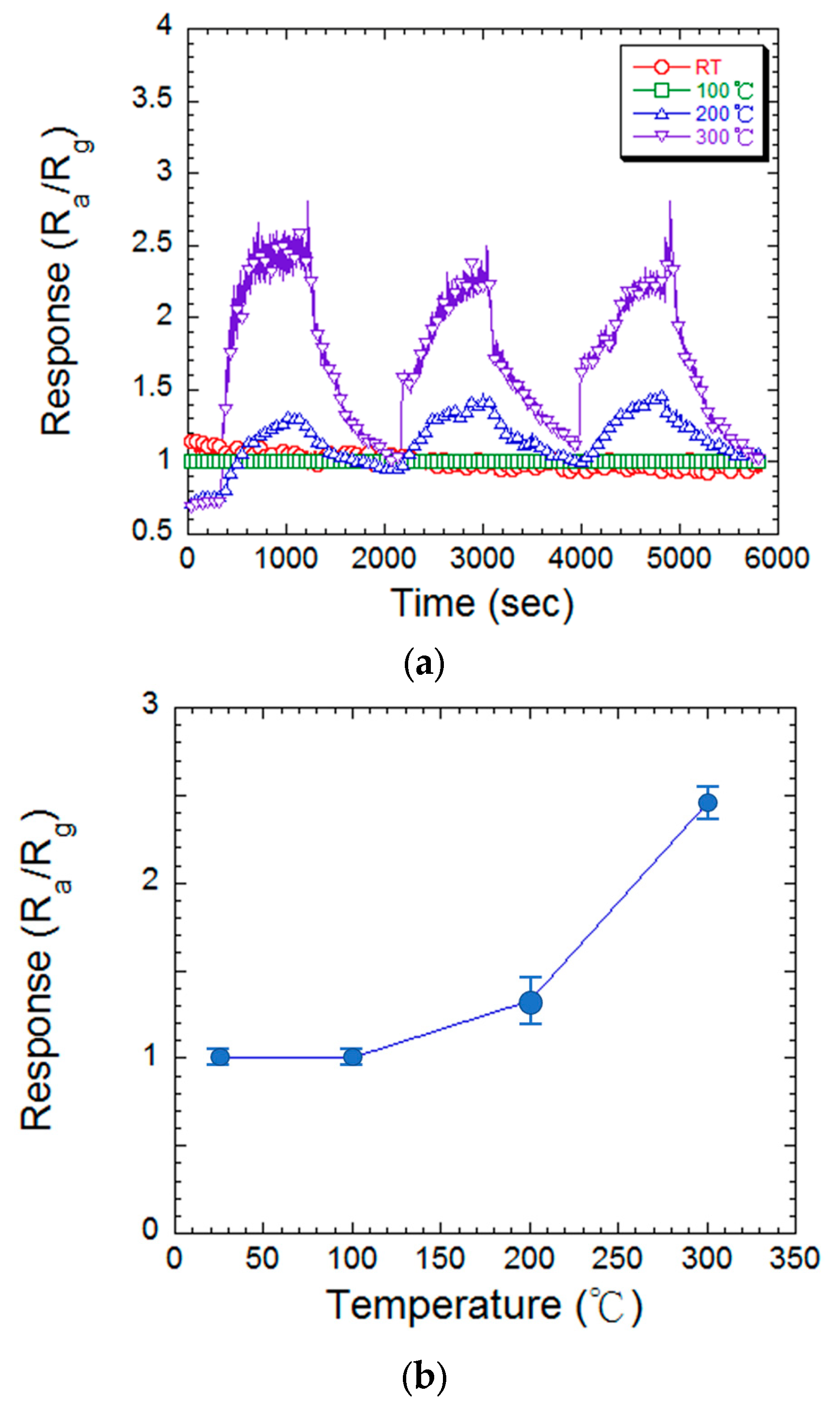
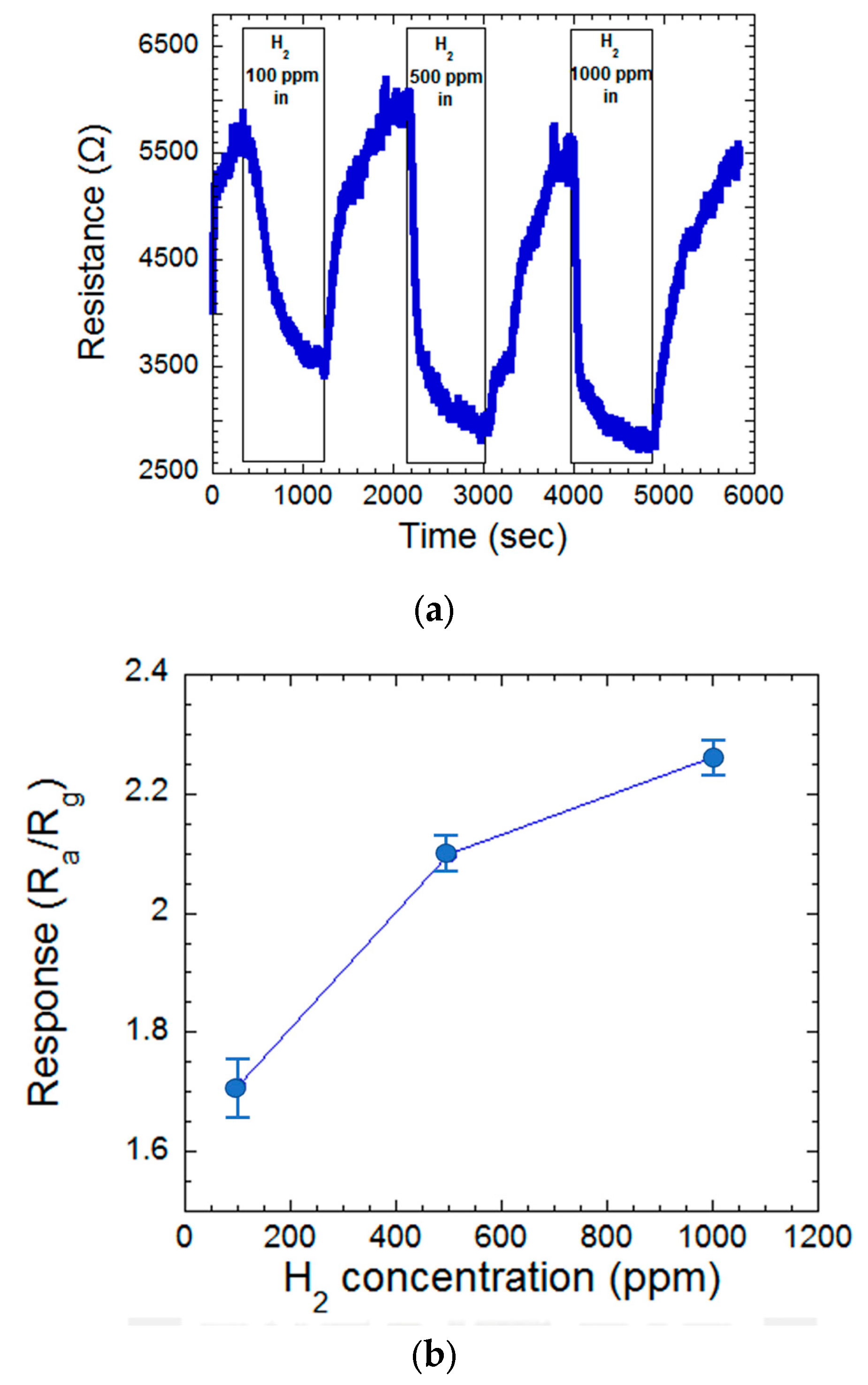
3.2. Temperature and Hydrogen Concentration Effect of MgZnO Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, B.; Cai, D.; Li, H.; Liu, Y.; Wang, D.; Wang, L.; Li, Q.; Wang, T. Room-temperature hydrogen sensor based on grain-boundary controlled Pt decorated In2O3 nanocubes. Sens. Actuators B Chem. 2014, 201, 351–359. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. Room-temperature gas sensor using carbon nanotube with cobalt oxides. Sens. Actuators B Chem. 2014, 204, 596–601. [Google Scholar] [CrossRef]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Romain, A.C.; Nicolas, J. Long-term stability of metal oxide-based gas sensors for e-nose environmental applications: An overview. Sens. Actuators B Chem. 2010, 146, 502–506. [Google Scholar] [CrossRef]
- Garcia, M.L.; Masson, M. Environmental and geologic application of solid-state methane sensors. Environ. Geol. 2004, 46, 1059–1063. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of water vapor on Pd-Loaded SnO2 nanoparticles gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Highly sensitive and selective CO gas sensor based on a hydrophobic SnO2/CuO bilayer. RSC Adv. 2016, 6, 47178–47184. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cai, D.; Liu, Y.; Wang, D.; Wang, L.; Wang, Y.; Li, H.; Li, Q.; Wang, T. Improved room-temperature hydrogen sensing performance of directly formed Pd/WO3 nanocomposite. Sens. Actuators B Chem. 2014, 193, 28–34. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Chandra, R. Highly sensitive and selective hydrogen gas sensor using sputtered grown Pd decorated MnO2 nanowalls. Sens. Actuators B Chem. 2016, 234, 8–14. [Google Scholar] [CrossRef]
