Microstructure Refinement via Nucleation of Intragranular Acicular Ferrite Stimulated by Ti-Containing Core–Shell Structured Particles in Low-Carbon Steel
Abstract
1. Introduction
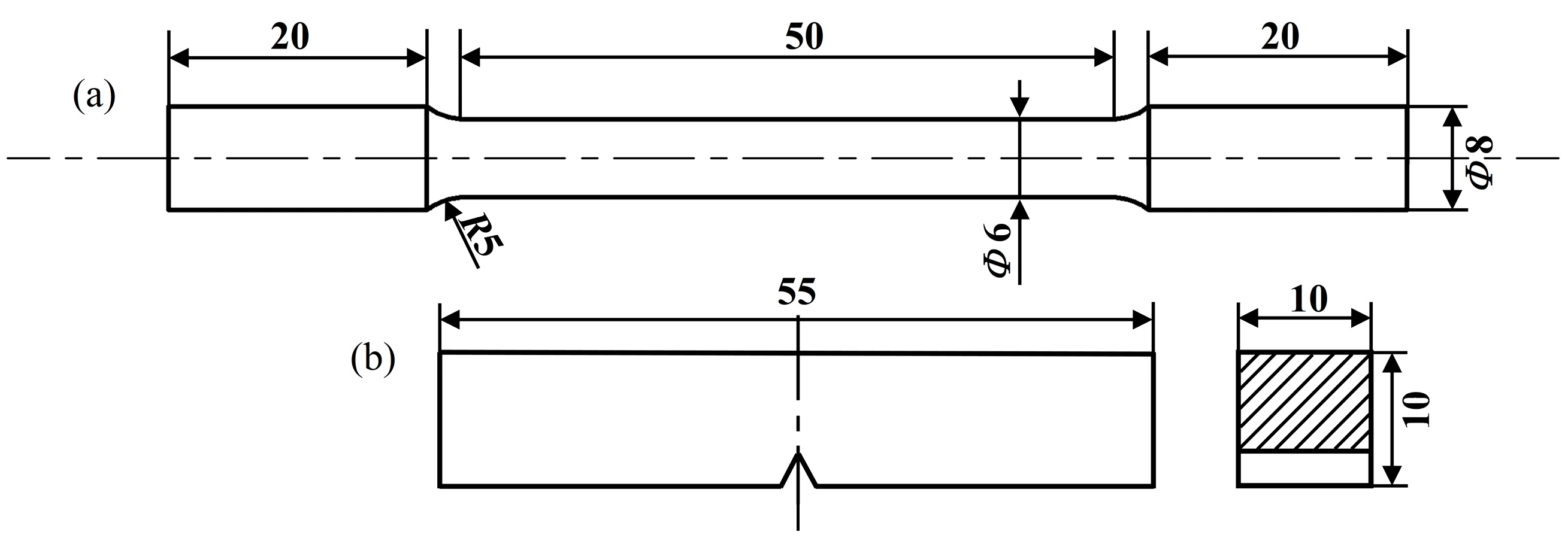
2. Experimental Materials and Methods
3. Results
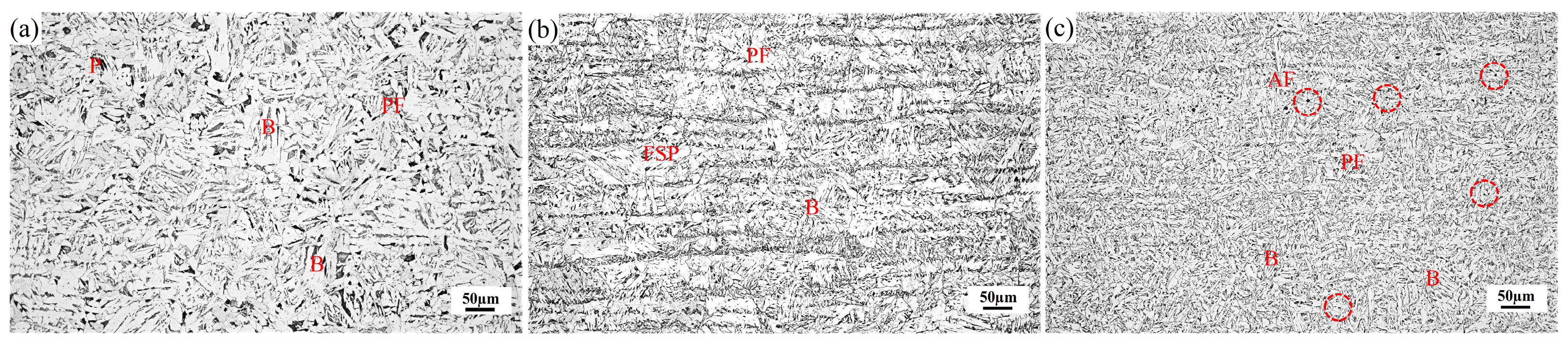
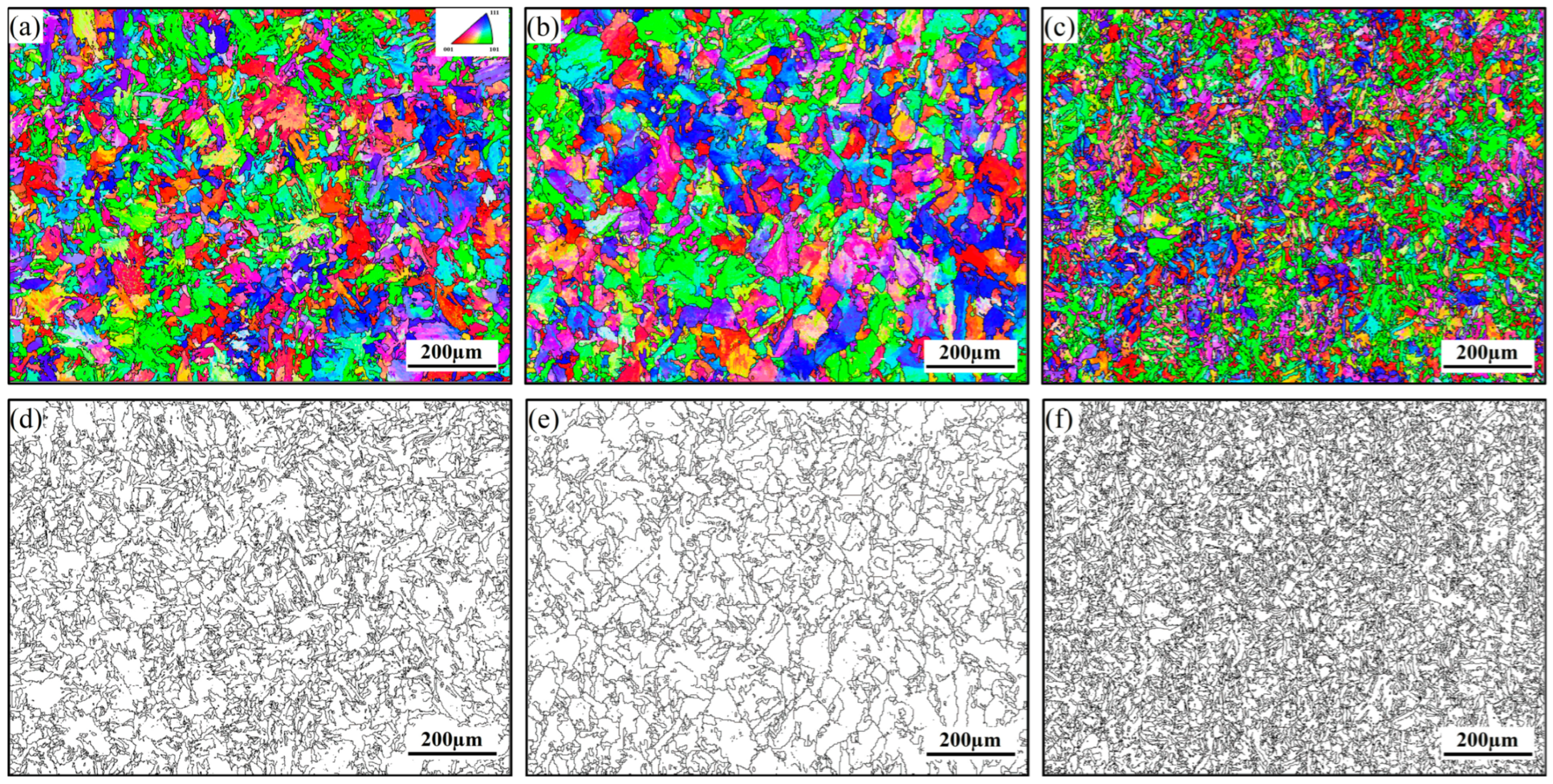
3.1. Microstructure of Hot-Rolled Steel Plates and Particles Characteristics
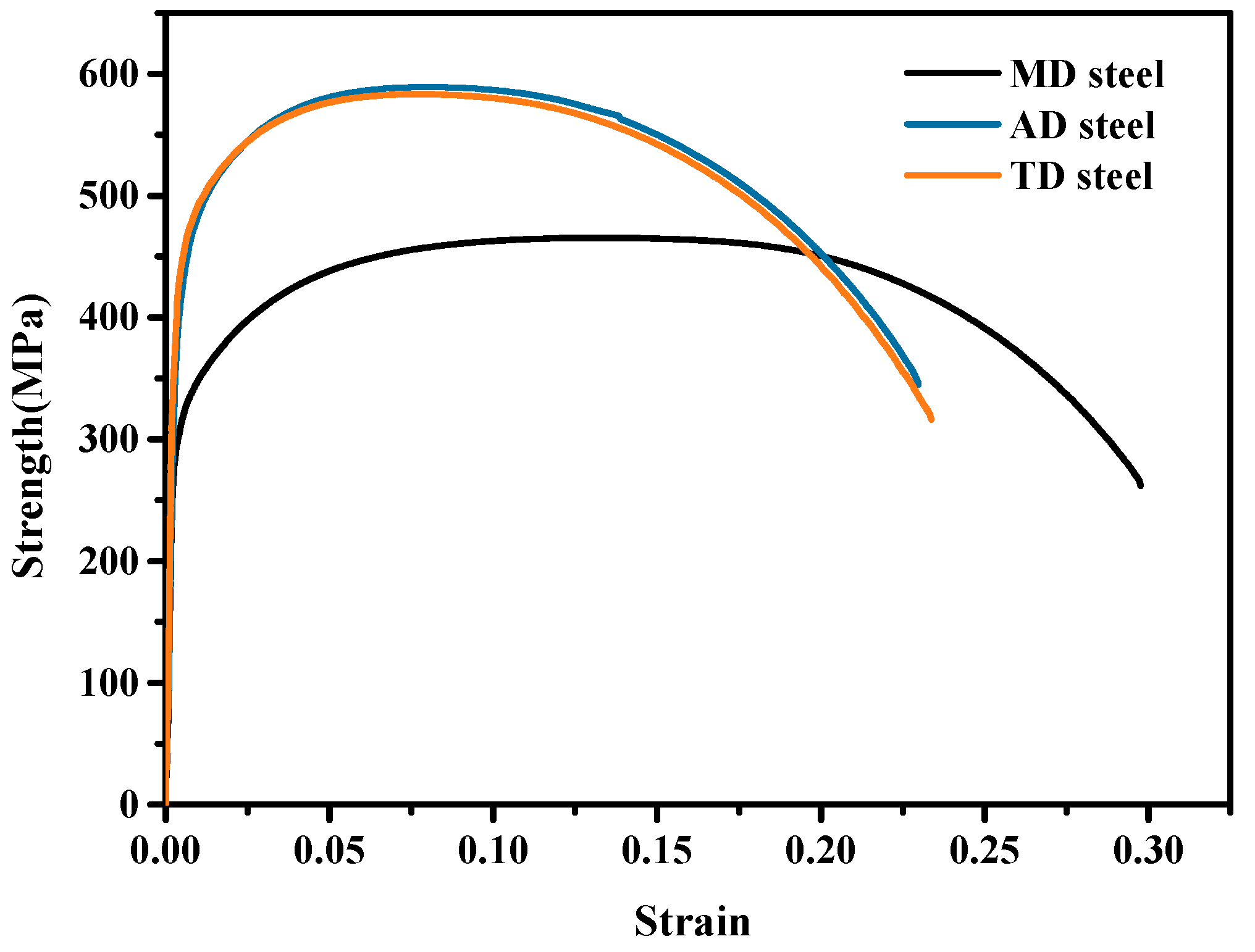
3.2. Mechanical Properties of the Experimental Steels
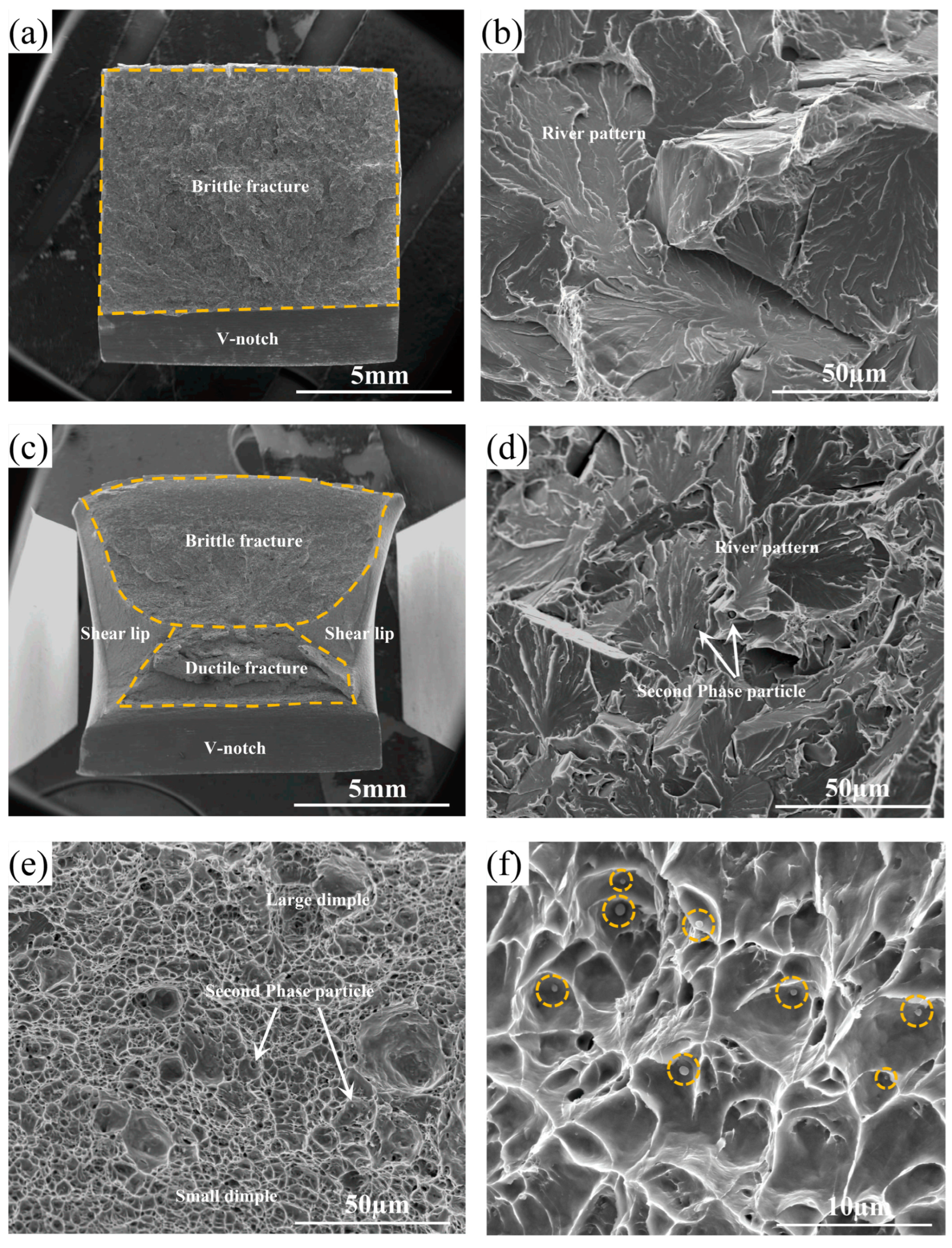
3.3. Fracture Morphologies of the Experimental Steels
4. Discussion
4.1. Interaction between Microstructure and Toughness
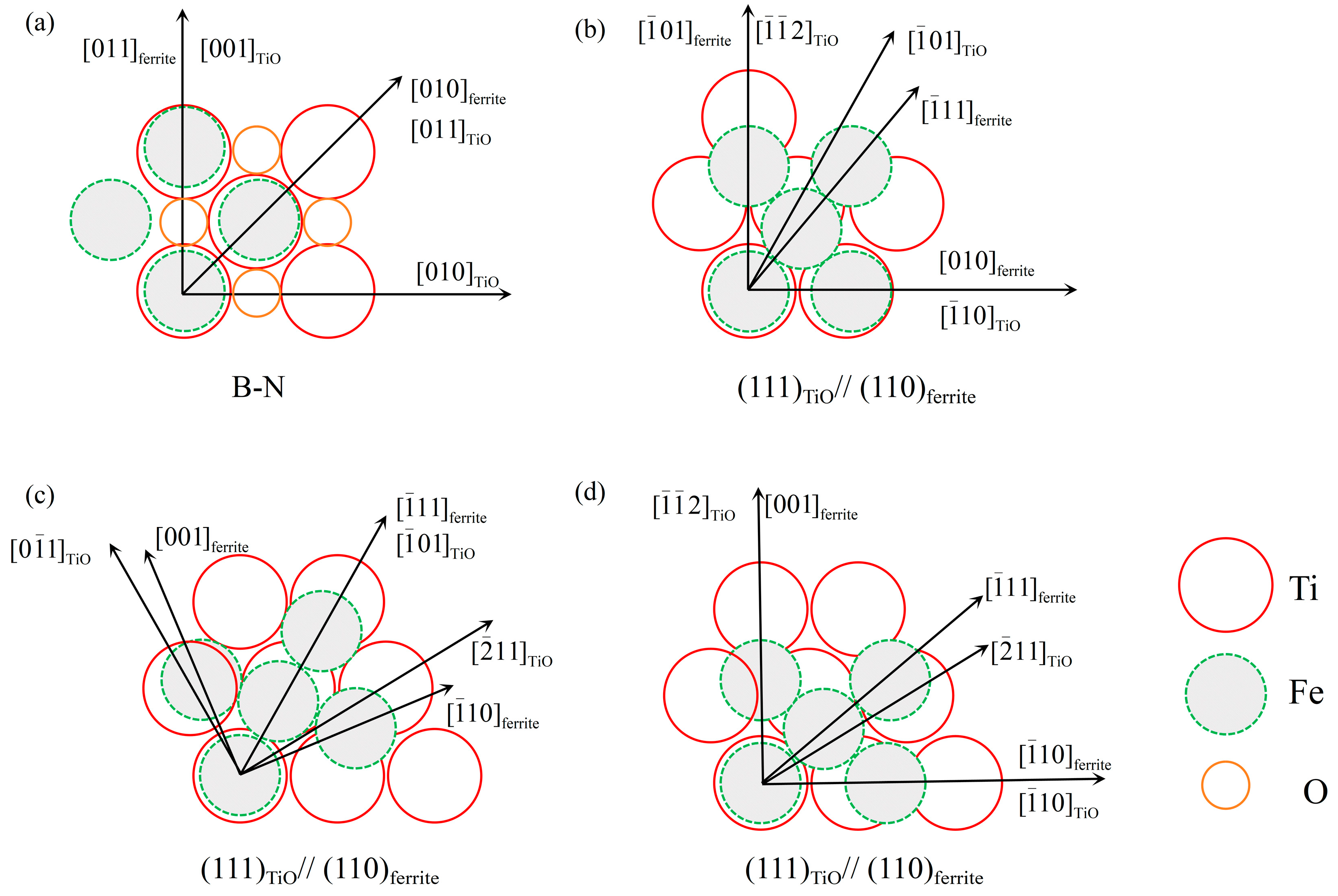
4.2. Mechanism of AF Nucleation Stimulated by Ti-Containing Particles
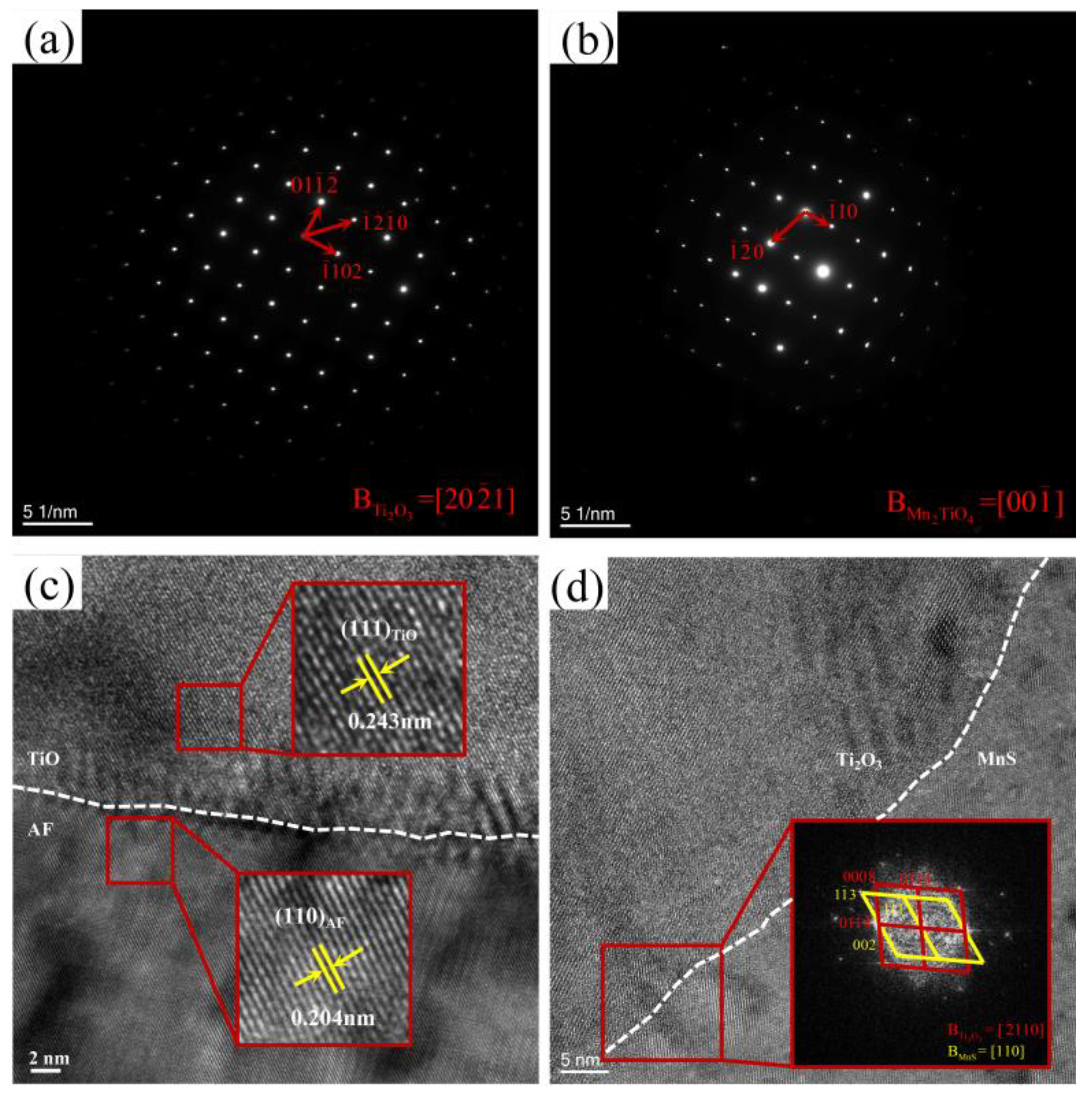
4.2.1. Existing Epistemic Relationship between the Nucleant and Nuclei
