Atomistic Insights into Impact-Induced Energy Release and Deformation of Core–Shell-Structured Ni/Al Nanoparticle in an Oxygen Environment
Abstract
1. Introduction
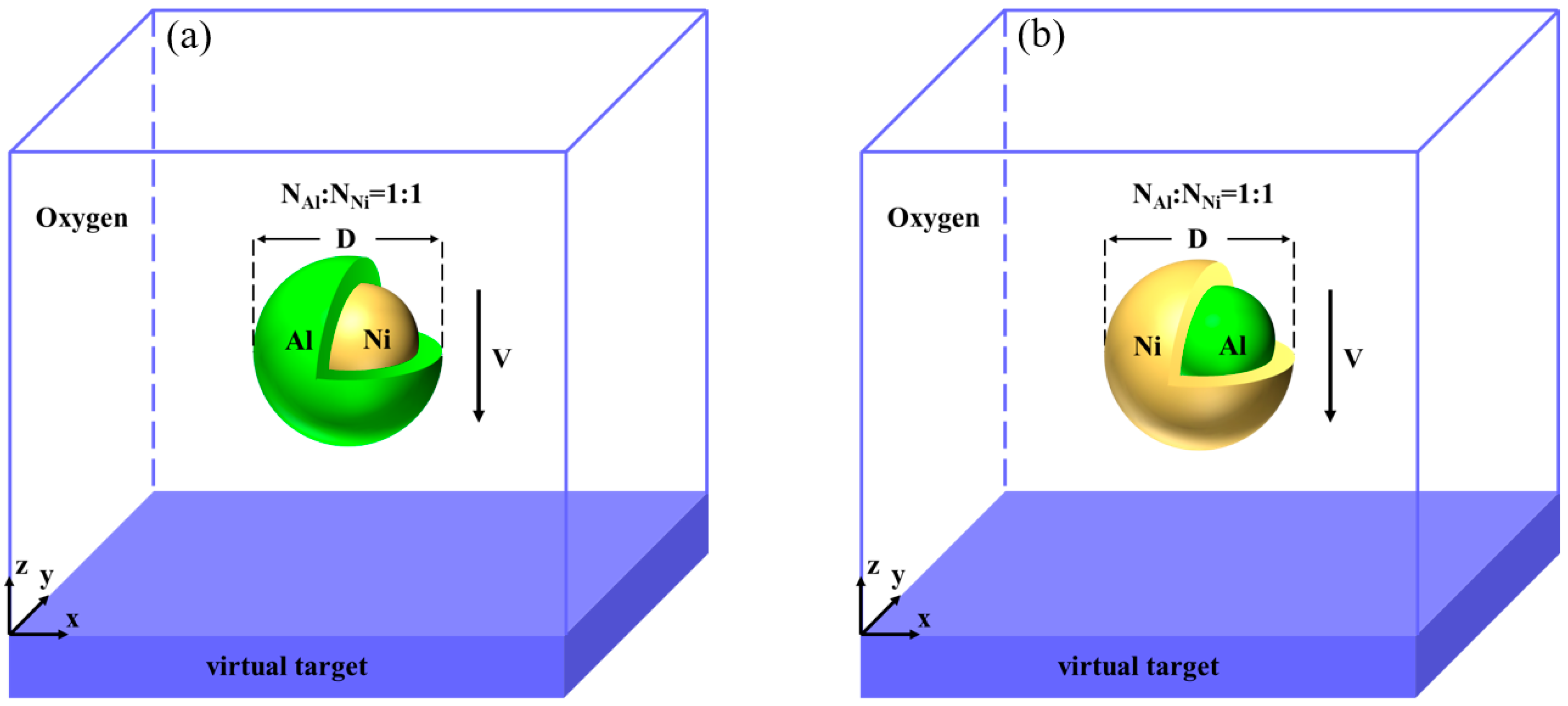
2. Materials and Methods
3. Results and Discussion
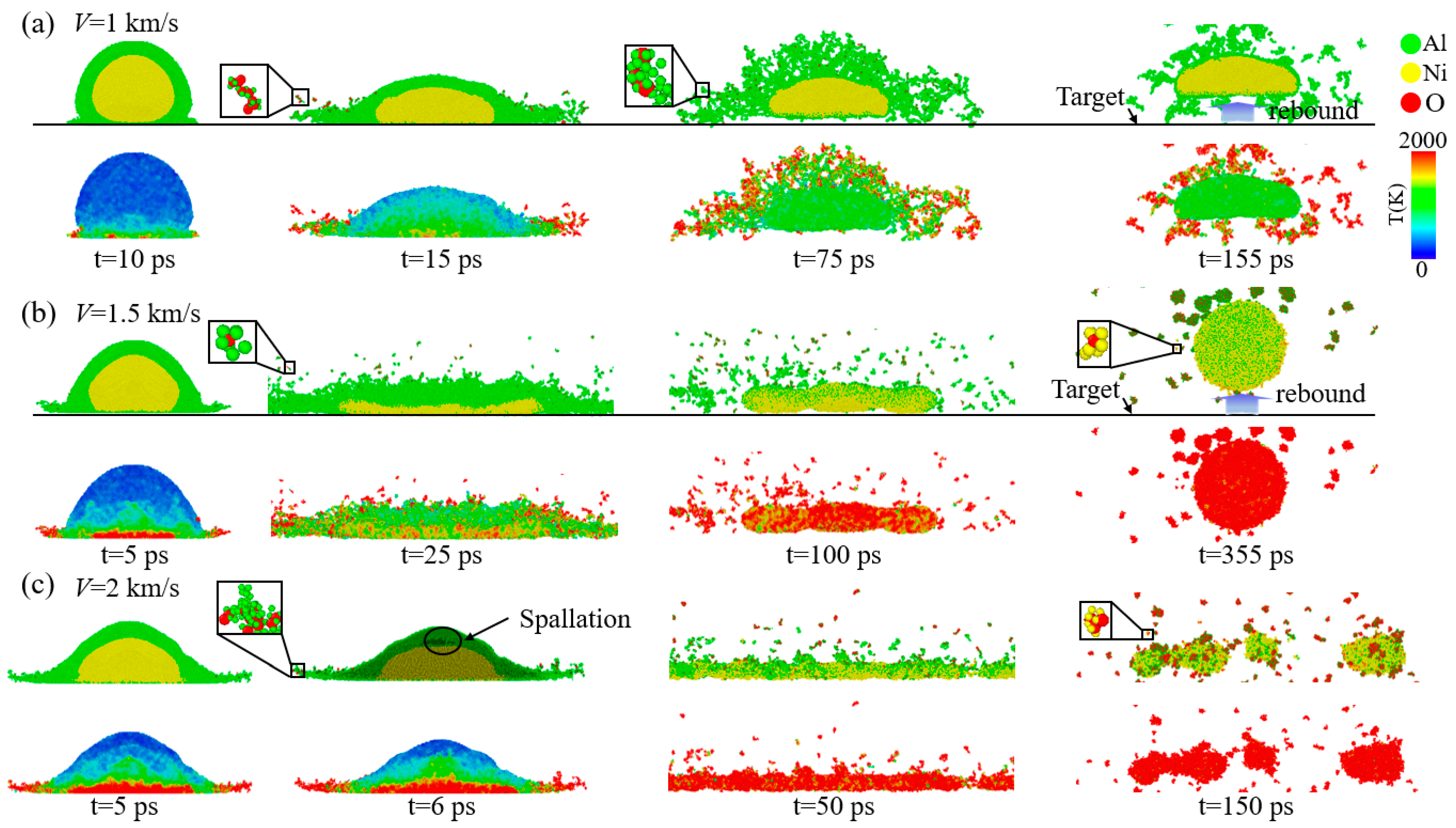
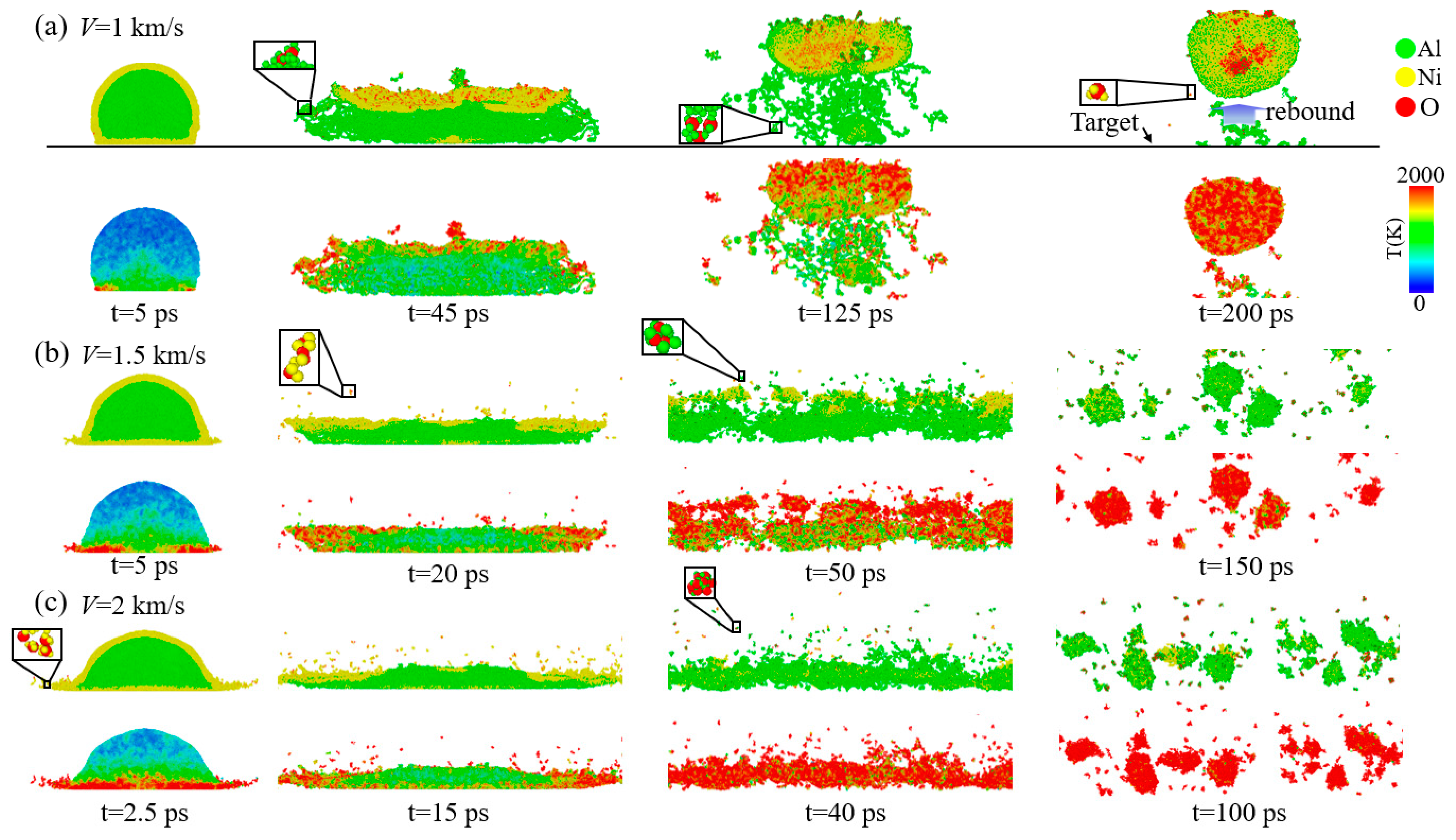
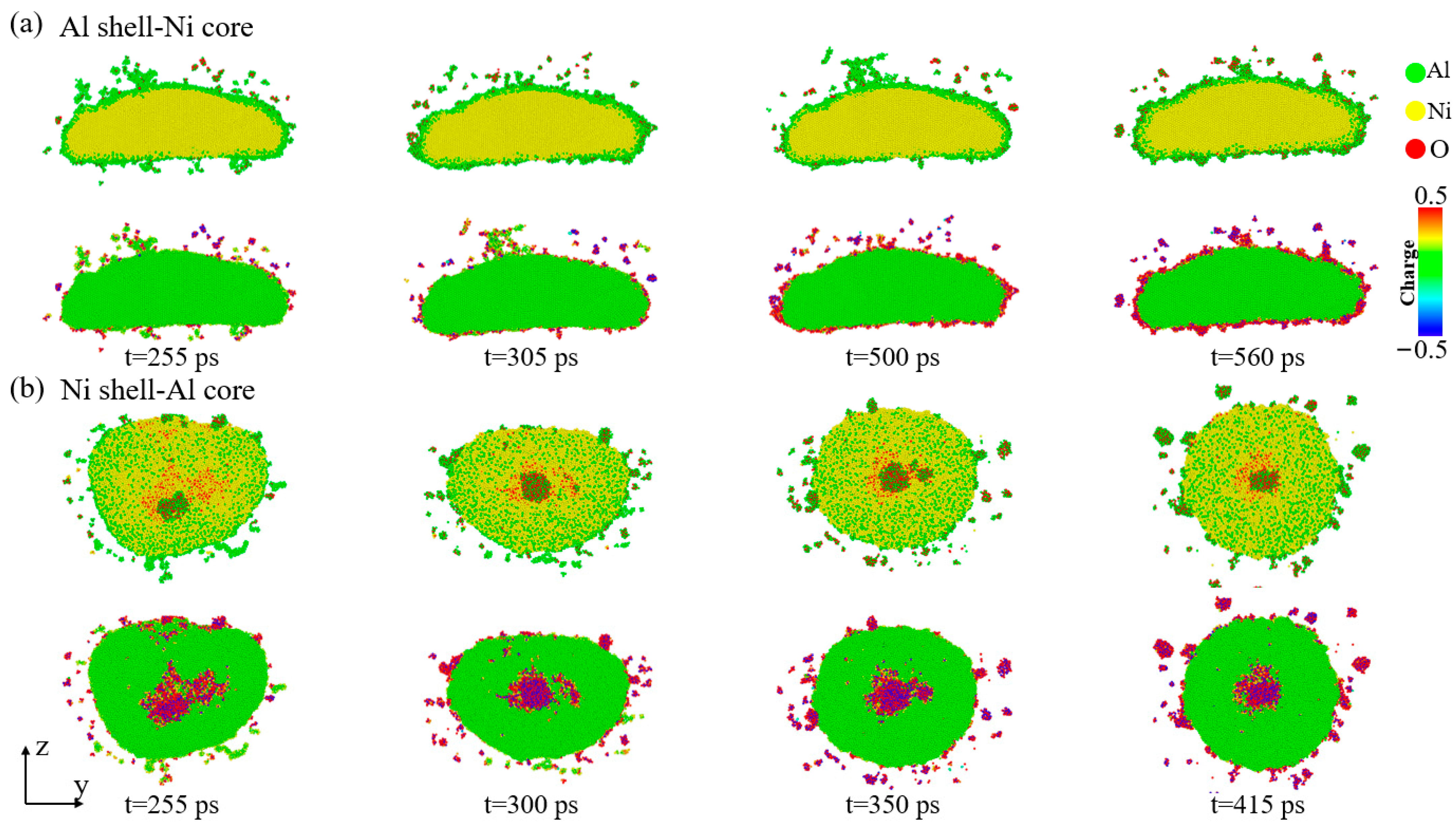
3.1. Analysis of Nanoparticle Deformation and Damage
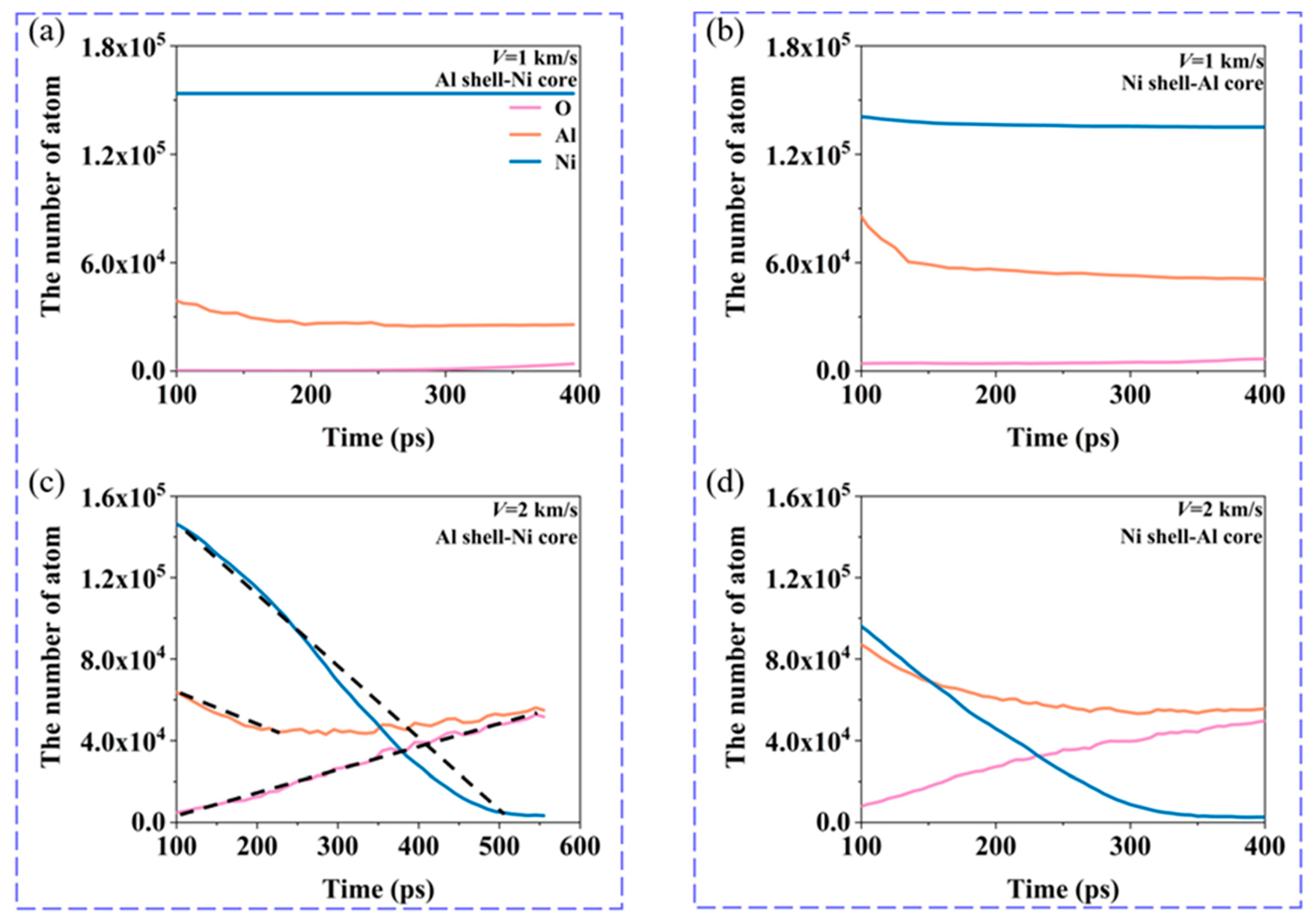
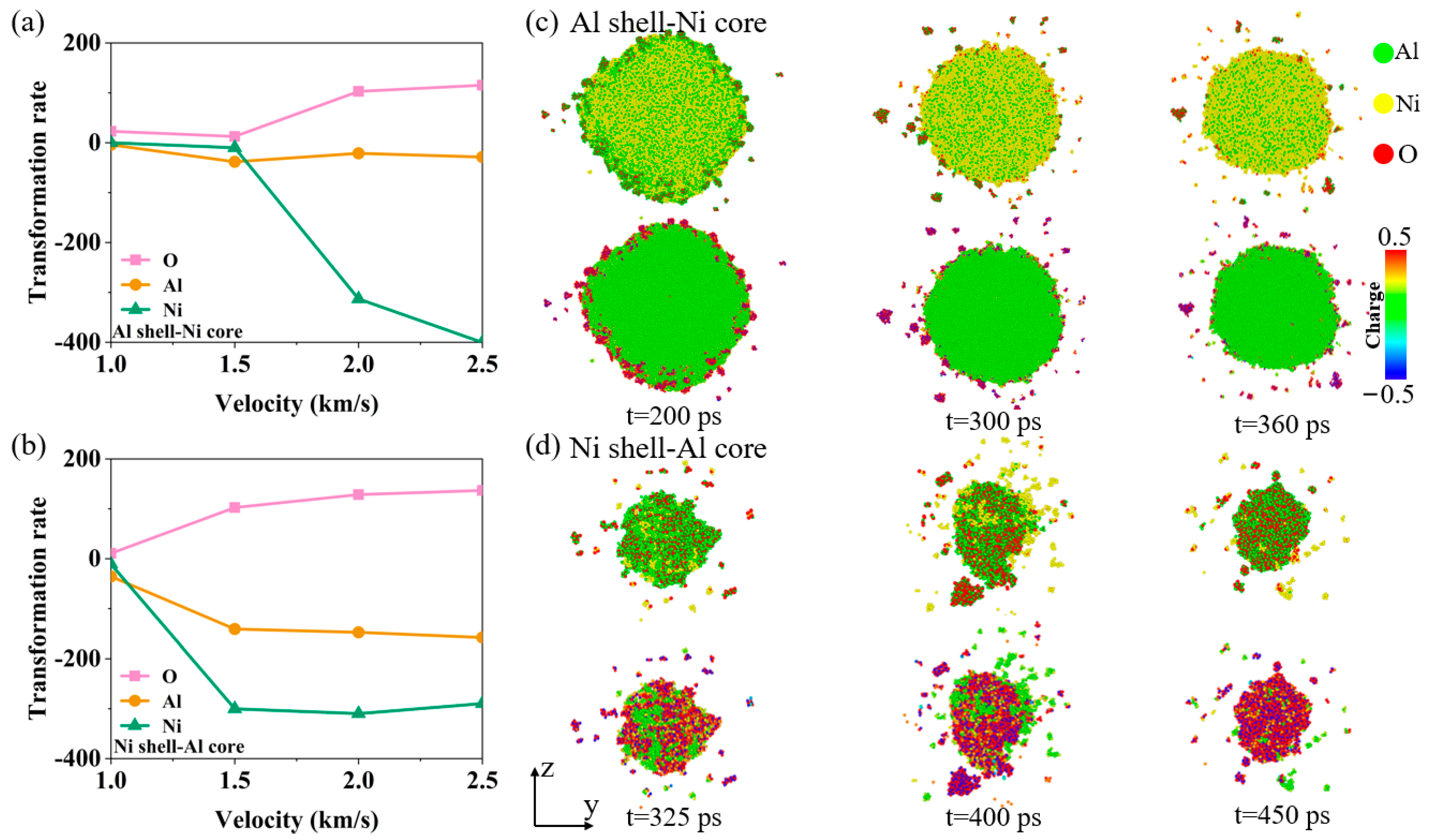
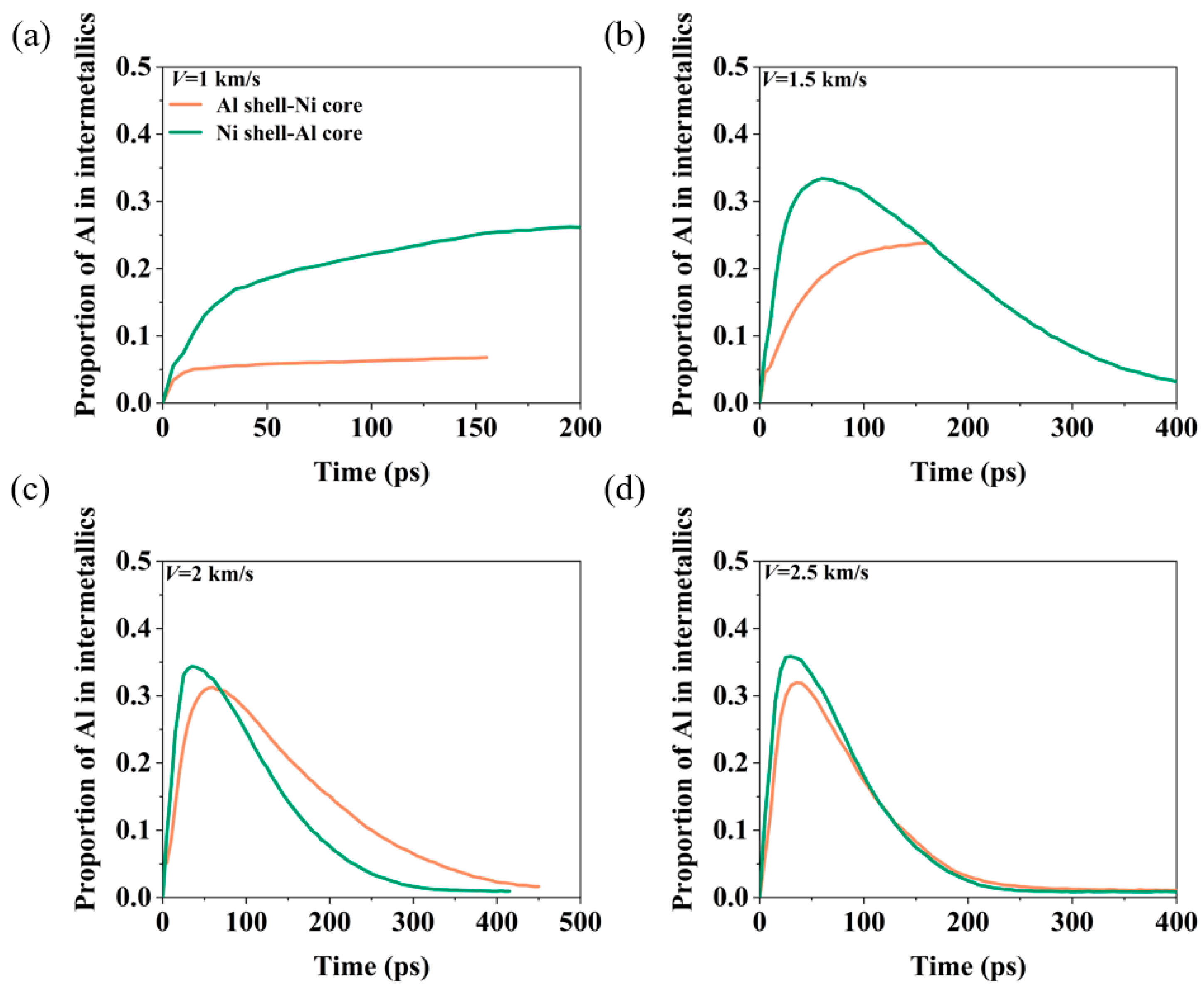
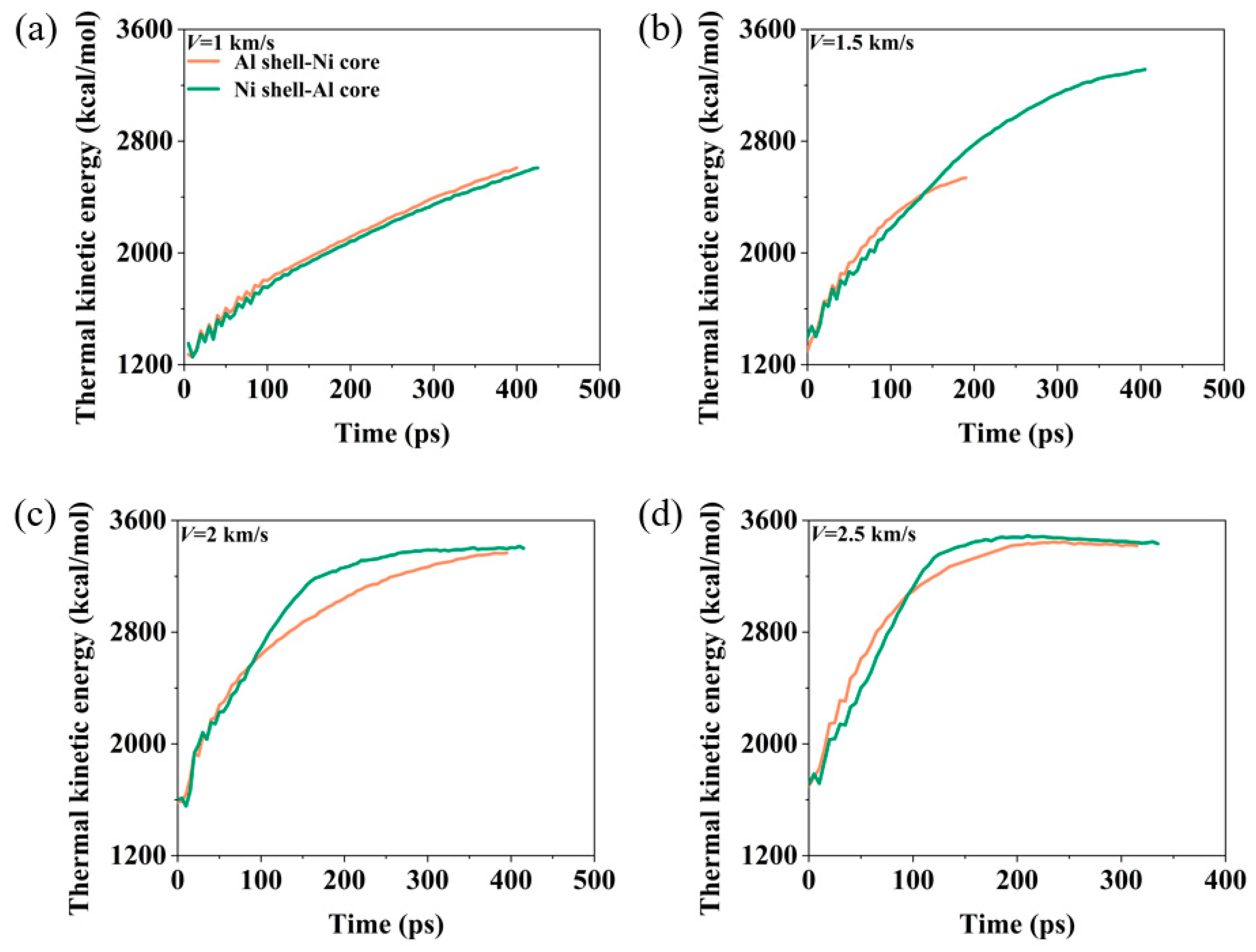
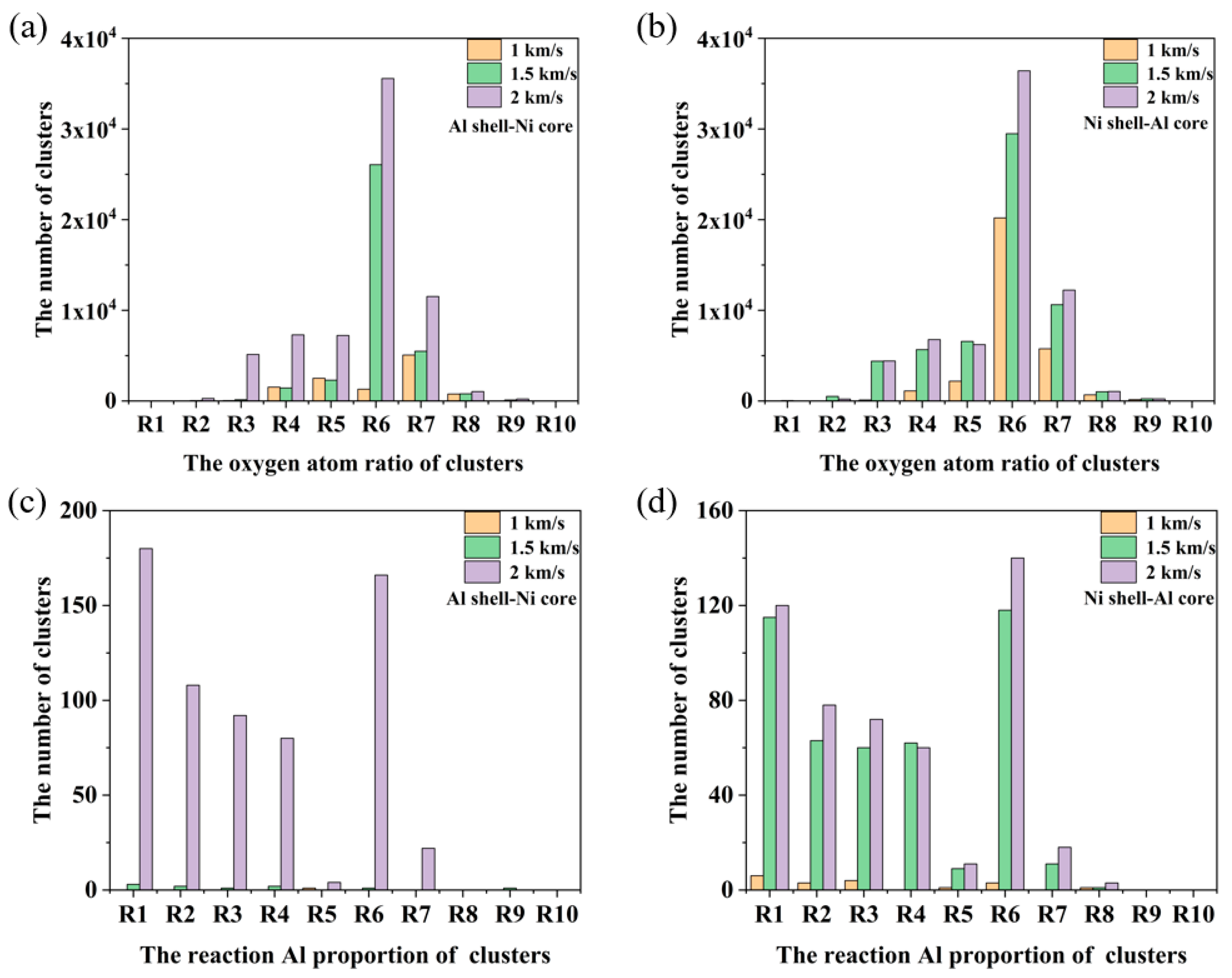
3.2. Energy Release Characteristics
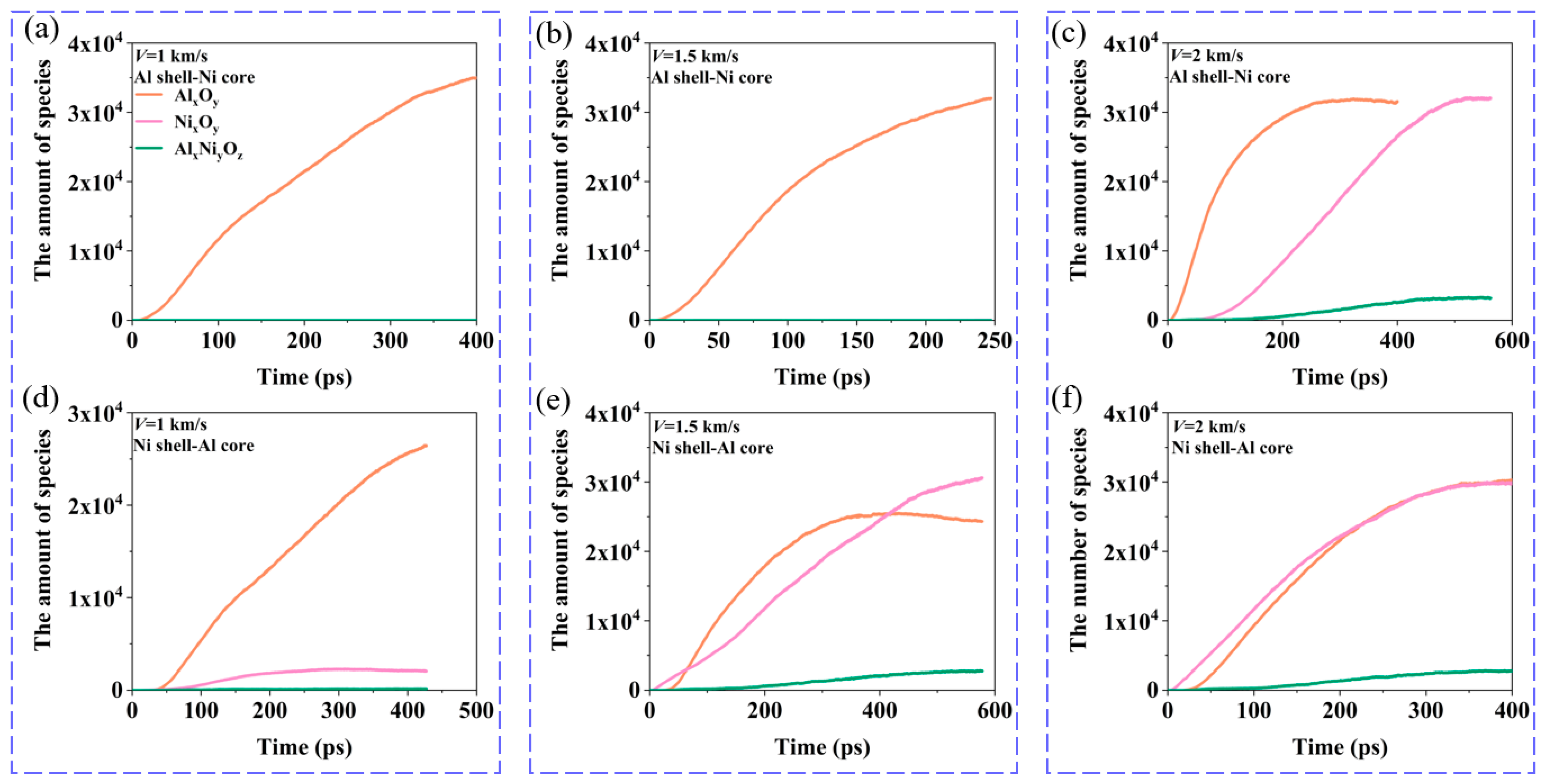
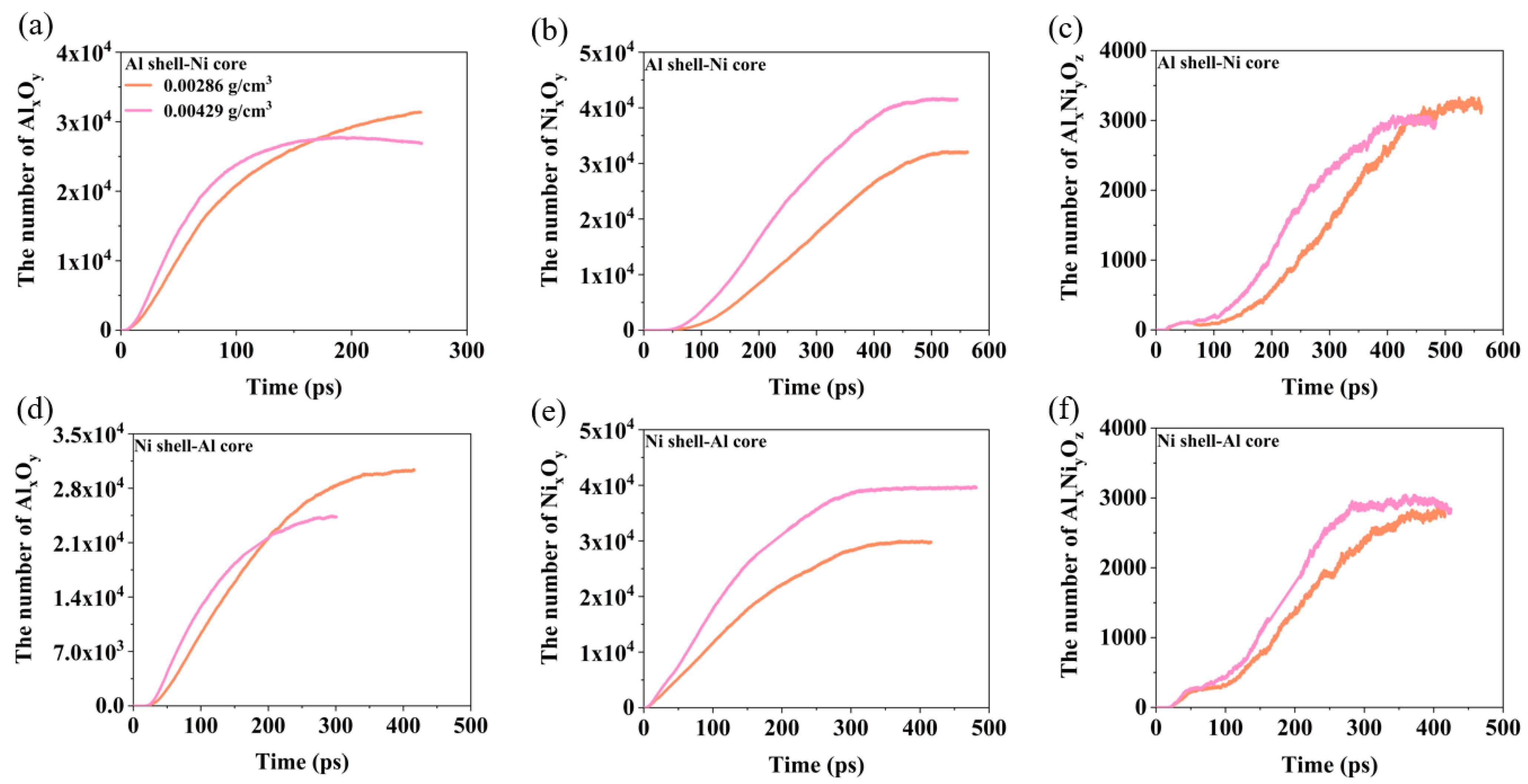
3.3. The Effect of Oxygen Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hastings, D.L.; Dreizin, E.L. Reactive structural materials: Preparation and characterization. Adv. Eng. Mater. 2018, 20, 1700631. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, Q.; Wang, B.; Zhou, B.; Chen, P.; Liu, R. Fabrication and characterization of the Ni–Al energetic structural material with high energy density and mechanical properties. J. Alloys Compd. 2020, 832, 154894. [Google Scholar] [CrossRef]
- Qu, J.; Deng, J.; Luo, Z.M.; Xiao, Y.; Shu, C.M. Thermal reaction characteristics and microstructure evolution of aluminium nano-powder in various mixtures of oxygen and nitrogen atmosphere. Process Saf. Environ. Protect. 2023, 170, 45–53. [Google Scholar] [CrossRef]