- Duoc, V.T.; Nguyen, H.; Ngoc, T.M.; Xuan, C.T.; Hung, C.M.; Duy, N.V.; Hoa, N.D. Hydrogen gas sensor based on the self-heating effect of SnO2/Pt thin film with ultralow power consumption. Int. J. Hydrogen Energy 2024, 61, 774–782. [Google Scholar] [CrossRef]
- Pai, S.H.S.; Mondal, A.; Barathy, R.; Ajitha, T.B.; Samuel, J.J.; Reddy, E.Y.A.K. Effect of calcination temperature on NiO for hydrogen gas sensor performance. Int. J. Hydrogen Energy 2024, 50, 928–941. [Google Scholar] [CrossRef]
- Huang, B.R.; Kathiravan, D.; Hsieh, H.R.; Saravanan, A. Heterostructure interfaces and dimensionally transformed GaO/ZnO nanostructures for room-temperature hydrogen gas sensors. Int. J. Hydrogen Energy 2024, 64, 889–895. [Google Scholar] [CrossRef]
- Amu-Darko, J.N.O.; Hussain, S.; Issaka, E.; Wang, M.; Alothman, A.A.; Lei, S.; Qiao, G.; Liu, G. Nanosheet assembled NiO-doped-ZnO flower-like sensors for highly sensitive hydrogen sulfide gas detection. Ceram. Int. 2024, 50, 17681–17690. [Google Scholar] [CrossRef]
- Huang, W.C.; Lin, T.C.; Hsien, T.L.; Tsai, M.H.; Horng, C.T.; Kuo, T.L. Ag Contact on sol-gel processed MgZnO film. Microelectron. Eng. 2013, 107, 205–209. [Google Scholar] [CrossRef]
- Kasapoglu, A.E.; Habashyani, S.; Baltakesmez, A.; Iskenderoglu, D.; Gur, E. The effect of the change in the amount of Sb doping in ZnO nanorods for hydrogen gas sensors. Int. J. Hydrogen Energy 2021, 46, 21715–21725. [Google Scholar] [CrossRef]
- Bai, J.; Kong, Y.; Liu, Z.; Yang, H.; Li, M.; Xu, D.; Zhang, Q. Ag modified Tb-doped double-phase In2O3 for ultrasensitive hydrogen gas sensor. Appl. Surf. Sci. 2022, 583, 152521–152531. [Google Scholar] [CrossRef]
- Huang, W.C.; Lin, Y.H.; Zhang, Y.H.; Chang, C.W.; Wang, D.Y.; Chuang, C.C.; Huang, Y.H.; Lin, T.C.; Sermon Wu, Y.C.; Chen, H. Investigation of ZnO/V2O5 hybrid nanocomposite-based ultraviolet photodetector and hydrogen gas sensor. J. Mater. Sci. Mater. Electron. 2023, 34, 398. [Google Scholar] [CrossRef]
