Impact of Thermochemical Treatments on Electrical Conductivity of Donor-Doped Strontium Titanate Sr(Ln)TiO3 Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
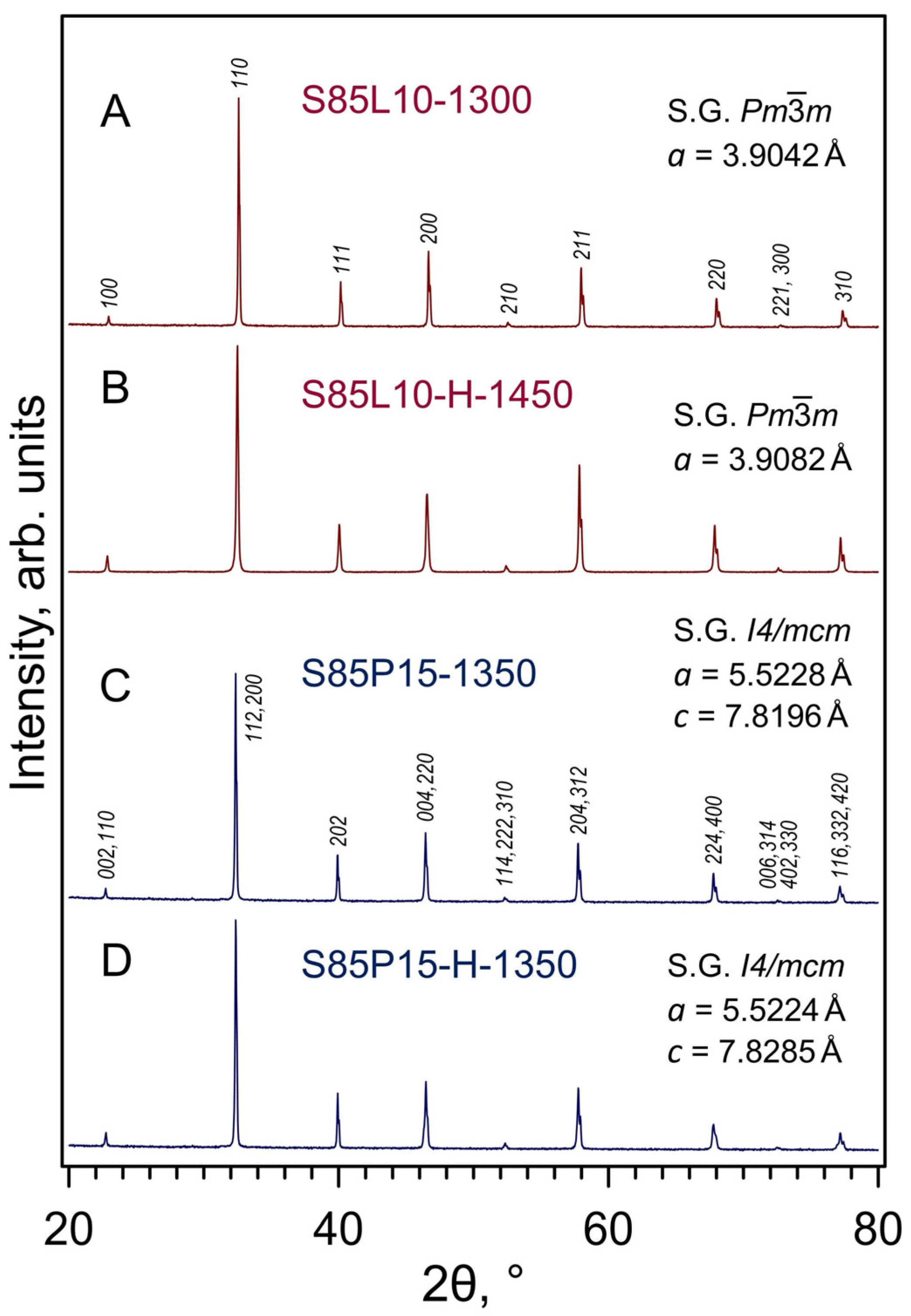
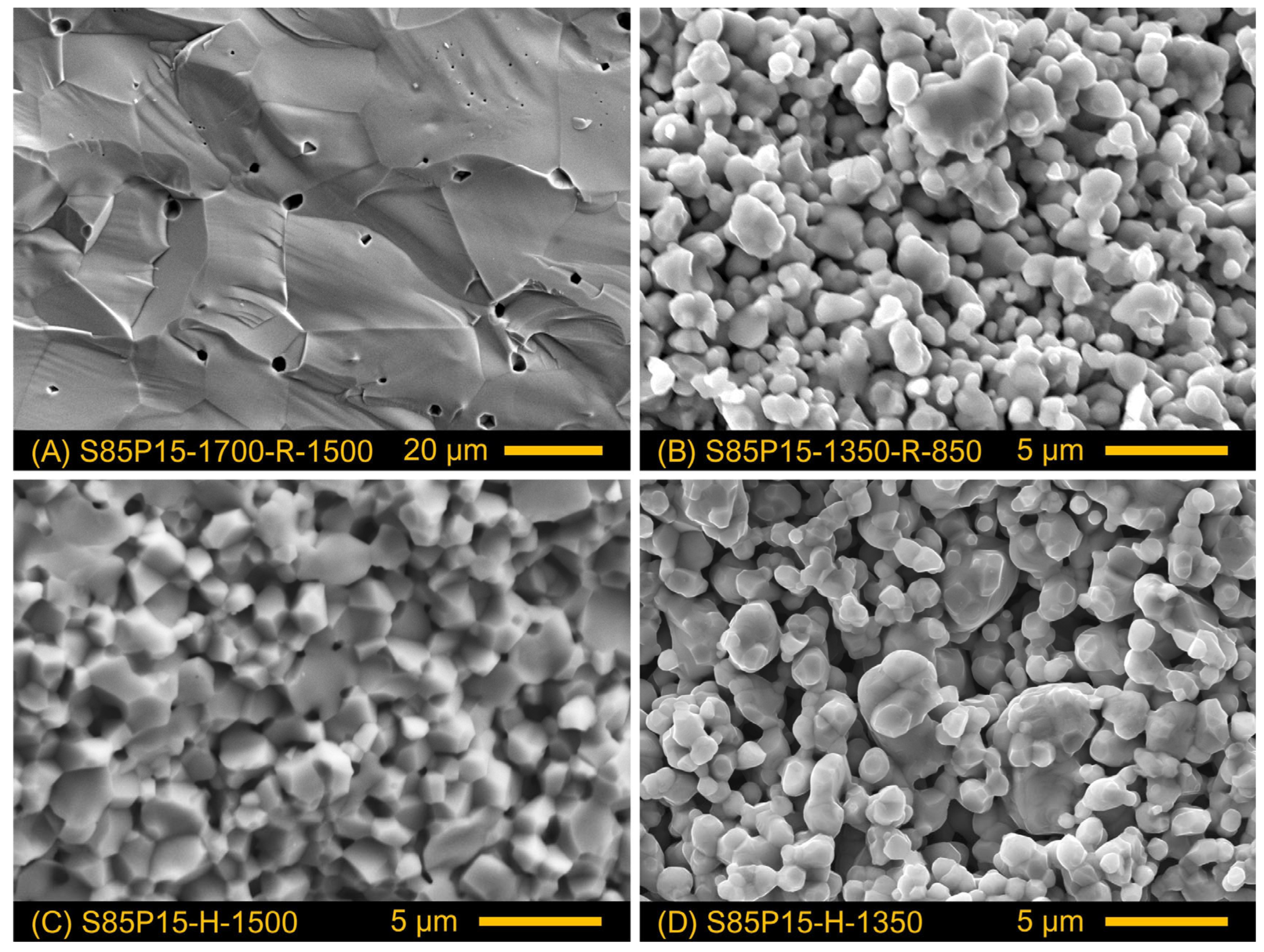
3.1. Crystal Structure and Microstructure of Prepared Ceramics
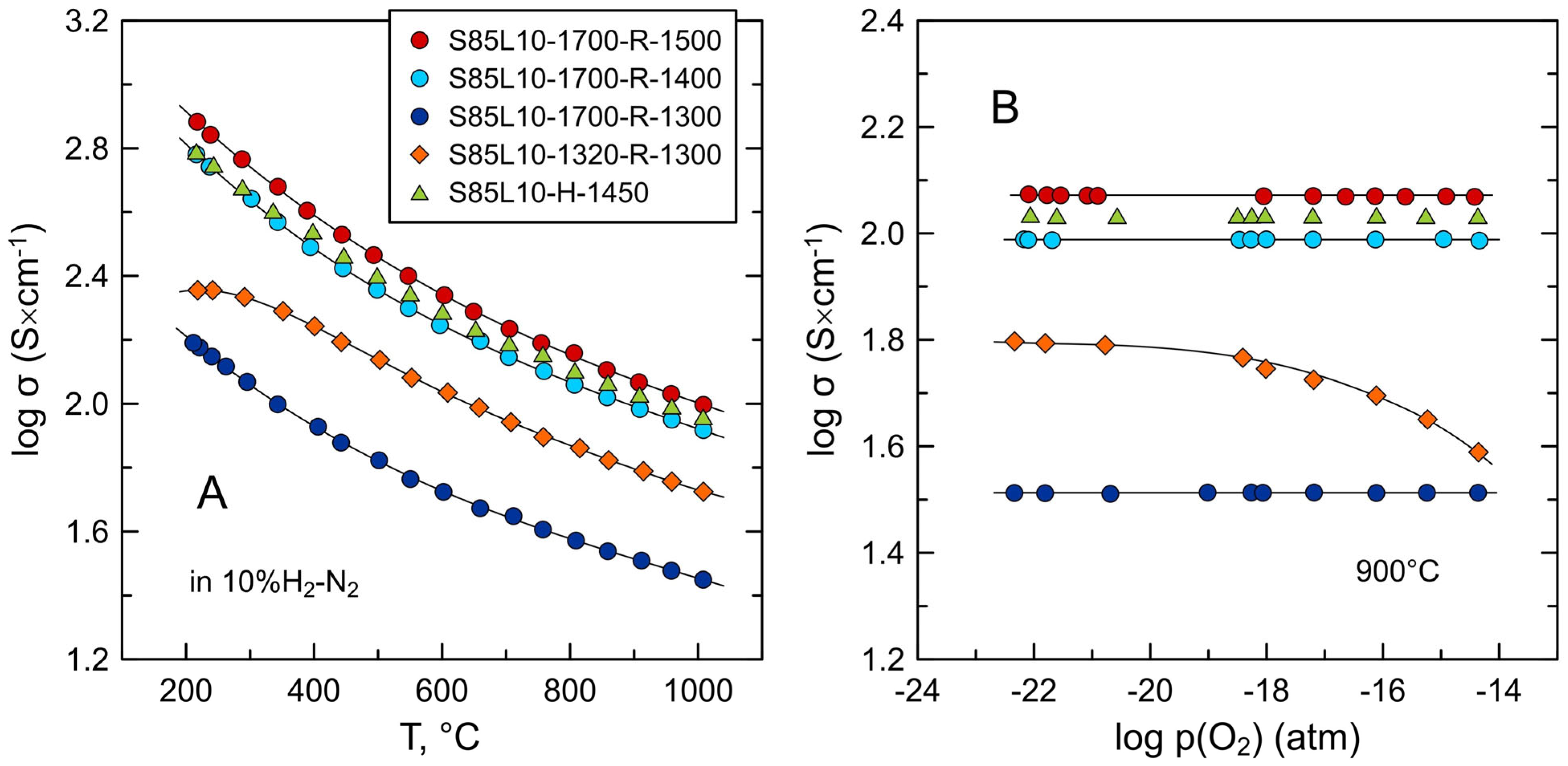
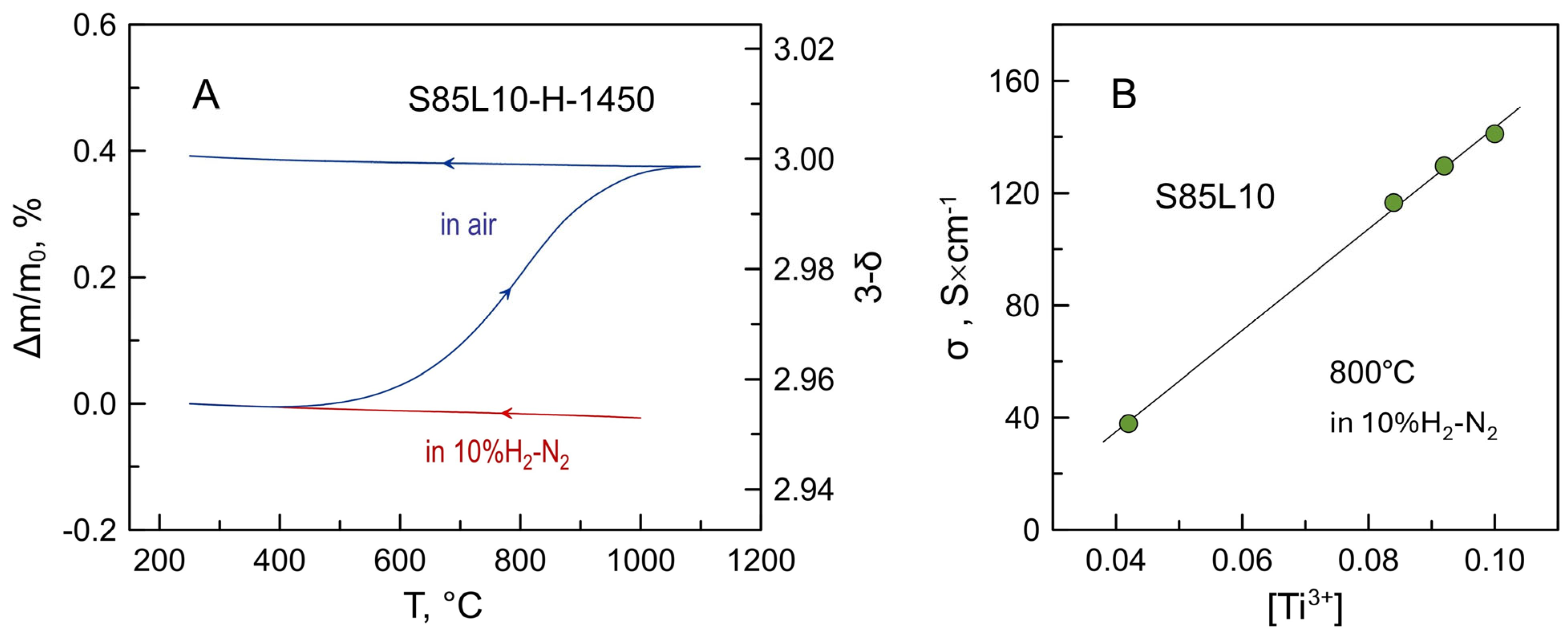
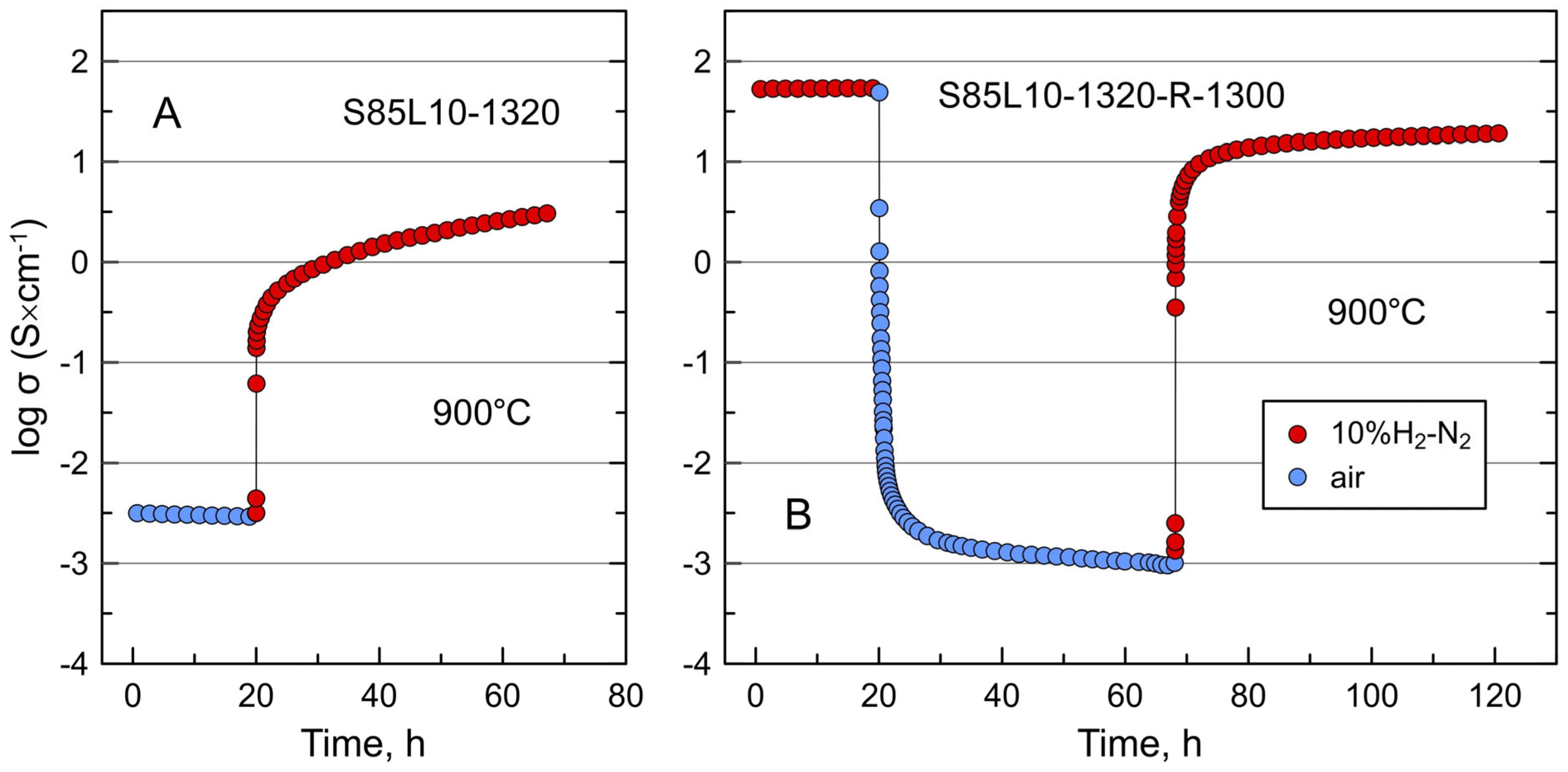
3.2. Electrical Conductivity of S85L10 Ceramics
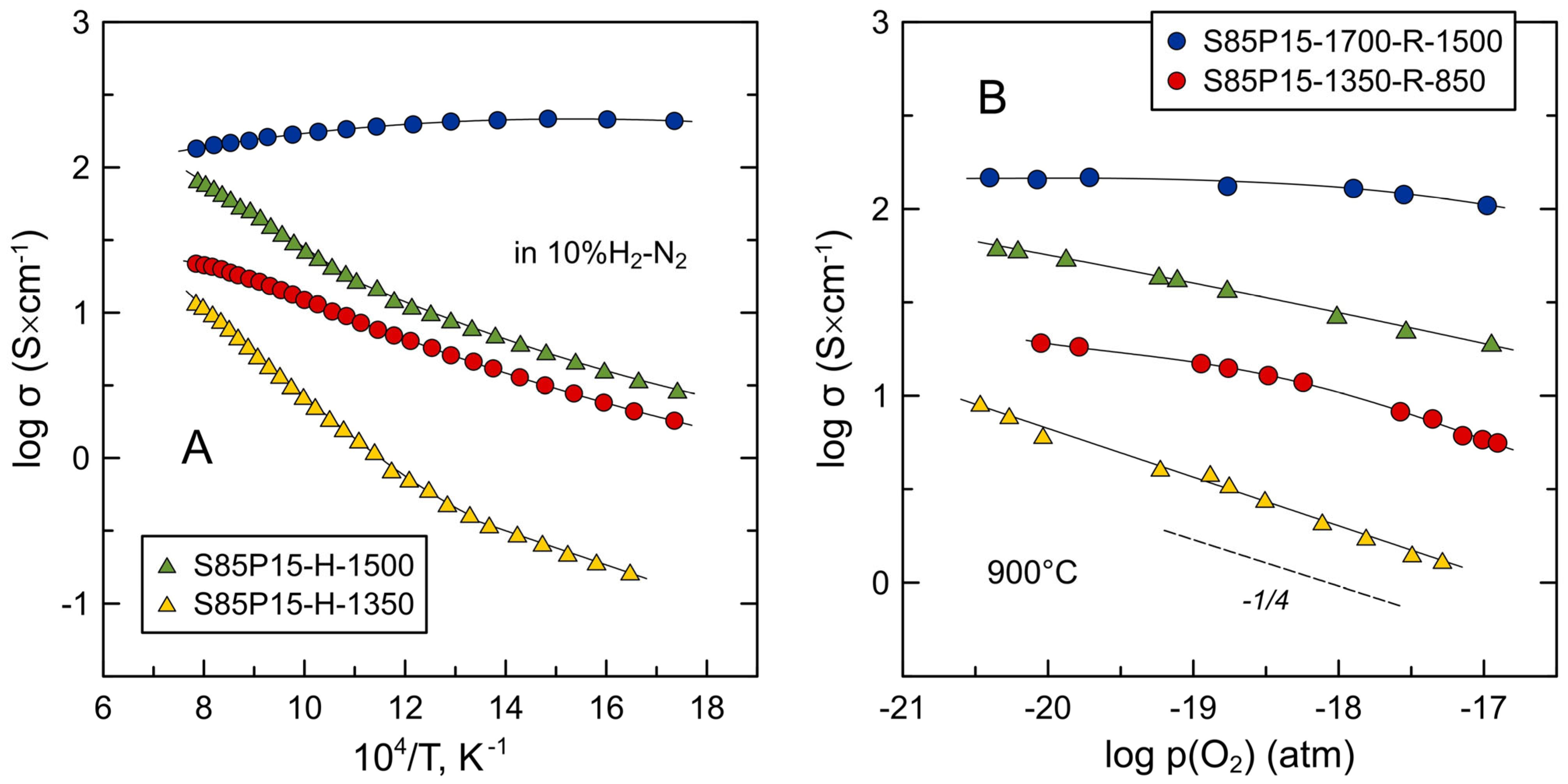
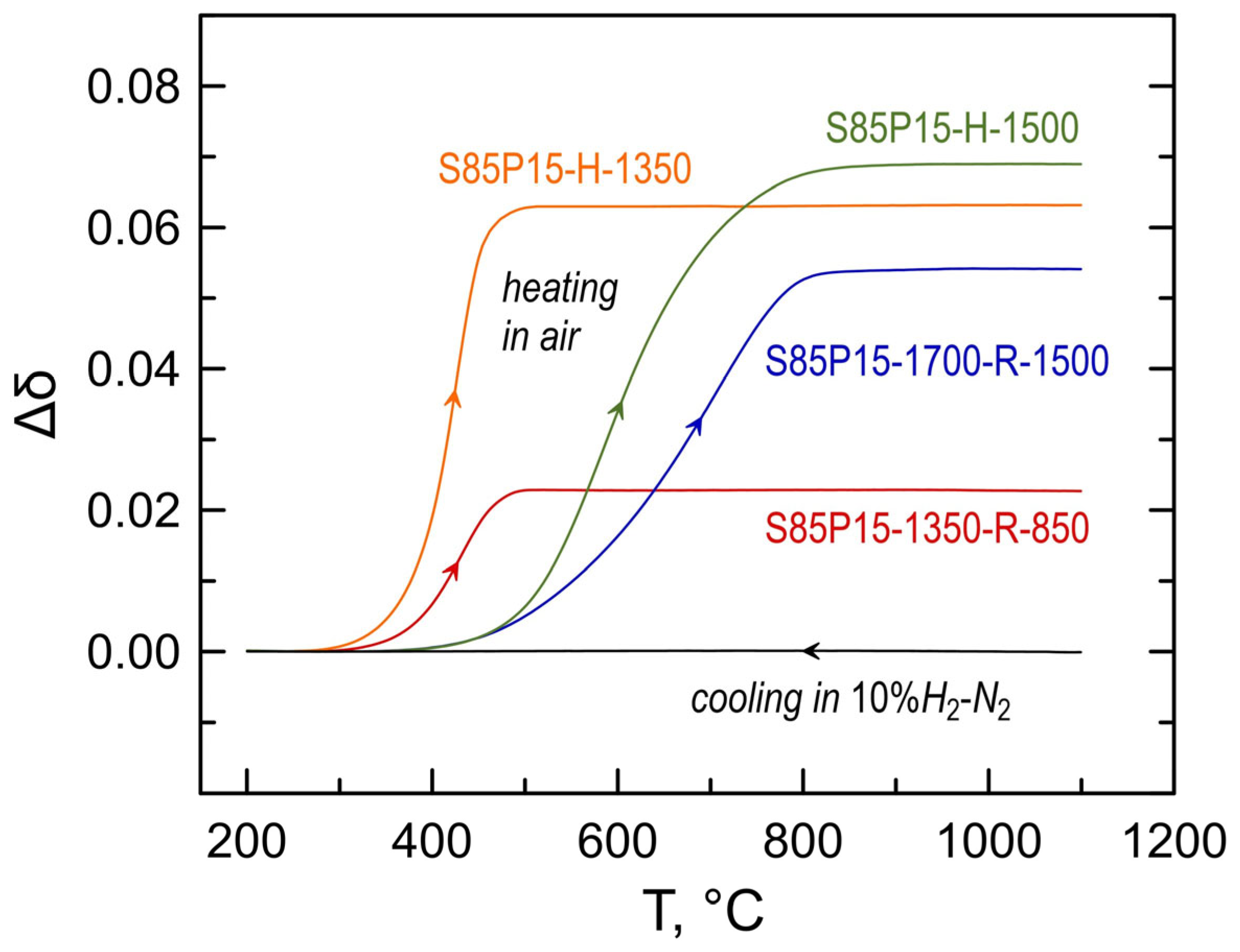
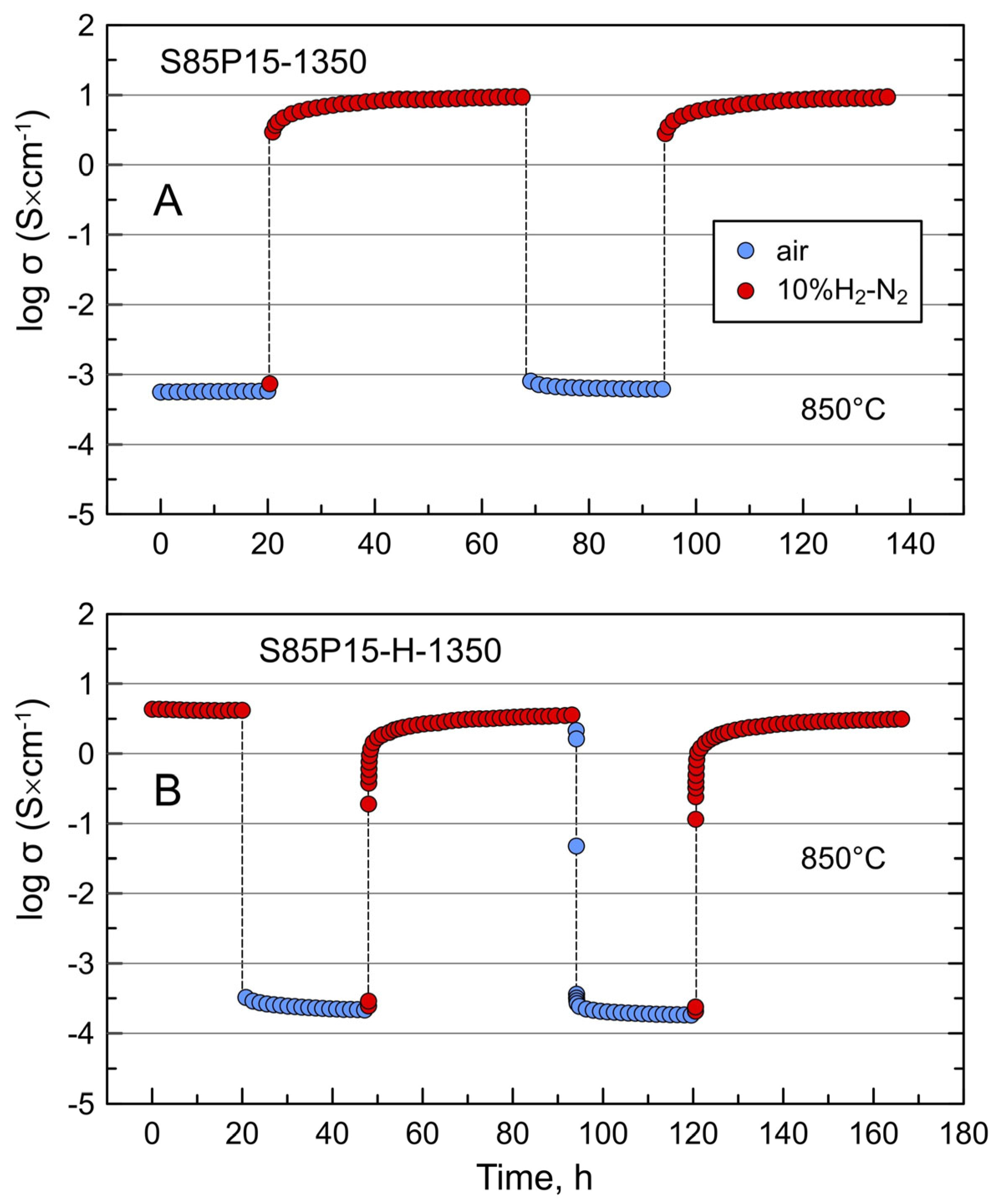
3.3. Electrical Conductivity of S85P15 Ceramics
4. Conclusions
- (i)
- Reduction of dense donor-doped SrTiO3 ceramics is limited by sluggish oxygen diffusion in the crystal lattice even at temperatures as high as 1300 °C. A higher degree of reduction and higher conductivity can be obtained for porous structures under similar thermochemical treatment conditions.
- (ii)
- Metallic-like conductivity of dense reduced Sr0.85La0.10TiO3−δ is proportional to the concentration of Ti3+ and corresponds to the state quenched from the processing temperature. Due to poor oxygen diffusivity in the bulk at temperatures below 1000 °C, the oxygen exchange processes in dense ceramics are essentially limited to the surface. As a result, dense Sr0.85La0.10TiO3−δ ceramics remain redox inactive and maintain high levels of conductivity under reducing conditions, at least in the short term.
- (iii)
- A similar behavior is characteristic of dense Sr0.85Pr0.15TiO3+δ ceramics with large grain size (10–40 µm) provided by sintering at temperatures of around 1700 °C. Reducing grain size down to 1–3 µm results in the increasing role of grain boundaries in this material, even for the samples reduced at a high temperature of 1500 °C. Regardless of the degree of reduction, resistive grain boundaries define semiconducting behavior and a lower total electrical conductivity of Sr0.85Pr0.15TiO3+δ ceramics with fine grain size. The resistive nature of grain boundaries is likely to be associated with the specific defect chemistry of nominally cation-stoichiometric Sr1−xLnxTiO3±δ titanates.
- (iv)