- Peuker, J.M.; Krier, H.; Glumac, N. Particle size and gas environment effects on blast and overpressure enhancement in aluminized explosives. Proc. Combust. Inst. 2013, 34, 2205–2212. [Google Scholar] [CrossRef]
- Risha, G.A.; Son, S.F.; Yetter, R.A.; Yang, V.; Tappan, B.C. Combustion of nano-aluminum and liquid water. Proc. Combust. Inst. 2007, 31, 2029–2036. [Google Scholar] [CrossRef]
- Ohkura, Y.; Rao, P.M.; Zheng, X. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Tang, Y.; Kong, C.; Zong, Y.; Li, S.; Zhuo, J.; Yao, Q. Combustion of aluminum nanoparticle agglomerates: From mild oxidation to microexplosion. Proc. Combust. Inst. 2017, 36, 2325–2332. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, C.; Xia, Y.; Duan, Y.; Li, Y.; Wang, Z.; Li, H. Atomistic origin of the complex morphological evolution of aluminum nanoparticles during oxidation: A chain-like oxide nucleation and growth mechanism. ACS Nano 2019, 13, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Niu, L.; Hao, W.; Liu, Y.; Zhang, C. Atomistic insight into the microexplosion-accelerated oxidation process of molten aluminum nanoparticles. Combust. Flame 2020, 214, 238–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Zhou, Z.; Zhao, H.; Tang, C.; Gao, Y. Study on the effect of particle size on early oxidation characteristics of aluminum nanoparticles. Powder Technol. 2022, 400, 117227. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Shao, J.L.; Chen, P.W.; Liu, R. Transportation and deformation of high-speed aluminum nanoparticles in inert gas with molecular dynamics study. Phys. Fluids 2023, 35, 042004. [Google Scholar]