- Yang, A.L.; Wei, H.Y.; Liu, X.L.; Song, H.P.; Fan, H.B.; Zhang, P.F.; Zheng, G.L.; Yang, S.Y.; Zhu, Q.S.; Wang, Z.G. Characterization of ZnMgO hexagonal-nano towers/films on m-plane sapphire synthesized by metal-organic chemical vapor deposition. J. Phys. D Appl. Phys. 2008, 41, 205–416. [Google Scholar] [CrossRef]
- Li, H.; Pan, X.; Qiao, M.; Zhang, Y.; Wang, T.; Xie, E. The influence of ambient conditions on properties of MgxZn1−xO films by sputtering. Vacuum 2008, 82, 459–462. [Google Scholar] [CrossRef]
- Liu, Y.; Hang, T.; Xie, Y.; Bao, Z.; Song, J.; Zhang, H.; Xie, E. Effect of Mg doping on the hydrogen-sensing characteristics of ZnO thin films. Sens. Actuators B Chem. 2011, 160, 266–270. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Renitta, A.; Karthick, K. Growth of high-quality ZnO:Mg films on ITO coated glass substrates for enhanced H2 sensing. Ceram. Int. 2014, 40, 6171–6177. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Karthick, K. Growth of highly c-axis oriented Mg:ZnO nanorods on Al2O3 substrate towards high-performance H2 sensing. Int. J. Hydrogen Energy 2014, 39, 7165–7172. [Google Scholar] [CrossRef]
- Adachi, Y. Enchancement of H2 gas sensing properties of ZnO films by Mg alloying. Surf. Interfaces 2022, 28, 101597–101605. [Google Scholar] [CrossRef]
- Wang, M.; Kim, E.J.; Kim, S.; Chung, J.S.; Yoo, I.K.; Shin, E.W.; Hahn, S.H.; Park, C. Optical and structural properties of sol–gel prepared MgZnO alloy thin films. Thin Solid Films 2008, 516, 1124–1129. [Google Scholar] [CrossRef]
- Galvani, M.; Freddi, S.; Sangaletti, L. Disclosing fast detection opportunities with nanostructured chemiresistor gas sensors based on metal oxides, carbon, and transition metal dichalcogenides. Sensors 2024, 24, 584. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Saha, H.; Basu, S. Pd sensitized porous silicon hydrogen sensor-influence of ZnO thin films. Sens. Actuators B 2010, 147, 128–136. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. Pinecone-shaped ZnO nanostrucutres: Growth, optical and gas sensor properties. Sens. Actuators B 2011, 157, 98–102. [Google Scholar] [CrossRef]
