Facilely Promoting the Concentration of Baicalin in Polylactic Acid Fiber for UV Shielding and Antibacterial Functions: A Customized and Sustainable Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Properties of Baicalin in Aqueous Solution
2.3. Fabric Treatment
2.4. CCD Experiment
2.5. Measurement
2.5.1. UV–Visible Spectroscopy and FTIR
2.5.2. UV-Protective and Antibacterial Performance
3. Results
3.1. Spectroscopy Analysis
3.1.1. UV–Vis Adsorption Spectroscopy
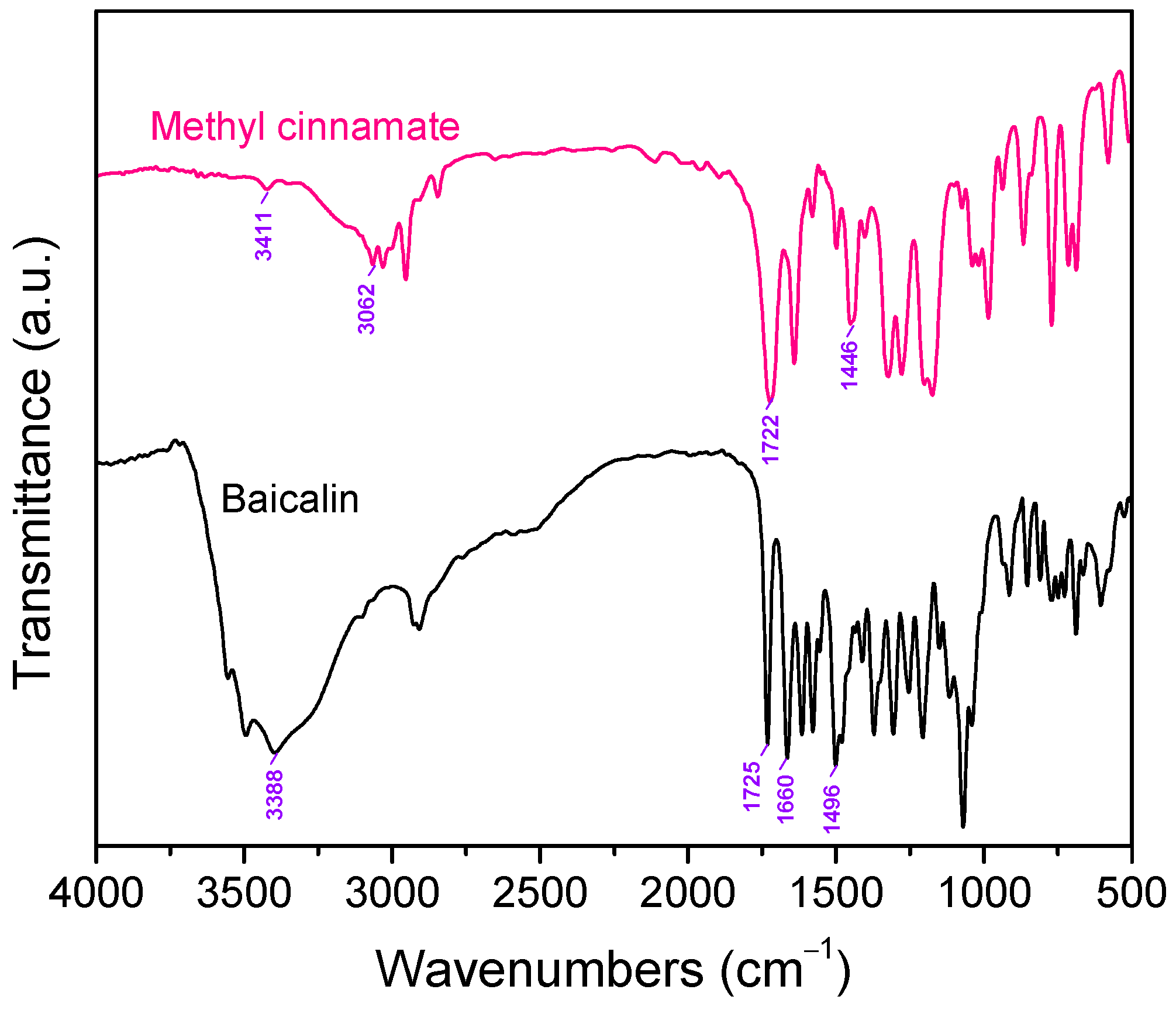
3.1.2. FTIR
3.2. Methyl-Cinnamate-Assisted Finishing
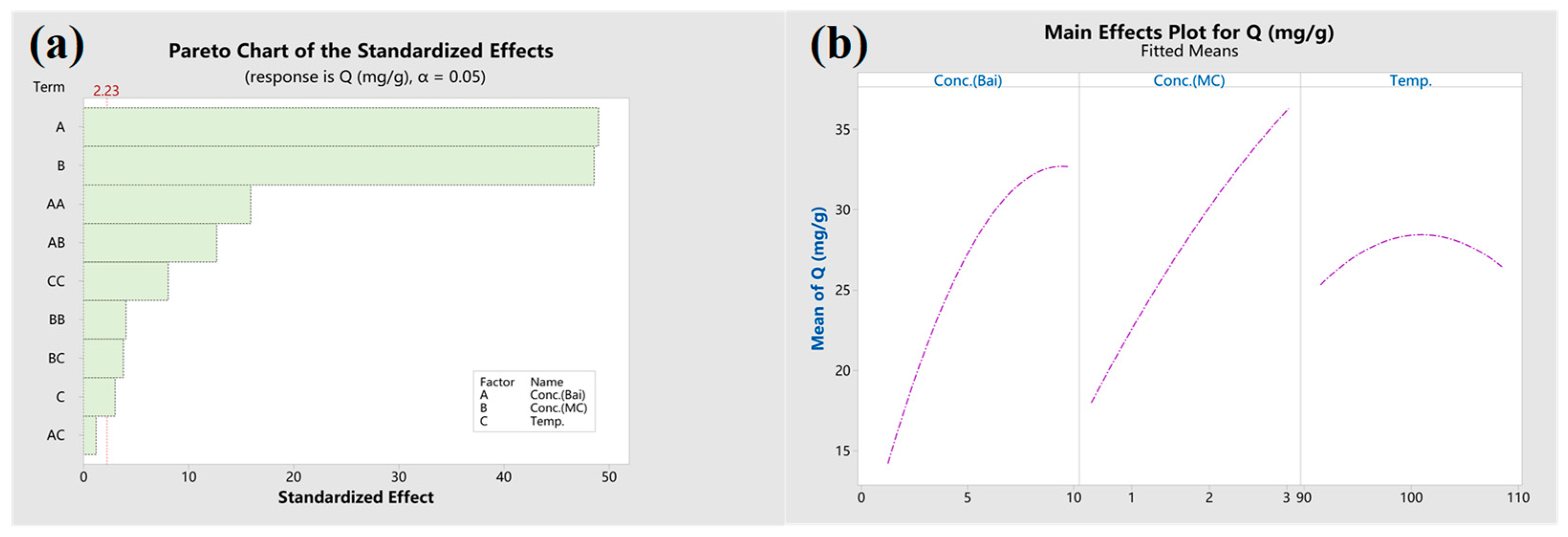
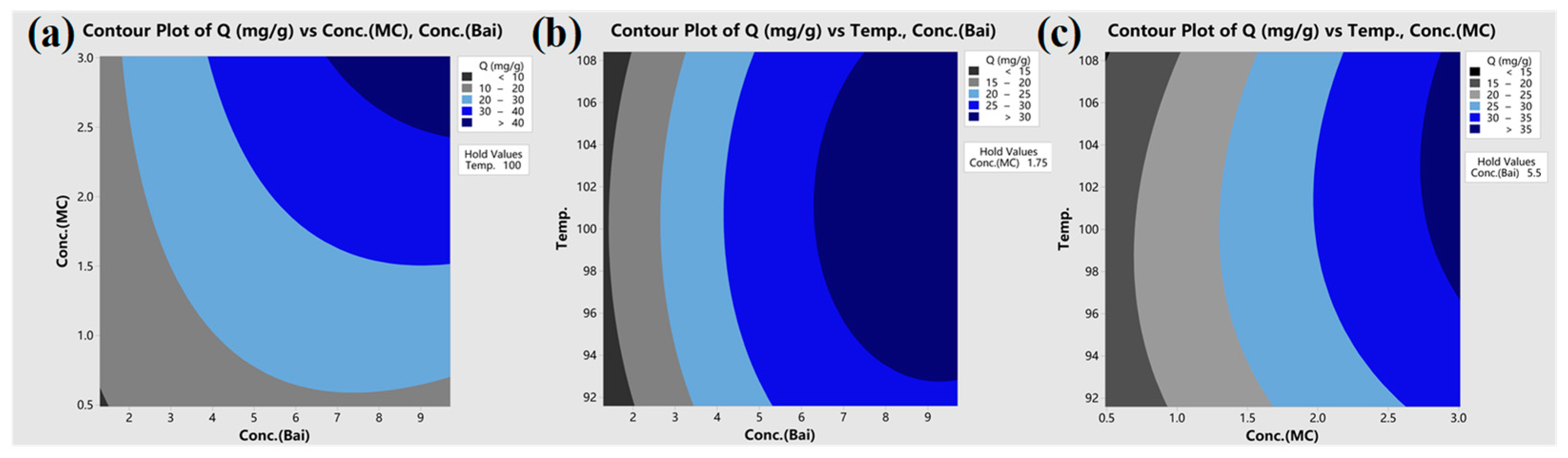
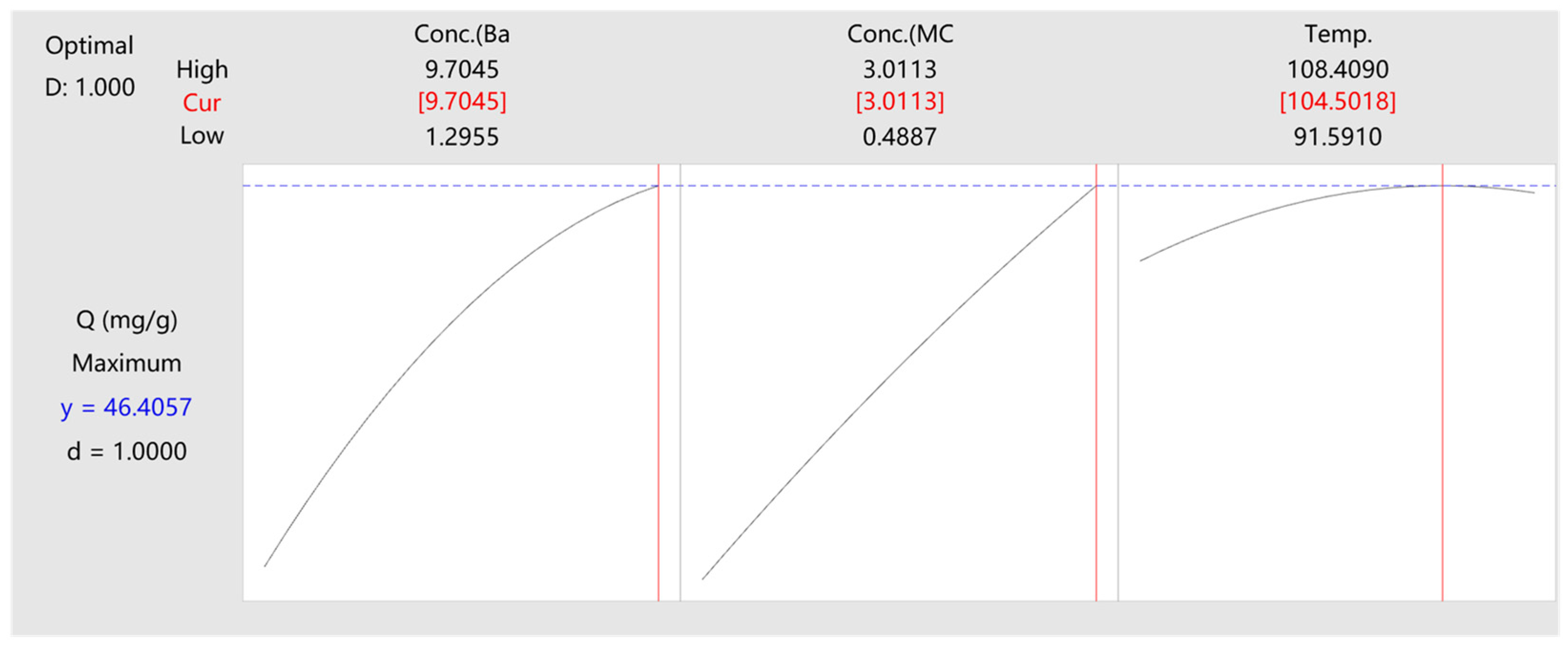
3.3. CCD Experiment
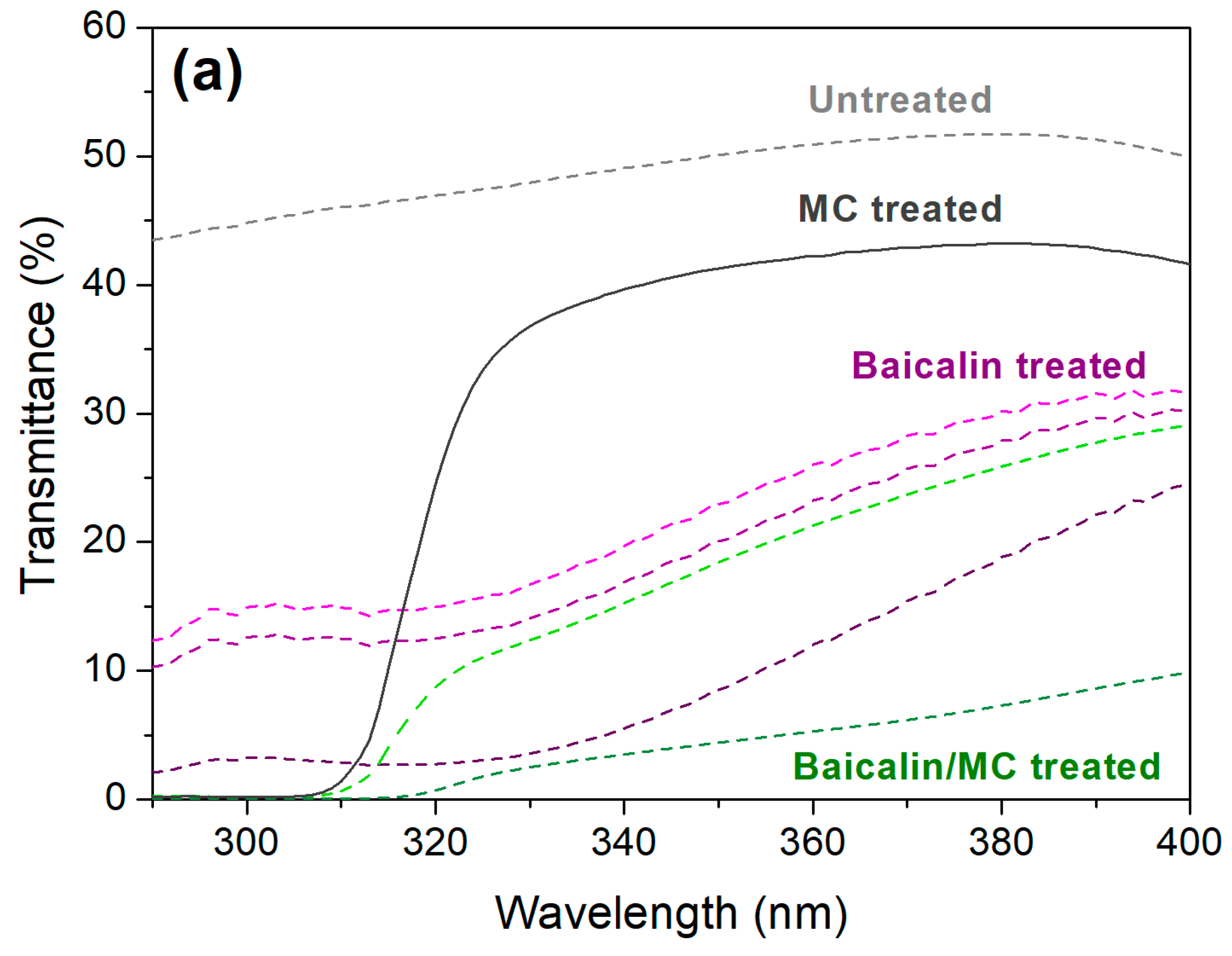
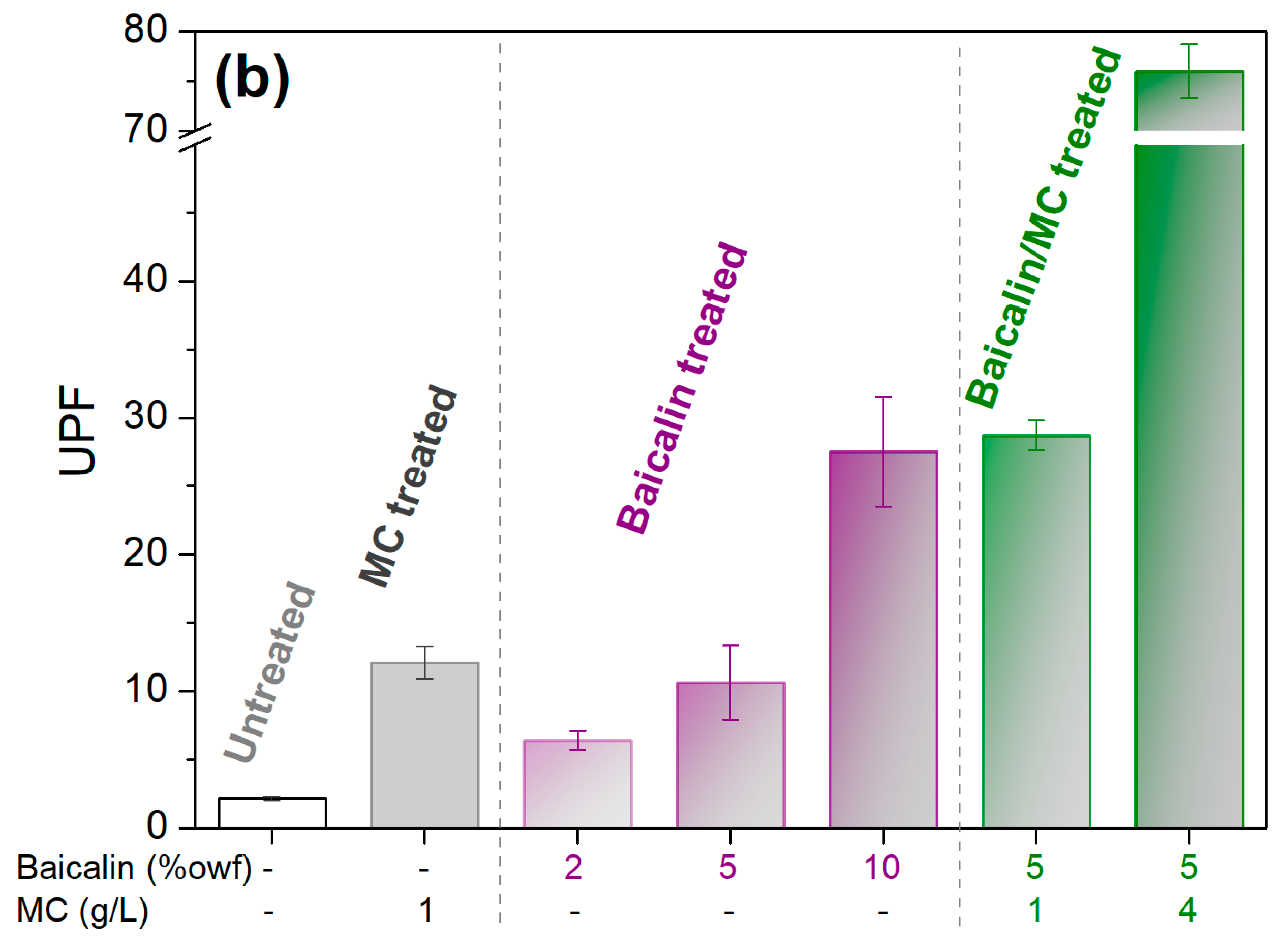
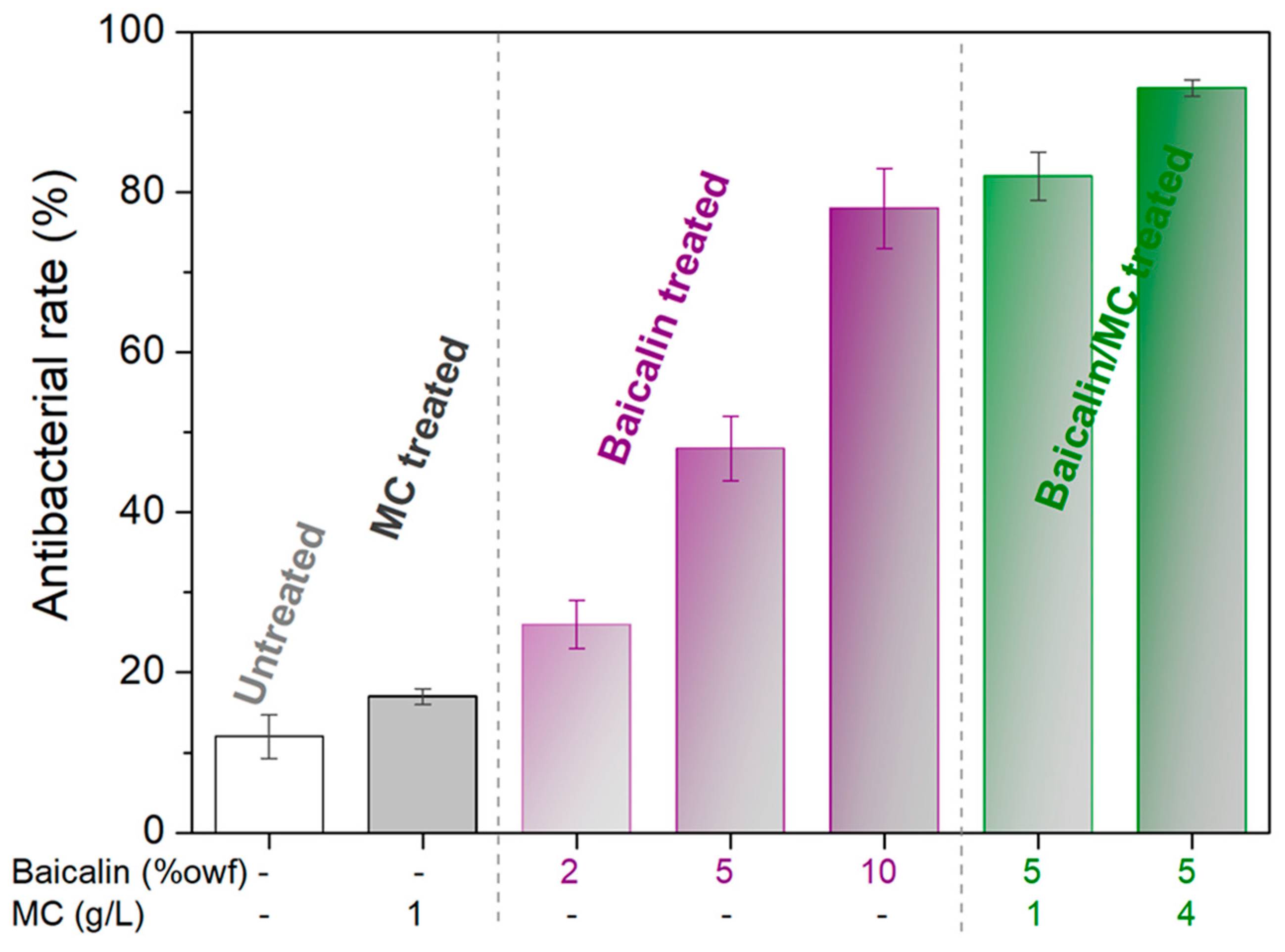
3.4. Functionality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)—Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tausif, M.; Ashraf, M. A review of progress in the dyeing of eco-friendly aliphatic polyester-based polylactic acid fabrics. J. Clean. Prod. 2015, 108, 476–483. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; López de Dicastillo, C.; Velásquez, E.; Villegas, C.; Faba, S.; Rivera, P.; Guarda, A.; Romero, J.; Galotto, M. Foaming with scCO2 and Impregnation with Cinnamaldehyde of PLA Nanocomposites for Food Packaging. Processes 2022, 10, 376. [Google Scholar] [CrossRef]
- Saadati Ardestani, N.; Rojas, A.; Esfandiari, N.; Galotto, M.J.; Babhadiashar, A.; Sajadian, S.A. Supercritical Fluid Extraction from Zataria multiflora Boiss and Impregnation of Bioactive Compounds in PLA for the Development of Materials with Antibacterial Properties. Processes 2022, 10, 1787. [Google Scholar] [CrossRef]
- Nagy, B.; Török, F.; Tomasek, S.; Miskolczi, N. Vegetable oil based additives to enhance the properties of PLA/Starch composites: The effect of reaction parameters. Ind. Crops Prod. 2023, 191, 116025. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B.; Chang, F.; Chen, L.; Cao, X.; He, G. Improved compatibility of PLA/Starch blends with Binary functional monomers through UV-induced reactive extrusion. Ind. Crops Prod. 2023, 197, 116635. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Li, Y.; Wang, W.; Gilchrist, M.D.; Zhang, N. Toward the Scalable Fabrication of Fully Bio-Based Antimicrobial and UVB-Blocking Transparent Polylactic Acid Films That Incorporate Natural Coatings and Nanopatterns. ACS Appl. Mater. Interfaces 2022, 14, 54338–54348. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Ni, L.; Wei, Z.; Quan, H.; Zhou, Y. One-pot high efficiency low temperature ultrasonic-assisted strategy for fully bio-based coloristic, anti-pilling, antistatic, bioactive and reinforced cashmere using grape seed proanthocyanidins. J. Clean. Prod. 2021, 315, 128148. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.Y.; Tang, R.C. Bioactive and UV protective silk materials containing baicalin—The multifunctional plant extract from Scutellaria baicalensis Georgi. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 336–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W. Nucleophilic modification of flavonoids for enhanced solubility and photostability towards uniform colouration, bio-activation and ultraviolet-proof finishing of silk fabric. Arab. J. Chem. 2022, 15, 104343. [Google Scholar] [CrossRef]