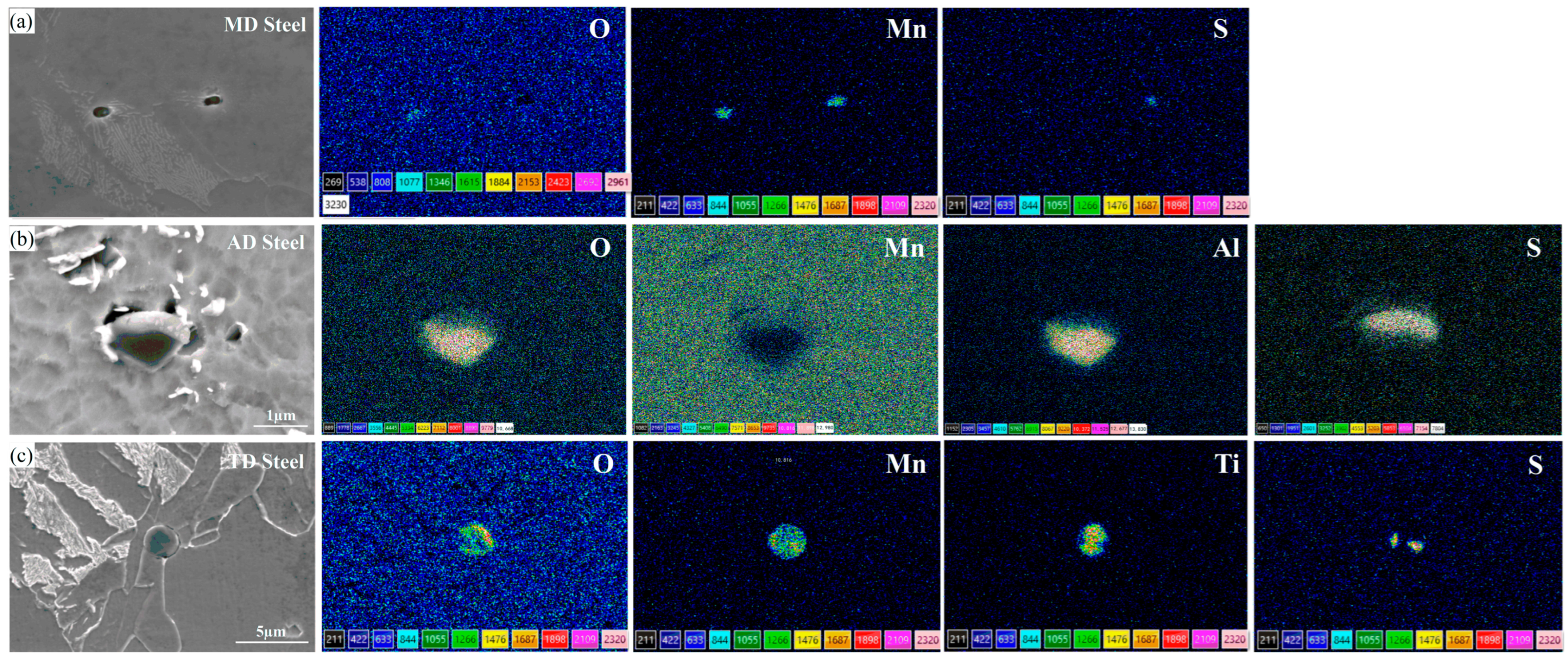
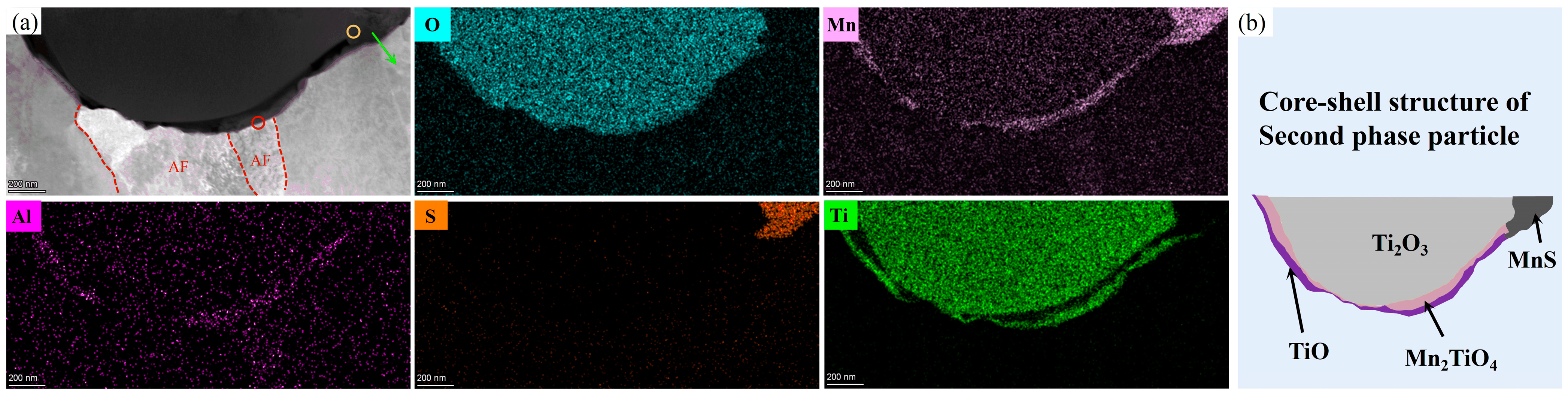
4.2.2. MDZ around MnS Precipitates on Ti Oxide Particles
5. Conclusions
- (1)
- Compared with Al and Mn deoxidized steels, the impact toughness of Ti deoxidized steel at −20 °C was significantly increased by nearly 130 J. The introduced Ti-containing second-phase particles can more effectively promote AF transformation, which contributes to microstructural refinement, and interlocking AF plates with high angular grain boundaries effectively improve the toughness.
- (2)
- TiO, the outermost layer of the second phase containing Ti core–shell particles, can effectively promote the nucleation of AF. TiO has a good semi-coherent relationship with AF and reduces the lattice mismatch, thus effectively stimulating the nucleation of AF.
- (3)
