Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
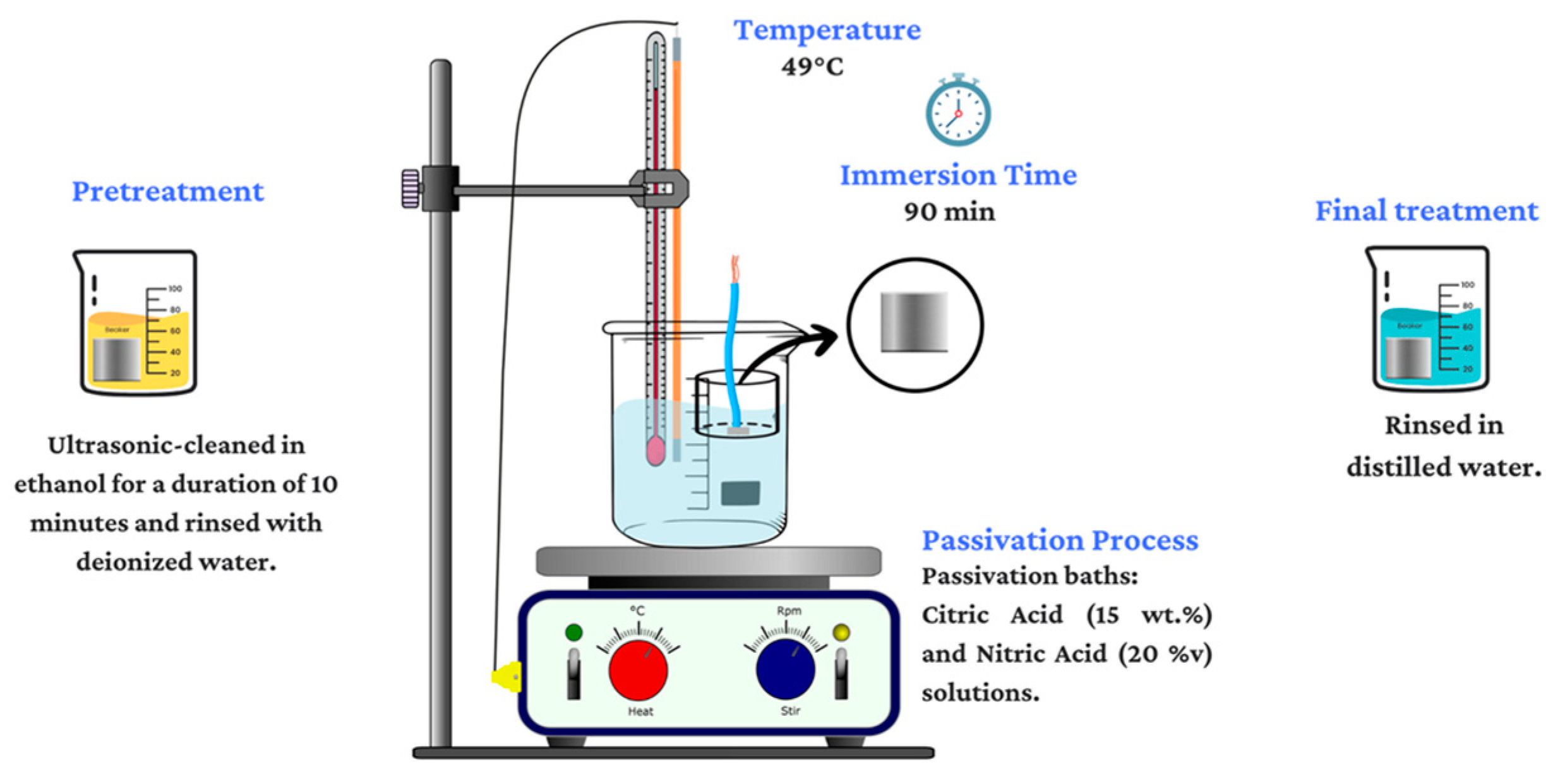
2.2. Passivation Treatment
- The samples were cleaned ultrasonically according to ASTM A380-17 [41]. This cleaning process involved immersing the samples in ethanol for a duration of 10 min; after that, they were rinsed with deionized water to remove any residual contaminants. Ultrasonic cleaning is a highly effective method for removing any impurities that could compromise subsequent treatments [42].
- Two passivation solutions were prepared for the treatment of the samples. The first solution consisted of citric acid (C6H8O7) at a concentration of 15 wt.% and a pH of 1.51. The second solution comprised nitric acid (HNO3) at a concentration of 20 v/v% and a pH of 0.41.
