The Influence of Adding B4C and CeO2 on the Mechanical Properties of Laser Cladding Nickel-Based Coatings on the Surface of TC4 Titanium Alloy
Abstract
:1. Introduction
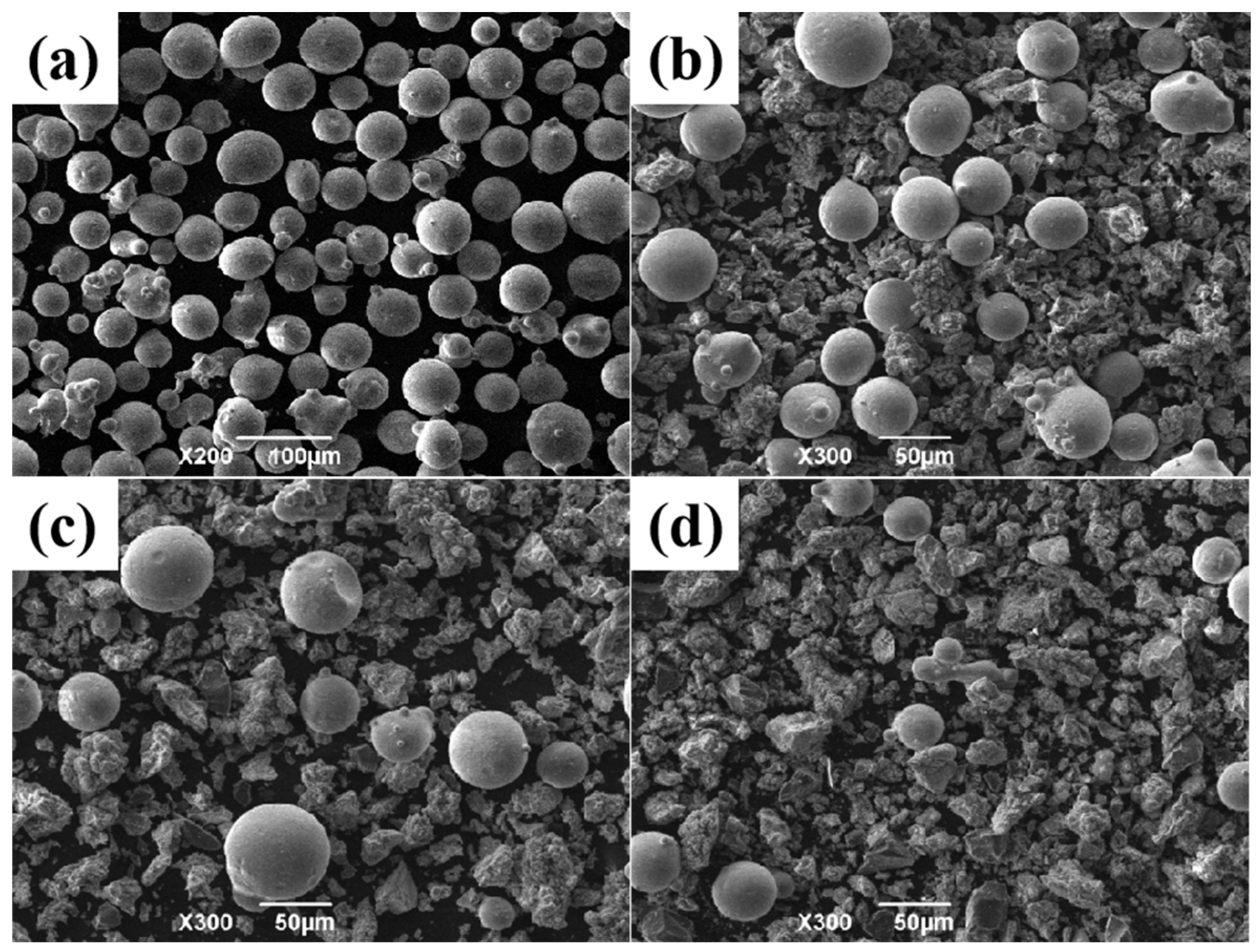
2. Materials and Methods
3. Results and Discussion
3.1. The Effect of B4C Content
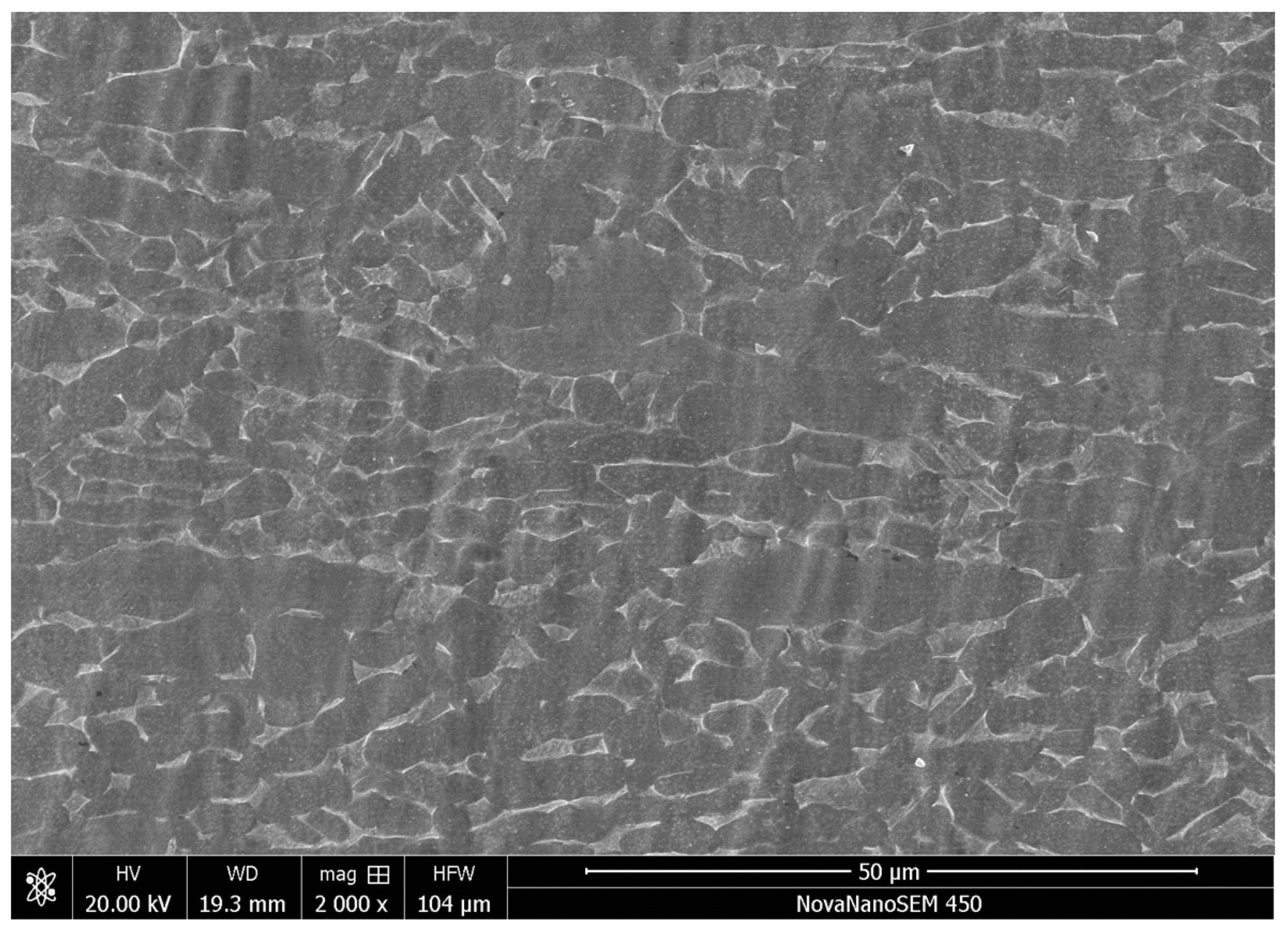
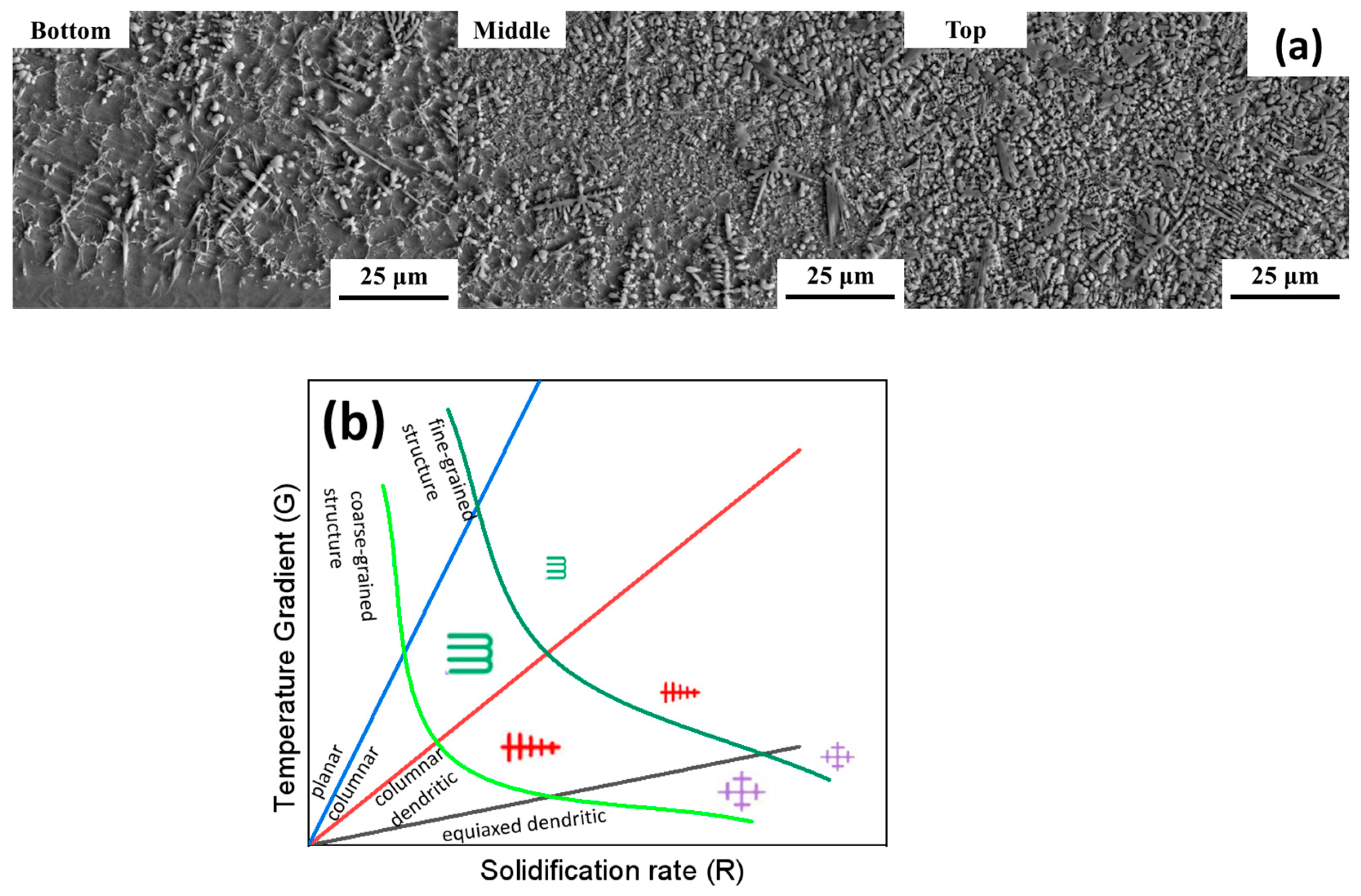
3.1.1. Microstructure of the Cladding by B4C Adding
3.1.2. Microhardness
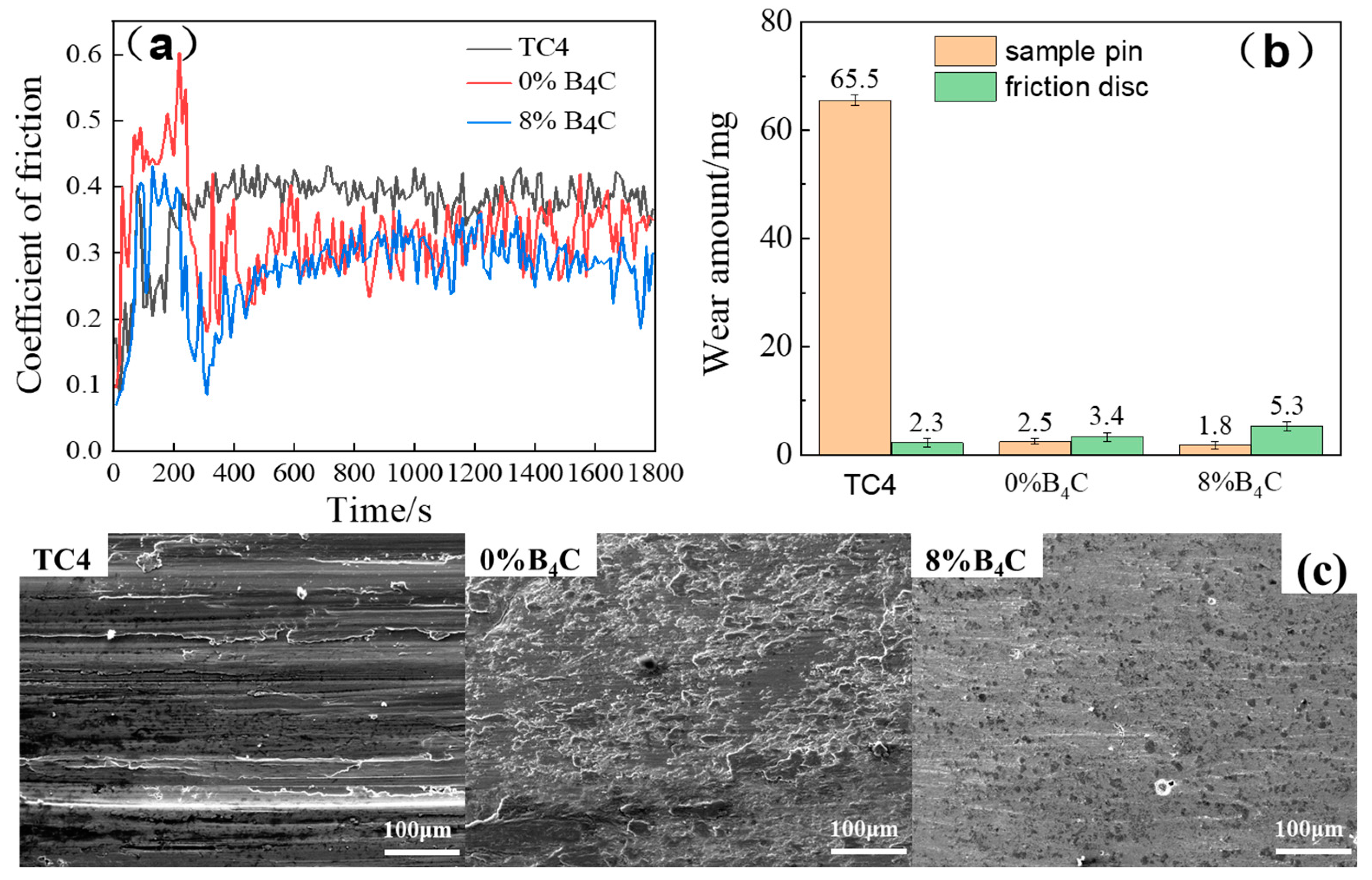
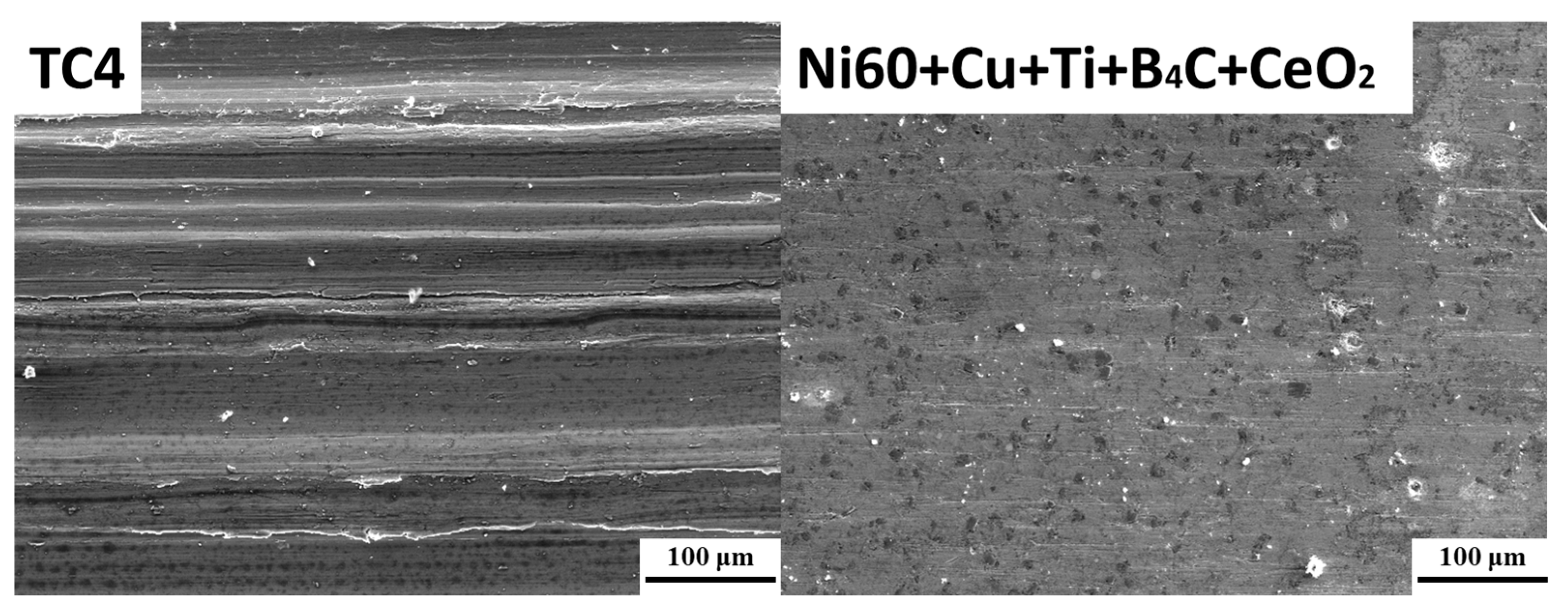
3.1.3. Friction and Wear Property
3.2. The Effect of CeO2 Content
3.2.1. Microstructure of the Cladding by CeO2 Adding
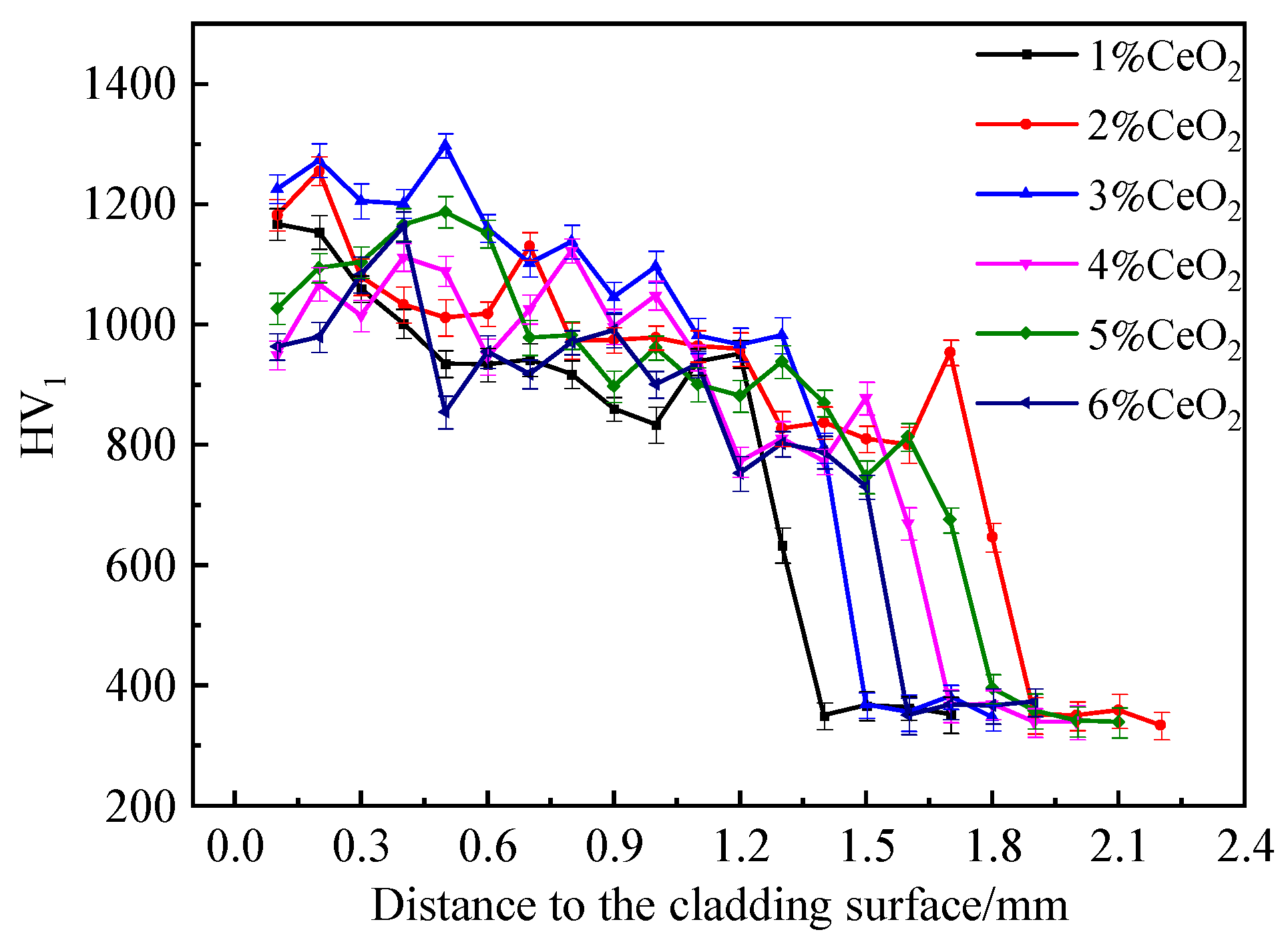
3.2.2. Microhardness, Friction, and Wear Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Li, Y.; Liu, H.; Shi, T.; Zhang, Q.; Zhang, Z.; Yu, X.; Guo, S.; Jiang, Y.; Yang, F.; et al. Comparative Analysis of Friction and Wear Properties of Titanium Alloy Drill Pipe and Steel Drill Pipe. J. Phys. Conf. Ser. 2023, 2587, 012001. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, J.; Zhao, M.; Li, N.; Hu, F. Comparative Study on Tribology Behavior of Titanium Alloy Drill Pipe Under Simulated Working Conditions in Ultra-deep Well Drilling Conditions. Rare Met. Mater. Eng. 2023, 52, 195–205. [Google Scholar]
