Study on Microscopic Oil Displacement Mechanism of Alkaline–Surfactant–Polymer Ternary Flooding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Method
2.2.1. Interfacial Tension Test
2.2.2. Bulk Viscosity Test
2.2.3. Emulsifying Property
2.2.4. Microscopic Visualization Oil Displacement Experiment
3. Results
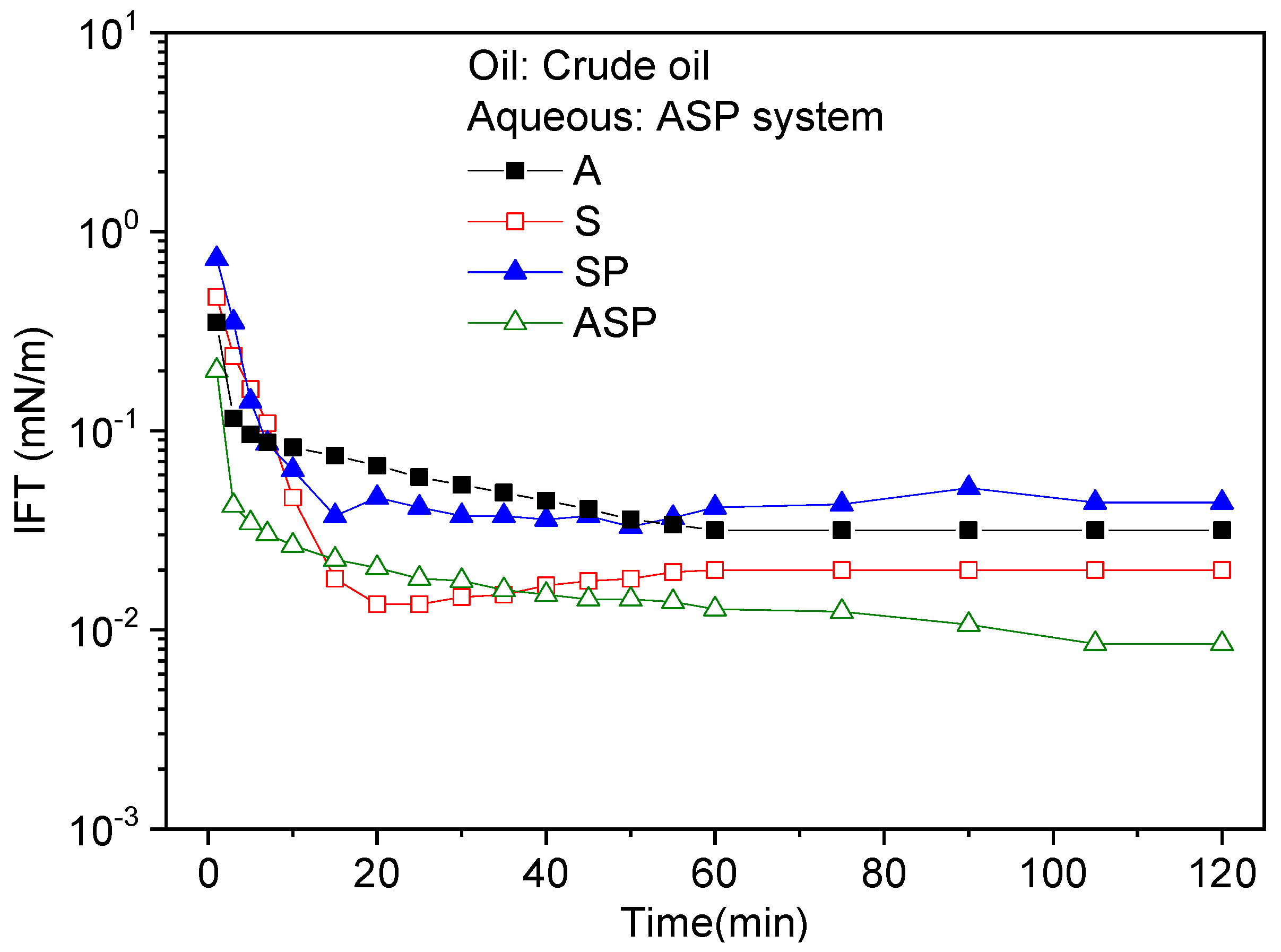
3.1. Oil/Water Interfacial Tensions
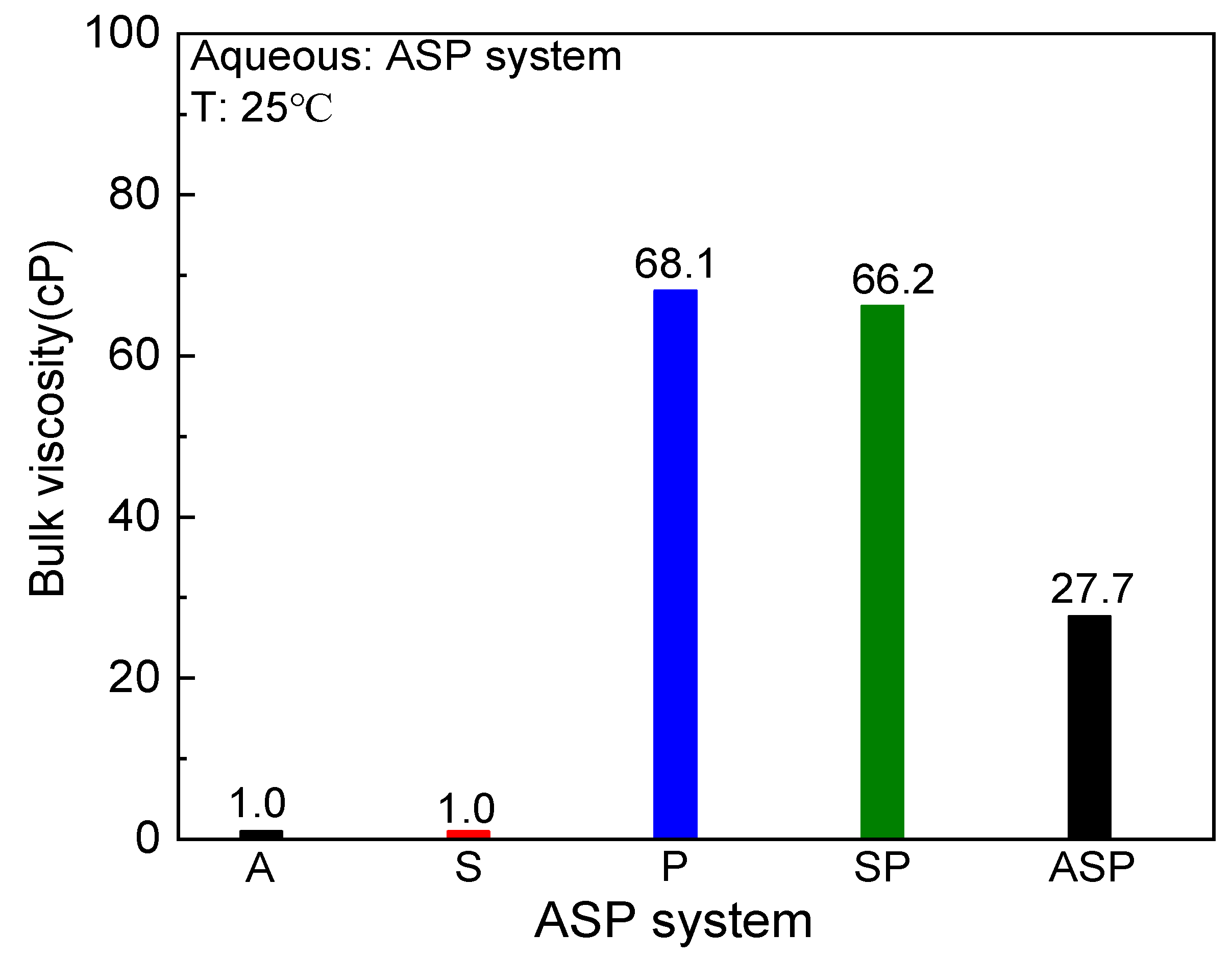
3.2. Bulk Viscosity
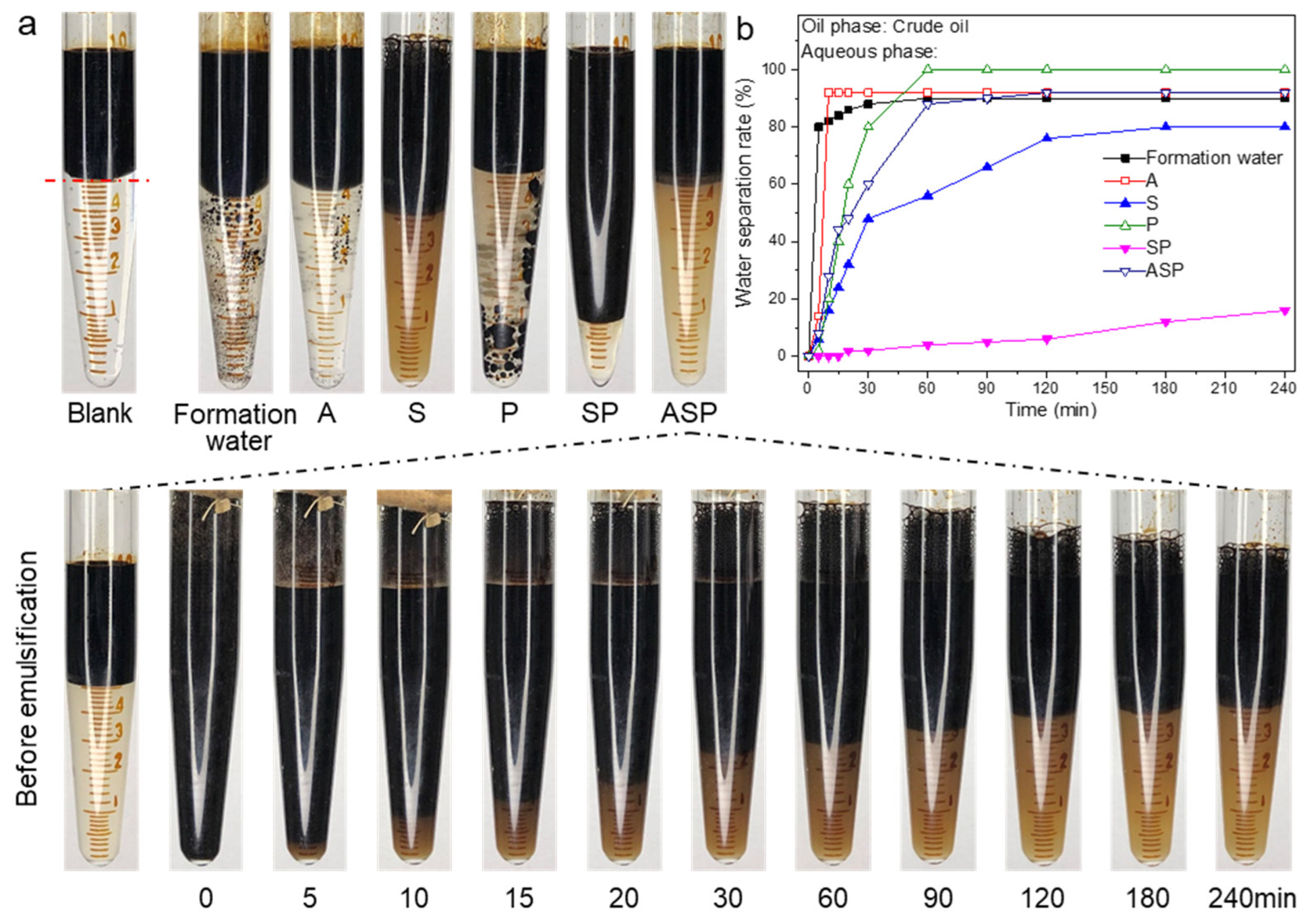
3.3. Emulsification Performance and Emulsion Stability Test
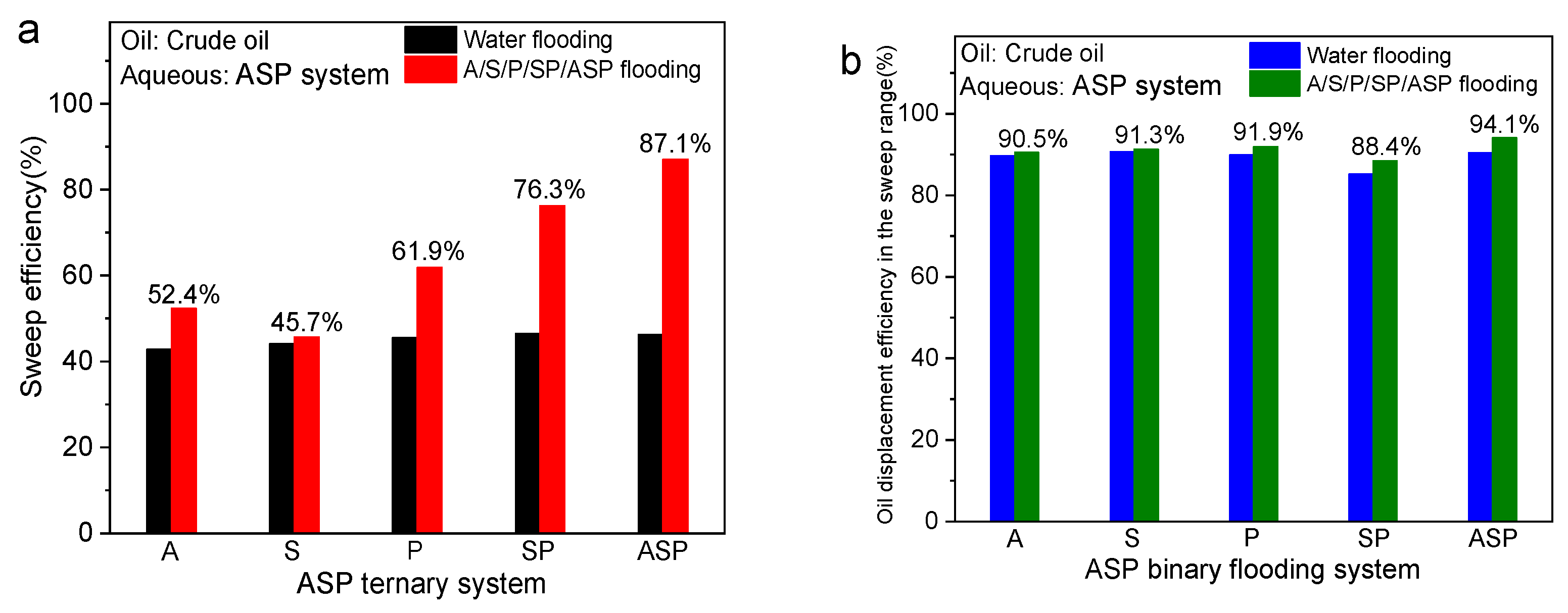
3.4. Oil Displacement Effect
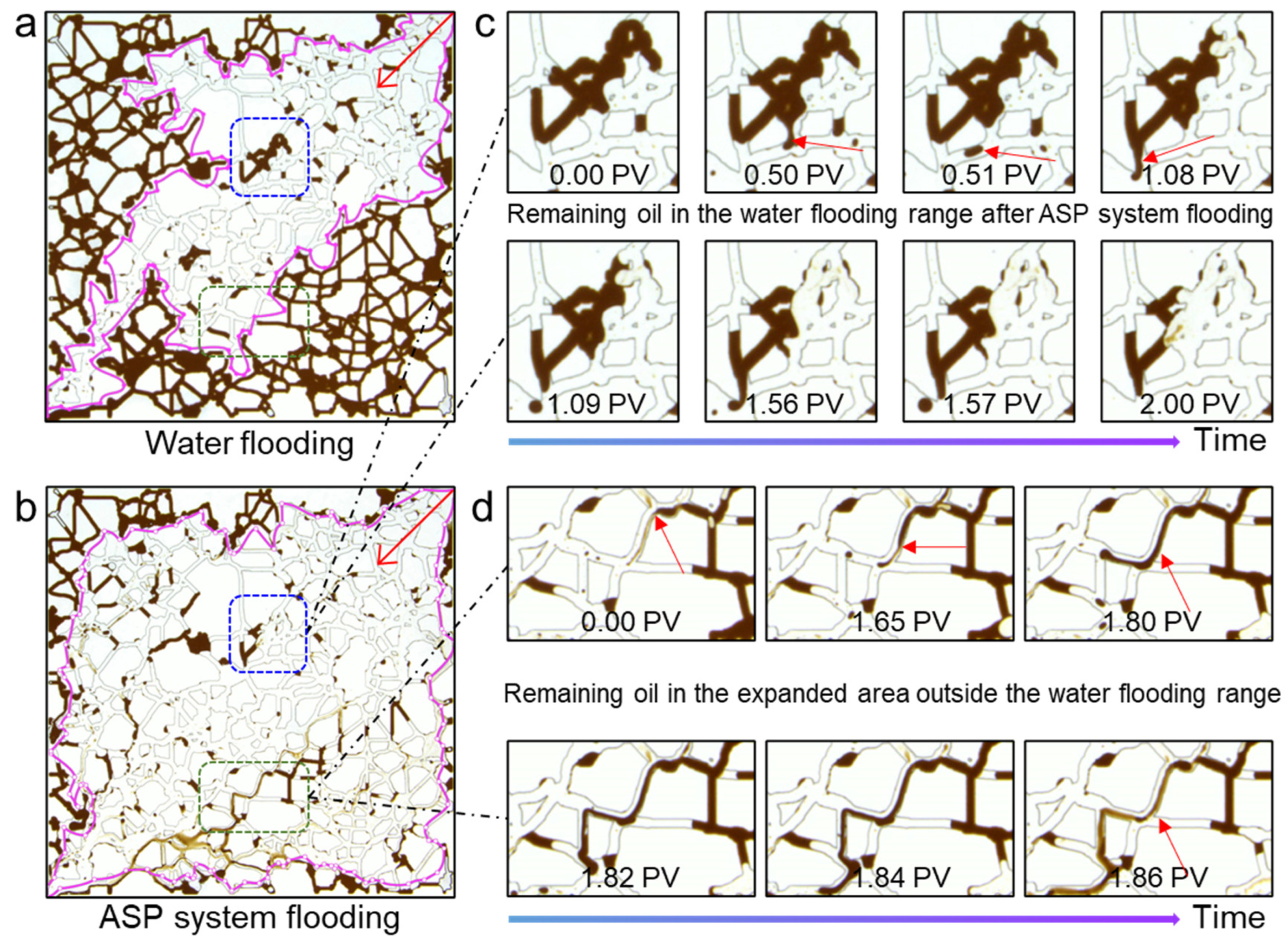
3.5. Oil Displacement Mechanism of ASP Ternary System
4. Conclusions
- (1)
- In the ASP system, the active substances generated by the alkali synergize with the surfactant molecules to form a more compact interfacial film. Meanwhile, the polymer and surfactant can form a mixed adsorption layer with a higher surface charge. This enables the ASP system to obtain ultra-low interfacial tension;
- (2)
- Alkali not only plays the role of salt to reduce the viscosity of the system but also tends to break the polymer chain. Therefore, the bulk viscosity of the ASP system is larger than that of the A and S systems but smaller than that of the P and SP systems;
- (3)
- The low interfacial tension of the ASP system facilitates the emulsification of crude oil. Alkali decreases the viscosity of the ASP system but weakens the stability of the emulsion. The ASP system has a strong emulsifying ability, fast emulsification rate, uniform emulsion, and the ability to emulsify stripping crude oil;