- Liao, J.; Li, Z.; Wang, G.; Chen, C.; Lv, S.; Li, M. ZnO nanorod/porous silicon nanowire hybrid structures as highly-sensitive NO2 gas sensors at room temperature. Phys. Chem. Chem. Phys. 2016, 18, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Ma, S.; Sun, A.; Xu, X.; Yang, G.; Yan, S.; Gao, J.; Zhang, Z.; Zhu, H. Improvement of acetone gas sensing performance of ZnO nanoparticles. J. Alloys Compd. 2016, 658, 629–635. [Google Scholar] [CrossRef]
- Castaneda, L.; Moreno-Valenzuela, J.; Torres-Torres, C. Chemisorptive detection by electrical and nonlinear optical absorption properties of a nanostructured ruthenium-doped zinc oxide film. Optik 2013, 124, 5209–5213. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-C.; Wu, J.-Y.; Mendez, A.J.J.; Salazar, N.; Hsu, H.-L.; Huang, W.-C. A Study of MgZnO Thin Film for Hydrogen Sensing Application. Materials 2024, 17, 3677. https://doi.org/10.3390/ma17153677
Lin T-C, Wu J-Y, Mendez AJJ, Salazar N, Hsu H-L, Huang W-C. A Study of MgZnO Thin Film for Hydrogen Sensing Application. Materials. 2024; 17(15):3677. https://doi.org/10.3390/ma17153677
Chicago/Turabian StyleLin, Tien-Chai, Jyun-Yan Wu, Andres Joseph John Mendez, Nadir Salazar, Hao-Lin Hsu, and Wen-Chang Huang. 2024. "A Study of MgZnO Thin Film for Hydrogen Sensing Application" Materials 17, no. 15: 3677. https://doi.org/10.3390/ma17153677
APA StyleLin, T.-C., Wu, J.-Y., Mendez, A. J. J., Salazar, N., Hsu, H.-L., & Huang, W.-C. (2024). A Study of MgZnO Thin Film for Hydrogen Sensing Application. Materials, 17(15), 3677. https://doi.org/10.3390/ma17153677