- Tian, H.F.; Tantai, F.L. Research on the Microstructure and properties of Oil Drill Pipe Prepared by Laser Additive Manufacturing. Therm. Spray Technol. 2018, 10, 75–82. [Google Scholar]
- Zhao, H.; Zhao, C.; Xie, W.; Wu, D.; Du, B.; Zhang, X.; Wen, M.; Ma, R.; Li, R.; Jiao, J.; et al. Research Progress of Laser Cladding on the Surface of Titanium and Its Alloys. Materials 2023, 16, 3250. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, S.; Gong, Y. Research Progress of Laser Cladding Technology. Surf. Technol. 2020, 49, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Zhang, W.; Wang, X.; Dai, J.; Xu, J. Research Progress of Laser Cladding Coating on Titanium Alloy Surface. Surf. Technol. 2020, 49, 97–104. [Google Scholar] [CrossRef]
- Zhao, X.J.; Fang, S.Q.; Lyu, P.Z.; Fang, J.S.; Jiang, Y.X.; Ren, P.H.; Peng, Z.W.; Chen, L.M.; Xiao, L.R.; Liu, S.N. Microstructure and anti-ablation of laser cladding Ti-Zr-B-C coating on TC11 titanium alloy. J. Alloys Compd. 2024, 990, 174498. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J.; Chen, Y.; Hou, Z.; Zhao, Q.; Li, Y.; Zhu, L.; Zhang, F.; Zhao, Y. On enhancing wear resistance of titanium alloys by laser cladded WC-Co composite coatings. Int. J. Refract. Met. Hard Mater. 2022, 107, 105902. [Google Scholar] [CrossRef]
