Polyurethane Foam and Algae-Based Activated Carbon Biocomposites for Oil Spill Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocomposite Synthesis
2.3. Characterization of Samples
- water: density of water;
- dapparent: apparent density of the sample.
- dair: density of air;
- doil: density of oil;
- dreal: real density of the sample;
- A: corrective factor taking into account the Archimedes’ buoyancy force on the setup.
2.4. Kinetics Study
3. Results and Discussion
3.1. Characterization of Blank Foam and Biocomposite
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Thermogravimetric Analysis TGA
3.1.3. Density
3.2. Adsorption Isotherms and Kinetic Study of Gasoil
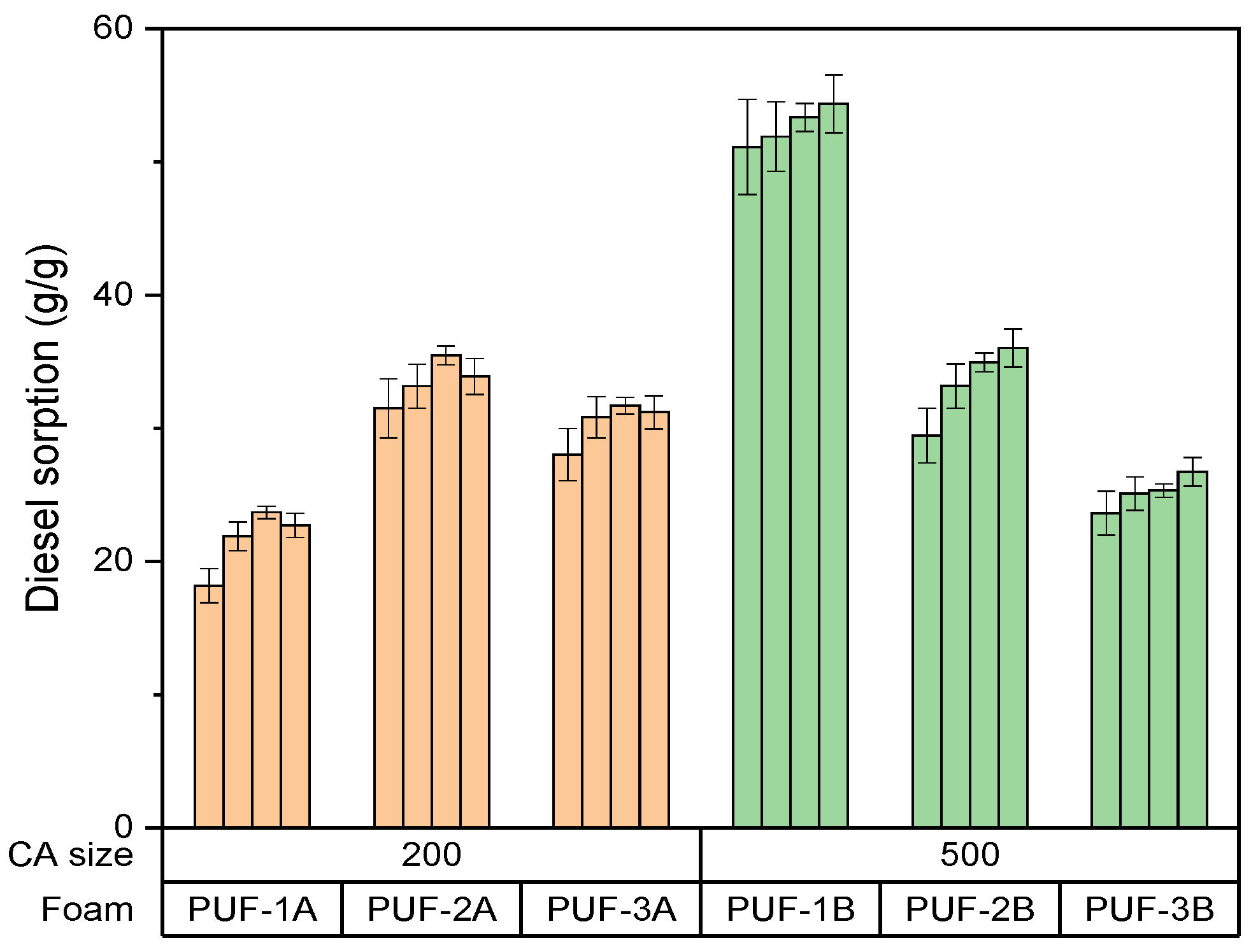
3.2.1. Parameter Effects on Gasoil Adsorption Capacity
- Effect of algae activated carbon content in foam on gasoil adsorption capacity
- Effect of particle diameter of algae activated carbon used in foam on gasoil adsorption capacity
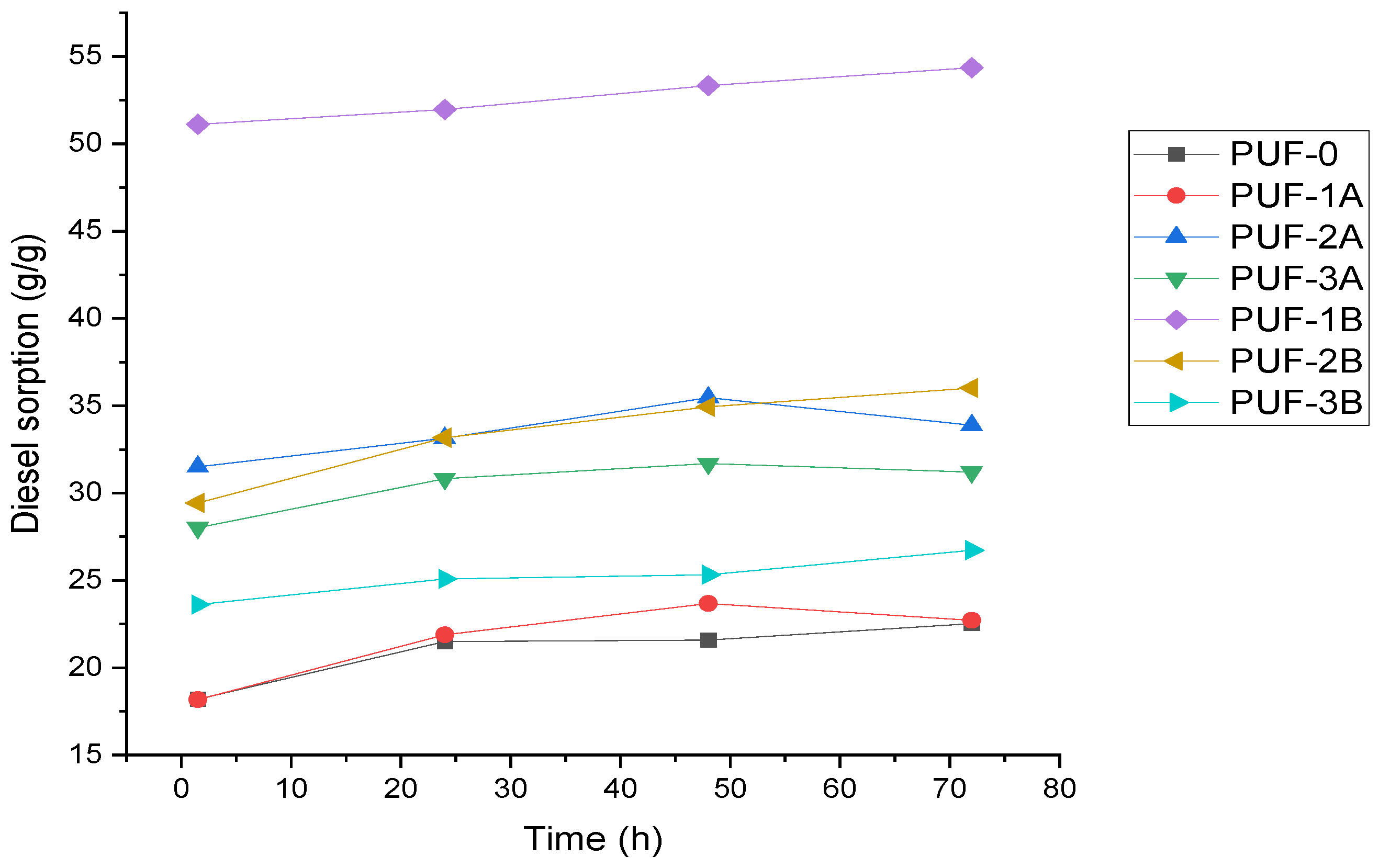