- Oxidized porous Sr0.85Pr0.15TiO3+δ ceramics exhibit faster kinetics of reduction compared to the Sr0.85La0.10TiO3−δ counterpart at temperatures below 1000 °C. This underlines the important role of extended defects in the structure of cation-stoichiometric Sr1−xLnxTiO3±δ titanates. On the other hand, the reductive pretreatment of porous Sr0.85La0.10TiO3−δ ceramics at elevated temperatures (1300 °C) facilitates low-temperature equilibration kinetics on redox cycling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, S.; Chi, B.; Tu, Z. Technological limitations and recent developments in a solid oxide electrolyzer cell: A review. Int. J. Hydrogen Energy 2024, 50, 548–591. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, J.; Huang, X.; Liao, Z.; Liu, Y.; Tu, Z. Recent development in reversible solid oxide fuel cells: Theory, integration and prospective. ChemElectroChem 2023, 11, e202300593. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrogen Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, N.; Chuang, K.T.; Luo, J. Progress in La-doped SrTiO3 (LST)-based anode materials for solid oxide fuel cells. RSC Adv. 2014, 4, 118–131. [Google Scholar] [CrossRef]
- Verbraeken, M.C.; Ramos, T.; Agersted, K.; Ma, Q.; Savaniu, C.D.; Sudireddy, B.R.; Irvine, J.T.S.; Holtappels, P.; Tietz, F. Modified strontium titanates: From defect chemistry to SOFC anodes. RSC Adv. 2015, 5, 1168–1180. [Google Scholar] [CrossRef]
- Alvarado Flores, J.J.; Avalos Rodríguez, M.L.; Andrade Espinosa, G.; Alcaraz Vera, J.V. Advances in the development of titanates for anodes in SOFC. Int. J. Hydrogen Energy 2019, 44, 12529–12542. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.; Liu, J.; Zhou, J.; Xu, L. A review on the electrical properties of doped SrTiO3 as anode materials for solid oxide fuel cells. Mater. Res. Express 2019, 6, 102006. [Google Scholar] [CrossRef]
- Yoo, K.B.; Park, B.H.; Choi, G.M. Stability and performance of SOFC with SrTiO3-based anode in CH4 fuel. Solid State Ionics 2012, 225, 104–107. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhou, X.W.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.N.; Zhang, L.Z.; Hua, B.; Li, J.; Li, J.H.; Luo, J.L. An ingenious Ni/Ce co-doped titanate based perovskite as a coking-tolerant anode material for direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 22830–22838. [Google Scholar] [CrossRef]
