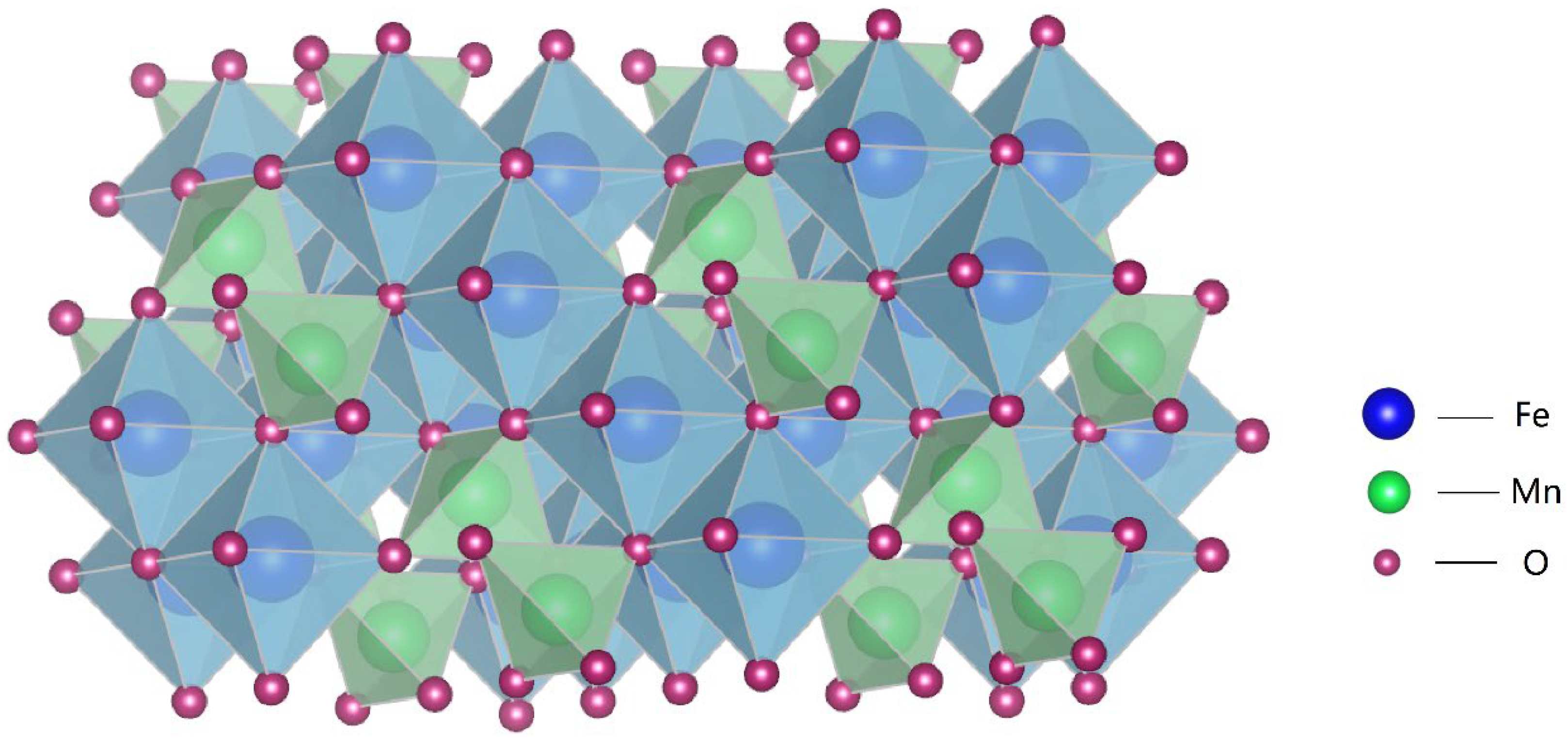
Synthesis of MnFe2O4 Spinel on Rusted Q235 Steel Surface and Its Corrosion Resistance Properties
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Electrochemical Measurements
2.2.1. Electrode Preparation
2.2.2. Measuring Condition
3. Results and Discussion
3.1. The Impact of Electrolysis Time on the Corrosion Resistance of MnFe2O4 Spinel Coatings