- (4)
- The ASP system simultaneously has the advantages of interfacial reaction of alkali, low interfacial tension of surfactant, and viscosity-increasing effect of the polymer, which significantly improves the sweep effect and oil washing effect. The ASP system has the highest EOR (38.0%) compared with other systems;
- (5)
- The interfacial reaction of the alkali facilitates the mobilization of residual oil after water flooding, and the low tension of the surfactant and the rheology of the polymer can emulsify, strip, and entrain the remaining oil in the expanded area outside of water flooding, which improves the crude oil recovery of the ASP system;
- (6)
- Ternary flooding can be used in combination with CO2/water alternating flooding, thus reducing the use and emission of CO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lv, W.F.; Zhou, Z.H.; Zhang, Q.; Zhang, X.J.; Zhang, L. Study on the mechanism of surfactant flooding: Effect of betaine structure. Adv. Geo-Energy Res. 2023, 10, 146–158. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Mohammadi, M.; Sedighi, M. Introduction to chemical enhanced oil recovery. In Chemical Methods; Gulf Professional Publishing: Oxford, UK, 2022; pp. 1–32. [Google Scholar]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Sedaghat, M.; Mohammadzadeh, O.; Kord, S.; Chatzis, I. Heavy oil recovery using ASP flooding: A pore-level experimental study in fractured five-spot micromodels. Can. J. Chem. Eng. 2016, 94, 779–791. [Google Scholar] [CrossRef]