- After preconditioning the passivating solutions at 49 °C, the samples were carefully submerged. The immersion time was 90 min, ensuring adequate exposure to the passivation solution for effective treatment.
- Finally, rinsing in deionized water and air drying were carried out.
2.3. SEM Microstructural Characterization
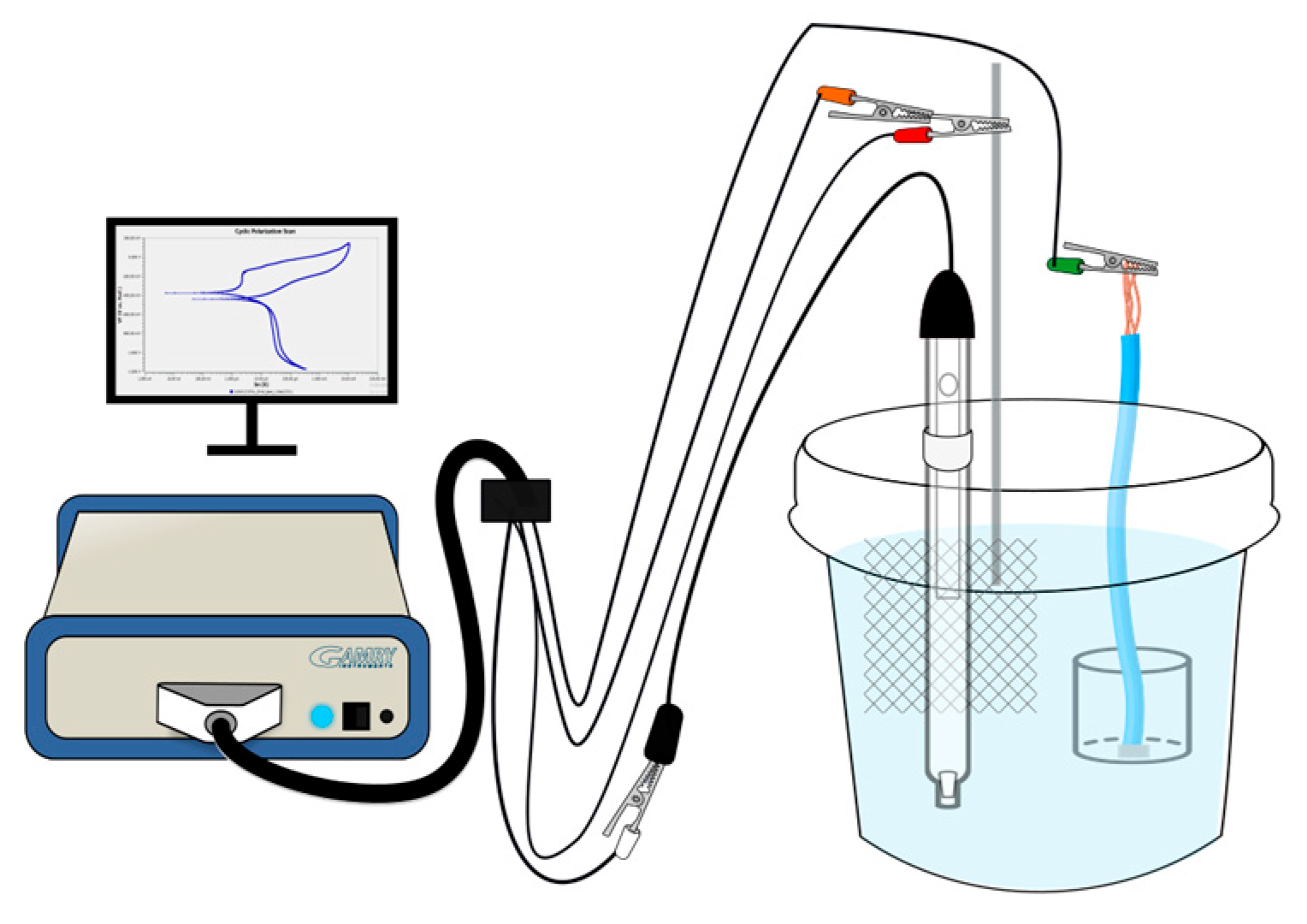
2.4. Electrochemical Testing
2.5. X-ray Photoelectron Spectroscopy (XPS) Characterization
3. Results and Discussion
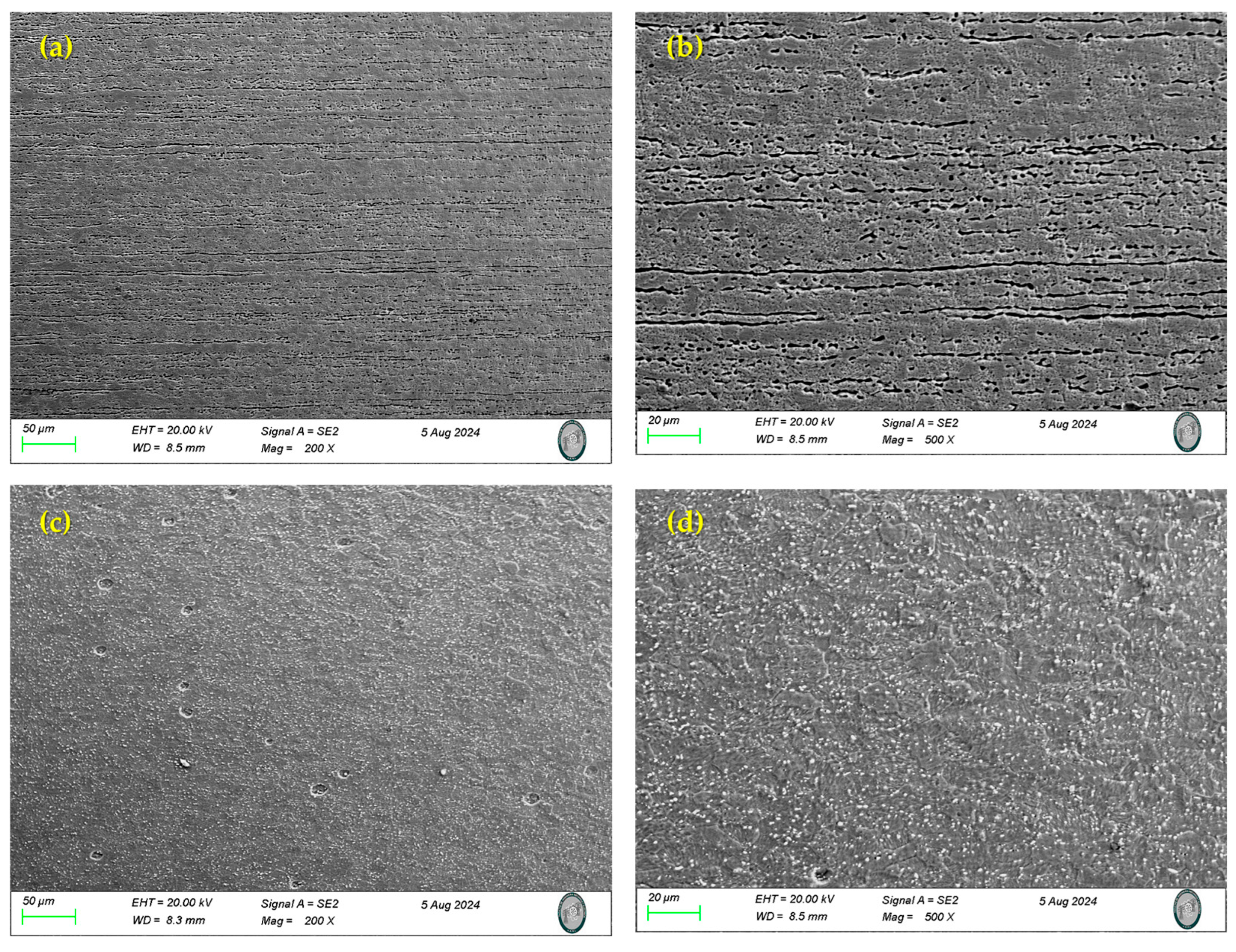
3.1. Scanning Electron Microscopy (SEM) and Microstructural Analysis
3.2. Pitting Resistance Equivalent Number (PREN)
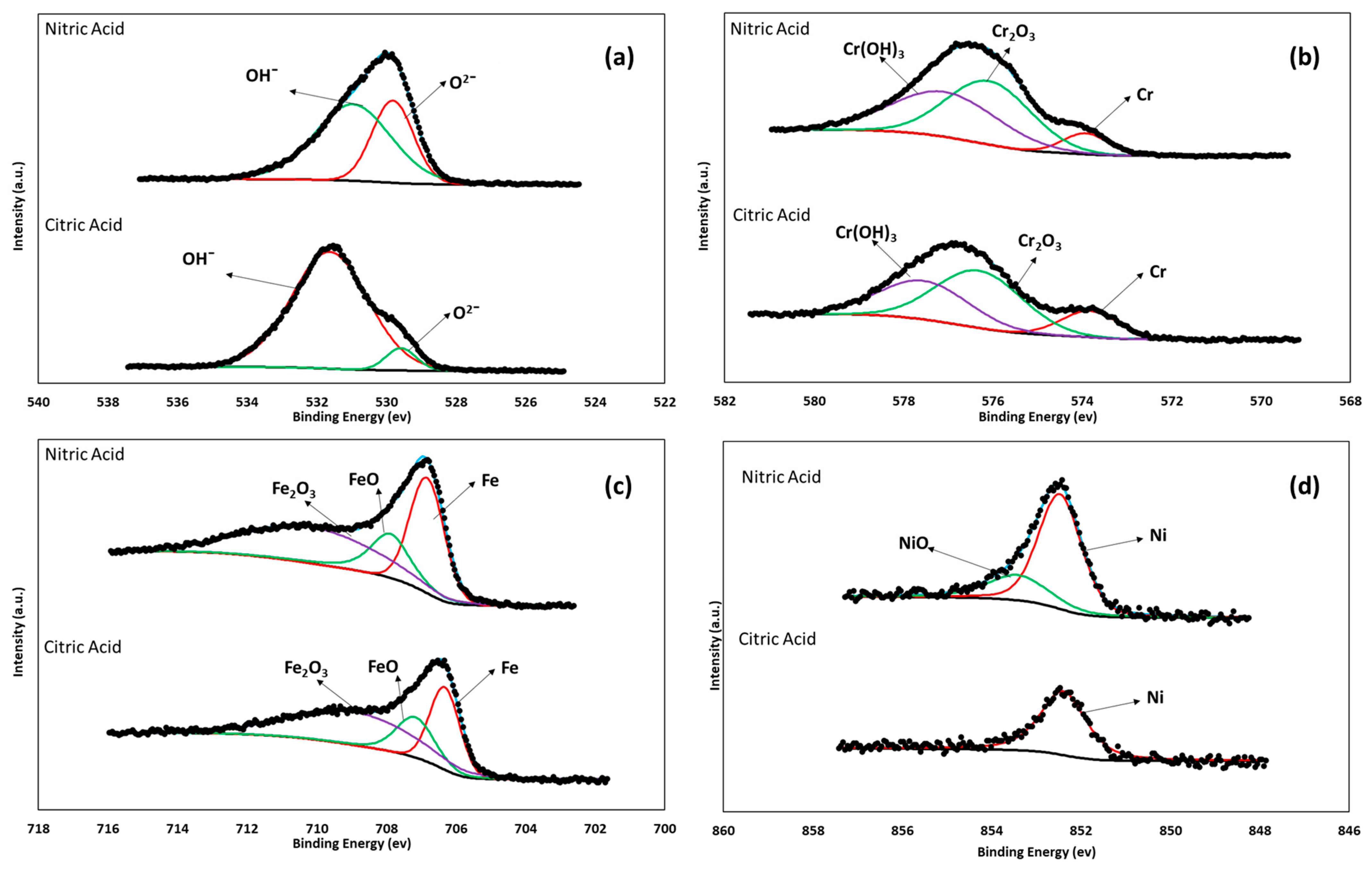
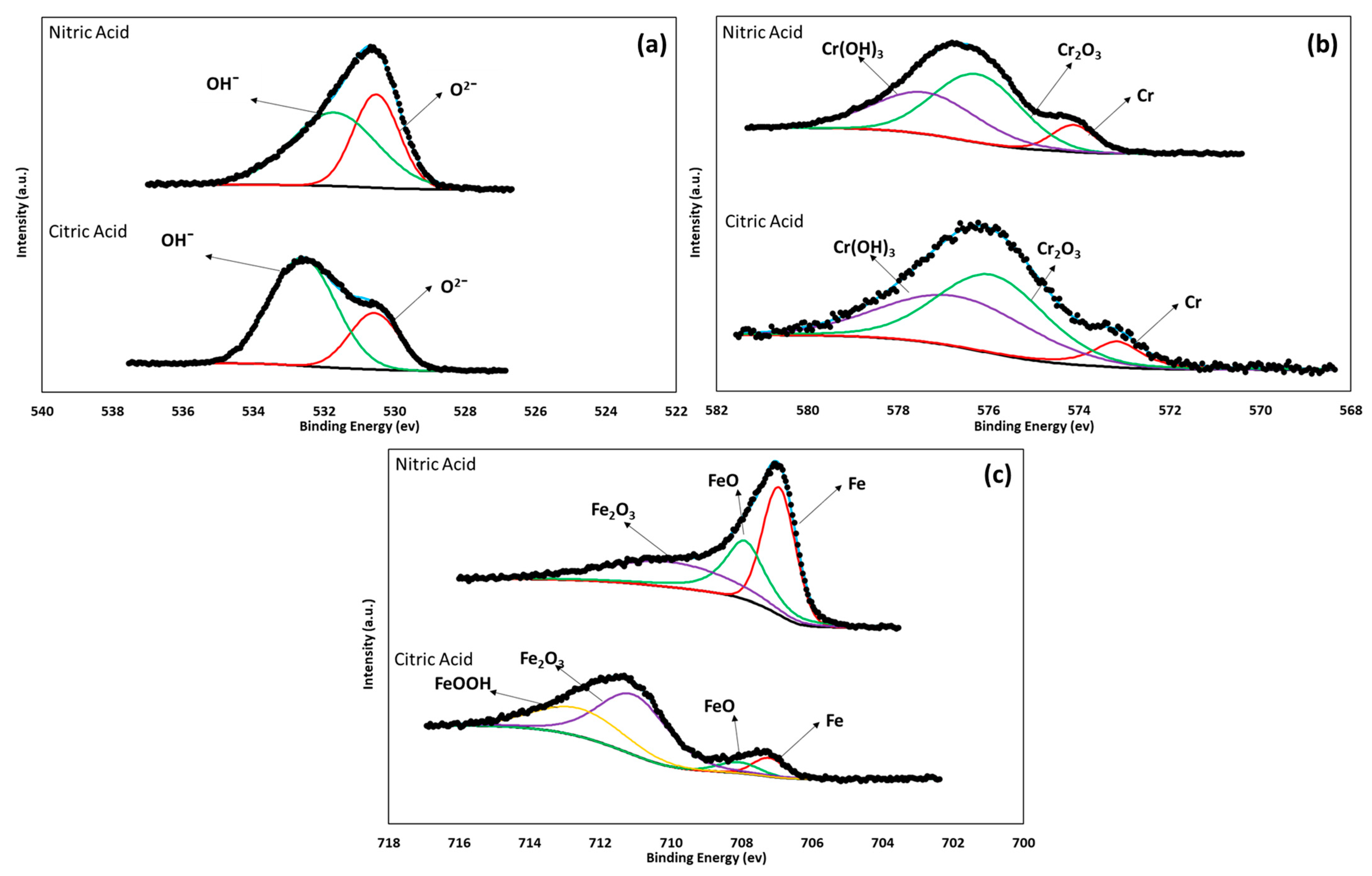
3.3. XPS Characterization
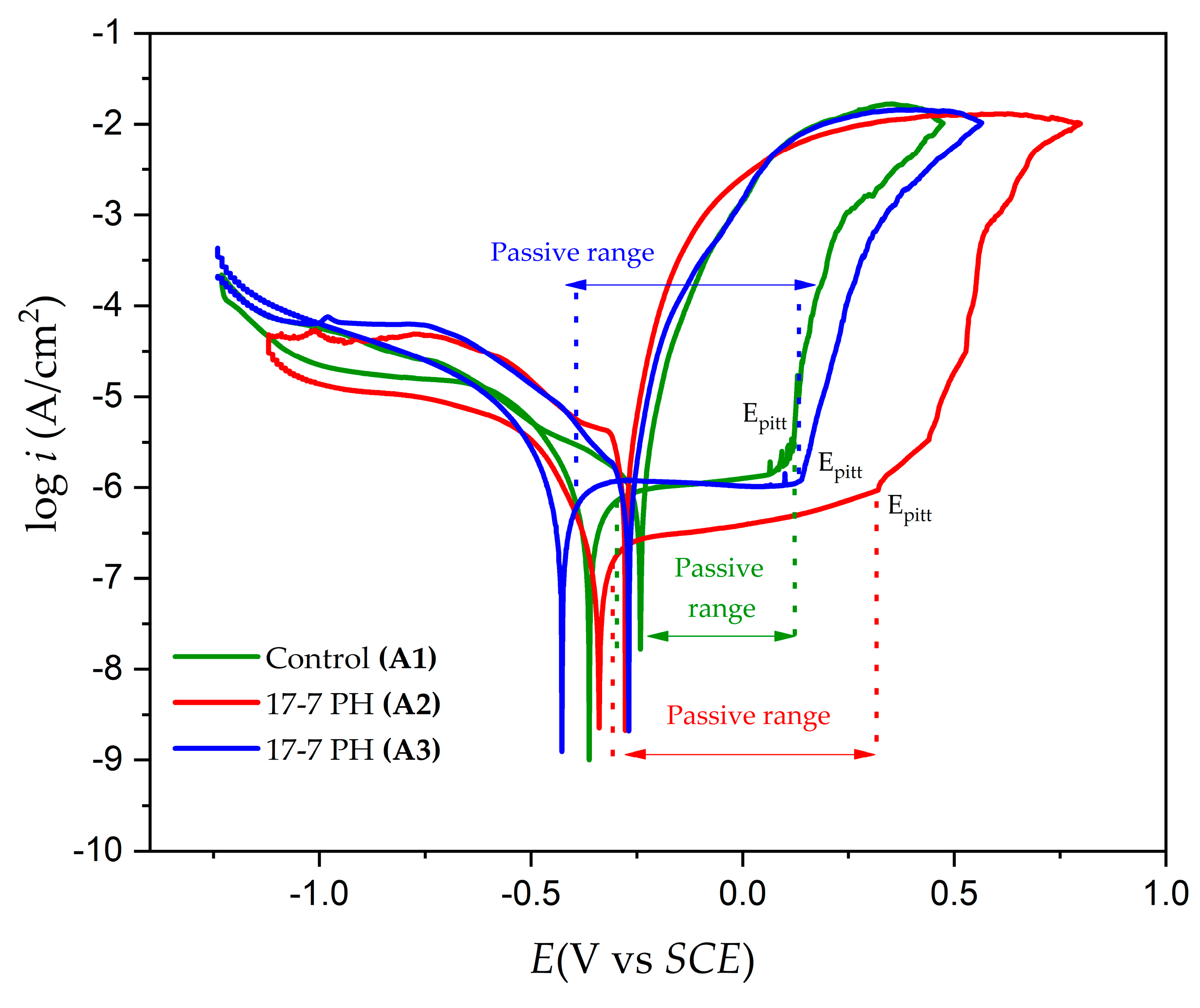
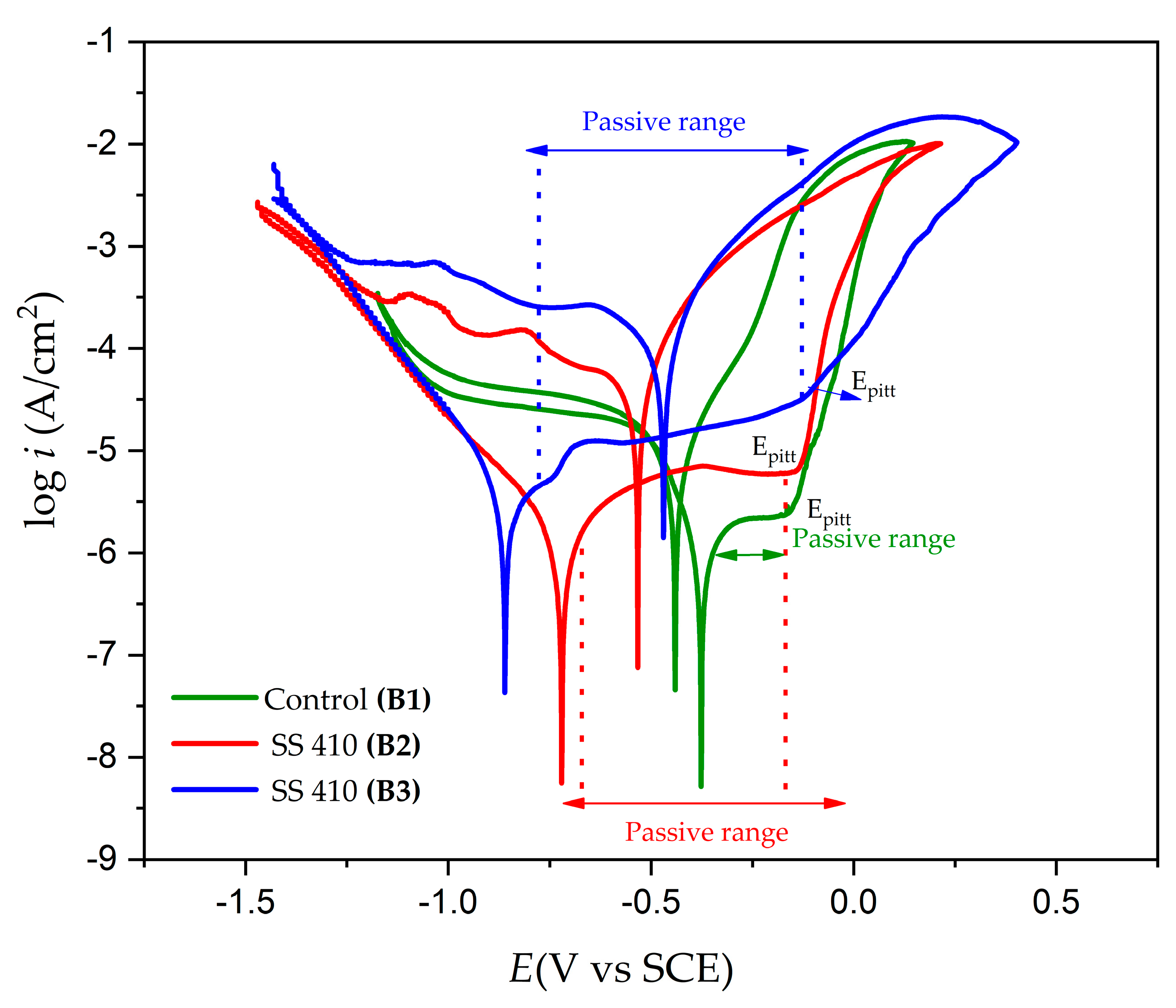