3.1.1. XRD Analysis of Samples with Different Electrolysis Times
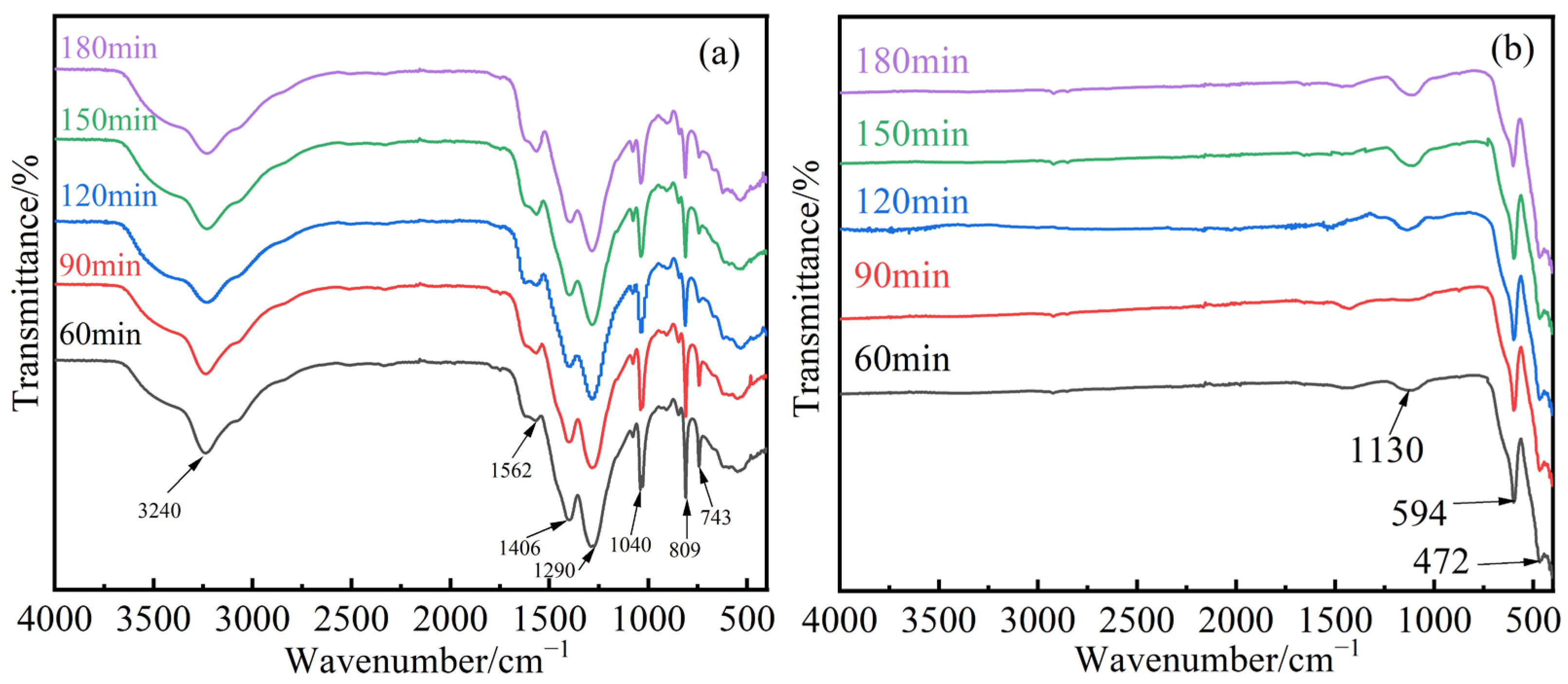
3.1.2. FT-IR Analysis of Samples with Different Electrolysis Times
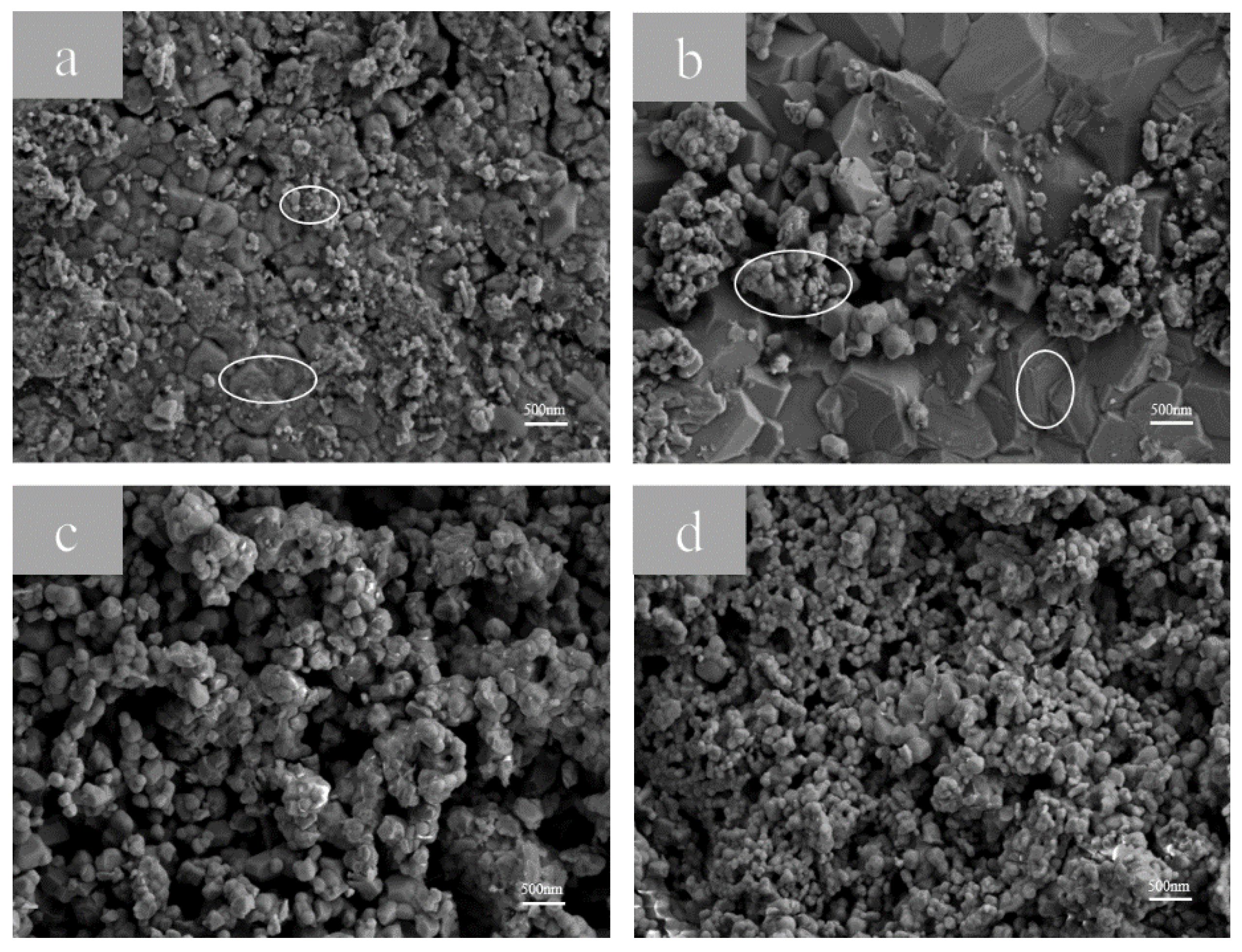
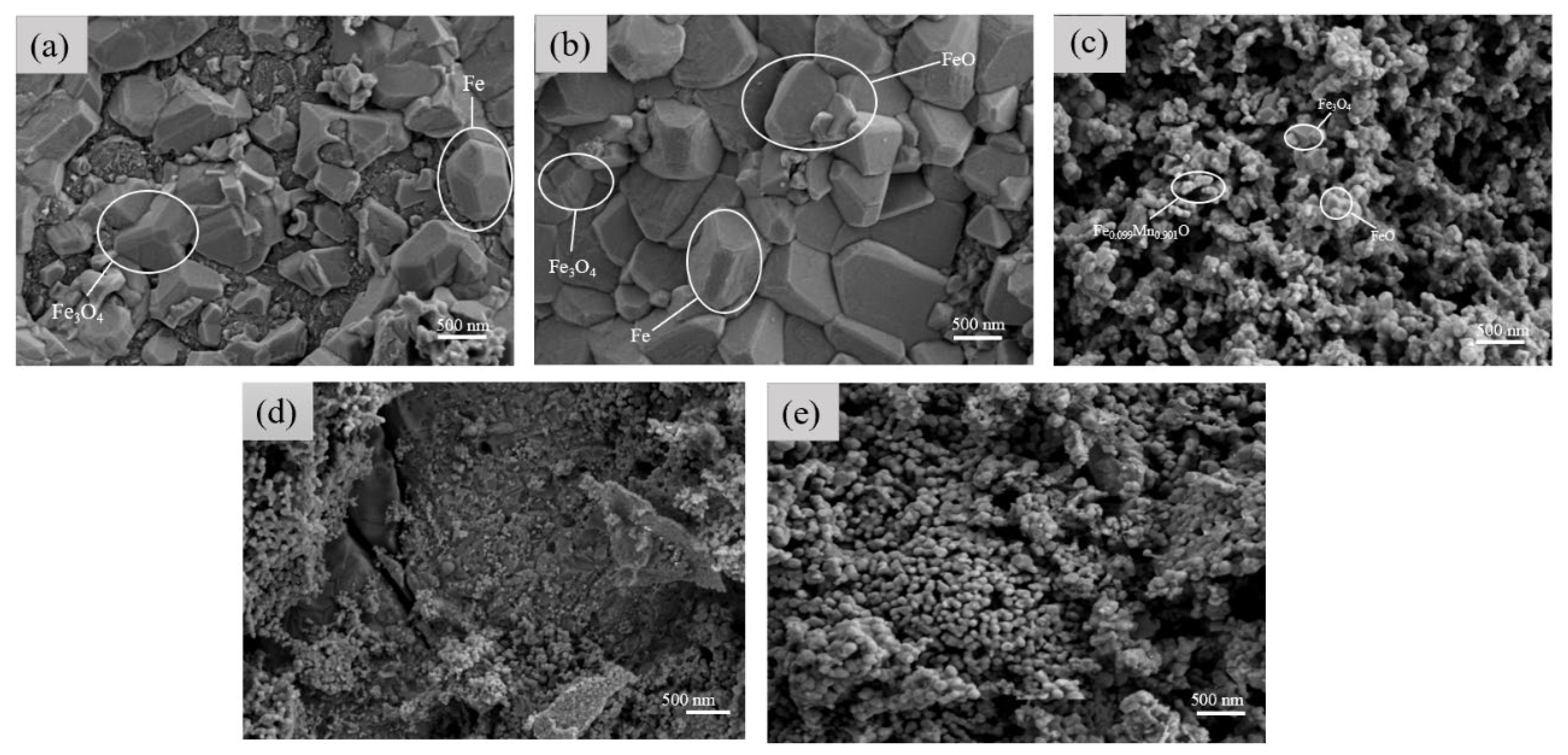
3.1.3. Surface Morphology Analysis of Samples with Different Electrolysis Times
