Hierarchically Graphitic Carbon Structure Derived from Metal Ions Impregnated Harmful Inedible Seaweed as Energy-Related Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
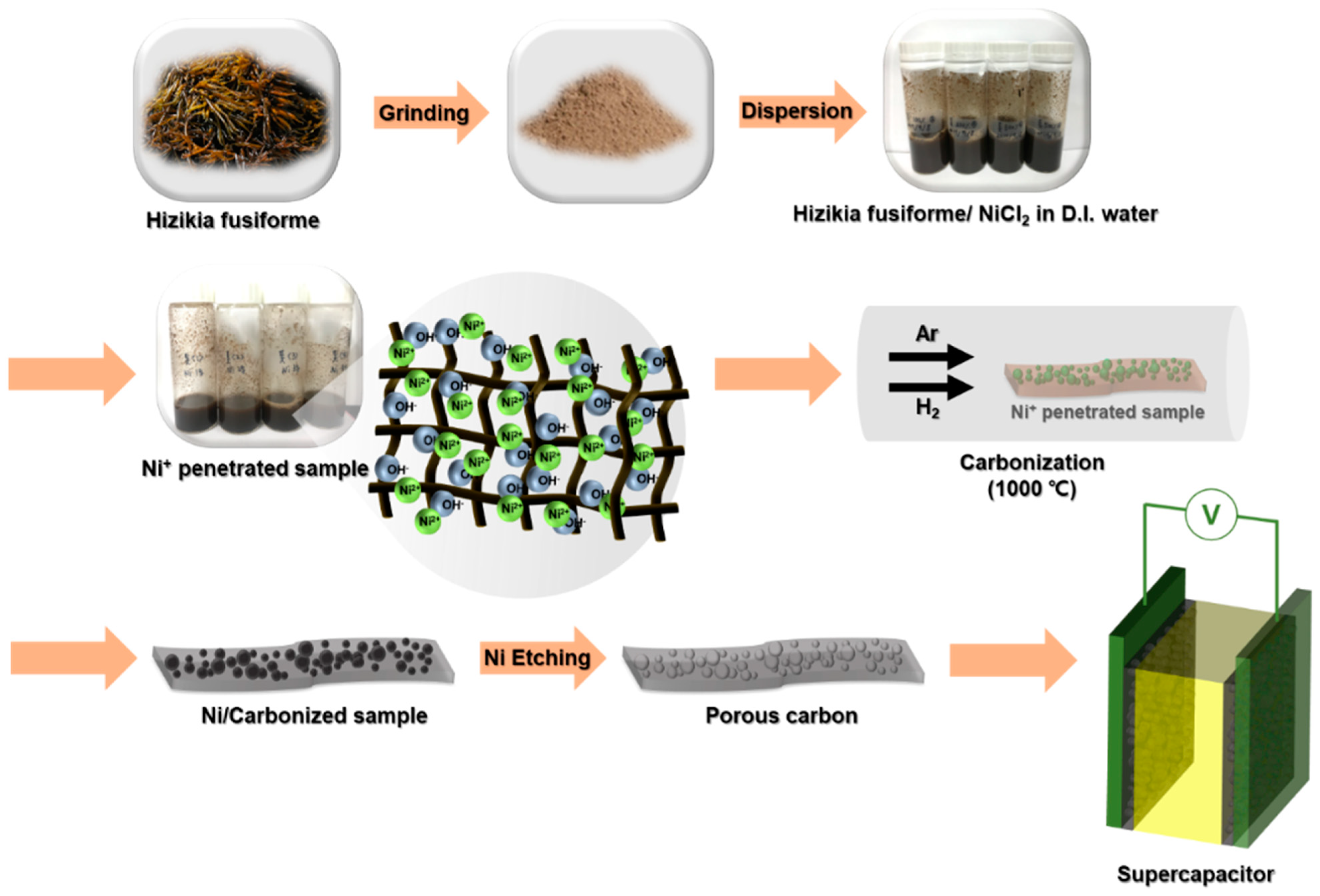
2.2. Preparation of Nickel Ion-Impregnated Seaweed (Sw/Ni+)
2.3. Fabrication of HGCs
2.4. Characterization
2.5. Electrochemical Characterization
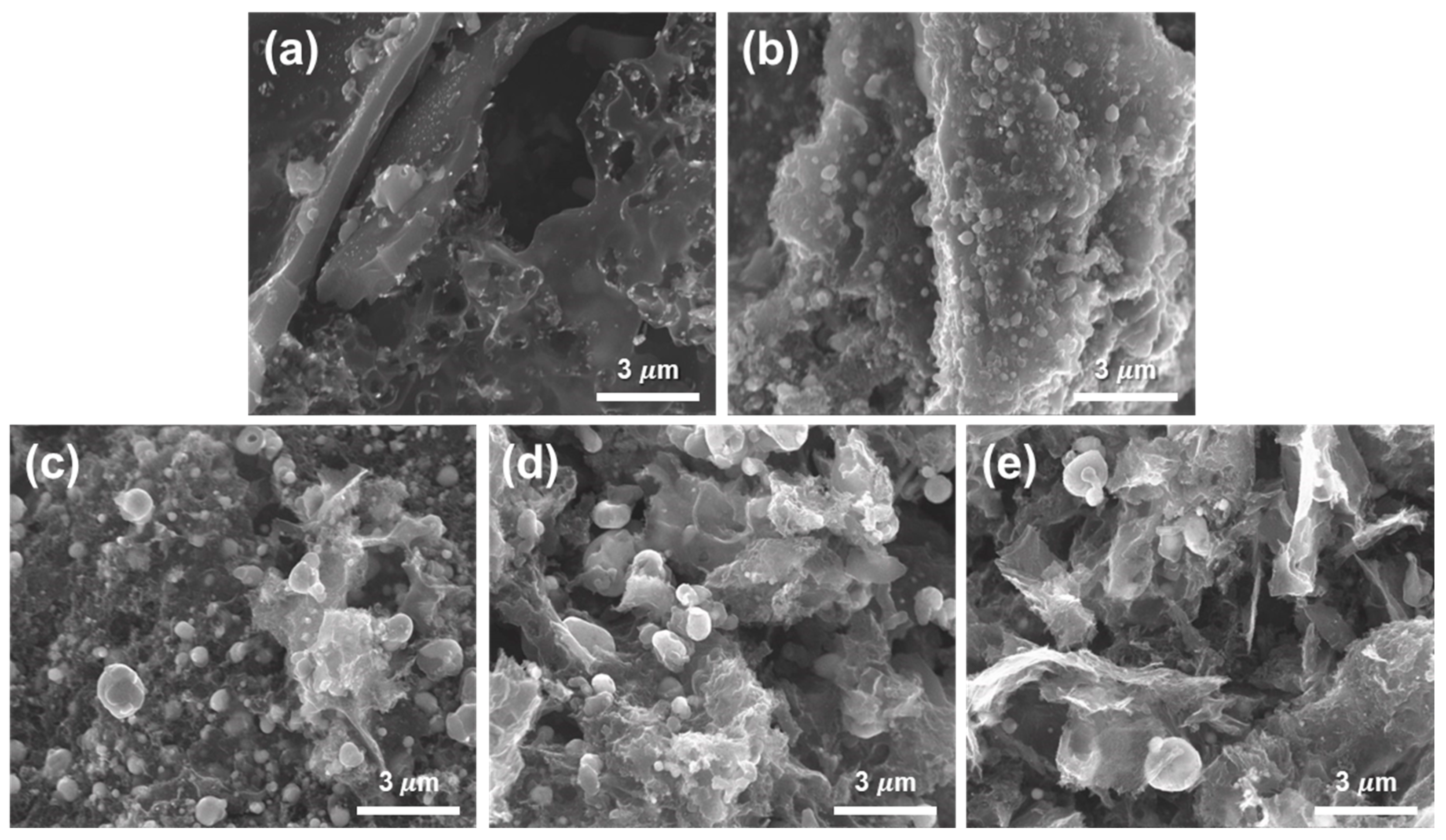
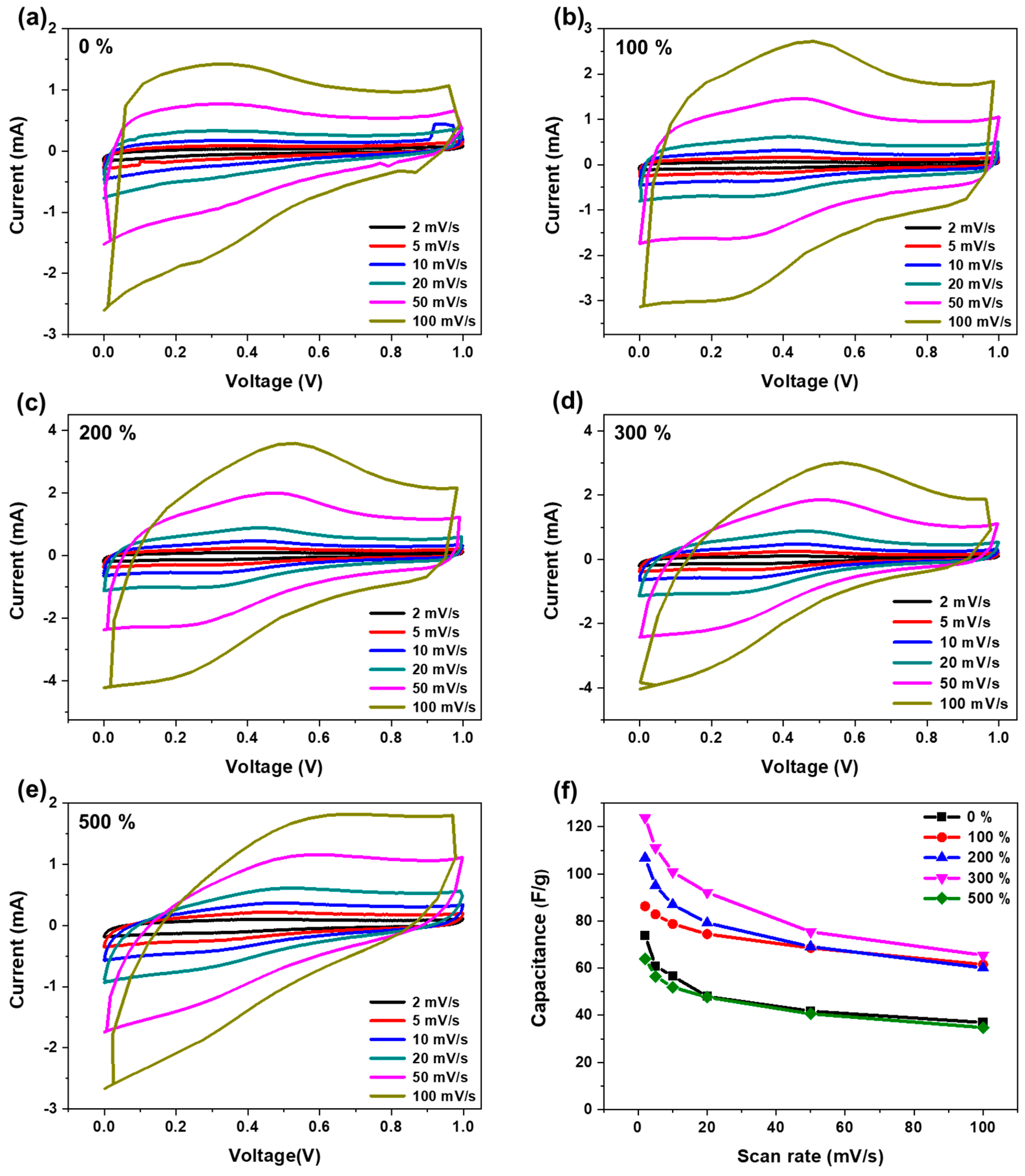
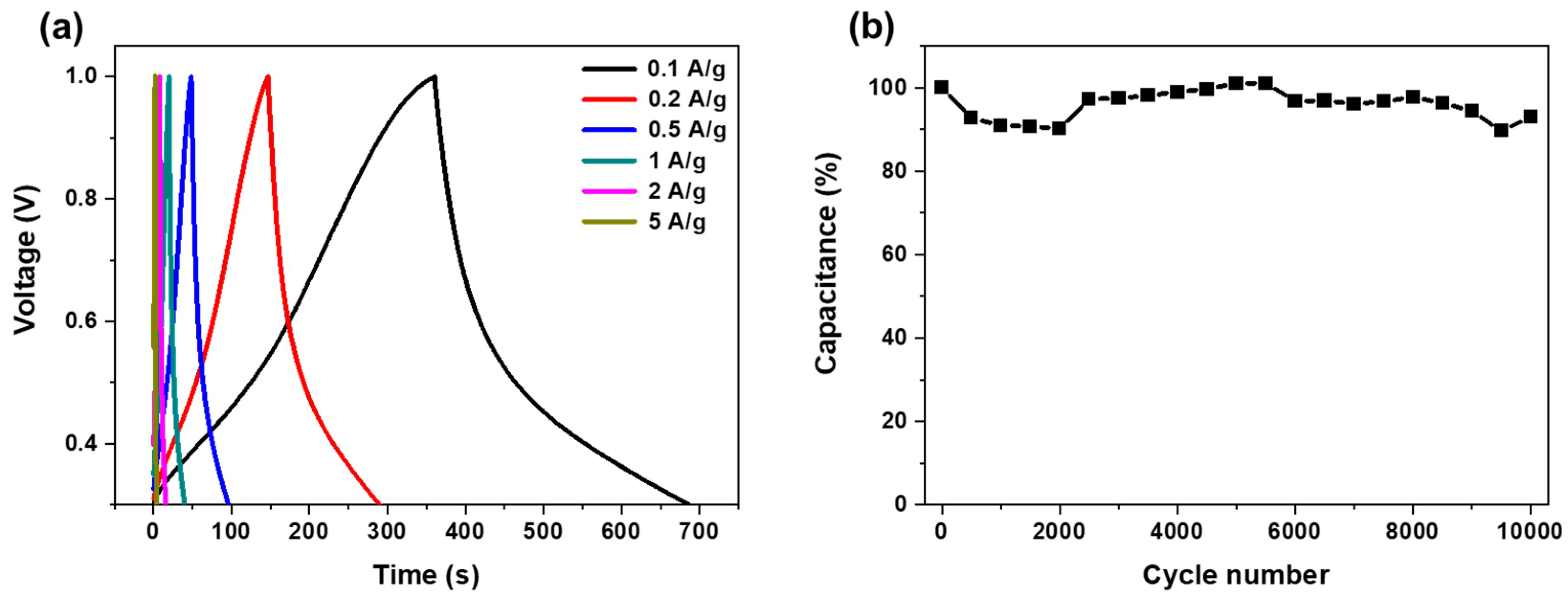
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Yan, J.; Wang, Y.; Wei, T.; Zhang, M.; Jing, X.; Fan, Z. Three-dimensional flower-like and hierarchical porous carbon materials as high-rate performance electrodes for supercapacitors. Carbon 2014, 67, 119–127. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery energy storage technology for power systems—An overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.-K.; Kim, T. Activated Biomass-derived Graphene-based Carbons for Supercapacitors with High Energy and Power Density. Sci. Rep. 2018, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, A.; Zhou, X.; Blaisdell, D.; Yang, S. Carbon nanofibers (CNFs) supported cobalt- nickel sulfide (CoNi2S4) nanoparticles hybrid anode for high performance lithium ion capacitor. Sci. Rep. 2018, 8, 1602. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Kandalkar, S.G.; Gunjakar, J.L.; Lokhande, C.D. Preparation of cobalt oxide thin films and its use in supercapacitor application. Appl. Surf. Sci. 2008, 254, 5540–5544. [Google Scholar] [CrossRef]