- Błaszczak, P.; Łapiński, M.; Wang, S.F.; Jasiński, P.; Bochentyn, B. Exsolution of Ni nanoparticles on the surface of cerium and nickel co-doped lanthanum strontium titanate as a new anodic layer for DIR-SOFC. Anti-coking potential and H2S poisoning resistance of the prepared material. Int. J. Hydrogen Energy 2020, 45, 29186–29200. [Google Scholar] [CrossRef]
- Christensen, J.O.; Longo, G.; Bausinger, H.; Mai, A.; Sudireddy, B.R.; Hagen, A. Performance and sulfur tolerance of a short stack with solid oxide cells using infiltrated strontium titanate based anodes. J. Power Sources 2023, 580, 233458. [Google Scholar] [CrossRef]
- Kulkarni, A.; Giddey, S.; Badwal, S.P.S.; Paul, G. Electrochemical performance of direct carbon fuel cells with titanate anodes. Electrochim. Acta 2014, 121, 34–43. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, H.; Wang, Z.; Sun, W.; Li, H.; Sun, K. Enhancing the catalytic activity of Y0.08Sr0.92TiO3−δ anodes through in situ Cu exsolution for direct carbon solid oxide fuel cells. Ind. Eng. Chem. Res. 2020, 59, 13105–13112. [Google Scholar] [CrossRef]
- Cai, W.; Cao, D.; Zhou, M.; Yan, X.; Li, Y.; Wu, Z.; Lü, S.; Mao, C.; Xie, Y.; Zhao, C.; et al. Sulfur-tolerant Fe-doped La0.3Sr0.7TiO3 perovskite as anode of direct carbon solid oxide fuel cells. Energy 2020, 211, 118958. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Gan, Y.; Xie, K.; Meng, G. Electrolysis of H2O and CO2 in an oxygen-ion conducting solid oxide electrolyzer with a La0.2Sr0.8TiO3+δ composite cathode. J. Power Sources 2012, 218, 244–249. [Google Scholar] [CrossRef]
- Qi, W.; Ruan, C.; Wu, G.; Zhang, Y.; Wang, Y.; Xie, K.; Wu, Y. Reversibly in-situ anchoring copper nanocatalyst in perovskite titanate cathode for direct high-temperature steam electrolysis. Int. J. Hydrogen Energy 2014, 39, 5485–5496. [Google Scholar] [CrossRef]
- Zhou, C.; Ling, R.; Wang, M.; Yang, C.; Qi, W. Efficient H2O/CO2 co-electrolysis with NixCu1−x alloy nanocatalysts modified perovskite-type titanate cathodes. Ceram. Int. 2024, 50, 29398–29405. [Google Scholar] [CrossRef]