- Rai, A.; Park, K.; Zhou, L.; Zachariah, M.R. Understanding the mechanism of aluminium nanoparticle oxidation. Combust. Theory Model. 2006, 10, 843–859. [Google Scholar] [CrossRef]
- Park, K.; Lee, D.; Rai, A.; Mukherjee, D.; Zachariah, M.R. Size-resolved kinetic measurements of aluminum nanoparticle oxidation with single particle mass spectrometry. J. Phys. Chem. B 2005, 109, 7290–7299. [Google Scholar] [CrossRef] [PubMed]
- Levitas, V.I.; Asay, B.W.; Son, S.F.; Pantoya, M. Melt dispersion mechanism for fast reaction of nanothermites. Appl. Phys. Lett. 2006, 89, 071909. [Google Scholar] [CrossRef]
- Levitas, V.I.; Pantoya, M.L.; Dikici, B. Melt dispersion versus diffusive oxidation mechanism for aluminum nanoparticles: Critical experiments and controlling parameters. Appl. Phys. Lett. 2008, 92, 011921. [Google Scholar] [CrossRef]
- Levitas, V.I. Mechanochemical mechanism for reaction of aluminium nano-and micrometre-scale particles. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2013, 371, 20120215. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Chu, Q.; Chen, D. Shock-induced anisotropic metal combustion. J. Phys. Chem. C 2020, 124, 13206–13214. [Google Scholar] [CrossRef]
- Chang, X.; Chu, Q.; Chen, D. Revealing pressure effects in the anisotropic combustion of aluminum nanoparticles. J. Phys. Chem. C 2021, 125, 28100–28107. [Google Scholar] [CrossRef]
- Chang, X.; Chen, D.; Chu, Q. Anisotropic combustion of aluminum nanoparticles in carbon dioxide and water flows. J. Therm. Sci. 2022, 31, 867–881. [Google Scholar] [CrossRef]