- Wu, J.; Guo, H.; Ke, J.; Fan, J. Studies on kinetic and thermodynamic parameters of natural dye curcumin on PLA fibre. Indian J. Fibre Text. Res. 2013, 38, 424–426. [Google Scholar]
- Karst, D.; Yang, Y. Using the solubility parameter to explain disperse dye sorption on polylactide. J. Appl. Polym. Sci. 2005, 96, 416–422. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.F.; Lin, J.X. Studies on the ultrasonic-assisted dyeing of poly(trimethylene terephthalate) fabric. Color. Technol. 2010, 126, 243–248. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Wellington, G.A.; Cocchiara, J.; Lalko, J.; Letizia, C.S.; Api, A.M. Fragrance material review on methyl cinnamate. Food Chem. Toxicol. 2007, 45, 113–119. [Google Scholar] [CrossRef]
- Fujiwara, G.M.; Annies, V.; de Oliveira, C.F.; Lara, R.A.; Gabriel, M.M.; Betim, F.C.; Nadal, J.M.; Farago, P.V.; Dias, J.F.; Miguel, O.G.; et al. Evaluation of larvicidal activity and ecotoxicity of linalool, methyl cinnamate and methyl cinnamate/linalool in combination against Aedes aegypti. Ecotoxicol. Environ. Saf. 2017, 139, 238–244. [Google Scholar] [CrossRef]