- Sharma, S.; Stanley, R.; Tiwari, P.; Basu, S.; Kumari, N. In situ exsolution of ceria nanoparticles in perovskite cathode for elevating CO2 reduction performance of solid oxide electrolysis cells (SOECs). J. Electroanal. Chem. 2024, 962, 118254. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, D.W.; Song, R.H.; Lee, S.B.; Lim, T.H.; Park, S.J.; Park, C.O.; Lee, J.W. Design of a dual-layer ceramic interconnect based on perovskite oxides for segmented-in-series solid oxide fuel cells. J. Power Sources 2015, 300, 318–324. [Google Scholar] [CrossRef]
- Chen, X.; Kou, C.C.; Liao, X.J.; Li, C.X.; Yang, G.J.; Huang, K.; Li, C.J. Plasma-sprayed lanthanum-doped strontium titanate as an interconnect for solid oxide fuel cells: Effects of powder size and process conditions. J. Alloy Compd. 2021, 876, 160212. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, P.; Xu, N.; Jia, L.; Zhang, L.A.; Huang, K. Dense and low oxygen permeability bilayer ceramic interconnect for tubular anode-support solid oxide cells. ACS Appl. Energy Mater. 2021, 4, 341–349. [Google Scholar] [CrossRef]
- Xie, J.; Han, F.Z.; Zhang, J.H.; Liu, S.; Tang, Y.H.; Li, C.X.; Zhang, S.L. Dense La0.3Sr0.7TiO3 interconnect sintered in air atmosphere at a reduced temperature with improved conductivity for solid oxide electrochemical cells. J. Eur. Ceram. Soc. 2024, 44, 3191–3199. [Google Scholar] [CrossRef]
- Li, S.; Tu, H.; Yu, L.; Anwar, M.T. Fabrication and performance of solid oxide fuel cells with La0.2Sr0.7TiO3−δ as anode support and La2NiO4+δ as cathode. Fuel Cells 2016, 16, 822–828. [Google Scholar] [CrossRef]
- Lu, L.; Ni, C.; Cassidy, M.; Irvine, J.T.S. Demonstration of high performance in a perovskite oxide supported solid oxide fuel cell based on La and Ca co-doped SrTiO3. J. Mater. Chem. A 2016, 4, 11708–11718. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Miller, E.; Liu, Q.; Senn, D.; Barnett, S. Tape casting of high-performance low-temperature solid oxide cells with thin La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolytes and impregnated nano anodes. ACS Appl. Mater. Interfaces 2017, 9, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Kawabata, T.; Sasaki, K. Redox-stable Sr0.9La0.1TiO3-supported SOFC single cells. Int. J. Hydrogen Energy 2017, 42, 6941–6949. [Google Scholar] [CrossRef]