- Nakamura, R.; Lee, J.G.; Mori, H.; Nakajima, H. Oxidation behaviour of Ni nanoparticles and formation process of hollow NiO. Philos. Mag. 2008, 88, 257–264. [Google Scholar] [CrossRef]
- Railsback, J.G.; Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Size-dependent nanoscale Kirkendall effect during the oxidation of nickel nanoparticles. ACS Nano 2010, 4, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wen, D.; Guo, Z.X.; Korakianitis, T. Oxidation investigation of nickel nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 5057–5065. [Google Scholar] [CrossRef] [PubMed]
- Jeangros, Q.; Hansen, T.W.; Wagner, J.B.; Dunin-Borkowski, R.E.; Hébert, C.; Hessler-Wyser, A. Oxidation mechanism of nickel particles studied in an environmental transmission electron microscope. Acta Mater. 2014, 67, 362–372. [Google Scholar] [CrossRef]
- Knez, D.; Thaler, P.; Volk, A.; Kothleitner, G.; Ernst, W.E.; Hofer, F. Transformation dynamics of Ni clusters into NiO rings under electron beam irradiation. Ultramicroscopy 2017, 176, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sainju, R.; Rathnayake, D.; Tan, H.; Bollas, G.; Dongare, A.M.; Suib, S.L.; Zhu, Y. In situ studies of single-nanoparticle-level nickel thermal oxidation: From early oxide nucleation to diffusion-balanced oxide thickening. ACS Nano 2022, 16, 6468–6479. [Google Scholar] [CrossRef] [PubMed]
- Baras, F.; Turlo, V.; Politano, O.; Vadchenko, S.G.; Rogachev, A.S.; Mukasyan, A.S. SHS in Ni/Al nanofoils: A review of experiments and molecular dynamics simulations. Adv. Eng. Mater. 2018, 20, 1800091. [Google Scholar] [CrossRef]