- Yamazaki, K.; Miyazaki, Y.; Harabuchi, Y.; Taketsugu, T.; Maeda, S.; Inokuchi, Y.; Kinoshita, S.N.; Sumida, M.; Onitsuka, Y.; Kohguchi, H.; et al. Multistep intersystem crossing pathways in cinnamate-based UV-B sunscreens. J. Phys. Chem. Lett. 2016, 7, 4001–4007. [Google Scholar] [CrossRef]
- Kanellis, V.G.; Kanellis, A.L. Availability of Hats That Meet Australian Sun-safety Standards at a Major Canberra Shopping Complex. Photochem. Photobiol. 2020, 96, 945–948. [Google Scholar] [CrossRef]
- GB/T 20944.3-2008; Valuation of Antibacterial Properties of Textiles. Standardization Administration of the People’s Republic of China: Beijing, China, 2008; pp. 1–9. (In Chinese)
- Zhou, Y.; Tang, R.-C. Modification of curcumin with a reactive UV absorber and its dyeing and functional properties for silk. Dye. Pigment. 2016, 134, 203–211. [Google Scholar] [CrossRef]
- Splechtna, R.; Elshehaly, M.; Gracanin, D.; Ɖuras, M.; Bühler, K.; Matković, K. Interactive interaction plot. Vis. Comput. 2015, 31, 1055–1065. [Google Scholar] [CrossRef]