- Hua, K.; Ding, H.; Sun, L.; Cao, Y.; Li, X.; Wu, H.; Wang, H. Enhancing high-temperature fretting wear resistance of TC21 titanium alloys by laser cladding self-lubricating composite coatings. J. Alloys Compd. 2024, 977, 173360. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Y.; Yan, M.; Tu, B.; Li, Y. Microstructure and mechanical properties of surface coating prepared on grade 2 titanium by Ni-B composite electroplating and laser cladding. Opt. Laser Technol. 2023, 164, 109498. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Yang, Y.; Zhang, W.; Cui, H.; Zheng, X.; Mu, X.; Xu, C. Effect of graphene and niobium carbide on microstructure and mechanical properties of Ni60 composite coatings prepared by laser cladding. J. Mater. Res. Technol. 2024, 30, 8277–8286. [Google Scholar] [CrossRef]
- Luo, F.; Wang, S.; Shi, W.; Zhao, Y.; Huang, J. Wear behavior and corrosion resistance of laser-clad Ni60-1 % carbon nanotubes coating. Surf. Coat. Technol. 2024, 482, 130686. [Google Scholar] [CrossRef]
- Lu, P.-Z.; Jia, L.; Zhang, C.; Heng, X.; Xi, X.-C.; Duan, M.-F.; Lu, Z.-L.; Zhou, Y.-X. Optimization on laser cladding parameters for preparing Ni60 coating along with its friction and wear properties. Mater. Today Commun. 2023, 37, 107162. [Google Scholar] [CrossRef]