- Samanta, A.; Ojha, K.; Mandal, A. Interactions between acidic crude oil and alkali and their effects on enhanced oil recovery. Energy Fuels 2011, 24, 1642–1649. [Google Scholar] [CrossRef]
- Dong, M.Z.; Ma, S.Z.; Li, A.F. Sweep efficiency improvement by alkaline flooding for pelican lake heavy oil. In Proceedings of the Canadian Unconventional Resources Conference, Calgary, AB, Canada, 15–17 November 2011; p. SPE-148971-MS. [Google Scholar]
- Sharma, H.; Weerasooriya, U.; Pope, G.A.; Mohanty, K.K. Ammonia-Based ASP Processes for Gypsum-Containing Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014. [Google Scholar]
- Kumar Sharma, V.; Singh, A.; Tiwari, P. An experimental study of pore-scale flow dynamics and heavy oil recovery using low saline water and chemical flooding. Fuel 2023, 334, 126756. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers 2019, 11, 419. [Google Scholar] [CrossRef]
- Yun, W.; Kovscek, A.R. Microvisual investigation of polymer retention on the homogeneous pore network of a micromodel. J. Pet. Sci. Eng. 2015, 128, 115–127. [Google Scholar] [CrossRef]
- Torrealba, V.A.; Hoteit, H. Improved polymer flooding injectivity and displacement by considering compositionally-tuned slugs. J. Pet. Sci. Eng. 2019, 178, 14–26. [Google Scholar] [CrossRef]
- Juan, Z.L.; An, Y.X.; Qiao, G.F. Micro-mechanisms of residual oil mobilization by viscoelastic fluids. Pet. Sci. 2008, 5, 56–61. [Google Scholar]