- The MnS precipitation on the Ti2O3 core adopts a particular orientation relationship of ||(002)MnS, ||[110]MnS, promoting the formation of MDZ near MnS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation/Symbol | Comment | |
| AF | Acicular ferrite | |
| MDZ | Mn-depleted zone | |
| TMCP | Thermo-mechanical control process | |
| IAF | Intragranular acicular ferrite | |
| PSN | Particle-stimulated nucleation | |
| MDZs | Mn-depleted zones | |
| MD | Mn deoxidized steel | |
| AD | Al deoxidized steel | |
| TD | Ti deoxidized steel | |
| OM | Optical microscope | |
| SEM | Scanning electron microscope | |
| EBSD | Electron backscatter diffraction | |
| STEM | Scanning transmission electron microscopy | |
| FIB | Focused ion beam | |
| HRTEM | High-resolution electron microscopy | |
| SAED | Selected area electron diffraction | |
| B | bainite | |
| PF | Polygonal ferrite | |
| P | Pearlite | |
| FSP | Ferrite side plate | |
| AEGS | Average effective grain size | |
| LAGBs | Low-angle grain boundaries | |
| HAGBs | High-angle grain boundaries | |
| FFT | Fast Fourier transform | |
| IFFT | Inverse fast Fourier transform | |
| Low-index crystallographic plane of the new phase | ||
| Low-index crystallographic plane of the core substrate | ||
| Low-index crystallographic direction on the crystallographic plane (hkl)n | ||
| Low-index crystallographic direction on the crystallographic plane (hkl)s | ||
| Atomic spacing along the crystallographic direction [uvw]n | ||
| Atomic spacing along the crystallographic direction [uvw]s | ||
| Angle between crystallographic directions [uvw]s and [uvw]n | ||
| Activation energy | ||
| Interfacial energy of AF/austenite phase | ||
| Driving force of the transformation | ||
| Strain energy | ||
| Shape factor | ||
| Contact angle of nucleus with respect to the precipitation interface | ||
| Interfacial energy of AF/particle | ||
| Interfacial energy of austenite phase/particle |