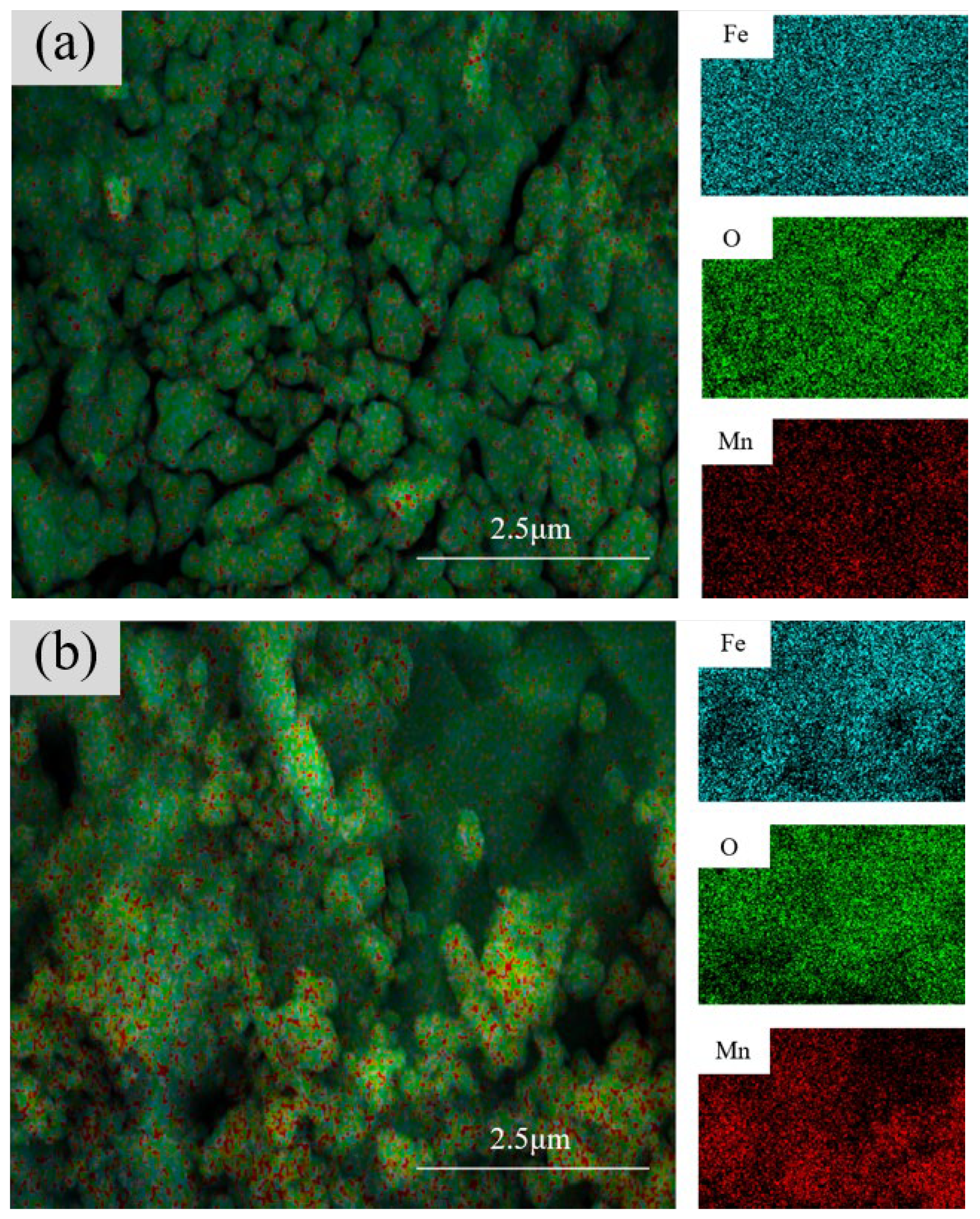
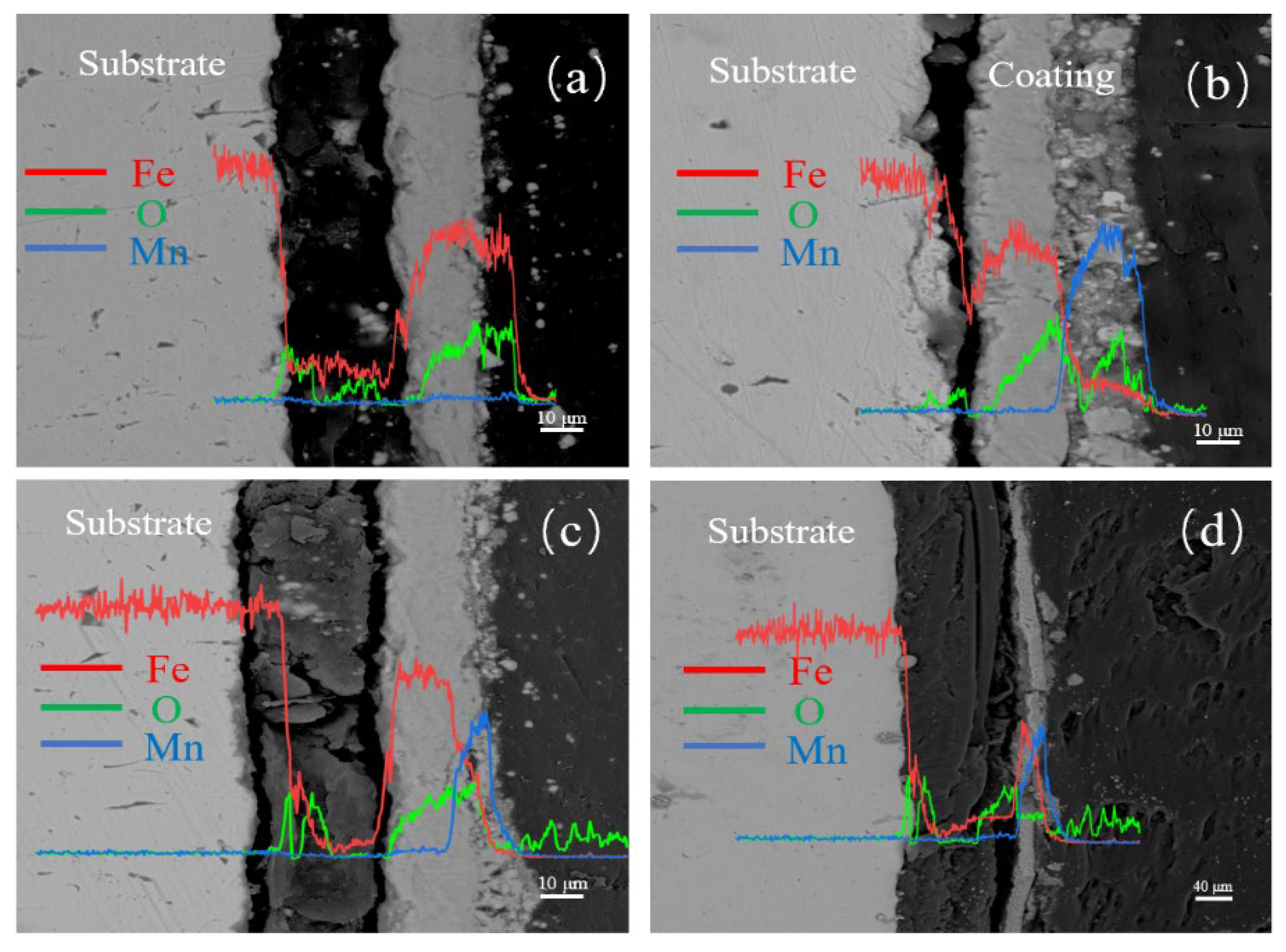
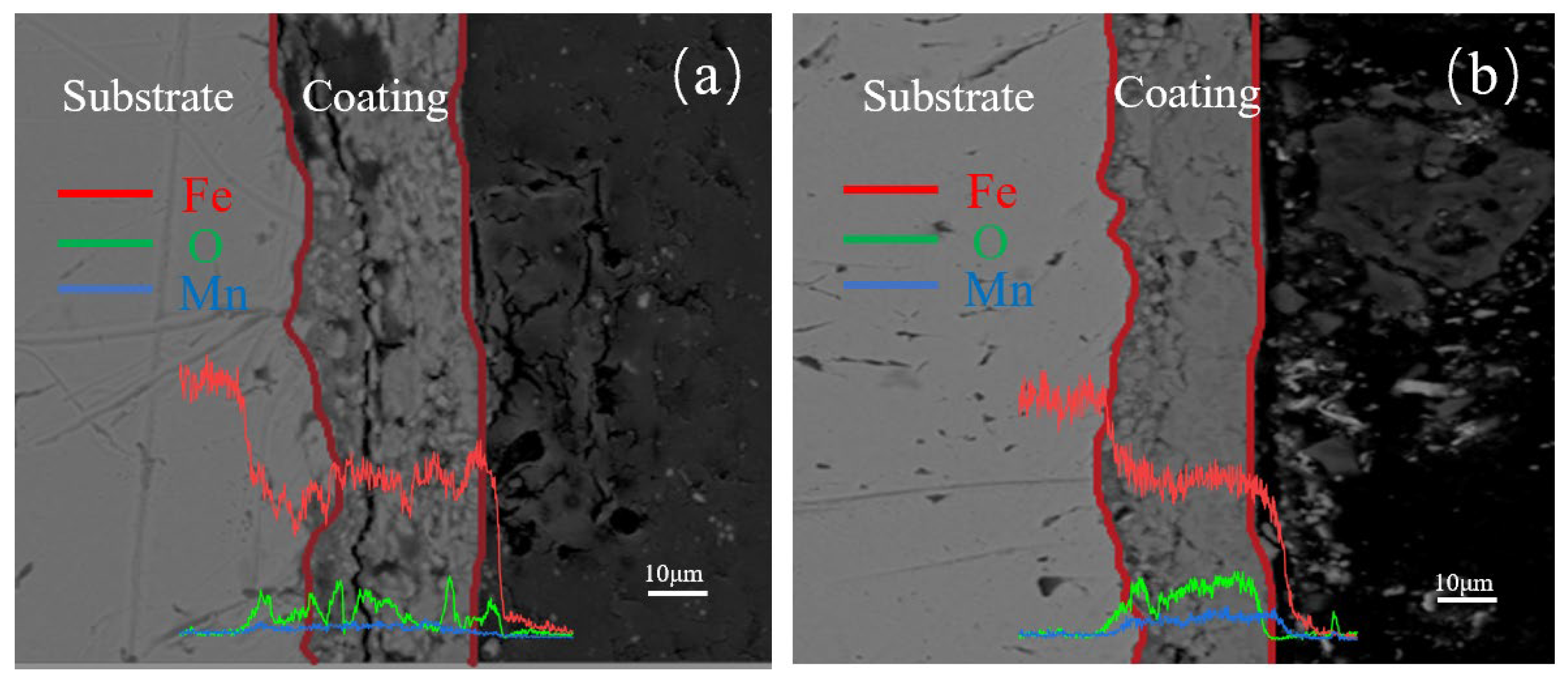
3.1.4. Cross-Sectional Morphology Analysis of Samples with Different Electrolysis Times
3.2. The Effect of Mn2+ Concentration on the Corrosion Resistance of MnFe2O4 Spinel Coatings
3.2.1. XRD Analysis of Samples with Different Mn2+ Concentrations