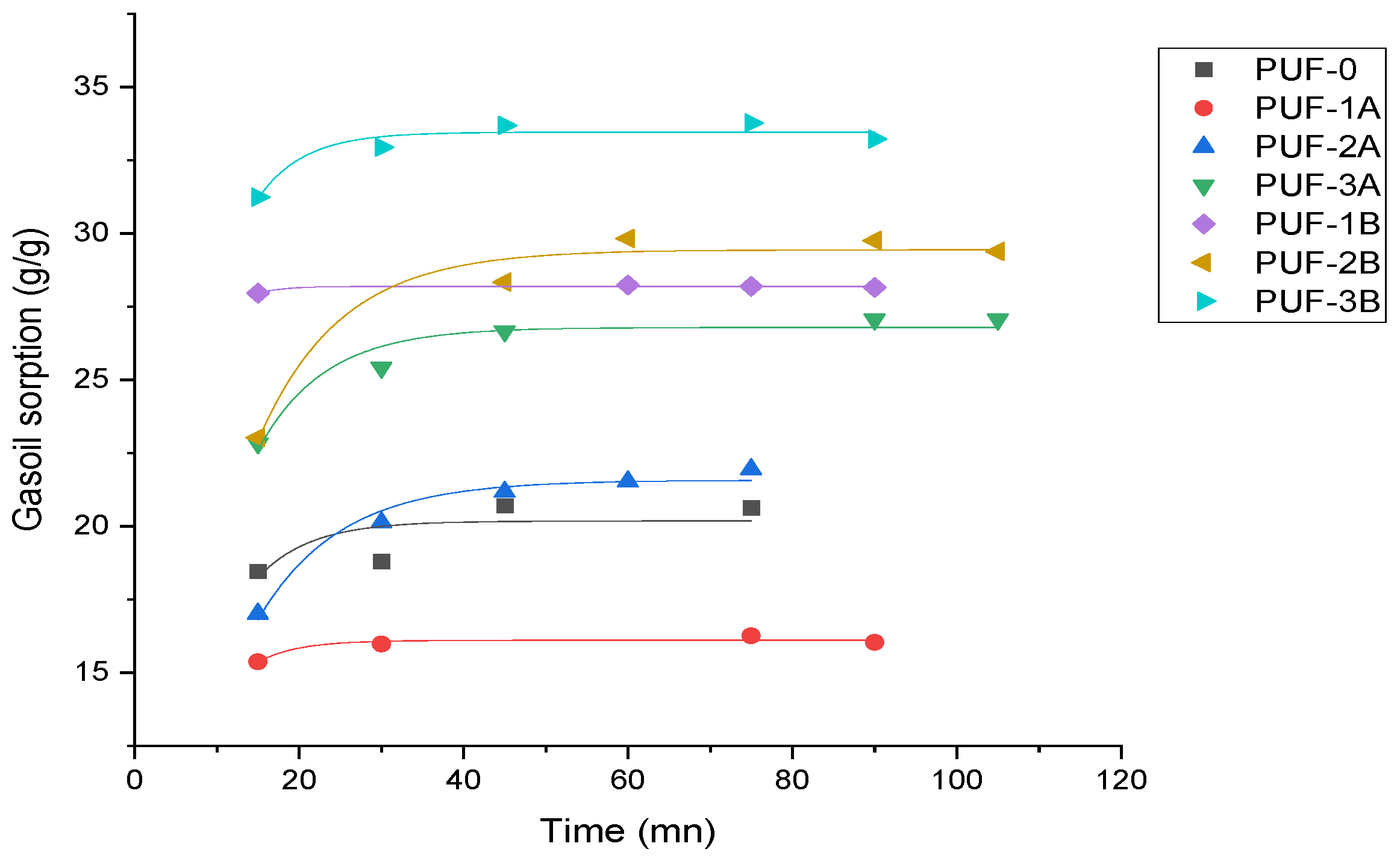
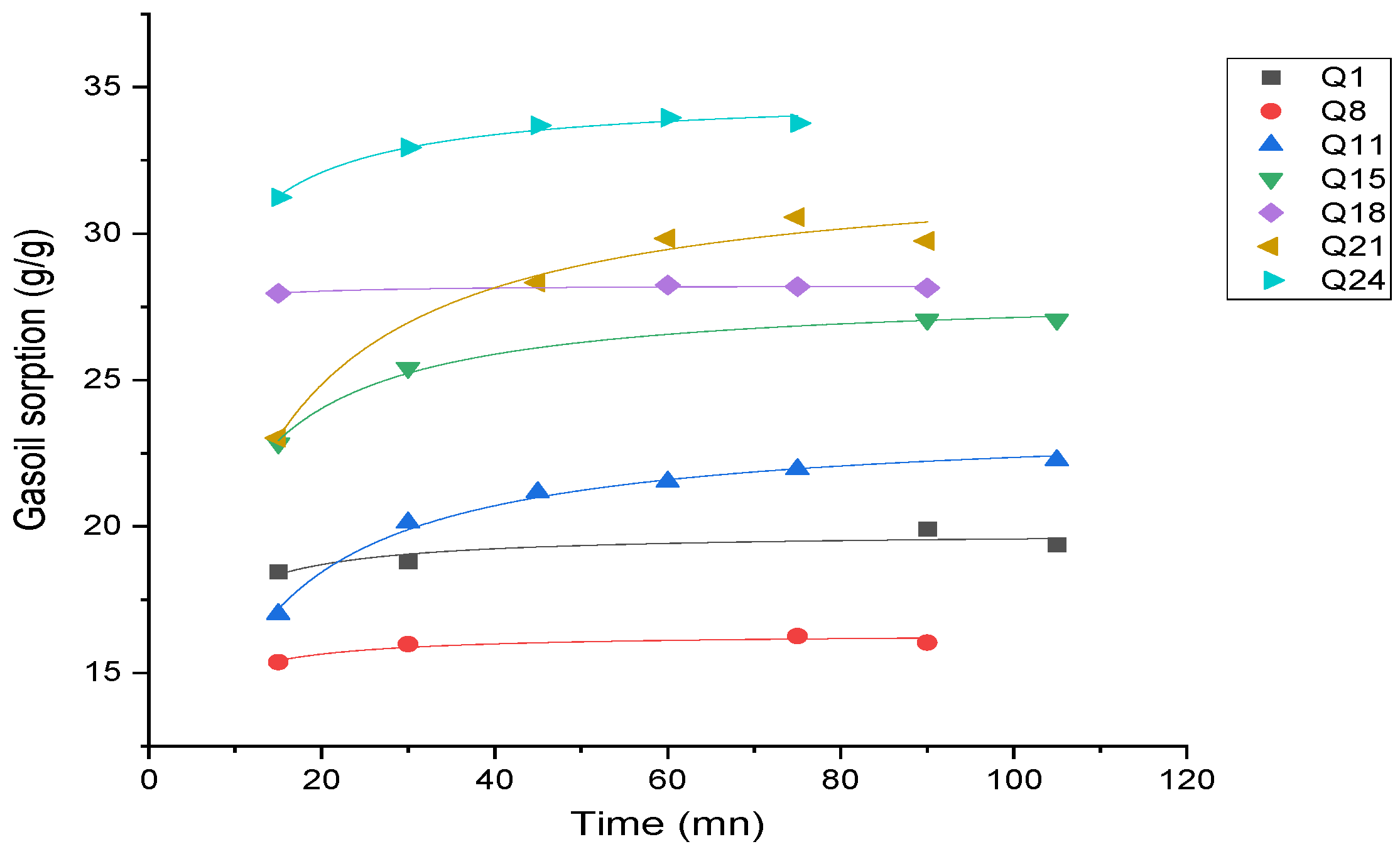
- Effect of time on gasoil adsorption capacity
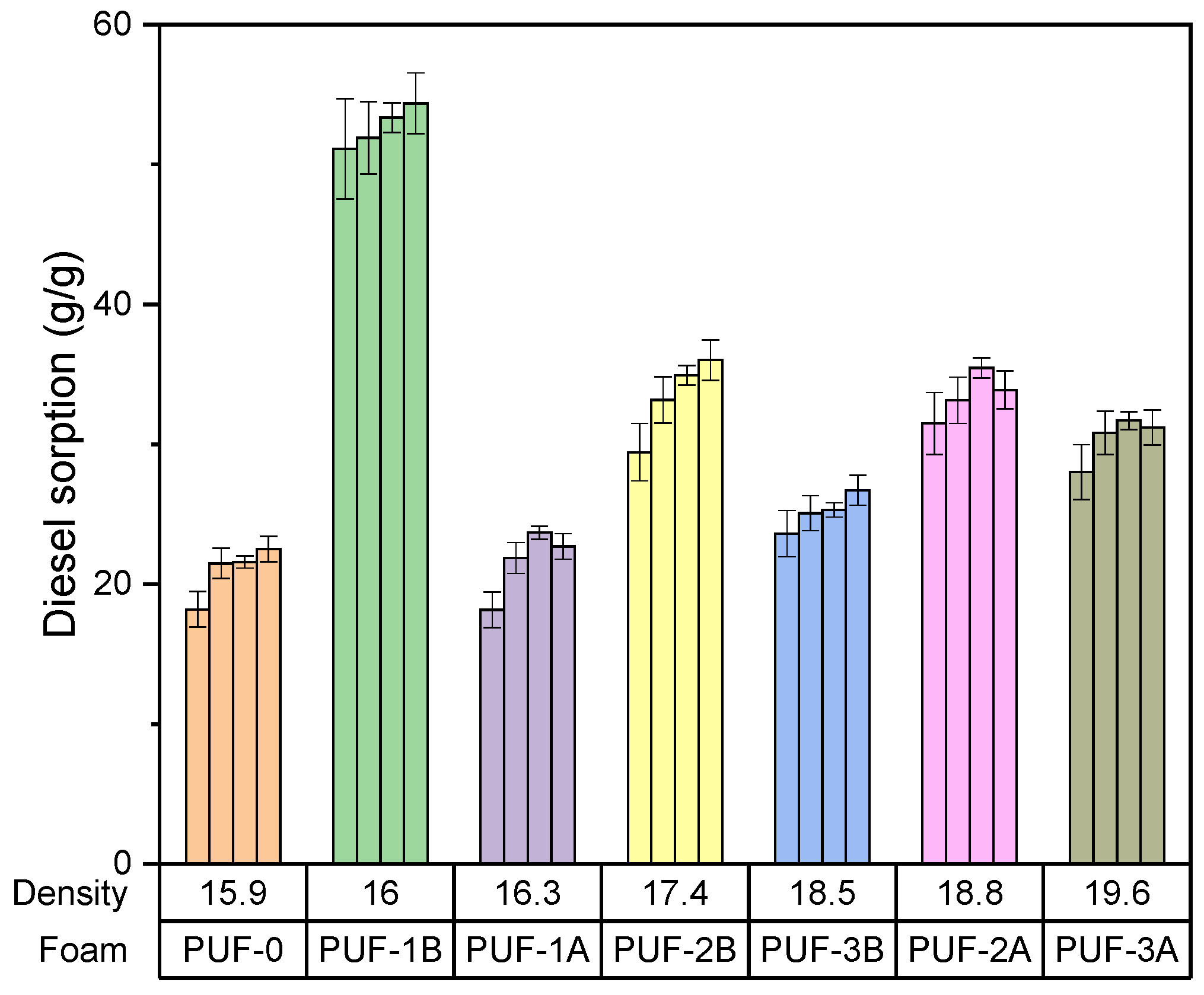
- Effect of density of algae activated carbon–foam composite on gasoil adsorption capacity
- -
- PUF1B (1.14 mass% of 500 µm algae carbon) shows the highest initial gasoil sorption capacity at 50 g/g, indicating the impact of larger particle size on adsorption efficiency.
- -
- PUF1A (1.14 mass% of 200 µm algae carbon) shows a sorption capacity of around 25 g/g, significantly lower than PUF1B.
- -
- PUF2B (3.34 mass% of 500 µm algae carbon) has a sorption capacity of around 45 g/g, similar to PUF1B but slightly lower.
- -
- PUF3B (4.41 mass% g of 500 µm algae carbon) has a gasoil sorption capacity of around 40 g/g, slightly lower than PUF2B.
- -
- For short-term adsorption, PUF1B (1.14 mass% of 500 µm algae carbon) is most effective, with the highest initial sorption capacity of 50 g/g.