- Ye, J.; Li, X.; Shen, H.; Xu, Z. Research on Crack Regulation of Laser-cladding Ni60 Alloy Coatings. Welded Pipe Tube 2023, 46, 28–33. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, X.; Jin, G.; Song, Q.; Yuan, C.; Fang, Y.; Wen, X. Microstructure and tribology performance of laser cladded Ni60+h-BN coatings on Ti-6Al-4V alloy at high temperature. Tribol. Trans. 2019, 22, 332–341. [Google Scholar] [CrossRef]
- Yao, X.; Sun, Q.; Xiao, L.; Sun, J. Effect of Ti2Cu precipitates on mechanical behavior of Ti-2.5Cu alloy subjected to different heat treatments. J. Alloys Compd. 2009, 484, 196–202. [Google Scholar] [CrossRef]
- Zhao, X.; Lyu, P.; Fang, S.; Li, S.; Tu, X.; Ren, P.; Liu, D.; Chen, L.; Xiao, L.; Liu, S. Microstructure and wear behavior of Ti-xFe-SiC in situ composite ceramic coatings on TC4 substrate from laser cladding. Materials 2024, 17, 100. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, S.; Zhang, S.; Xu, C.; Qin, G. Microstructure and wear resistance of Ti-Cu-N composite coating prepared via laser cladding/laser nitriding technology on Ti-6Al-4V alloy. Appl. Phys. A 2017, 123, 4741–4749. [Google Scholar] [CrossRef]