3.2.2. FT-IR Analysis of Samples with Different Mn2+ Concentrations
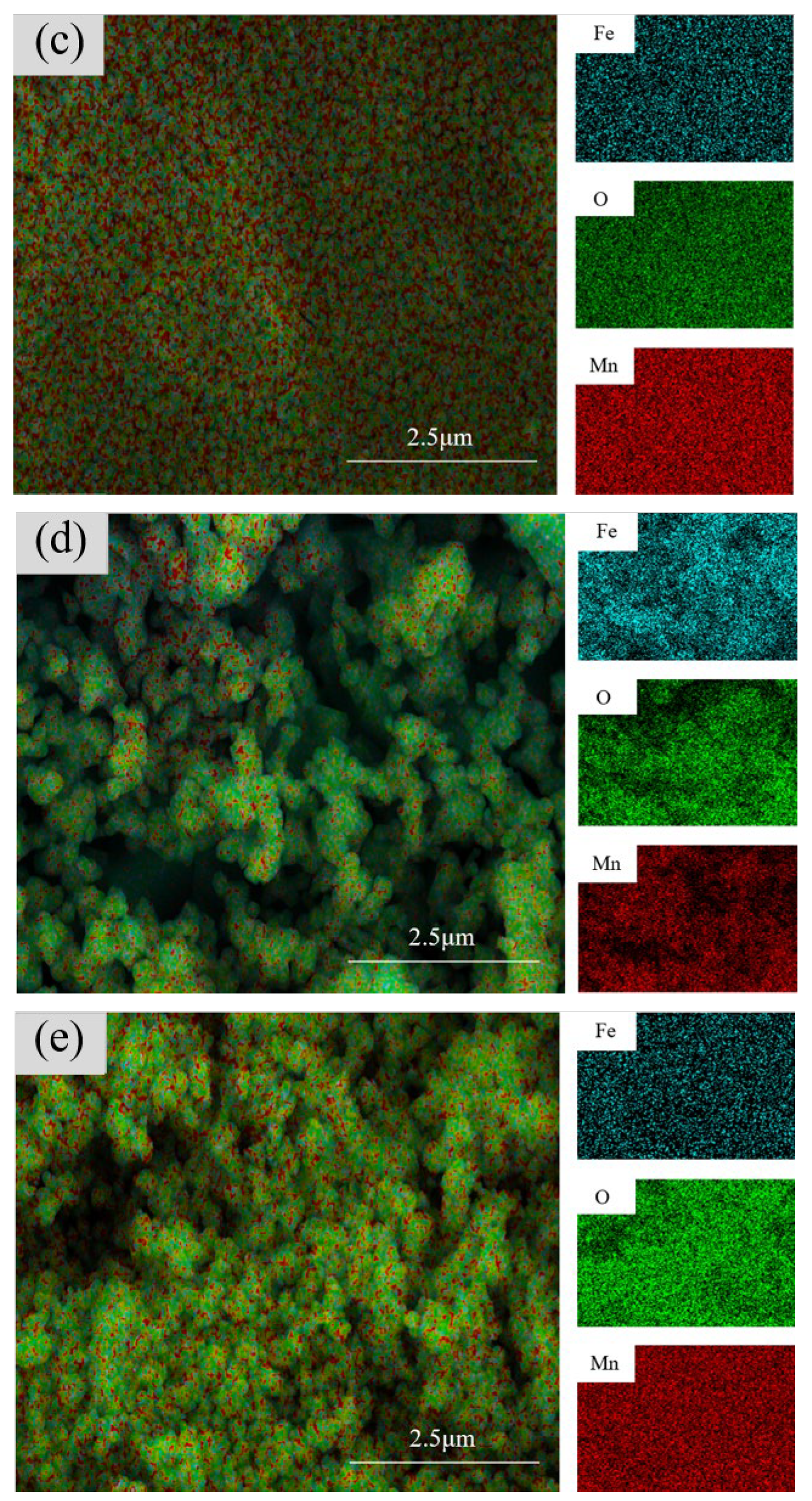
3.2.3. Surface Morphology Analysis of Samples with Different Mn2+ Concentrations
3.2.4. Cross-Sectional Morphology Analysis of Samples with Different Mn2+ Concentrations
3.3. Corrosion Resistance Testing of MnFe2O4 Spinel Coatings
3.3.1. Potentiodynamic Polarization Measurement