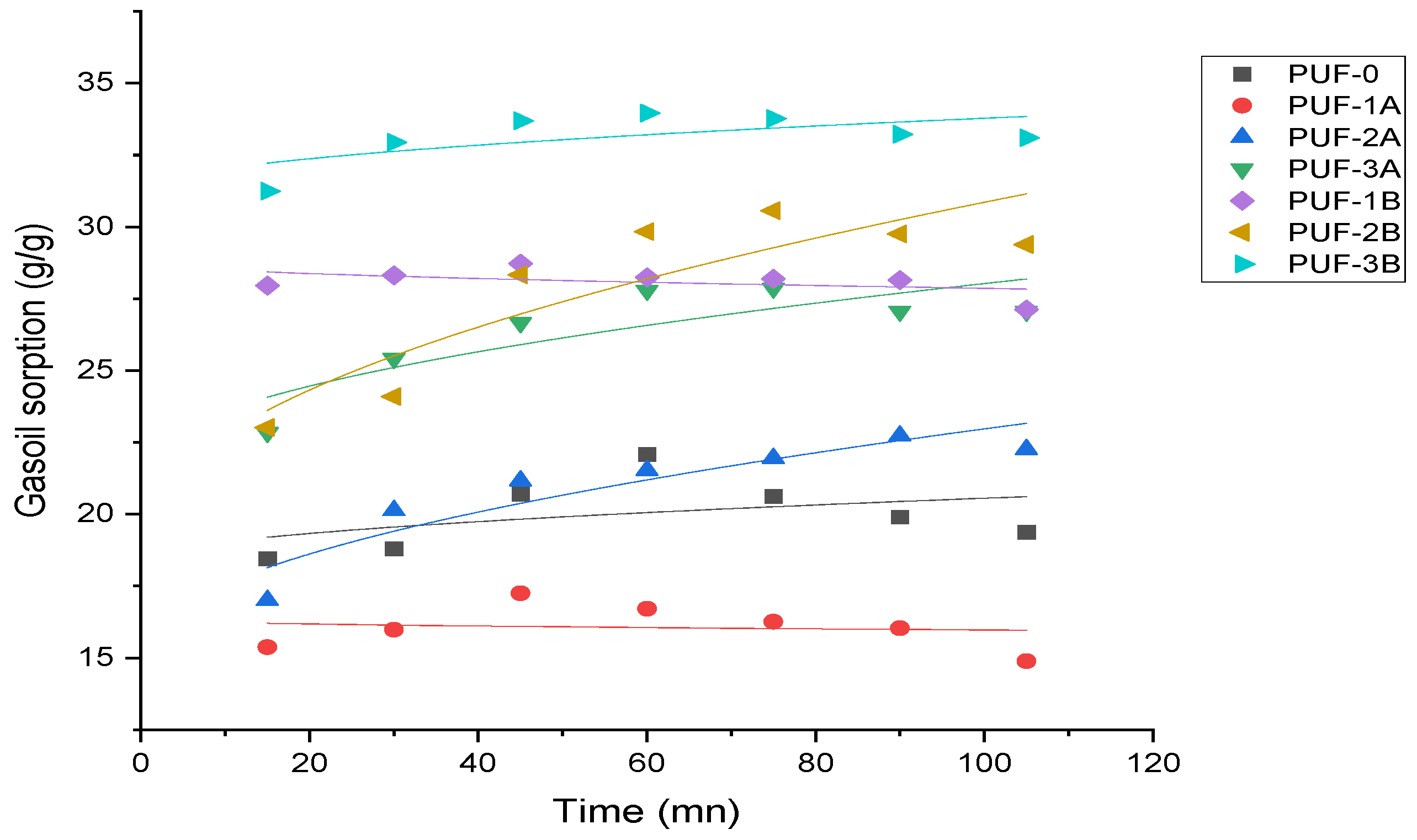
3.2.2. Kinetic Study of Gasoil
- Pseudo-first-order model
- Pseudo-second-order model
- Intraparticle diffusion model
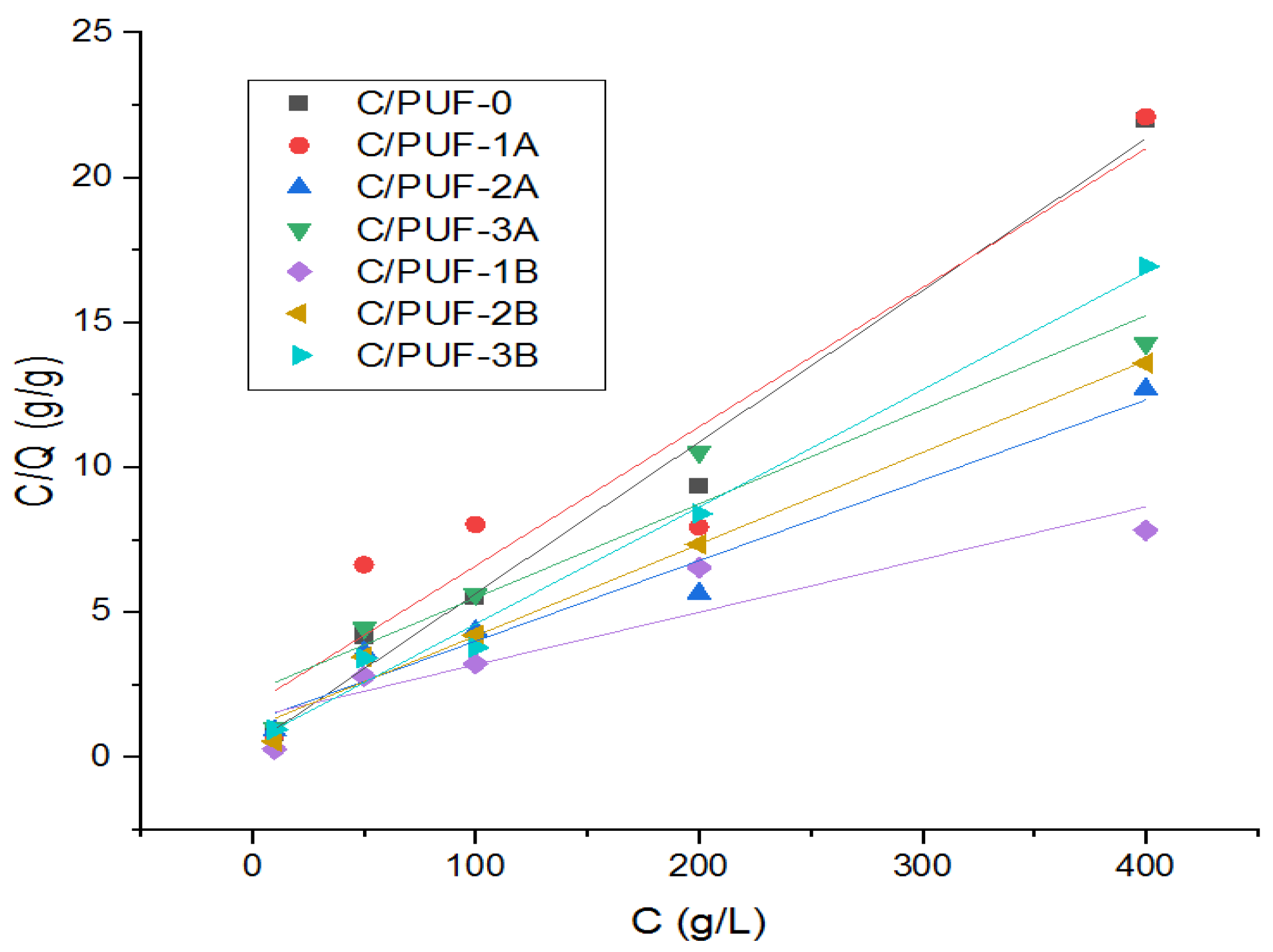
3.2.3. Adsorption Isotherm
- Langmuir isotherm
- Freundlich isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingston, P.F. Long-term environmental impact of oil spills. Spill Sci. Technol. Bull. 2002, 7, 53–61. [Google Scholar]
- Medjahdi, M.; Benderdouche, N.; Kherroub, D.E.; Reinert, L.; Baillis, D.; Bestani, B.; Duclaux, L. Mechanical analysis of powdered activated carbon-filled polyurethane foam composites for oil spill cleanup. Desalin. Water Treat. 2019, 140, 253–258. [Google Scholar]
- Boyd, J.N.; Scholz, D.; Walker, A.H. Effects of oil and chemically dispersed oil in the environment. Int. Oil Spill Conf. Proc. 2001, 1, 1213–1216. [Google Scholar]
- Walton, W.D.; Jason, N.H. In Situ Burning Oil Spill Workshop Proceedings: New Orleans, Louisiana, 2–4 November 1998; United States Minerals Management Service; National Institute of Standards and Technology (U.S.): Gaithersburg, MD, USA, 1999; 114p.
- Zhong, L.; Wu, J.; Wen, Y.; Yang, B.; Grifoll, M.; Hu, Y.; Zheng, P. Analysis of Factors Affecting the Effectiveness of Oil Spill Clean-Up: A Bayesian Network Approach. Sustainability 2023, 15, 4965. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.; Dai, Y.; Yue, X.; Rong, J.; Qiu, F.; Pan, J. Enhanced oils and organic solvents absorption by polyurethane foams composites modified with MnO2 nanowires. Chem. Eng. J. 2017, 309, 7–14. [Google Scholar]
- Zhou, S.; Hao, G.; Zhou, X.; Jiang, W.; Wang, T.; Zhang, N.; Yu, L. One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil-water separation. Chem. Eng. J. 2016, 302, 155–162. [Google Scholar]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar]
- Muhammad Imran, S.; Go, G.-M.; Hussain, M.; Al-Harthi, M.A. Multiwalled Carbon Nanotube-Coated Poly-Methyl Methacrylate Dispersed Thermoplastic Polyurethane Composites for Pressure-Sensitive Applications. Macromolecules 2022, 2, 211–224. [Google Scholar]
- Abdullah, M.M.S.; Al-Lohedan, H.A. Fabrication of Environmental-Friendly Magnetite Nanoparticle Surface Coatings for the Efficient Collection of Oil Spill. Nanomaterials 2021, 11, 3081. [Google Scholar] [CrossRef]