- Osório, W.R.; Cremasco, A.; Andrade, P.N.; Garcia, A.; Caram, R. Electrochemical behavior of centrifuged cast and heat treated Ti-Cu alloys for medical applications. Electrochim. Acta 2010, 55, 759–770. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Zhang, M.; Lin, H.; Yan, D.; Lin, Q.; Kang, X.; Wang, X. Gradient Coating of Laser Cladding TiB2/Ti-Based Alloy on Titanium Alloy Surface. Coatings 2023, 13, 743. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.; Lou, C.; Kang, L.; Hou, Q.; Ma, C. Influence of WC ceramic particles on structures and properties of laser cladding Ni50-WC coatings. J. Mater. Res. Technol. 2023, 26, 14–21. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Wu, W.; Wang, M. An in situ formed tic particle reinforcement composite coating induced by laser melting on surface of alloy Ti6Al4V and its wearing performance. Acta Metall. Sin. 2001, 37, 315–320. [Google Scholar]
- Gao, Y.; Liu, R.; Wu, T.; Ma, W.; Yun, M.; Zhu, Y. Microstructure and wear resistance of laser clad TiAlSi + xB4C coatings on Ti-6Al-4V alloy. Heat Treat. Met. 2021, 46, 196–200. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, R.; Niu, W.; Zhang, T.; Lei, Y. Effects of CeO2 on microstructure and properties of TiC/Ti2Ni reinforced Ti-based laser cladding composite coatings. Opt. Laser Eng. 2019, 120, 84–94. [Google Scholar] [CrossRef]