3.4. Electrochemical Measurements
3.5. SEM Surface Characterization
4. Conclusions
- SEM characterization under the initial conditions and conducted on the samples in an as-received state indicated that the 17-7PH presented a microstructure with a martensitic (α′) phase, while 410 SS contained a microstructure consisting of austenite (γ), a delta (δ) ferrite phase, and chromium carbides.
- The PREN results indicate that 17-7PH (18.92) steel has better resistance to pitting corrosion than 410 SS (13.5).
- According to the cyclic potentiodynamic polarization results, 17-7PH steel passivated in citric acid exhibited lower corrosion rate values (in the order of ×10−3 mm/yr).
- The application of nitric acid passivation caused the surface to become susceptible to localized corrosion.
- Passivation of 410 SS in acid nitric showed a trend in the cyclic potentiodynamic polarization curves, although it was not fully defined.
- The passivated 17-7PH and 410 SS steels exhibited positive hysteresis, which indicates that they are susceptible to localized corrosion.
- The results obtained following SEM analysis of the electrochemically tested samples indicated the presence of localized corrosion (pitting), and for the 410 SS, a higher density of pits compared to that for 17-7PH stainless steel was found, showing 50 and 100 μm pit sizes, respectively.
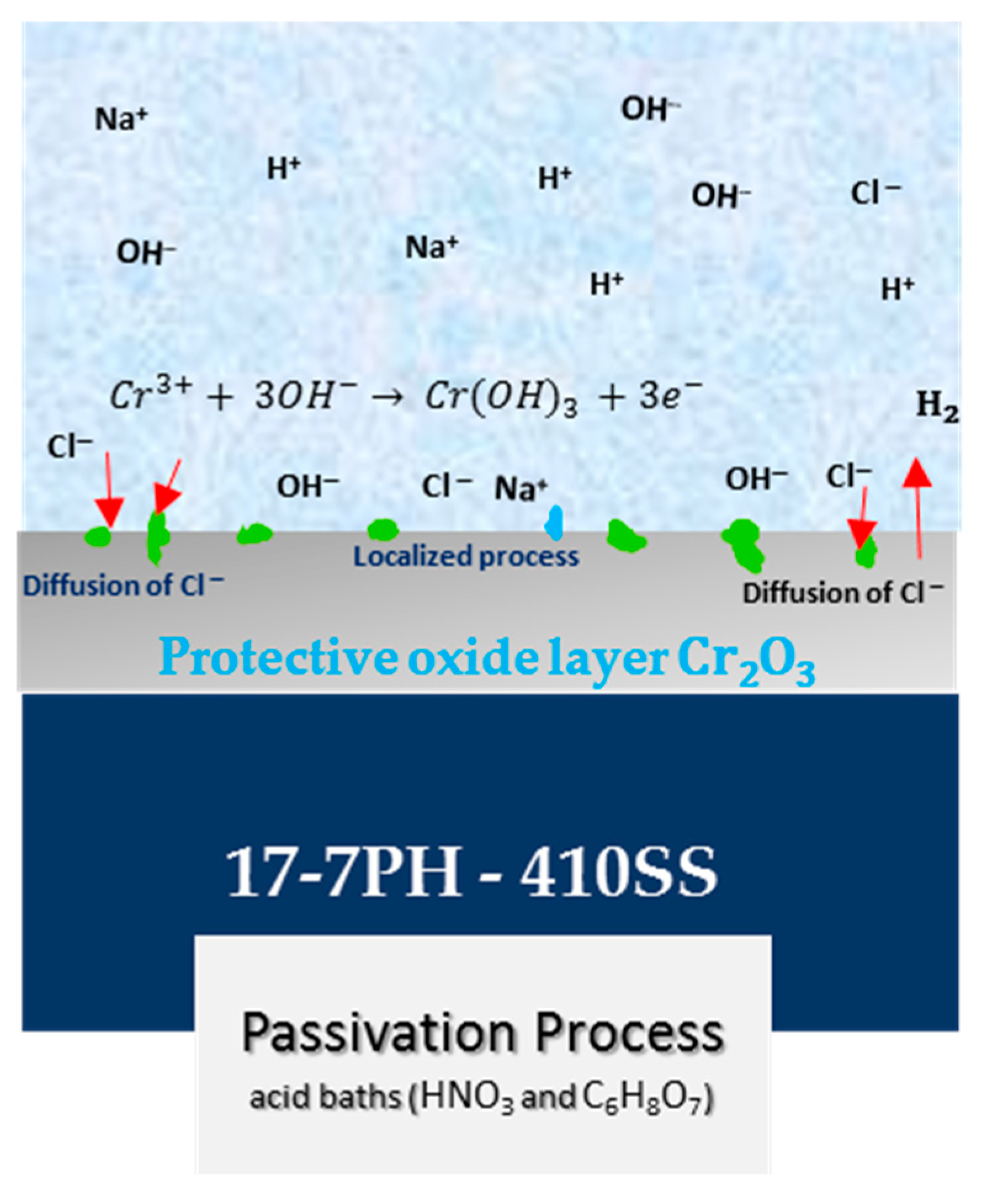
- The XPS analysis indicated different chemical species on the surface films of the 17-7PH and 410 SSs, such as Cr2O3, Cr (OH)3, FeOOH, and Fe2O3. The passive films contained iron chromium oxide and hydroxide.