- Merillas, B.; Villafañe, F.; Rodríguez-Pérez, M.Á. Improving the Insulating Capacity of Polyurethane Foams through Polyurethane Aerogel Inclusion: From Insulation to Superinsulation. Nanomaterials 2022, 12, 2232. [Google Scholar] [CrossRef]
- Medjahdi, M.; Benderdouche, N.; Bestani, B.; Duclaux, L.; Reinert, L. Modeling of the sorption of crude oil on a polyurethane foam-powdered activated carbon composite. Desalin. Water Treat. 2016, 57, 22311–22320. [Google Scholar]
- Li, H.; Liu, L.; Yang, F. Oleophilic Polyurethane Foams for Oil Spill Cleanup. Procedia Environ. Sci. 2013, 18, 528–533. [Google Scholar]
- Fang, Z.; Huang, L.; Fu, J. Research Status of Graphene Polyurethane Composite Coating. Coatings 2022, 12, 264. [Google Scholar] [CrossRef]
- Medjahdi, M.; Mahida, B.; Benderdouche, N.; Mechab, B.; Bestani, B.; Reinert, L.; Duclaux, L.; Baillis, D. Development of a Hydrophobic Carbon Sponge Nanocomposite for Oil Spill Cleanup. Materials 2022, 15, 8389. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Yang, F. Hydrophobic modification of polyurethane foam for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 1648–1653. [Google Scholar] [PubMed]
- Tiwary, A.; Kumar, R.; Chohan, J.S. A review on characteristics of composite and advanced materials used for aerospace applications. Mater. Today Proc. 2022, 51, 865–870. [Google Scholar]
- Madaleno, L.; Pyrz, R.; Crosky, A.; Jensen, L.R.; Rauhe, J.C.M.; Dolomanova, V.; de Barros Timmons, A.M.M.V.; Cruz Pinto, J.J.; Norman, J. Processing and characterization of polyurethane nanocomposite foam reinforced with montmorillonite-carbon nanotube hybrids. Compos. Part A Appl. Sci. Manuf. 2013, 44, 1–7. [Google Scholar]
- Bukowczan, A.; Łukaszewska, I.; Pielichowski, K. Thermal degradation of non-isocyanate polyurethanes. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Ciecierska, E.; Jurczyk-Kowalska, M.; Bazarnik, P.; Kowalski, M.; Krauze, S.; Lewandowska, M. The influence of carbon fillers on the thermal properties of polyurethane foam. J. Therm. Anal. Calorim. 2016, 123, 283–291. [Google Scholar]
- Auguścik-Królikowska, M.; Ryszkowska, J.; Ambroziak, A.; Szczepkowski, L.; Oliwa, R.; Oleksy, M. The structure and properties of viscoelastic polyurethane foams with fillers from coffee grounds. Polimery 2020, 65, 708–718. [Google Scholar]
- Kherroub, D.E.; Bouhadjar, L.; Medjahdi, M. Development of novel conductive copolymer based on furan with improved solubility and thermal properties. J. Mol. Struct. 2021, 1225, 129174. [Google Scholar]