- Gao, Z.; Geng, H.; Qiao, Z.; Sun, B.; Gao, Z.; Zhang, C. In situ TiBX/TiXNiY/TiC reinforced Ni60 composites by laser cladding and its effect on the tribological properties. Ceram. Int. 2023, 49, 6409–6418. [Google Scholar] [CrossRef]
- Feng, S.-R.; Tang, H.-B.; Zhang, S.-Q.; Wang, H.-M. Microstructure and wear resistance of laser clad TiB–TiC/TiNi–Ti2Ni intermetallic coating on titanium alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 1667–1673. [Google Scholar] [CrossRef]
- Jiang, H.; Su, H.; Shen, Z.; Zhao, D.; Liu, Y.; Yu, M.; Liu, Y.; Zhang, Z. Research Progress on Laser Additive Manufacturing and Solidification Defect Control of Ultra-High Temperature Oxide Ceramics. Aeronaut. Manuf. Technol. 2023, 66, 61–71. [Google Scholar] [CrossRef]
- Hou, L.N. Influence of content of B4C on the hardness of Braze Coatings of the B4C/Ni-based Powder Composite Coating. Surf. Technol. 2012, 41, 49–51. [Google Scholar] [CrossRef]
- Oliveira, J.P.; LaLonde, A.D.; Ma, J. Processing parameters in laser powder bed fusion metal additive manufacturing. Mater. Des. 2020, 193, 108762. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhang, C. Effect of nano-CeO2 on microstructure properties of TiC/TiN + nTi(CN) reinforced composite coating. Bull. Mater. Sci. 2013, 36, 541–546. [Google Scholar]