- Zhu, H.J.; Luo, J.H.; Klaus, O.; Fan, Y.Z. The impact of extensional viscosity on oil displacement efficiency in polymer flooding. Colloids Surf. A 2012, 414, 498–503. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Ayoub Mohammed, M.A.; Yekeen, N.; Negash, B.M. Alternative chemical agents for alkalis, surfactants and polymers for enhanced oil recovery: Research trend and prospects. J. Pet. Sci. Eng. 2020, 187, 106828. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, T. Design and performance evaluation of Alkaline-Surfactant-Polymer slug of a natural surfactant of fenugreek in saline media for Foaming/Emulsification applications. J. Mol. Liq. 2024, 395, 123912. [Google Scholar] [CrossRef]
- Fu, L.P.; Zhang, G.C.; Ge, J.J.; Liao, K.L.; Pei, H.H.; Jiang, P.; Li, X.Q. Study on organic alkali-surfactant-polymer flooding for enhanced ordinary heavy oil recovery. Colloids Surf. A 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bera, A.; Raipuria, V.; Kumar, A.; Mandal, A.; Kumar, T. Numerical simulation of enhanced oil recovery by alkali-surfactant-polymer floodings. Pet. Sci. Technol. 2015, 33, 1229–1237. [Google Scholar] [CrossRef]
- Mohammadi, H.; Delshad, M.; Pope, G.A. Mechanistic modeling of alkaline/surfactant/polymer floods. SPE Reserv. Eval. Eng. 2009, 12, 518–527. [Google Scholar] [CrossRef]
- Sun, Z.; Kang, X.; Lu, X.; Li, Q.; Jiang, W. Effects of crude oil composition on the ASP flooding: A case from Saertu, Xingshugang and Lamadian Oilfield in Daqing. Colloids Surf. A 2018, 555, 586–594. [Google Scholar] [CrossRef]
- Sun, L.; Wu, X.; Zhou, W.; Li, X.; Han, P. Technologies of enhancing oil recovery by chemical flooding in Daqing Oilfield, NE China. Pet. Explor. Dev. 2018, 45, 673–684. [Google Scholar] [CrossRef]