- Zhang, T.; Xiao, C.; Zhao, J.; Liu, X.; Huang, Y.; Ji, D. Graphite powder coated polyurethane sponge hollow tube as a highefficiency and cost-effective oil-removal materials for continuous oil collection from water surface. J. Appl. Polym. Sci. 2020, 137, 48921. [Google Scholar]
- Martins, L.S.; Zanini, N.C.; Maia, L.S.; Souza, A.G.; Barbosa, R.F.S.; Rosa, D.S.; Mulinari, D.R. Crude oil and S500 diesel removal from seawater by polyurethane composites reinforced with palm fiber residues. Chemosphere 2021, 267, 129288. [Google Scholar] [PubMed]
- Sarup, R.; Sharma, M.; Behl, K.; Kumar Avasthi, D.; Kumar, P.; Ojha, S.; Nigam, S.; Joshi, M. Fabrication of superhydrophobic polyurethane sponge coated with oil sorbent derived from textile sludge for oily wastewater remediation. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100675. [Google Scholar]
- Li, B.; Liu, X.; Zhang, X.; Zou, J.; Chaia, W.; Louc, Y. Rapid adsorption for oil using superhydrophobic and superoleophilic polyurethane sponge. J. Chem. Technol. Biotechnol. 2015, 90, 2106. [Google Scholar]
- De Nino, A.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and Fast Removal of Oils from Water Surfaces via Highly Oleophilic Polyurethane Composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef]
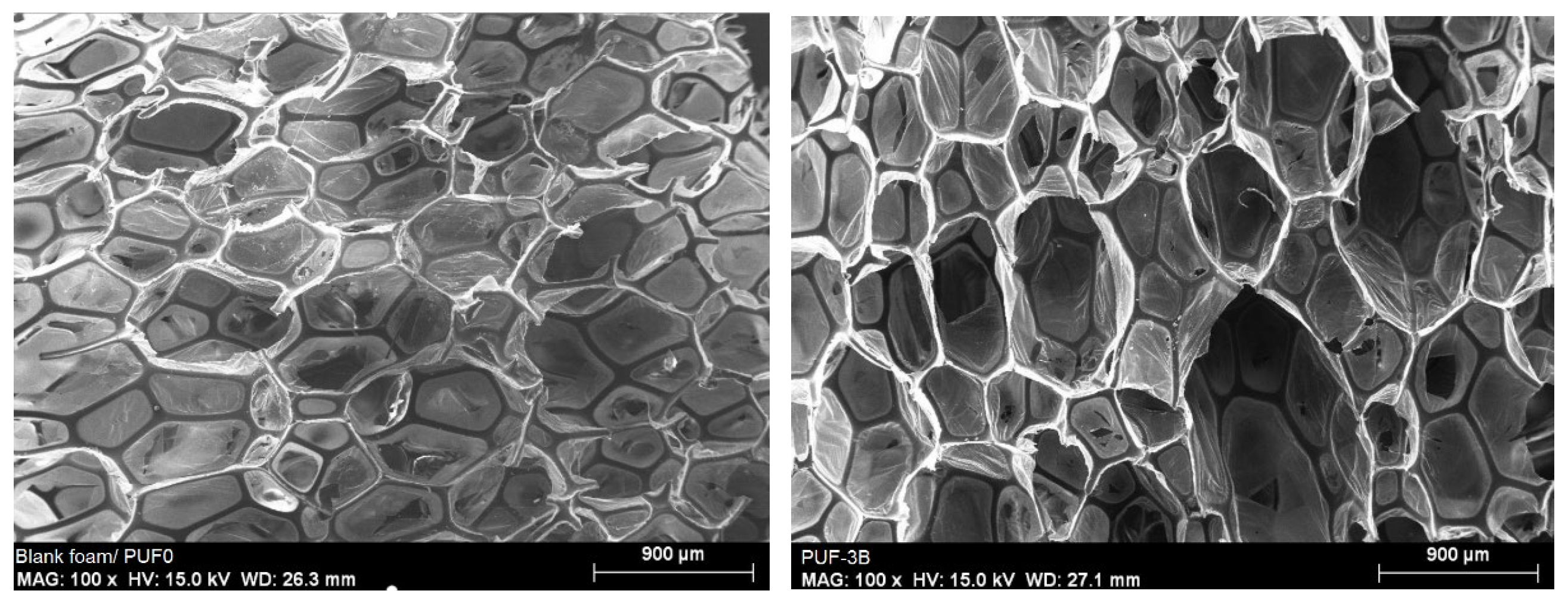




| Iodine index | 609.794 mg/g |
| Methylene blue index | 945.26 mg/g |
| moisture content | 1.972% |
| Decomposition Stage | First Stage | Second Stage | Final Residue | |||
|---|---|---|---|---|---|---|
| PUF0 | PUF3 | PUF0 | PUF3 | PUF0 | PUF3 | |
| Onset Temperature (°C) | ~310 °C | ~300 °C | ~390 °C | ~370 °C | 600 °C | 600 °C |
| Endset Temperature (°C) | ~390 °C | ~370 °C | ~450 °C | ~420 °C | / | / |
| Weight Loss (%) | ~30% | ~40% | ~50% | ~45% | / | / |
| Mass Remaining at Endset (%) | ~70% | ~60% | ~20% | ~15% | ~20% | ~10% |
| Material | Modification Type | Sorption Capacity (g/g) | Reference |
|---|---|---|---|
| PU sponge hollow tube–graphite | Coating | 20 | [23] |
| PU–Australian palm residues | added during the polyol and isocyanate mixing | 28.9 | [24] |
| PU–textile sludge and PDM | Dip coating method | 26.88 | [25] |
| PU–Lauryl methacrylate | grafting | 37.64 | [16] |
| PU-ZnO and palmitic acid | coating | 33 | [26] |