- Wang, H.; Zuo, D.; Li, X.; Chen, K.; Huang, M. Effects of CeO2 nanoparticles on microstructure and properties of laser cladded NiCoCrAlY coatings. J. Rare Earths 2010, 28, 246–250. [Google Scholar] [CrossRef]
- Chen, T.; Liu, D.; Wu, F.; Wang, H. Effect of CeO2 on microstructure and wear resistance of TiC bioinert coatings on Ti6Al4V alloy by laser cladding. Materials 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, J.; Li, J. Effect of rare earth oxide on the properties of laser cladding layer and machining vibration suppressing in side milling. Appl. Surf. Sci. 2014, 321, 387–395. [Google Scholar] [CrossRef]
- Quazi, M.; Fazal, M.; Haseeb, A.; Yusof, F.; Masjuki, H.; Arslan, A. Effect of rare earth elements and their oxides on tribo-mechanical performance of laser claddings: A review. J. Rare Earths 2016, 34, 549–564. [Google Scholar] [CrossRef]




| Al | V | O | Fe | H | C | N | Ti | |
|---|---|---|---|---|---|---|---|---|
| TC4 | 6.71 | 4.23 | 0.14 | 0.12 | 0.03 | 0.03 | 0.02 | Balance |
| Cr | Fe | Si | B | C | Cu | Ti | Ni |
|---|---|---|---|---|---|---|---|
| 9 | 2.4 | 2.1 | 2.1 | 0.5 | 15 | 25 | Balance |
| No. | Ni60-Ti-Cu | B4C |
|---|---|---|
| 1 | 100 | 0 |
| 2 | 98 | 2 |
| 3 | 96 | 4 |
| 4 | 94 | 6 |
| 5 | 92 | 8 |
| 6 | 90 | 10 |
| No. | Chemical Equation of Reaction | Products |
|---|---|---|