- Lang, J.-W.; Kong, L.-B.; Wu, W.-J.; Luo, Y.-C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 4213–4215. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef]
- Guo, H.; Gao, Q. Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor. J. Power Sources 2009, 186, 551–556. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Cao, G.; Wu, F.; Yang, Y. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Huang, Q.; Gamboa, S.; Sebastian, P.J. Studies on preparation and performances of carbon aerogel electrodes for the application of supercapacitor. J. Power Sources 2006, 158, 784–788. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.-C. Graphene and nanostructured MnO2 composite electrodes for supercapacitors. Carbon 2011, 49, 2917–2925. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Li, X.; Zang, X.; Li, Z.; Li, X.; Li, P.; Sun, P.; Lee, X.; Zhang, R.; Huang, Z.; Wang, K.; et al. Large-Area Flexible Core–Shell Graphene/Porous Carbon Woven Fabric Films for Fiber Supercapacitor Electrodes. Adv. Funct. Mater. 2013, 23, 4862–4869. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Saadallah, S.; Jurewicz, K.; Frackowiak, E.; Reda, M.; Parmentier, J.; Patarin, J.; Beguin, F. Supercapacitor electrodes from new ordered porous carbon materials obtained by a templating procedure. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2004, 108, 148–155. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Zhu, B.; Qiu, K.; Shang, C.; Guo, Z. Naturally derived porous carbon with selective metal- and/or nitrogen-doping for efficient CO2 capture and oxygen reduction. J. Mater. Chem. A 2015, 3, 5212–5222. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Qin, Y. 1—Seaweed Bioresources. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–24. [Google Scholar]
- Han, S.; Park, J.-S.; Umanzor, S.; Yarish, C.; Kim, J.K. Effects of extraction methods for a new source of biostimulant from Sargassum horneri on the growth of economically important red algae, Neopyropia yezoensis. Sci. Rep. 2022, 12, 11878. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.R.; Noman, M.; Kulkarni, R.; Patil, C.S.; Saqib, Q.M.; Chougale, M.Y.; Kim, J.; Ko, Y.; Jeon, Y.P.; Dongale, T.D.; et al. Unveiling the marine Sargassum horneri material for energy and active sensor devices: Towards multitasking approaches. Nano Today 2024, 57, 102379. [Google Scholar] [CrossRef]
- DeAngelo, J.; Saenz, B.T.; Arzeno-Soltero, I.B.; Frieder, C.A.; Long, M.C.; Hamman, J.; Davis, K.A.; Davis, S.J. Economic and biophysical limits to seaweed farming for climate change mitigation. Nat. Plants 2023, 9, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge? Angew. Chem.-Int. Edit. 2011, 50, 8510–8537. [Google Scholar] [CrossRef]