- Kharton, V.V.; Tsipis, E.V.; Marozau, I.P.; Viskup, A.P.; Frade, J.R.; Irvine, J.T.S. Mixed conductivity and electrochemical behavior of (La0.75Sr0.25)0.95Cr0.5Mn0.5O3−δ. Solid State Ionics 2007, 178, 101–113. [Google Scholar] [CrossRef]
- Kim, J.J.; Vu, A.D.; Cronauer, D.C.; Carter, J.D.; Hock, A.S.; Ingram, B.J. Influence of electronic transport on electrochemical performance of (Cu,Mn)3O4 solid oxide fuel cell cathodes. Int. J. Hydrogen Energy 2023, 48, 23706–23715. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nature Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef]
- Hodjati-Pugh, O.; Dhir, A.; Steinberger-Wilckens, R. The development of current collection in micro-tubular solid oxide fuel cells—A review. Appl. Sci. 2021, 11, 1077. [Google Scholar] [CrossRef]
- Chan, N.H.; Sharma, R.K.; Smyth, D.M. Nonstoichiometry in SrTiO3. J. Electrochem. Soc. 1981, 128, 1762–1769. [Google Scholar] [CrossRef]
- Abrantes, J.C.C.; Ferreira, A.A.L.; Labrincha, J.A.; Frade, J.R. Electrical conductivity of Srl−xTiO3−δ materials. Ionics 1997, 3, 436–441. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Patrício, S.G.; Frade, J.R. Thermochemical behavior and transport properties of Pr-substituted SrTiO3 as potential SOFC anode. J. Power Sources 2014, 245, 557–569. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Macías, J.; Kovalevsky, A.V.; Arias-Serrano, B.I.; Frade, J.R. Electrical conductivity and thermal expansion of Ln-substituted SrTiO3 for solid oxide cell electrodes and interconnects: The effect of rare-earth cation size. J. Power Sources 2020, 474, 228531. [Google Scholar] [CrossRef]
- Marina, O.A.; Canfield, N.L.; Stevenson, J.W. Thermal, electrical, and electrocatalytical properties of lanthanum-doped strontium titanate. Solid State Ionics 2002, 149, 21–28. [Google Scholar] [CrossRef]
- Moos, R.; Härdtl, K.H. Electronic transport properties of Sr1−xLaxTiO3 ceramics. J. Appl. Phys. 1996, 80, 393–400. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Xu, N.; Zhou, X.; Zhang, C.; Chen, N. Electrical conduction behavior of La, Co co-doped SrTiO3 perovskite as anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2009, 34, 6407–6414. [Google Scholar] [CrossRef]