- The 17-7PH SS samples passivated at 49 °C for 90 min in citric and nitric baths exhibited the best performance.
- The application of the citric acid passivation process to passivated stainless steels could be an environmentally friendly alternative to the frequently used nitric acid passivation process.
- The potentiodynamic polarization results indicated that 17-7PH stainless steel passivated in citric and nitric acid showed lower corrosion rate values (in the order of ×10−3 mm/yr).
- XPS analysis allowed us to determine that the surface film of the 17-7PH and 410 SS samples analyzed in this work consisted of different chemical species, such as Cr2O3 and Fe (OH)O.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys; Topics in Mining, Metallurgy and Materials Engineering; Bergmann, C.P., Ed.; Springer: Cham, Switzerland, 2020; ISSN 2364-3307. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, P.Y.; Payer, J.H. Chapter 1: Costs of corrosion in the United States. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew: Washington, DC, USA, 2005; pp. 3–24. [Google Scholar]
- Mouritz, P.A. Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Lara Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Cabral, M.J.A.; Estupinan, F.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion Behaviour of 304 Austenitic, 15-5PH and 17-4PH Passive Stainless Steels in acid solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Prasad Eswara, N.; Wanhill, R.J.H. Volume 2: Aerospace Materials and Technologies; Springer Science + Business Media: Singapore, 2017. [Google Scholar]
- Cobb, H.M. The History of Stainless Steel; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- NASA Technical Reports Sever. Available online: https://ntrs.nasa.gov/citations/19680010964 (accessed on 1 May 2024).
- Farrar, J. The Alloy Tree—A Guide to Low-Alloy Steels, Stainless Steels and Nickel-Base Alloys; Woodhead Publishing Limited: Cambridge, UK; CRC Press: Cambridge, UK, 2004. [Google Scholar]
- Almeraya-Calderón, F.; Samaniego-Gámez, O.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Olguín-Coca, J.; Jáquez-Muňoz, J.M.; Cabral-Miramontes, J.; Flores-De los Rios, J.P.; Bautista-Margulis, R.G.; Gaona-Tiburcio, C. Corrosion Behavior of Passivated Martensitic and Semi-Austenitic Precipitation Hardening Stainless Steel. Metals 2022, 12, 1033. [Google Scholar] [CrossRef]
- Outokumpu. Handbook of Stainless Steel; Outokumpu Oyj: Helsinki, Finland, 2017. [Google Scholar]
- ASM International. Introduction to Stainless Steel. In Alloy Digest Sourcebook—Stainless Steel, 3rd ed.; David, J.R., Ed.; ASM International: Materials Park, OH, USA, 2000; pp. 2–3. [Google Scholar]
- AIST Steel Glossary. Available online: https://apps.aist.org/asp/glossary/ (accessed on 1 April 2024).
- Yu, Y.; Shironita, S.; Souma, K.; Umeda, M. Effect of chromium content on the corrosion resistance of ferritic stainless steels in sulfuric acid solution. Heliyon 2018, 4, e00958. [Google Scholar] [CrossRef]
- Gaydos, S.P. Passivation of aerospace stainless parts with citric acid solutions. Plat. Surf. Finish. 2003, 90, 20–25. [Google Scholar]
- Schweitzer, P.A. Metallic Materials: Physical, Mechanical, and Corrosion Properties; M. Dekker: New York, NY, USA, 2003. [Google Scholar]
- Benavides, S. Corrosion Control in the Aerospace Industry; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- ASTM A967-17; Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts. ASTM International: West Conshohocken, PA, USA, 1999.
- Martínez-Villafañe, A.; Almeraya-Calderón, M.F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Shibata, T. Stochastic studies of passivity breakdown. Corros. Sci. 1990, 31, 413–423. [Google Scholar] [CrossRef]
- Isselin, J.; Kasada, R.; Kimura, A. Effects of aluminum on the corrosion behavior of 16% Cr ODS ferritic steels in a nitric acid solution. J. Nucl. Sci. Techmol. 2011, 48, 169–171. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Seifzadeh, D.; Raghibi-Boroujeni, M. Analysis of electrochemical noise data in both time and frequency domains to evaluate the effect of ZnO nanopowder addition on the corrosion protection performance of epoxy coatings. Arab. J. Chem. 2016, 9, S1320–S1327. [Google Scholar] [CrossRef]
- Lewis, P.; Kolody, M. Alternative to Nitric Acid Passivation of Stainless Steel Alloys. In Technology Evaluation for Environmental Risk Mitigation Compendium, Proceedings of the NASA Technology Evaluation for Environmental Risk Mitigation Principal Center (TEERM), Orlando, FL, USA, 13 January 2008; Department of Defense (DoD) and NASA: Merritt Island, FL, USA, 2008. [Google Scholar]
- Yasensky, D.; Reali, J.; Larson, C.; Carl, C. Citric acid passivation of stainless steel. In Proceedings of the Aircraft Airworthiness and Sustainment Conference, San Diego, CA, USA, 18–21 April 2009. [Google Scholar]
- Suresh, G.U.; Kamachi, M.S. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E, M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef] [PubMed]
- Bragaglia, M.; Cherubini, V.; Cacciotti, I.; Rinaldi, M.; Mori, S.; Soltani, P.; Nanni, F.; Kaciulis, S.; Montesperelli, G. Citric Acid Aerospace Stainless Steel Passivation: A Green Approach. In Proceedings of the CEAS Aerospace Europe Conference, Delft, The Netherlands, 7–11 September 2015. [Google Scholar]
- Marcelin, S.; Pébèrea, N.; Régnierb, S. Electrochemical characterisation of a martensitic stainless steel in a neutral chloride solution. Electrochim. Acta 2013, 87, 32–40. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Ghoneim, A.; Fekry, A. Stability of spontaneous passive films on high strength Mo-containing stainless steels in aqueous solutions. J. Appl. Electrochem. 2007, 37, 405–413. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Ameer, M.A.; El-Aziz, A.M.; Fekry, A.M. Electrochemical behavior of Mo-containing austenitic stainless steel in buffer solutions. Mat.-Wiss. Werkstofftech. 2004, 35, 407–413. [Google Scholar] [CrossRef]
- Fakić, B.; Ćubela, D. Review of the Development of Research in the Design of Semi Austenitic Stainless Steel 17-7PH. In Proceedings of the 17th International Research/Expert Conference Trends in the Development of Machinery and Associated Technology, TMT 2013, Istanbul, Turkey, 10–11 September 2013; pp. 113–116. [Google Scholar]
- Warren, J.; Wei, D.Y. The low cycle fatigue behavior of the controlled transformation stainless steel alloy AM355 at 121, 204 and 315 °C. Mater. Sci. Eng. A 2008, 475, 148–156. [Google Scholar] [CrossRef]
- Tseng, K.H.; Chen, Y.C.; Chen, Y.C. Micro Plasma Arc Welding of AM 350 Precipitation Hardening Alloys. Appl. Mech. Mater. 2012, 121, 2681–2685. [Google Scholar] [CrossRef]
- Favor, R.J.; Deel, O.L.; Achbach, W.P. Design Information on AM-350 Stainless Steel for Aircraft and Missiles; Defense Metals Information Center, Battelle Memorial Institute: Columbus, OH, USA, 1961; Volume 156. [Google Scholar]
- Shaikh, H.; George, G.; Khatak, H.S. Failure analysis of an AM 350 steel bellows. Fail. Anal. 2001, 8, 571–576. [Google Scholar] [CrossRef]
- Lin, C.K.; Chu, C.C. Mean stress effects on low-cycle fatigue for a precipitation-hardening martensitic stainless steel in different tempers. Fatigue Fract. Eng. Mater. Struct. 2000, 23, 545–553. [Google Scholar] [CrossRef]
- Kumar, T.S.; Ramanujam, R.; Vignesh, M.; Rohith, D.; Manoj, V.; Sankar, P.H. Comparative machining studies on custom 450 alloy with TiCN, TiAlN coated and uncoated carbide tools using Taguchi-Fuzzy logic approach. Mater. Res. Express 2019, 6, 066411. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.D.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti-6Al-4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Mollapour, Y.; Poursaeidi, E. Experimental and numerical analysis of Pitting Corrosion in CUSTOM 450 Stainless Steel. Eng. Fail. Anal. 2021, 128, 105589. [Google Scholar] [CrossRef]
- Samaniego-Gámez, O.; Almeraya-Calderón, F.; Chacón-Nava, J.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Flores-De los Rios, J.P.; Jáquez-Muñoz, J.M.; Delgado, A.D.; Gaona-Tiburcio, C. Corrosion Behavior of Passivated CUSTOM450 and AM350 Stainless Steels for Aeronautical Applications. Metals 2022, 12, 666. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- ASTM A380-17; Standard Practice for Cleaning, Descaling and Passivation of Stainless-Steel Parts, Equipment, and Systems. ASTM International: West Conshohocken, PA, USA, 1999.