- Tsuji, Y.; Fujihara, T. Carbon dioxide as a carbon source in organic transformation: Carbon–carbon bond forming reactions by transition-metal catalysts. Chem. Commun. 2012, 48, 9956–9964. [Google Scholar] [CrossRef]
- Homma, Y.; Kobayashi, Y.; Ogino, T.; Takagi, D.; Ito, R.; Jung, Y.J.; Ajayan, P.M. Role of Transition Metal Catalysts in Single-Walled Carbon Nanotube Growth in Chemical Vapor Deposition. J. Phys. Chem. B 2003, 107, 12161–12164. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Ruch, P.W.; Hahn, M.; Scheifele, W.; Kötz, R.; Novák, P. In situ Raman spectroscopy of insertion electrodes for lithium-ion batteries and supercapacitors: First cycle effects. J. Phys. Chem. Solids 2008, 69, 1232–1237. [Google Scholar] [CrossRef]
- Labat, S.; Gergaud, P.; Thomas, O.; Gilles, B.; Marty, A. Interdependence of elastic strain and segregation in metallic multilayers: An x-ray diffraction study of (111) Au/Ni multilayers. J. Appl. Phys. 2000, 87, 1172–1181. [Google Scholar] [CrossRef]
- Lee, J.-S.; Ahn, H.-J.; Yoon, J.-C.; Jang, J.-H. Three-dimensional nano-foam of few-layer graphene grown by CVD for DSSC. Phys. Chem. Chem. Phys. 2012, 14, 7938–7943. [Google Scholar] [CrossRef] [PubMed]