- Li, J.; Niu, L.; Lu, X. Performance of ASP compound systems and effects on flooding efficiency. J. Pet. Sci. Eng. 2019, 178, 1178–1193. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.Q.; Wang, F.Y.; Gu, Y.Y. Comparison of strong-alkali and weak-alkali ASP-flooding field tests in Daqing oil field. SPE Prod. Oper. 2017, 33, 353–362. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Shu Tang, G.; Qiang, G. Recent progress and evaluation of ASP flooding for EOR in Daqing oil field. In Proceedings of the SPE EOR Conference at Oil & Gas West Asia, Muscat, Oman, 11–13 April 2010; p. SPE-127714-MS. [Google Scholar]
- Denney, D. Progress and effects of ASP flooding. J. Pet. Technol. 2013, 65, 77–81. [Google Scholar] [CrossRef]
- Li, H.F.; Xu, D.P.; Jiang, J.; Du, X.W.; Hong, J.C.; Jiang, Y.; Xu, Y.S. Performance and effect analysis of ASP commercial flooding in central Xing 2 area of Daqing oil field. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 19–23 April 2008. [Google Scholar]
- Jie Cheng, C.; Dian Ping, X.; Wen Guang, B. Commercial ASP flood test in Daqing oil field. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 3–6 November 2008. [Google Scholar]
- Zhu, Y.Y.; Hou, Q.F.; Jian, G.Q.; Ma, D.S.; Wang, Z. Current development and application of chemical combination flooding technique. Pet. Explor. Dev. 2013, 40, 96–103. [Google Scholar] [CrossRef]