References
- Al Hajeri, K.F.; Garcia, C.I.; Hua, M.; Deardo, A.J. Particle-stimulated nucleation of ferrite in heavy steel sections. ISIJ Int. 2006, 46, 1233–1240. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Kang, J.; Yuan, G.; Misra, R.D.K.; Wang, G.D. Improved toughness of double-pass welding heat affected zone by fine Ti-Ca oxide inclusions for high-strength low-alloy steel. Mater. Sci. Eng. A 2020, 780, 139198. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Kang, J.; Wang, G.D.; Misra, D.; Yuan, G. Relationship between impact toughness and microstructure for as-rolled and simulated HAZ of low-carbon steel containing Ti-Ca oxide particles. Metall. Trans. A 2020, 51, 2927–2938. [Google Scholar] [CrossRef]
- Babu, S.S.; Bhadeshia, H.K.D.H. Mechanism of the transition from bainite to acicular ferrite. Mater. Trans. 2007, 32, 679–688. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Babu, S.S.; Bhadeshia, H.K.D.H. Transition from bainite to acicular ferrite in reheated Fe-Cr-C weld deposits. Mater. Sci. Technol. 1990, 10, 1005–1020. [Google Scholar] [CrossRef]
- Thewlis, G. Transformation kinetics of ferrous weld metals. Mater. Sci. Technol. 1994, 10, 110–125. [Google Scholar] [CrossRef]
- Wang, X.M.; He, X.L. Effect of boron addition on structure and properties of low carbon bainitic steels. ISIJ Int. 2002, 42, S38–S46. [Google Scholar] [CrossRef]
- Morral, J.E.; Cameron, T.B. A model for ferrite nucleation applied to boron hardenability. Metall. Trans. A 1977, 8, 1817–1819. [Google Scholar] [CrossRef]
- Mu, W.Z.; Jönsson, P.G.; Nakajima, K. Recent aspects on the effect of inclusion characteristics on the intragranular ferrite formation in low alloy steels: A review. High. Temp. Mat. Process. 2017, 36, 309–325. [Google Scholar] [CrossRef]