- Lv, X.; Chen, G.; Wei, K.; Dai, R.; Wang, M.; Yu, K.; Geng, S. Influence of La doping concentration and A-site deficiency on electrical conductivity of La substituted SrTiO3 and its chemical compatibility with ScSZ. Ceram. Int. 2022, 48, 27527–27535. [Google Scholar] [CrossRef]
- Wang, Z.; Mori, M. Sintering characteristics and electrical conductivity of (Sr1−xLax)TiO3 synthesized by the citric acid method. J. Fuel Cell Sci. Technol. 2011, 8, 051018. [Google Scholar] [CrossRef]
- Niwa, E.; Sato, K.; Yashiro, K.; Mizusaki, J. Conductivities and Seebeck coefficients of donor-doped SrTiO3 oxide ceramics. ECS Trans. 2009, 25, 2631–2638. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, J.W.; Lee, S.B.; Lim, T.H.; Park, S.J.; Song, R.H.; Shin, D.R. Synthesis and electrical properties of strontium titanate-based materials for solid oxide fuel cells. ECS Trans. 2011, 35, 2553–2559. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kindermann, L.; Poulsen, F.W.; Mogensen, M. A study on the structural and electrical properties of lanthanum-doped strontium titanate prepared in air. J. Alloy Compd. 2005, 397, 245–249. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Naumovich, E.N.; Patrício, S.G.; Merkulov, O.V.; Patrakeev, M.V.; Frade, J.R. Rare-earth—Substituted strontium titanate: Insight into local oxygen-rich structures and redox kinetics. Inorg. Chem. 2016, 55, 4836–4849. [Google Scholar] [CrossRef] [PubMed]
- Flandermeyer, B.F.; Agarwal, A.K.; Anderson, H.U.; Nasrallah, M.M. Oxidation-reduction behaviour of La-doped SrTiO3. J. Mater. Sci. 1984, 19, 2593–2598. [Google Scholar] [CrossRef]
- Moos, R.; Hardtl, K.H. Defect chemistry of donor-doped and undoped strontium titanate ceramics between 1000° and 1400 °C. J. Am. Ceram. Soc. 1997, 80, 2549–2562. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Image Processing Program ImageJ. Available online: https://imagej.net (accessed on 28 July 2024).
- Lu, Z.; Zhang, H.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-figure-of-merit thermoelectric La-doped A-site-deficient SrTiO3 ceramics. Chem. Mater. 2016, 28, 925–935. [Google Scholar] [CrossRef]
- Garg, R.; Senyshyn, A.; Boysen, H.; Ranjan, R. Structure of the noncubic phase in the ferroelectric state of Pr-substituted SrTiO3. Phys. Rev. B 2009, 79, 144122. [Google Scholar] [CrossRef]