3.3.2. Electrochemical Impedance Spectroscopy Analysis
4. Conclusions
- (1)
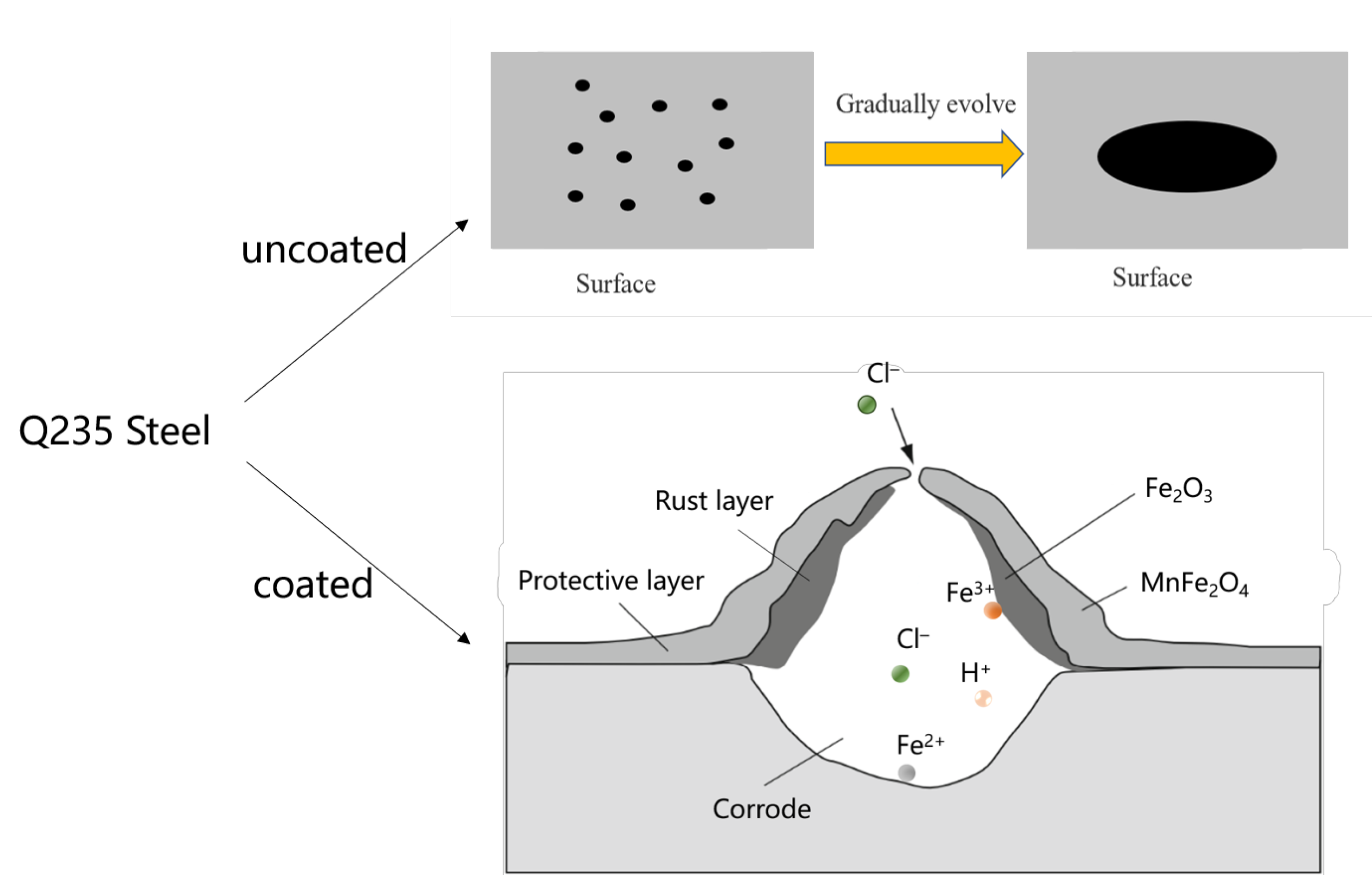
- Q235 steel undergoes electrolytic rust enhancement to form a stable rust layer. The precursor can then be prepared in situ using the sol-gel method, and MnFe2O4 spinel corrosion-resistant coatings can be fabricated through high-temperature in situ processing.