- Gallego-Juárez, J.A.; Graff, K.F. Power Ultrasonics—Applications of High-Intensity Ultrasound; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- ASTM G44–99; Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5% Sodium Chloride Solution. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 1999.
- Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Chacon-Nava, J.; Matutes-Aquino, A.M.; Martinez-Villafañe, A. Electrochemical response of permanent magnets in different solutions. J. Alloys Compd. 2004, 369, 78–80. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- 17-7PH Technical Data. Available online: https://www.mcmaster.com/products/17-7-ph-stainless-steel-sheets/ (accessed on 4 August 2024).
- 410 SS Technical Data. Available online: https://www.mcmaster.com/products/sheets/material~410-stainless-steel/ (accessed on 4 August 2024).
- Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G.; Campillo, B.; Gaona-Tiburcio, C.; Dominguez-Patiño, G.; Leduc Lezama, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. An electrochemical study of the corrosion behavior of a dual phase steel in 0.5 m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786–1798. [Google Scholar] [CrossRef]
- The British Stainless Steel Association. 2022. Available online: https://bssa.org.uk/bssa_articles/calculation-of-pren/ (accessed on 2 June 2022).
- Schweitzer, P. Fundamentals of Metallic Corrosion: Atmospheric and Media Corrosion of Metals; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 10:0-8493-8243-2. [Google Scholar]
- Natarajan, R.; Palaniswamy, N.; Natesan, M.; Muralidharan, V.S. XPS analysis of passive film on stainless steel. Open Corros. J. 2009, 2, 114–124. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Rokicki, R. Electrochemical and XPS studies of AISI 316L stainless steel after electropolishing in a magnetic field. Corros. Sci. 2008, 50, 2676–2681. [Google Scholar] [CrossRef]
- Hermas, A.A. XPS analysis of the passive film formed on austenitic stainless steel coated with conductive polymer. Corros. Sci. 2008, 50, 2498–2505. [Google Scholar] [CrossRef]
- Jung, R.H.; Tsuchiya, H.; Fujimoto, S. XPS characterization of passive films formed on Type 304 stainless steel in humid atmosphere. Corros. Sci. 2012, 58, 62–68. [Google Scholar] [CrossRef]
- Mesquita, T.J.; Chauveau, E.; Mantel, M.; Nogueira, R.P. A XPS study of the Mo effect on passivation behaviors for highly controlled stainless steels in neutral and alkaline conditions. Appl. Surf. Sci. 2013, 270, 90–97. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T.; Wang, C.; Dong, L. Comparison of corrosion properties of passive films formed on coarse grained and ultrafine grained AISI 2205 duplex stainless steels. J. Electroanal. Chem. 2015, 757, 263–269. [Google Scholar]
- Feng, Z.; Cheng, X.; Dong, C.; Xu, L.; Li, X. Passivity of 316L stainless steel in borate buffer solution studied by Mott–Schottky analysis, atomic absorption spectrometry and X-ray photoelectron spectroscopy. Corros. Sci. 2010, 52, 3646–3653. [Google Scholar] [CrossRef]
- Pellegrini-Cervantes, M.J.; Almeraya-Calderon, F.; Borunda-Terrazas, A.; Bautista-Margulis, R.G.; Chacón-Nava, J.G.; Fajardo-San-Miguel, G.; Almaral-Sanchez, J.L.; Barrios-Durstewitz, C.; Martinez-Villafañe, A. Corrosion Resistance, Porosity and Strength of lended Portland Cement Mortar Containing Rice Husk Ash and Nano-SiO2. Int. J. Electrochem. Sci. 2013, 8, 10697–10710. [Google Scholar] [CrossRef]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II.—The kinetic interpretation on the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to pitting corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V alloys for aeronautical applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Martinez-Villafane, A.; Chacon-Nava, J.G.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Dominguez-Patino, G.; Gonzalez-Rodriguez, J.G. Oxidation performance of a Fe–13Cr alloy with additions of rare earth elements. Mater. Sci. Eng. A 2003, 363, 15–19. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; de Oca, N.G.M.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Bojinov, M.; Fabricius, G.; Laitinen, T.; Saario, T. The mechanism of the transpassive dissolution of chromium in acidic sulfate solutions. J. Electrochem. Soc. 1998, 145, 2043–2050. [Google Scholar] [CrossRef]