- Krokidi, K.M.; Turner, M.A.P.; Pearcy, P.A.J.; Stavros, V.G. A systematic approach to methyl cinnamate photodynamics. Mol. Phys. 2020, 119, e1811910. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C. Natural flavonoid-functionalized silk fiber presenting antibacterial, antioxidant, and UV protection performance. ACS Sustain. Chem. Eng. 2017, 5, 10518–10526. [Google Scholar] [CrossRef]


| Variables | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| A: Conc.(Bai) (% owf) | 1.3 | 3 | 5.5 | 8 | 9.7 |
| B: Conc.(MC) (g/L) | 0.49 | 1 | 1.75 | 2.5 | 3.01 |
| C: Temp. (°C) | 91.6 | 95 | 100 | 105 | 108.4 |
| Run | Conc.(Bai) (% owf) | Conc.(MC) (g/L) | Temp. (°C) | Quantity (mg/g) | |
|---|---|---|---|---|---|
| Predicted | Actual | ||||
| 1 | 3 | 1 | 95 | 16.79 | 16.68 |
| 2 | 8 | 1 | 95 | 23.63 | 23.21 |
| 3 | 3 | 2.5 | 95 | 22.79 | 22.48 |
| 4 | 8 | 2.5 | 95 | 36.98 | 36.64 |
| 5 | 3 | 1 | 105 | 16.01 | 15.90 |
| 6 | 8 | 1 | 105 | 23.55 | 23.41 |
| 7 | 3 | 2.5 | 105 | 24.21 | 24.17 |
| 8 | 8 | 2.5 | 105 | 39.10 | 38.75 |
| 9 | 1.3 | 1.75 | 100 | 14.42 | 14.54 |
| 10 | 9.7 | 1.75 | 100 | 32.70 | 33.22 |
| 11 | 5.5 | 0.49 | 100 | 18.12 | 18.37 |
| 12 | 5.5 | 3.01 | 100 | 36.24 | 36.64 |
| 13 | 5.5 | 1.75 | 91.6 | 25.39 | 25.87 |
| 14 | 5.5 | 1.75 | 108.4 | 26.52 | 26.68 |