- Zhao, S.; Germann, T.C.; Strachan, A. Melting and alloying of Ni∕Al nanolaminates induced by shock loading: A molecular dynamics simulation study. Phys. Rev. B 2007, 76, 104105. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, J.L.; Liu, R.; Chen, P. Chemical reaction of Ni/Al interface associated with perturbation growth under shock compression. Phys. Fluids 2022, 34, 044111. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, J.L.; Liu, R.; Chen, P. Atomic insights into shock-induced alloying reaction of premixed Ni/Al nanolaminates. J. Chem. Phys. 2023, 159, 174702. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Spolenak, R. Molecular dynamics study of the influence of microstructure on reaction front propagation in Al–Ni multilayers. Appl. Phys. Lett. 2021, 119, 133901. [Google Scholar] [CrossRef]
- Evteev, A.V.; Levchenko, E.V.; Hagel, F.A.; Belova, I.V.; Murch, G.E. Molecular dynamics study of reaction pathways in an Al-coated Ni nanoparticle. Intermetallics 2011, 19, 934–941. [Google Scholar] [CrossRef]
- Levchenko, E.V.; Evteev, A.V.; Riley, D.P.; Belova, I.V.; Murch, G.E. Molecular dynamics simulation of the alloying reaction in Al-coated Ni nanoparticle. Comput. Mater. Sci. 2010, 47, 712–720. [Google Scholar] [CrossRef]
- Zhu, K.; Xie, Y.; Shao, J.L.; Chen, P. Deformation, damage, and reaction characteristics during the collision between Ni and Al nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 27654–27667. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, C.; Chen, J.; Tang, Y.; Bai, S. Effect of W on the impact-induced energy release behavior of Al–Ni energetic structural materials. Metals 2021, 11, 1217. [Google Scholar] [CrossRef]