- Sluchinskaya, I.A.; Lebedev, A.I.; Erko, A. Crystal structure, local structure, and defect structure of Pr-doped SrTiO3. J. Appl. Phys. 2012, 112, 024103. [Google Scholar] [CrossRef]
- Kiessling, U.; Claus, J.; Borchardt, G.; Weber, S.; Scherrer, S. Oxygen tracer diffusion in lanthanum-doped single-crystal strontium titanate. J. Am. Ceram. Soc. 1994, 77, 2188–2190. [Google Scholar] [CrossRef]
- Hashimoto, S.; Poulsen, F.W.; Mogensen, M. Conductivity of SrTiO3 based oxides in the reducing atmosphere at high temperature. J. Alloy Compd. 2007, 439, 232–236. [Google Scholar] [CrossRef]
- Neagu, D.; Irvine, J.T.S. Structure and properties of La0.4Sr0.4TiO3 ceramics for use as anode materials in solid oxide fuel cells. Chem. Mater. 2010, 22, 5042–5053. [Google Scholar] [CrossRef]
- Abrantes, J.C.C.; Labrincha, J.A.; Frade, J.R. Behavior of strontium titanate ceramics in reducing conditions suggesting enhanced conductivity along grain contacts. J. Eur. Ceram. Soc. 2002, 22, 1683–1691. [Google Scholar] [CrossRef]
- Meyer, R.; Waser, R.; Helmbold, J.; Borchardt, G. Cationic surface segregation in donor-doped SrTiO3 under oxidizing conditions. J. Electroceram. 2002, 9, 101–110. [Google Scholar] [CrossRef]
- Meyer, R.; Waser, R.; Helmbold, J.; Borchardt, G. Observation of vacancy defect migration in the cation sublattice of complex oxides by 18O tracer experiments. Phys. Rev. Lett. 2003, 90, 105901. [Google Scholar] [CrossRef]
- Rahmati, B.; Fleig, J.; Sigle, W.; Bischoff, E.; Maier, J.; Rühle, M. Oxidation of reduced polycrystalline Nb-doped SrTiO3: Characterization of surface islands. Surf. Sci. 2005, 595, 115–126. [Google Scholar] [CrossRef]
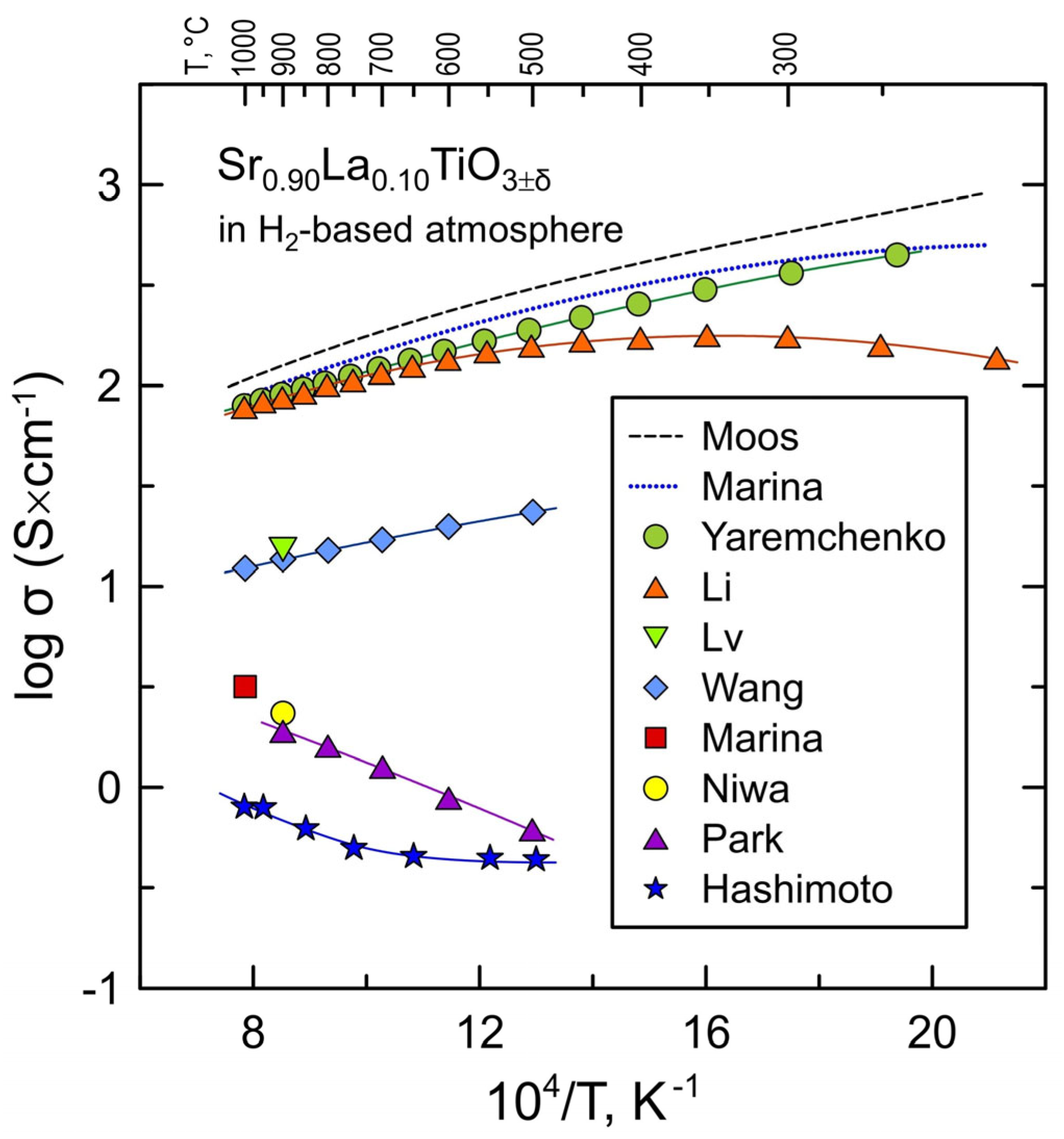

| Sintering | Reductive Treatment | Measurement Atmosphere | σ, S/cm (900 °C) | Ref. |
|---|---|---|---|---|
| dry H2, 1450 °C | dry H2 | 125 | Moos [37] | |
| 2%H2-N2, 1650 °C/8 h | 4%H2-Ar 1 | 104 | Marina [36] | |
| air, 1700 °C/10 h | 10%H2-N2, 1500 °C/10 h | 10%H2-N2 | 90 | Yaremchenko [35] |
| 5%H2-Ar, 1500 °C/10 h | humidified 5%H2-Ar | 87 | Li [38] | |
| air, 1450 °C/10 h | H2 + 1.2%H2O, 900 °C/10 h | H2 + 1.2%H2O | 15 | Lv [39] |