- Han, L.; Luan, H.X.; Ren, J.; Zhang, Q.; Xu, C.J.; Li, G.; Xiao, H.Y.; Zhou, Z.H.; Zhang, L.; Zhang, L. Effect of double chain anionic surfactant on the dynamic interfacial tensions of betaine solutions. J. Mol. Liq. 2023, 382, 121866. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, S.Y.; Zhou, Z.H.; Li, Z.X.; Lu, W.H.; Zhang, L.; Zhu, Y.; Zhang, L. Effect of crude oil fractions on the interfacial tensions of alkali-betaine mixed solutions. J. Pet. Sci. Eng. 2017, 159, 474–482. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, F.T.; Han, L.; Zhu, X.Y.; Zhang, F.; Ma, G.Y.; Zhang, L.; Zhou, Z.H.; Zhang, L. The synergistic effects between sulfobetaine and hydrophobically modified polyacrylamide on properties related to enhanced oil recovery. Molecules 2023, 28, 1787. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, M.Z.; Xu, Z.C.; Gong, Q.T.; Zhang, L.; Zhang, L. Study on oil displacement mechanism of alkyl carboxyl betaine. China Surfactant Deterg. Cosmet. 2024, 54, 123–130. [Google Scholar]
- Zhang, X.J.; Zhou, Z.H.; Han, L.; Zhang, Y.Q.; Zhang, Q.; Ma, D.S.; Ma, W.J.; Zhang, L.; Zhang, L. Mechanism responsible for the reduction of interfacial tension by extended surfactants. Colloids Surf. A 2022, 634, 128013. [Google Scholar] [CrossRef]
- Alsahhaf, T.; Ahmed, A.; Elkamel, A. Producing ultralow interfacial tension at the oil/water interface. Pet. Sci. Technol. 2002, 20, 773–788. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.Y.; Gong, H.J.; Bai, Y.; Tan, Y.B. Interaction between sodium oleate and partially hydrolyzed polyacrylamide: A rheological study. Colloids Surf. A 2008, 326, 1–9. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.Q.; Gao, W.B.; Cao, J.X.; Sun, B.Y.; Zhang, J. Experimental investigation on the effect of interfacial properties of chemical flooding for enhanced heavy oil recovery. Colloids Surf. A 2023, 677, 132335. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lin, X.Y.; Yu, T.Y.; Zhou, N.; Zhong, H.Y.; Zhu, J.Y. Formation and rupture mechanisms of visco-elastic interfacial films in polymer-stabilized emulsions. J. Dispers. Sci. Technol. 2019, 40, 612–626. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.Z.; Yue, X.A.; Hou, J.R. Synergy of alkali and surfactant in emulsification of heavy oil in brine. Colloids Surf. A 2006, 273, 219–228. [Google Scholar] [CrossRef]
- She, Y.; Mahardika, M.A.; Hu, Y.; Patmonoaji, A.; Matsushita, S.; Suekane, T.; Nagatsu, Y. Three-dimensional visualization of the alkaline flooding process with in-situ emulsification for oil recovery in porous media. J. Pet. Sci. Eng. 2021, 202, 108519. [Google Scholar] [CrossRef]
- Sedaghat, M.H.; Ghazanfari, M.H.; Masihi, M.; Rashtchian, D. Experimental and numerical investigation of polymer flooding in fractured heavy oil five-spot systems. J. Pet. Sci. Eng. 2013, 108, 370–382. [Google Scholar] [CrossRef]
- Dong, M.Z.; Liu, Q.; Li, A.F. Displacement mechanisms of enhanced heavy oil recovery by alkaline flooding in a micromodel. Particuology 2012, 10, 298–305. [Google Scholar] [CrossRef]
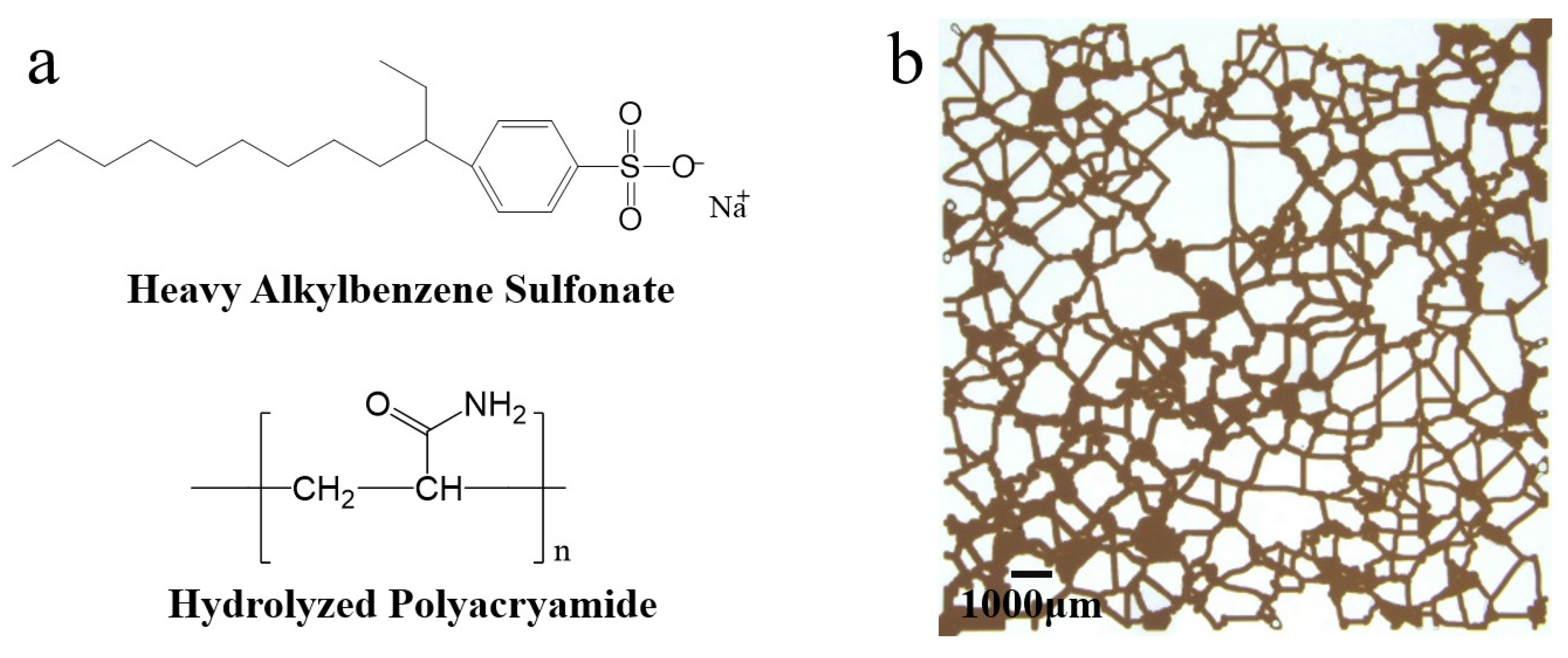

| Sample | Molecular Weight (×104) | Degree of Hydrolysis (mol %) | Solid Content (wt %) |
|---|---|---|---|
| Polymer | 1510.0 | 24.1 | 90.7 |
| TDS (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | CO32− (mg/L) | HCO3− (mg/L) |
|---|---|---|---|---|---|---|---|