- Tian, H.; Sun, F.; Chu, F.; Wang, L.; Wang, X.; Cui, X. Passivation behavior and surface chemistry of 316 SS in the environment containing Cl− and NH4. J. Electroanal. Chem. 2021, 886, 115138. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, Q.; Liu, P.; Zhang, J.; Cao, F. Quasi-simultaneous electrochemical/chemical imaging of local Fe2+ and pH distributions on 316 L stainless steel surface. J. Electroanal. Chem. 2020, 871, 114107. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Choudhary, S.; Qiu, Y.; Thomas, S.; Birbilis, N. Element-resolved electrochemical analysis of transpassive dissolution and repassivation behavior of the multi-principal element alloy AlTiVCr. Electrochim. Acta 2020, 362, 137104. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Estupiñán, F.; Zambrano, R.P.; Martínez-Villafañe, A.; Borunda, T.A.; Colás, O.R.; Gaona-Tiburcio, C. Análisis de los transitorios de ruido electroquímico para aceros inoxidables 316 y-DUPLEX 2205 en NaCl y FeCl. Rev. Metal. 2012, 4, 147–156. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Han, E.H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high-temperature alkaline environments. Corros. Sci. 2010, 52, 3444–3452. [Google Scholar] [CrossRef]
- Calinski, C.; Strehblow, H.H. ISS depth profiles of the passive layer on Fe/Cr alloys. J. Electrochem. Soc. 1989, 136, 1328–1331. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Luo, J.-L.; Huet, F. Review—Electrochemical Noise Applied in Cor-rosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Taheri Shoja, S.; Heydari Zebardast, B.; Mohamadian Samim, P. An Electrochemical Impedance Spectroscopic Study of the Passive State on AISI 304 Stainless Steel. Int. J. Electrochem. 2011, 2011, 8. [Google Scholar] [CrossRef]
- Castro, B.E.; Vilche, R.J. Investigation of passive layers on iron and iron-chromium alloys by electrochemical impedance spectroscopy. Electrochim. Acta 1993, 38, 1567–1572. [Google Scholar] [CrossRef]
- Luo, Z.G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.F.; Liao, B.K.; Guo, X.P. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Alireza, R.; Abdolreza, F.; Avni, B.; Alireza, S.; Mikhail, A.V.; Valbonë, M.; Zhong, X.; Samira, Y.; Richard, D. Novel sucrose derivative as a thermally stable inhibitor for mild steel corrosion in 15% HCl medium: An experimental and computational study. Chem. Eng. J. 2022, 446, 136938. [Google Scholar] [CrossRef]
- Cheng, M.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. A comparative study of primary and secondary passive films formed on AM355 stainless steel in 0.1 M NaOH. Appl. Surf. Sci. 2018, 427, 763–773. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Saatchi, A.; Golozar, M.A.; Raeissi, K. The transpassive dissolution mechanism of 316L stainless steel. Electrochim. Acta 2009, 54, 3645–3650. [Google Scholar] [CrossRef]
- Bojinov, M.; Betova, I.; Fabricius, G.; Laitinen, T.; Saario, T. The stability of the passive state of iron–chromium alloys in sulphuric acid solution. Corros. Sci. 1999, 41, 1557–1584. [Google Scholar] [CrossRef]
- Núñez-Jaquez, R.E.; Buelna-Rodríguez, J.E.; Barrios-Durstewitz, C.P.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion of modified concrete with sugar cane bagasse ash. Int. J. Corros. 2012, 2012, 451864. [Google Scholar] [CrossRef]