- Takahagi, T.; Ishitani, A. XPS study on the surface structure of carbon fibers using chemical modification and C1s line shape analysis. Carbon 1988, 26, 389–395. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Wachtler, M.; Béguin, F. Carbon Nanotubes as Nanotexturing Agents for High Power Supercapacitors Based on Seaweed Carbons. ChemSusChem 2011, 4, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-C.; Lee, J.-S.; Kim, S.-I.; Kim, K.-H.; Jang, J.-H. Three-Dimensional Graphene Nano-Networks with High Quality and Mass Production Capability via Precursor-Assisted Chemical Vapor Deposition. Sci. Rep. 2013, 3, 1788. [Google Scholar] [CrossRef]
- Long, Y.; An, X.; Zhang, H.; Yang, J.; Liu, L.; Tian, Z.; Yang, G.; Cheng, Z.; Cao, H.; Liu, H.; et al. Highly graphitized lignin-derived porous carbon with hierarchical N/O co-doping “core-shell” superstructure supported by metal-organic frameworks for advanced supercapacitor performance. Chem. Eng. J. 2023, 451, 138877. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Hou, Y.; Hu, C.; Liu, L.; Wu, Y. Electrode materials for aqueous asymmetric supercapacitors. RSC Adv. 2013, 3, 13059–13084. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric Supercapacitors Based on Graphene/MnO2 and Activated Carbon Nanofiber Electrodes with High Power and Energy Density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.-I.; Yoon, J.-C.; Jang, J.-H. Chemical Vapor Deposition of Mesoporous Graphene Nanoballs for Supercapacitor. ACS Nano 2013, 7, 6047–6055. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Vasant Kumar, R.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal–organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef]