| 15 | 5.5 | 1.75 | 100 | 28.58 | 28.39 |
| 16 | 5.5 | 1.75 | 100 | 28.58 | 29.22 |
| 17 | 5.5 | 1.75 | 100 | 28.58 | 27.99 |
| 18 | 5.5 | 1.75 | 100 | 28.58 | 29.59 |
| 19 | 5.5 | 1.75 | 100 | 28.58 | 28.68 |
| 20 | 5.5 | 1.75 | 100 | 28.58 | 27.49 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 885.146 | 98.350 | 227.32 | <0.001 |
| Linear | 3 | 800.961 | 266.987 | 617.11 | <0.001 |
| Conc.(Bai) | 1 | 403.018 | 403.018 | 931.53 | <0.001 |
| Conc.(MC) | 1 | 396.407 | 396.407 | 916.24 | <0.001 |
| Temp. | 1 | 1.536 | 1.536 | 3.55 | 0.089 |
| Square | 3 | 54.522 | 18.174 | 42.01 | <0.001 |
| Conc.(Bai)×Conc.(Bai) | 1 | 45.390 | 45.390 | 104.91 | <0.001 |
| Conc.(MC)×Conc.(MC) | 1 | 3.510 | 3.510 | 8.11 | 0.017 |
| Temp.×Temp. | 1 | 12.402 | 12.402 | 28.67 | <0.001 |
| 2-Way Interaction | 3 | 29.663 | 9.888 | 22.85 | <0.001 |
| Conc.(Bai)×Conc.(MC) | 1 | 26.998 | 26.998 | 62.40 | <0.001 |
| Conc.(Bai)×Temp. | 1 | 0.246 | 0.246 | 0.57 | 0.468 |
| Conc.(MC)×Temp. | 1 | 2.420 | 2.420 | 5.59 | 0.040 |
| Error | 10 | 4.326 | 0.433 | ||
| Lack-of-Fit | 5 | 1.322 | 0.264 | 0.44 | 0.806 |
| Pure Error | 5 | 3.004 | 0.601 | ||