- Azghan, M.A.; Alizadeh, A. Effects of W on the thermal induced-energy release behavior and mechanical properties of 2Al–Ni and Al–Ni composites fabricated by mechanical alloying. Intermetallics 2023, 161, 107980. [Google Scholar] [CrossRef]
- Huang, C.; Chen, J.; Bai, S.; Li, S.; Tang, Y.; Liu, X.; Ye, Y. Enhancement of energy release performance of Al–Ni composites by adding CuO. J. Alloys Compd. 2020, 835, 155271. [Google Scholar] [CrossRef]
- Purja Pun, G.P.; Mishin, Y. Development of an interatomic potential for the Ni-Al system. Philos. Mag. 2009, 89, 3245–3267. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Zou, C.; Shin, Y.K.; Van Duin, A.C.; Fang, H.; Liu, Z.K. Molecular dynamics simulations of the effects of vacancies on nickel self-diffusion, oxygen diffusion and oxidation initiation in nickel, using the ReaxFF reactive force field. Acta Mater. 2015, 83, 102–112. [Google Scholar] [CrossRef]
- Hong, S.; Van Duin, A.C. Atomistic-scale analysis of carbon coating and its effect on the oxidation of aluminum nanoparticles by ReaxFF-molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 9464–9474. [Google Scholar] [CrossRef]
- DorMohammadi, H.; Pang, Q.; Árnadóttir, L.; Isgor, O.B. Atomistic simulation of initial stages of iron corrosion in pure water using reactive molecular dynamics. Comput. Mater. Sci. 2018, 145, 126–133. [Google Scholar] [CrossRef]