| 5683.26 | 1741.54 | 60.12 | 9.12 | 801.96 | 54.03 | 371.64 | 2644.85 |
| Oil Displacement System | IFT (mN·m−1) | Bulk Viscosity (cP) | Water Flooding (%) | A/S/P/SP/ASP Flooding (%) | EOR (%) |
|---|---|---|---|---|---|
| 1.2% A | 0.032 | 1.0 | 39.3% | 45.6% | 6.3% |
| 0.3% S | 0.020 | 1.0 | 40.5% | 41.4% | 0.9% |
| 0.2% P | >10 | 68.1 | 41.6% | 54.8% | 13.2% |
| 0.3% S + 0.2% P | 0.044 | 66.2 | 40.0% | 61.0% | 20.9% |
| 1.2% A + 0.3% S + 0.2% P | 0.0085 | 27.7 | 41.7% | 79.7% | 38.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhou, Z.; Fan, J.; Zhang, F.; Zhao, J.; Zhang, Z.; Ding, W.; Zhang, L.; Zhang, L. Study on Microscopic Oil Displacement Mechanism of Alkaline–Surfactant–Polymer Ternary Flooding. Materials 2024, 17, 4457. https://doi.org/10.3390/ma17184457
Li G, Zhou Z, Fan J, Zhang F, Zhao J, Zhang Z, Ding W, Zhang L, Zhang L. Study on Microscopic Oil Displacement Mechanism of Alkaline–Surfactant–Polymer Ternary Flooding. Materials. 2024; 17(18):4457. https://doi.org/10.3390/ma17184457
Chicago/Turabian StyleLi, Guoqiao, Zhaohui Zhou, Jian Fan, Fan Zhang, Jinyi Zhao, Zhiqiu Zhang, Wei Ding, Lu Zhang, and Lei Zhang. 2024. "Study on Microscopic Oil Displacement Mechanism of Alkaline–Surfactant–Polymer Ternary Flooding" Materials 17, no. 18: 4457. https://doi.org/10.3390/ma17184457
APA StyleLi, G., Zhou, Z., Fan, J., Zhang, F., Zhao, J., Zhang, Z., Ding, W., Zhang, L., & Zhang, L. (2024). Study on Microscopic Oil Displacement Mechanism of Alkaline–Surfactant–Polymer Ternary Flooding. Materials, 17(18), 4457. https://doi.org/10.3390/ma17184457