- (2)
- Based on the XRD, FT-IR, morphological patterns of the samples, and cross-sectional morphology characterizations, the optimal conditions for precursor preparation are determined to be a Mn2+ concentration of 0.1 mol/L and an electrolysis time of 2 h.
- (3)
- The results of the polarization curves indicate that MnFe2O4 spinel corrosion-resistant coatings exhibit higher corrosion resistance in low mass fraction NaCl solutions. Notably, in a 1 wt.% NaCl solution, the corrosion current density of the MnFe2O4 spinel coating is 6.79 µA/cm2, which is significantly lower than the corrosion current density of Q235 steel under the same conditions at 24.8 µA/cm2. This demonstrates that the MnFe2O4 spinel coating enhances the corrosion resistance of Q235 steel.
- (4)
- The electrochemical impedance spectroscopy (EIS) results indicate that the MnFe2O4 spinel corrosion-resistant coating enhances corrosion resistance by inhibiting the charge transfer process. Compared to uncoated Q235 steel, the MnFe2O4 spinel coating significantly protects Q235 steel in corrosion solutions with wt.% NaCl = 1, 2, 3, 3.5, 4, and 5, exhibiting the strongest corrosion resistance at wt.% NaCl = 1.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IMPACT. The International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Kirchgeorg, T.; Weinberg, I.; Hörnig, M.; Baier, R.; Schmid, M.J.; Brockmeyer, B. Emissions from Corrosion Protection Systems of Offshore Wind Farms: Evaluation of the Potential Impact on the Marine Environment. Mar. Pollut. Bull. 2018, 136, 257–268. [Google Scholar] [CrossRef]
- Oldfield, J.W.; Todd, B. Environmental Aspects of Corrosion in MSF and RO Desalination Plants. Desalination 1997, 108, 27–36. [Google Scholar] [CrossRef]
- Sadeghi Meresht, E.; Shahrabi Farahani, T.; Neshati, J. Failure Analysis of Stress Corrosion Cracking Occurred in a Gas Transmission Steel Pipeline. Eng. Fail. Anal. 2011, 18, 963–970. [Google Scholar] [CrossRef]
- Mandke, J.S. Corrosion Causes Most Pipeline Failures in Gulf of Mexico. Oil Gas J. 1990, 88, 44. [Google Scholar]
- Panteleeva, M. Effective Modern Methods of Protecting Metal Road Structures from Corrosion. IOP Conf. Ser. Earth Environ. Sci. 2017, 90, 012119. [Google Scholar] [CrossRef]
- Aslam, J.; Aslam, R.; Lone, I.H.; Radwan, N.R.E.; Mobin, M.; Aslam, A.; Parveen, M.; Al-Freedi, A.A.; Alzulaibani, A.A. Inhibitory Effect of 2-Nitroacridone on Corrosion of Low Carbon Steel in 1 M HCl Solution: An Experimental and Theoretical Approach. J. Mater. Res. Technol. 2020, 9, 4061–4075. [Google Scholar] [CrossRef]
- Azam, M.A.; Sukarti, S.; Zaimi, M. Corrosion Behavior of API-5L-X42 Petroleum/Natural Gas Pipeline Steel in South China Sea and Strait of Melaka Seawaters. Eng. Fail. Anal. 2020, 115, 104654. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Zhou, N.; Luo, X.; Yang, H.; Chai, F.; Yang, C. Investigating the Corrosion Performance of Hull Steel with Different Microstructure in a Tropical Marine Atmosphere. J. Mater. Res. Technol. 2023, 27, 2600–2614. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Udosoro, I.I.; Udoh, A.P. Inhibitive and Adsorption Behaviour of Carboxymethyl Cellulose on Mild Steel Corrosion in Sulphuric Acid Solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the Corrosion of X70 and Q235 Steel in CO2 -Saturated Brine by Imidazoline-Based Inhibitor. J. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Yang, X.; Qing, W.; Feng, C.; Yin-Wei, M.; Dong-liang, Q. Sol-Gel Process and High-Temperature Property of SiO2/ZrO2-SiO2 Composites. Ceram. Int. 2017, 43, 854–859. [Google Scholar] [CrossRef]
- Chen, X.H.; Dong, J.H.; Han, E.H.; Ke, W. Effect of Al Alloying on Corrosion Performance of Steel. Corros. Eng. Sci. Technol. 2007, 42, 224–231. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, J.; Kang, J.; Duan, M.; Wang, Y. Rust Layer Adhesion Capability and Corrosion Behavior of Weathering Steel under Tension during Initial Stages of Simulated Marine Atmospheric Corrosion. Constr. Build. Mater. 2020, 234, 117393. [Google Scholar] [CrossRef]