| 1 | Ti(s) + Ni(s) = NiTi(s) | NiTi |
| 2 | 2Ti(s) + Ni(s) = NiTi2(s) | NiTi2 |
| 3 | 2Ti(s) + Cu(s) = Ti2Cu(s) | Ti2Cu |
| 4 | 3Ti(s) + B4C(s) = TiC(s) + 2TiB2(s) | TiC, TiB2 |
| 5 | 5Ti(s) + B4C(s) = TiC(s) + 4TiB(s) | TiC, TiB |
| Materials | Friction Coefficient | Wear Amount/mg |
|---|---|---|
| TC4 | 0.73 | 65.5 |
| Ni60 + Cu + Ti | 0.33 | 2.5 |
| Ni60 + Cu + Ti + 8%B4C | 0.28 | 1.8 |
| Ni60 + Cu + Ti + 8%B4C + 3%CeO2 | 0.25 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Han, K.; Wang, H.; Xi, Y.; Wang, L.; Dong, X. The Influence of Adding B4C and CeO2 on the Mechanical Properties of Laser Cladding Nickel-Based Coatings on the Surface of TC4 Titanium Alloy. Materials 2024, 17, 3823. https://doi.org/10.3390/ma17153823
Xu S, Han K, Wang H, Xi Y, Wang L, Dong X. The Influence of Adding B4C and CeO2 on the Mechanical Properties of Laser Cladding Nickel-Based Coatings on the Surface of TC4 Titanium Alloy. Materials. 2024; 17(15):3823. https://doi.org/10.3390/ma17153823
Chicago/Turabian StyleXu, Shanna, Keqi Han, Haili Wang, Yuntao Xi, Lei Wang, and Xikai Dong. 2024. "The Influence of Adding B4C and CeO2 on the Mechanical Properties of Laser Cladding Nickel-Based Coatings on the Surface of TC4 Titanium Alloy" Materials 17, no. 15: 3823. https://doi.org/10.3390/ma17153823