| air, 1650 °C/10 h | humidified 30%H2-N2 | 14 | Wang [40] | |
| air, 1650 °C/8 h | 4%H2-Ar 1 | 3.2 2 | Marina [36] | |
| air, 1550 °C/50 h | H2-Ar 3 | 2.3 | Niwa [41] | |
| air, 1400 °C/5 h | 30%H2-N2 | 1.9 | Park [42] | |
| air, 1450–1650 °C/10 h | 9%H2-N2 | 0.7 | Hashimoto [43] |
| Notation | Sintering | Reduction | Density, g/cm3 | Relative Density, % | Grain Size, µm |
|---|---|---|---|---|---|
| Sr0.85La0.10TiO3−δ | |||||
| S85L10-1700-R-1500 | air, 1700 °C, 10 h | 10%H2-N2, 1500 °C, 10 h | 4.89 | 96 | 5–31 |
| S85L10-1700-R-1400 | air, 1700 °C, 10 h | 10%H2-N2, 1400 °C, 10 h | 4.91 | 96 | 5–31 |
| S85L10-1700-R-1300 | air, 1700 °C, 10 h | 10%H2-N2, 1300 °C, 10 h | 4.88 | 95 | 5–31 |
| S85L10-1320-R-1300 | air, 1320 °C, 10 h | 10%H2-N2, 1300 °C, 10 h | 3.57 | 70 | 0.6–6.5 |
| S85L10-H-1450 | 10%H2-N2, 1450 °C, 10 h | 4.65 | 91 | 0.7–6.8 | |
| Sr0.85Pr0.15TiO3±δ | |||||
| S85P15-1700-R-1500 | air, 1700 °C, 10 h | 10%H2-N2, 1500 °C, 10 h | 4.80 | 90 | 10–38 |
| S85P15-1350-R-850 | air, 1350 °C, 10 h | 10%H2-N2, 850 °C, 24 h | 3.94 | 73 | 0.6–3.0 |
| S85P15-H-1500 | 10%H2-N2, 1500 °C, 10 h | 4.74 | 89 | 0.8–3.5 | |
| S85P15-H-1350 | 10%H2-N2, 1350 °C, 10 h | 3.44 | 65 | 0.6–3.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamburov, A.; Kravchenko, E.; Yaremchenko, A.A. Impact of Thermochemical Treatments on Electrical Conductivity of Donor-Doped Strontium Titanate Sr(Ln)TiO3 Ceramics. Materials 2024, 17, 3876. https://doi.org/10.3390/ma17153876
Bamburov A, Kravchenko E, Yaremchenko AA. Impact of Thermochemical Treatments on Electrical Conductivity of Donor-Doped Strontium Titanate Sr(Ln)TiO3 Ceramics. Materials. 2024; 17(15):3876. https://doi.org/10.3390/ma17153876
Chicago/Turabian StyleBamburov, Aleksandr, Ekaterina Kravchenko, and Aleksey A. Yaremchenko. 2024. "Impact of Thermochemical Treatments on Electrical Conductivity of Donor-Doped Strontium Titanate Sr(Ln)TiO3 Ceramics" Materials 17, no. 15: 3876. https://doi.org/10.3390/ma17153876
APA StyleBamburov, A., Kravchenko, E., & Yaremchenko, A. A. (2024). Impact of Thermochemical Treatments on Electrical Conductivity of Donor-Doped Strontium Titanate Sr(Ln)TiO3 Ceramics. Materials, 17(15), 3876. https://doi.org/10.3390/ma17153876