- Karim, M.R.A.; Daniel, S.; Ul Haq, E.; Ahmad, A.; Khan, K.I.; Raza, S.A. Improving the Corrosion Resistance of API X56 and API X70 Pipeline Steels by Hot Dip Aluminization. KEM 2021, 875, 315–321. [Google Scholar] [CrossRef]
- Nouri, M.; Sun, X.; Li, D.Y. Beneficial Effects of Yttrium on the Performance of Mg–3%Al Alloy during Wear, Corrosion and Corrosive Wear. Tribol. Int. 2013, 67, 154–163. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E.H. Influence of Yttrium Element on the Corrosion Behaviors of Mg–Y Binary Magnesium Alloy. J. Magnes. Alloys 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Feng, M.; Liu, Y.; Zhang, S.; Liu, Y.; Luo, N.; Wang, D. Carbon Quantum Dots (CQDs) Modified TiO2 Nanorods Photoelectrode for Enhanced Photocathodic Protection of Q235 Carbon Steel. Corros. Sci. 2020, 176, 108919. [Google Scholar] [CrossRef]
- Jamshidi, R.; Bayat, O.; Heidarpour, A. Tribological and Corrosion Behavior of Flame Sprayed Al–10 Wt.% Ti3SiC2 Composite Coating on Carbon Steel. Surf. Coat. Technol. 2019, 358, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Ou, J. Stable, Superfast and Self-Healing Fluid Coating with Active Corrosion Resistance. Adv. Colloid Interface Sci. 2021, 295, 102494. [Google Scholar] [CrossRef]
- Huang, C.; Su, X.; Song, Q.; Wang, X. Effects of Temperature on Acceleration and Simulation of Indoor Corrosion Test of Q235 Carbon Steel. Anti-Corros. Mater. Methods 2021, 68, 564–570. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahu, R.K.; Pramanick, A.K.; Singh, R. Development of Conversion Coating on Mild Steel Prior to Sol Gel Nanostructured Al2O3 Coating for Enhancement of Corrosion Resistance. Surf. Coat. Technol. 2011, 205, 4960–4967. [Google Scholar] [CrossRef]
- Kirtay, S. Preparation of Hybrid Silica Sol–Gel Coatings on Mild Steel Surfaces and Evaluation of Their Corrosion Resistance. Prog. Org. Coat. 2014, 77, 1861–1866. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, B.; Wang, Z.; Liu, Y.; Wang, L.; Zhang, Y.; Li, S. Successive Phase and Morphology Evolution of Galvannealed Coating in Hot Stamping and Diffusion Modeling of α-Fe(Zn)/Steel System Considering the Effect of Zn Concentration. Surf. Coat. Technol. 2019, 380, 125036. [Google Scholar] [CrossRef]
- Oladijo, O.P.; Mathabatha, M.H.; Popoola, A.P.I.; Ntsoane, T.P. Characterization and Corrosion Behaviour of Plasma Sprayed Zn-Sn Alloy Coating on Mild Steel. Surf. Coat. Technol. 2018, 352, 654–661. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kim, D.; Kim, D.H.; Kim, S.-J. Fe-Zn Reaction and Its Influence on Microcracks during Hot Tensile Deformation of Galvanized 22MnB5 Steel. Surf. Coat. Technol. 2019, 357, 1069–1075. [Google Scholar] [CrossRef]
- Imanieh, I.; Yousefi, E.; Dolati, A.; Mohammadi, M.R. Experiments Design for Hardness Optimization of the Ni-Cr Alloy Electrodeposited by Pulse Plating. Acta Metall. Sin. 2013, 26, 558–564. [Google Scholar] [CrossRef]
- Li, X.; Kong, G.; Zhang, J.; Lai, D.; Che, C.; Yang, B.; Zhang, S.; Gu, J. Corrosion Behavior of Q235 Steel in Graphene Oxide Aqueous Dispersion. Surf. Topogr. Metrol. Prop. 2022, 10, 015039. [Google Scholar] [CrossRef]
- Quan, B.; Li, J.; Chen, C. Effect of Sulfur on Corrosion Behavior of Q235 and 16Mn Steel in Sodium Aluminate Solutions. Mater. Res. Express 2020, 7, 035602. [Google Scholar] [CrossRef]
- Liu, P.; Li, G.; Liu, B.; Liao, C.; Baker, I.; Wu, H. Tribocorrosion Behavior of Additively-Manufactured Fe-Mn Alloys. Mater. Lett. 2023, 337, 133949. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.O.; Jeong, Y.J.; Lee, S.G.; Choi, J.K.; Kim, S.J. Long-Term Corrosion Behavior of Strong and Ductile High Mn-Low Cr Steel in Acidic Aqueous Environments. Materials 2022, 15, 1746. [Google Scholar] [CrossRef]
- Gu, D.-D.; Peng, J.; Wang, J.-W.; Liu, Z.-T.; Pan, F.-S. Effect of Mn Modification on the Corrosion Susceptibility of Mg–Mn Alloys by Magnesium Scrap. Acta Metall. Sin. 2021, 34, 1–11. [Google Scholar] [CrossRef]
- McAdam, G.; Young, D.J. Morphological Development of Oxide-Sulfide Scales on Iron and Iron-Manganese Alloys. Oxid. Met. 1987, 28, 165–181. [Google Scholar] [CrossRef]