| Total | 9 | 885.146 | 98.350 | 227.32 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Deng, P.; Chen, W. Facilely Promoting the Concentration of Baicalin in Polylactic Acid Fiber for UV Shielding and Antibacterial Functions: A Customized and Sustainable Approach. Materials 2024, 17, 3734. https://doi.org/10.3390/ma17153734
Zhou Y, Deng P, Chen W. Facilely Promoting the Concentration of Baicalin in Polylactic Acid Fiber for UV Shielding and Antibacterial Functions: A Customized and Sustainable Approach. Materials. 2024; 17(15):3734. https://doi.org/10.3390/ma17153734
Chicago/Turabian StyleZhou, Yuyang, Peng Deng, and Wei Chen. 2024. "Facilely Promoting the Concentration of Baicalin in Polylactic Acid Fiber for UV Shielding and Antibacterial Functions: A Customized and Sustainable Approach" Materials 17, no. 15: 3734. https://doi.org/10.3390/ma17153734
APA StyleZhou, Y., Deng, P., & Chen, W. (2024). Facilely Promoting the Concentration of Baicalin in Polylactic Acid Fiber for UV Shielding and Antibacterial Functions: A Customized and Sustainable Approach. Materials, 17(15), 3734. https://doi.org/10.3390/ma17153734