- Mazinanian, N.; Hedberg, Y.S. Metal Release Mechanisms for Passive Stainless Steel in Citric Acid at Weakly Acidic pH. J. Electrochem. Soc. 2016, 163, C686–C693. [Google Scholar] [CrossRef]
- Martínez-Aparicio, B.; Martínez-Bastidas, D.; Gaona-Tiburcio, C.; Martin, U.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Localized corrosion of 15-5 PH and 17-4 PH stainless steel in NaCl solution. J. Solid State Electrochem. 2023, 27, 2993–3001. [Google Scholar] [CrossRef]
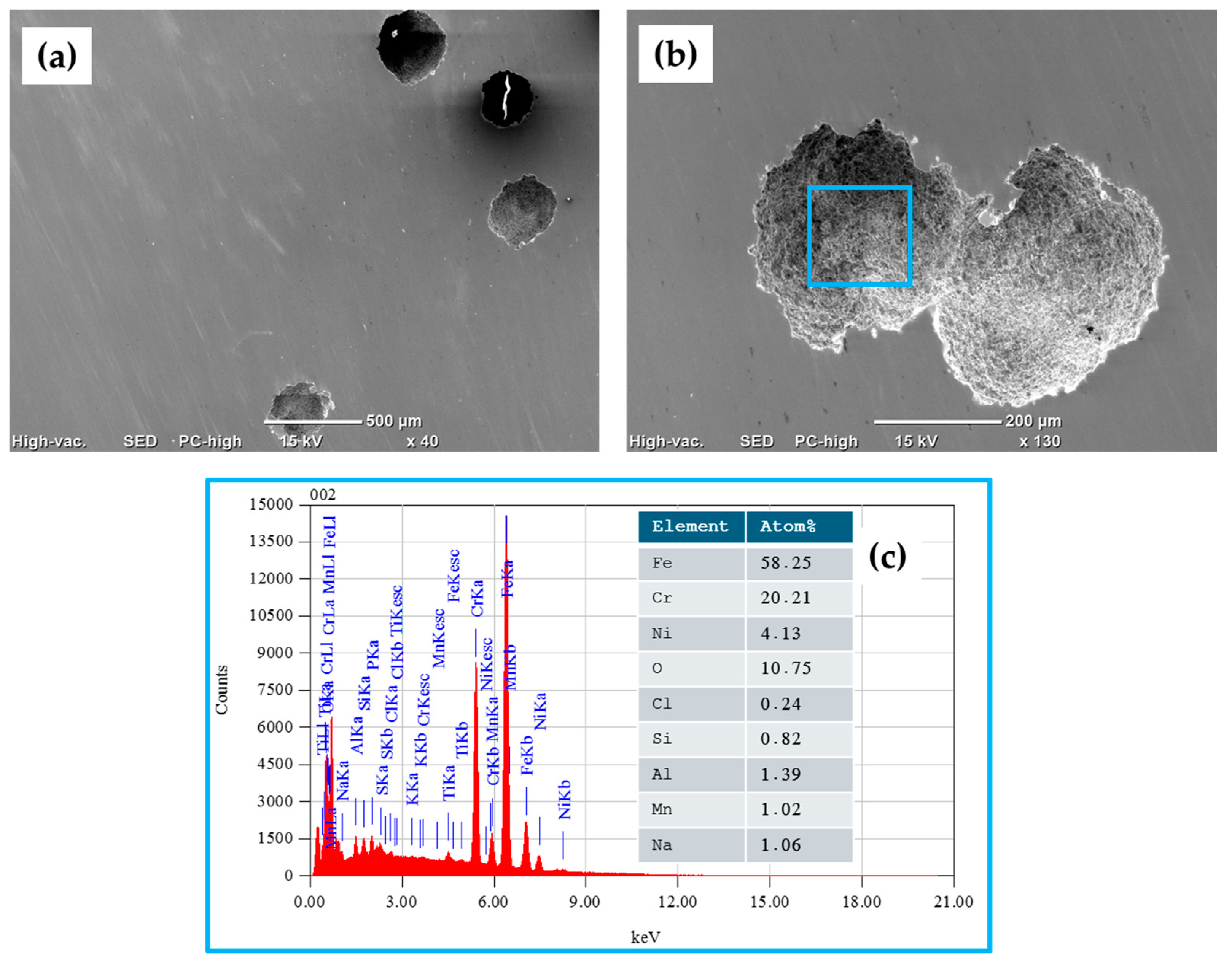
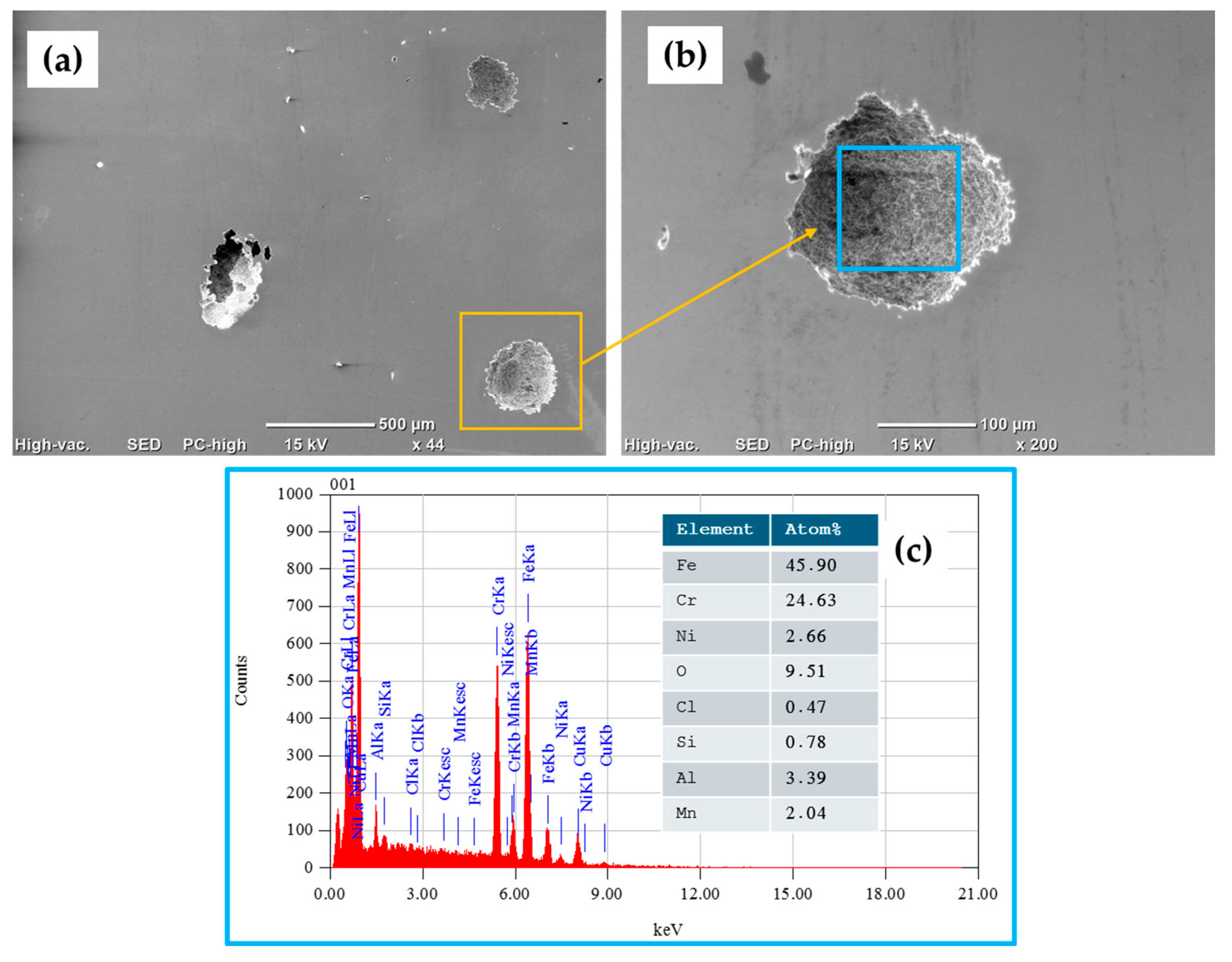

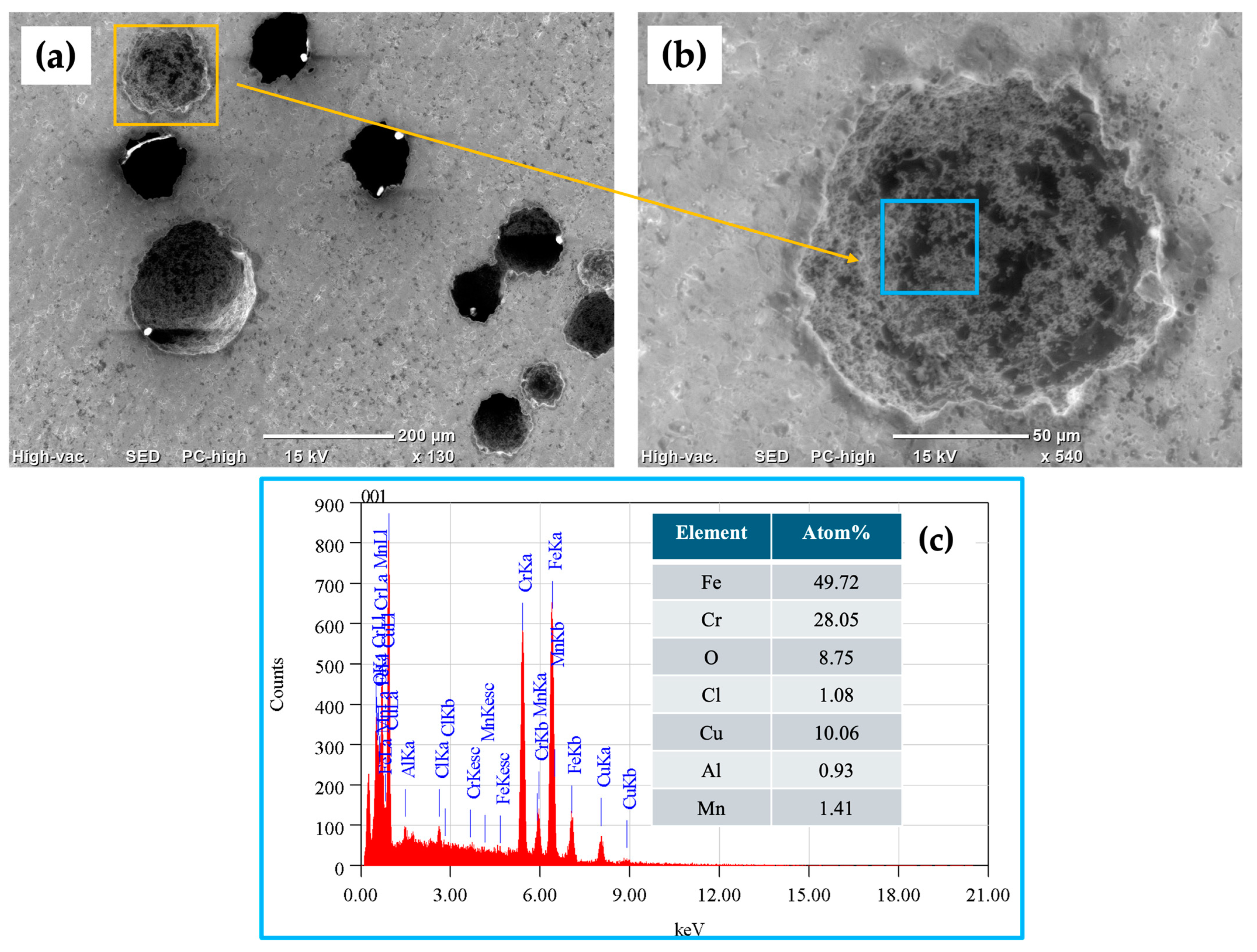
| Material | Elements (wt.%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Ni | Mo | Mn | Cu | Ti | N | Nb | W | V | Al | Fe | |
| 17-7PH | 0.09 | 16.64 | 7.17 | 0.19 | 0.88 | 0.24 | 0.10 | 0.10 | 0.034 | 0.034 | 0.106 | 1.1 | Balance |
| 410 SS | 0.15 | 13.5 | 0.75 | -- | 0.5 | -- | -- | -- | -- | -- | -- | 0.5 | Balance |
| Sample | Material | Temperature (°C) | Time (min) | Passivation Baths |
|---|---|---|---|---|
| A1 (Control) | 17-7PH | -- | -- | -- |
| B1 (Control) | 410 SS | -- | -- | -- |
| A2 | 17-7PH | 49 | 90 | Citric Acid C6H8O7 |
| B2 | 410 SS | 49 | 90 | |
| A3 | 17-7PH | 49 | 90 | Nitric Acid HNO3 |
| B3 | 410 SS | 49 | 90 |
| Sample | Cr | Mo | N | W | PREN |
|---|---|---|---|---|---|
| 17-7PH | 16.64 | 0.190 | 0.10 | 0.034 | 18.92 |
| 410SS | 13.5 | - | - | - | 13.5 |
| Samples: | 17-7 | 410 SS | ||||
|---|---|---|---|---|---|---|
| Element | Peak | Binding Energy (eV) | Citric | Nitric | Citric | Nitic |
| Fe 2p3/2 | Fe metal | 706.6 | 0.296 | 0.345 | 0.071 | 0.395 |
| FeO | 708.4 | 0.207 | 0.214 | 0.054 | 0.307 | |
| Fe3O4 | 709.8 | 0.497 | 0.441 | 0.573 | 0.298 | |
| FeOOH | 710.3 | - | - | 0.302 | - | |
| Cr 2p3/2 | Cr metal | 574.2 | 0.154 | 0.096 | 0.094 | 0.107 |
| Cr2O3 | 576.1 | 0.497 | 0.526 | 0.499 | 0.501 | |
| Cr (OH)3 | 577.3 | 0.349 | 0.378 | 0.407 | 0.392 | |
| Sample | Ecorr (Volts) | Epit (Volts) | Erp (Volts) | icorr (A/cm2) | σicorr (A/cm2) | ipass (A/cm2) | Passive Range (Volts) | CR (mm/year) | Hysteresis |
|---|---|---|---|---|---|---|---|---|---|
| Control A1 | −0.364 | 0.062 | −0.238 | 1.146 × 10−6 | ±4.137 × 10−3 | 1.023 × 10−6 | 0.323 | 1.325 × 10−2 | Positive |
| A2 | −0.341 | 0.311 | −0.276 | 1.8638 × 10−7 | ±4.292 × 10−3 | 4.4791 × 10−7 | 0.450 | 2.1345 × 10−3 | Positive |
| A3 | −0.420 | 0.133 | −0.263 | 2.5118 × 10−7 | ±4.184 × 10−3 | 1.1230 × 10−6 | 0.427 | 2.9131 × 10−3 | Positive |
| Control B1 | −0.380 | −0.170 | −0.440 | 1.113 × 10−6 | ±2.650 × 10−3 | 2.1682 × 10−6 | 0.492 | 1.2908 × 10−2 | Positive |
| B2 | −0.687 | −0.154 | −0.531 | 1.1945 × 10−6 | ±2.361 × 10−3 | 5.7451 × 10−6 | 0.646 | 1.3854 × 10−2 | Positive |
| B3 | −0.858 | −0.183 | −0.468 | 2.5971 × 10−6 | ±2.636 × 10−3 | 1.5502 × 10−5 | 0.857 | 3.0121 × 10−2 | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Aparicio, B.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Goldsberry, R.; Castaneda, H. Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution. Materials 2024, 17, 4060. https://doi.org/10.3390/ma17164060
Martínez-Aparicio B, Gaona-Tiburcio C, Almeraya-Calderon F, Goldsberry R, Castaneda H. Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution. Materials. 2024; 17(16):4060. https://doi.org/10.3390/ma17164060
Chicago/Turabian StyleMartínez-Aparicio, Brisa, Citlalli Gaona-Tiburcio, Facundo Almeraya-Calderon, Reece Goldsberry, and Homero Castaneda. 2024. "Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution" Materials 17, no. 16: 4060. https://doi.org/10.3390/ma17164060
APA StyleMartínez-Aparicio, B., Gaona-Tiburcio, C., Almeraya-Calderon, F., Goldsberry, R., & Castaneda, H. (2024). Evaluation of Passive Films on 17-7PH and 410 Stainless Steel Exposed to NaCl Solution. Materials, 17(16), 4060. https://doi.org/10.3390/ma17164060