- Santos Martinez, J.D.; Ladino, D.H.; Calderón Hernández, J.W.; Falleiros, N.A.; de Melo, H.G. Corrosion Characterization of New Low Manganese Microalloyed X65 Steels in Sour and NON-SOUR Synthetic Seawater. J. Mater. Res. Technol. 2022, 18, 3198–3214. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, S.O.; Park, J.S.; Lee, J.W.; Hwang, J.-K.; Lee, S.G.; Choi, J.K.; Kim, S.J. Strong and Ductile Fe-24Mn-3Cr Alloy Resistant against Erosion-Corrosion. NPJ Mater. Degrad. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Dehestani, M.; Trumble, K.; Wang, H.; Wang, H.; Stanciu, L.A. Effects of Microstructure and Heat Treatment on Mechanical Properties and Corrosion Behavior of Powder Metallurgy Derived Fe–30Mn Alloy. Mater. Sci. Eng. A 2017, 703, 214–226. [Google Scholar] [CrossRef]
- Yan, X.; Kang, S.; Xu, M.; Li, P. Corrosion Product Film of a Medium-Mn Steel Exposed to Simulated Marine Splash Zone Environment. Materials 2021, 14, 5652. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Morphology and Corrosion Properties of FeMn-Graphene Oxide Composite Coatings. J. Alloys Compd. 2020, 821, 153560. [Google Scholar] [CrossRef]
- Mobin, M.; Aslam, R.; Salim, R.; Kaya, S. An Investigation on the Synthesis, Characterization and Anti-Corrosion Properties of Choline Based Ionic Liquids as Novel and Environmentally Friendly Inhibitors for Mild Steel Corrosion in 5% HCl. J. Colloid Interface Sci. 2022, 620, 293–312. [Google Scholar] [CrossRef]
- Quan, B.; Li, J.; Chen, C. Effect of Corrosion Time on the Synergistic Corrosion of Q235 Steel in Sodium Aluminate Solutions. Metals 2021, 11, 753. [Google Scholar] [CrossRef]
- Quan, B.; Li, J.; Chen, C. Effect of Corrosion Temperature on the Corrosion of Q235 Steel and 16Mn Steel in Sodium Aluminate Solutions. ACS Omega 2021, 6, 25904–25915. [Google Scholar] [CrossRef]
- Chang, B.-Y. Conversion of a Constant Phase Element to an Equivalent Capacitor. J. Electrochem. Sci. Technol. 2020, 11, 318–321. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]




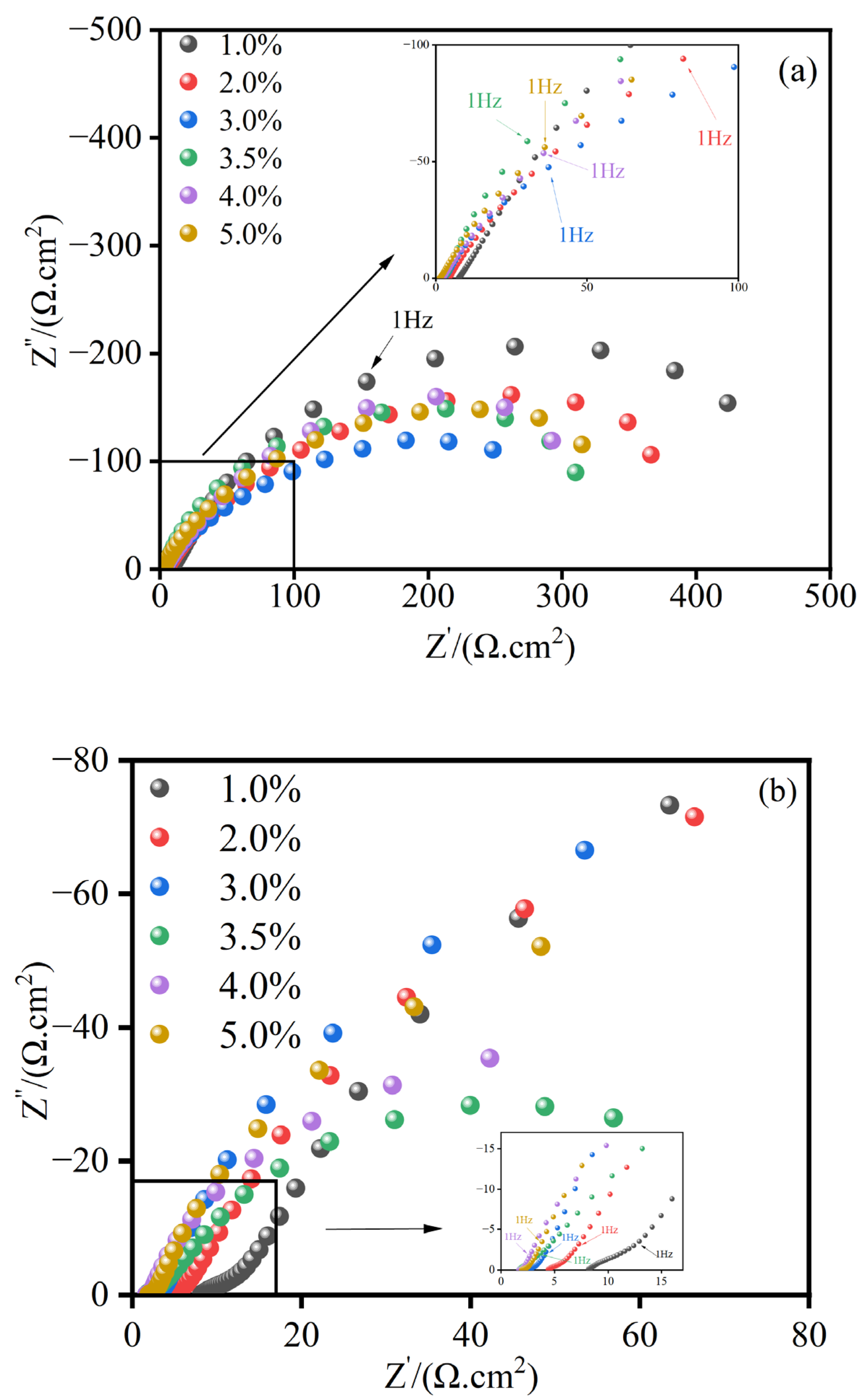
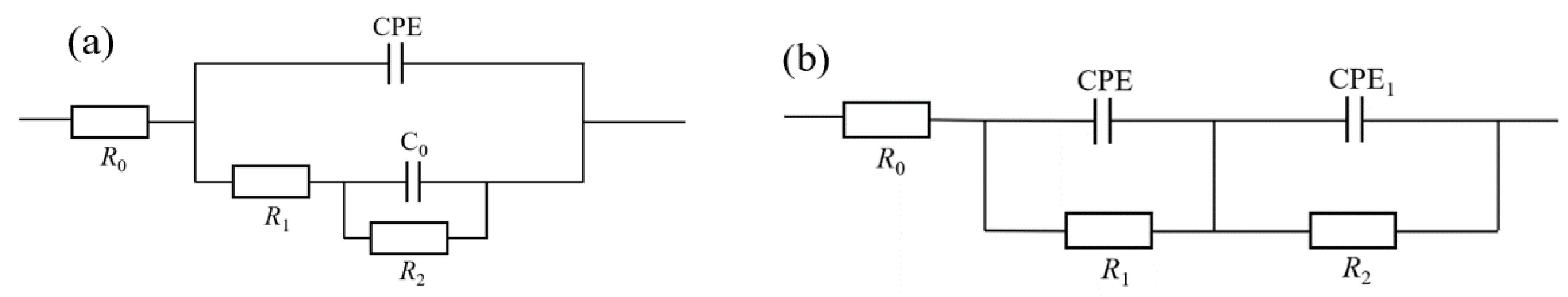

| NaCl wt.% | MnFe2O4 Spinel Coating | ||
|---|---|---|---|
| Ecorr (mV) | Icorr (µA/cm2) | Rp (kΩ·cm2) | |
| 1.0 | −169 | 6.79 | 2.330 |
| 2.0 | −231 | 10.6 | 1.860 |
| 3.0 | −249 | 12.4 | 1.830 |
| 3.5 | −253 | 15.6. | 1.390 |
| 4.0 | −247 | 41.0 | 0.561 |
| 5.0 | −235 | 40.0 | 0.473 |
| NaCl wt.% | Q235 | ||
|---|---|---|---|
| Ecorr (mV) | Icorr (µA/cm2) | Rp (kΩ·cm2) | |
| 1.0 | −385 | 24.8 | 0.857 |
| 2.0 | −396 | 29.4 | 0.586 |
| 3.0 | −395 | 53.2 | 0.518 |
| 3.5 | −379 | 72.3 | 0.292 |
| 4.0 | −400 | 62.5 | 0.351 |
| 5.0 | −397 | 55.7 | 0.497 |
| NaCl wt.% | MnFe2O4 Spinel Coating | |||||
|---|---|---|---|---|---|---|
| R0/Ω·cm2 | R1/Ω·cm2 | R2/Ω·cm2 | CPE/mF·cm−2 | n | C0/μF·cm−2 | |
| 1.0 | 7.76 | 77.7 | 536 | 762 | 0.780 | 67.4 |
| 2.0 | 4.26 | 3.08 | 523 | 679 | 0.712 | 52.4 |
| 3.0 | 2.51 | 6.37 | 476 | 638 | 0.637 | 285 |
| 3.5 | 2.25 | 5.75 | 466 | 722 | 0.721 | 670 |
| 4.0 | 2.35 | 17.7 | 553 | 709 | 0.754 | 44.2 |
| 5.0 | 1.35 | 8.61 | 501 | 712 | 0.712 | 127 |
| NaCl wt.% | Q235 | ||||||
|---|---|---|---|---|---|---|---|
| R0/Ω·cm2 | R1/Ω·cm2 | R2/Ω·cm2 | CPE/mF·cm−2 | n1 | CPE/mF·cm−2 | n2 | |
| 1.0 | 8.10 | 301 | 6.17 | 804 | 0.5 | 500 | 1 |
| 2.0 | 4.36 | 260 | 2.19 | 778 | 0.5 | 500 | 1 |
| 3.0 | 2.48 | 216 | 1.33 | 831 | 0.5 | 500 | 1 |
| 3.5 | 1.88 | 90.6 | 2.41 | 738 | 0.5 | 521 | 0.982 |
| 4.0 | 1.67 | 93.6 | 0.49 | 819 | 0.5 | 500 | 0.934 |
| 5.0 | 1.61 | 156 | 0.86 | 821 | 1 | 500 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.-A.; Miao, X.; Zhang, T.-A. Synthesis of MnFe2O4 Spinel on Rusted Q235 Steel Surface and Its Corrosion Resistance Properties. Materials 2024, 17, 4163. https://doi.org/10.3390/ma17164163
Feng B-A, Miao X, Zhang T-A. Synthesis of MnFe2O4 Spinel on Rusted Q235 Steel Surface and Its Corrosion Resistance Properties. Materials. 2024; 17(16):4163. https://doi.org/10.3390/ma17164163
Chicago/Turabian StyleFeng, Bai-Ao, Xu Miao, and Ting-An Zhang. 2024. "Synthesis of MnFe2O4 Spinel on Rusted Q235 Steel Surface and Its Corrosion Resistance Properties" Materials 17, no. 16: 4163. https://doi.org/10.3390/ma17164163