- Ai, L.; Huang, H.; Zhou, Y.; Chen, M.; Lü, Y. The oxidation of Fe/Ni alloy surface with supercritical water: A ReaxFF molecular dynamics simulation. Appl. Surf. Sci. 2021, 553, 149519. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J. Oxide growth characteristics on Al (100), (110), and (111) surfaces: A chemo-mechanical evaluation. Mater. Today Commun. 2021, 26, 102006. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, P.; Ma, Y.; Jiang, Y.; Li, H. Atomic-scale understanding of oxidation mechanisms of materials by computational approaches: A review. Mater. Des. 2022, 217, 110605. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Zheng, P.; Qian, J.; Wang, Y.; Jiang, Y.; Li, H. Oxidation of nickel with groove defects: Cation vacancies expansion mechanism and directional layer-by-layer oxidation. Appl. Surf. Sci. 2022, 593, 153384. [Google Scholar] [CrossRef]
- Qu, D.; Lei, C.; Zheng, M.; Chen, W.; Luo, D.; Yi, B.; Zhu, Z. A ReaxFF molecular dynamics study of the micro-dynamic oxidation behavior of Ni-Al alloys. Mater. Today Commun. 2024, 39, 108756. [Google Scholar] [CrossRef]
- Wu, B.; Wu, F.; Wang, P.; He, A.; Wu, H. Shock-induced ejecta transport and breakup in reactive gas. Phys. Chem. Chem. Phys. 2020, 22, 14857–14867. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Stukowski, A. Structure identification methods for atomistic simulations of crystalline materials. Model. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, X.; Xiao, S.; Deng, H.; Huang, B.; Zhu, W.; Hu, W. Effect of particle packing and density on shock response in ordered arrays of Ni+ Al nanoparticles. Phys. Chem. Chem. Phys. 2019, 21, 7272–7280. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, J.L.; Liu, R.; Chen, P. Atomistic study on reaction kinetics and reactivity of Ni/Al clad particles composites under shock loading. J. Chem. Phys. 2023, 158, 094706. [Google Scholar] [CrossRef] [PubMed]
- Cherukara, M.J.; Germann, T.C.; Kober, E.M.; Strachan, A. Shock loading of granular Ni/Al composites. Part 2: Shock-induced chemistry. J. Phys. Chem. C 2016, 120, 6804–6813. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Xie, Y.; Shao, J.-L.; Chen, P. Atomistic Insights into Impact-Induced Energy Release and Deformation of Core–Shell-Structured Ni/Al Nanoparticle in an Oxygen Environment. Materials 2024, 17, 4034. https://doi.org/10.3390/ma17164034
Zhu K, Xie Y, Shao J-L, Chen P. Atomistic Insights into Impact-Induced Energy Release and Deformation of Core–Shell-Structured Ni/Al Nanoparticle in an Oxygen Environment. Materials. 2024; 17(16):4034. https://doi.org/10.3390/ma17164034
Chicago/Turabian StyleZhu, Kexin, Yifan Xie, Jian-Li Shao, and Pengwan Chen. 2024. "Atomistic Insights into Impact-Induced Energy Release and Deformation of Core–Shell-Structured Ni/Al Nanoparticle in an Oxygen Environment" Materials 17, no. 16: 4034. https://doi.org/10.3390/ma17164034
APA StyleZhu, K., Xie, Y., Shao, J.-L., & Chen, P. (2024). Atomistic Insights into Impact-Induced Energy Release and Deformation of Core–Shell-Structured Ni/Al Nanoparticle in an Oxygen Environment. Materials, 17(16), 4034. https://doi.org/10.3390/ma17164034