- Fan, L.-Z.; Hu, Y.-S.; Maier, J.; Adelhelm, P.; Smarsly, B.; Antonietti, M. High Electroactivity of Polyaniline in Supercapacitors by Using a Hierarchically Porous Carbon Monolith as a Support. Adv. Funct. Mater. 2007, 17, 3083–3087. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-M.; Park, H.G.; Lee, J.-S. Hierarchically Graphitic Carbon Structure Derived from Metal Ions Impregnated Harmful Inedible Seaweed as Energy-Related Material. Materials 2024, 17, 4643. https://doi.org/10.3390/ma17184643
Song Y-M, Park HG, Lee J-S. Hierarchically Graphitic Carbon Structure Derived from Metal Ions Impregnated Harmful Inedible Seaweed as Energy-Related Material. Materials. 2024; 17(18):4643. https://doi.org/10.3390/ma17184643
Chicago/Turabian StyleSong, Yun-Mi, Hui Gyeong Park, and Jung-Soo Lee. 2024. "Hierarchically Graphitic Carbon Structure Derived from Metal Ions Impregnated Harmful Inedible Seaweed as Energy-Related Material" Materials 17, no. 18: 4643. https://doi.org/10.3390/ma17184643
APA StyleSong, Y.-M., Park, H. G., & Lee, J.-S. (2024). Hierarchically Graphitic Carbon Structure Derived from Metal Ions Impregnated Harmful Inedible Seaweed as Energy-Related Material. Materials, 17(18), 4643. https://doi.org/10.3390/ma17184643