| PU–microparticles of silica | coating | 20 | [27] |
| PU–activated carbon | coating | 29.5 | [27] |
| PU–algea activated carbon | added during the polyol and isocyanate mixing | 53 after 72 h | This study |
| Equations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PUF | qe (±0.2) | k (±0.01) | R2 | qe (±0.2) | k2 (±0.18) | R2 | Kdiff (±0.03) | C (±31.23) | R2 |
| 0 | 20.17 | 0.16 | 0.532 | 19.80 | 0.042 | 0.798 | 0.221 | 18.341 | 0.160 |
| 1A | 16.10 | 0.20 | 0.912 | 16.35 | 0.067 | 0.826 | 0.037 | 16.350 | 0.011 |
| 2A | 21.57 | 0.10 | 0.976 | 23.61 | 0.007 | 0.984 | 0.787 | 21.572 | 0.848 |
| 3A | 26.79 | 0.12 | 0.944 | 28.04 | 0.010 | 0.994 | 0.645 | 21.572 | 0.686 |
| 1B | 28.19 | 0.31 | 0.994 | 25.25 | 0.223 | 0.987 | 0.093 | 28.792 | 0.185 |
| 2B | 29.44 | 0.10 | 0.971 | 32.47 | 0.005 | 0.967 | 1.181 | 29.108 | 0.782 |
| 3B | 33.45 | 0.17 | 0.889 | 34.77 | 0.017 | 0.970 | 0.254 | 31.230 | 0.397 |
| Equations | ||||||
|---|---|---|---|---|---|---|
| 1/QmKl | 1/Qm | R2 | lnKf | 1/n | R2 | |
| PUF-0 | 0.420 ± 0.1 | 0.052 ± 0.03 | 0.985 | 2.118 ± 0.2 | 0.149 ± 0.01 | 0.647 |
| PUF-1A | 1.797 ± 11.79 | 0.049 ± 0.03 | 0.908 | 1.877 ± 0.1 | 0.172 ± 0.10 | 0.294 |
| PUF-2A | 1.225 ± 0.5 | 0.027 ± 0.04 | 0.905 | 1.544 ± 0.3 | 0.335 ± 0.10 | 0.870 |
| PUF-3A | 0.293 ± 0.1 | 0.093 ± 0.01 | 0.962 | 1.515 ± 0.2 | 0.283 ± 0.10 | 0.883 |
| PUF-1B | 1.362 ± 11.36 | 0.018 ± 0.01 | 0.875 | 2.054 ± 0.6 | 0.089 ± 0.10 | 0.109 |
| PUF-2B | 1.020 ± 11.02 | 0.016 ± 0.03 | 0.761 | 2.356 ± 0.3 | 0.15 ± 0.10 | 0.567 |
| PUF-3B | 0.544 ± 0.1 | 0.146 ± 0.02 | 0.852 | 1.829 ± 0.3 | 0.248 ± 0.10 | 0.518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baidar, L.A.; Medjahdi, M.; Mahida, B.; Mechab, B.; Baillis, D. Polyurethane Foam and Algae-Based Activated Carbon Biocomposites for Oil Spill Remediation. Materials 2024, 17, 4137. https://doi.org/10.3390/ma17164137
Baidar LA, Medjahdi M, Mahida B, Mechab B, Baillis D. Polyurethane Foam and Algae-Based Activated Carbon Biocomposites for Oil Spill Remediation. Materials. 2024; 17(16):4137. https://doi.org/10.3390/ma17164137
Chicago/Turabian StyleBaidar, Lokmane Abdelkaddous, Malika Medjahdi, Badra Mahida, Belaid Mechab, and Dominique Baillis. 2024. "Polyurethane Foam and Algae-Based Activated Carbon Biocomposites for Oil Spill Remediation" Materials 17, no. 16: 4137. https://doi.org/10.3390/ma17164137