- Lee, C.; Nambu, S.; Inoue, J.; Koseki, T. Ferrite formation behaviors from B1 compounds in steels. ISIJ Int. 2011, 51, 2036–2041. [Google Scholar] [CrossRef]
- Takada, A.; Komizo, Y.I.; Terasaki, H.; Yokota, T.; Oi, K.; Yasuda, K. Crystallographic analysis for acicular ferrite formation in low carbon steel weld metals. Weld. Int. 2014, 29, 254–261. [Google Scholar] [CrossRef]
- Shim, J.H.; Cho, Y.W.; Chung, S.H.; Shim, J.D.; Lee, D.N. Nucleation of intragranular ferrite at Ti2O3 particle in low carbon steel. Acta Mater. 1999, 47, 2751–2760. [Google Scholar] [CrossRef]
- Shim, J.H.; Oh, Y.J.; Suh, J.Y.; Cho, Y.W.; Shim, J.D.; Byun, J.S.; Lee, D.N. Ferrite nucleation potency of non-metallic inclusions in medium carbon steels. Acta Mater. 2001, 49, 2115–2122. [Google Scholar] [CrossRef]
- Shim, J.H.; Byun, J.S.; Cho, Y.W.; Oh, Y.J.; Shim, J.D.; Lee, D.N. Mn absorption characteristics of Ti2O3 inclusions in low carbon steels. Scripta Mater. 2001, 44, 49–54. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bhadeshia, H. Titanium-rich mineral phases and the nucleation of bainite. Metall. Trans. A 1994, 25, 1603–1611. [Google Scholar] [CrossRef]
- ASTM E23-12c; Standard Test Methods for Notched Bar Impact Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2012.
- Dowling, J.M.; Corbett, J.M.; Kerr, H.W. Inclusion phases and the nucleation of acicular ferrite in submerged arc welds in high strength low alloy steels. Metall. Trans. A 1986, 17, 1611–1623. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Wang, S.; Song, B. Intragranular ferrite formation mechanism and mechanical properties of non-quenched-and-tempered medium carbon steels. Steel Res. Int. 2008, 79, 390–395. [Google Scholar] [CrossRef]
- Li, X.; Min, Y.; Liu, C.; Jiang, M. Study on the formation of intragranular acicular ferrite in a Zr-Mg-Al deoxidized low carbon steel. Steel Res. Int. 2016, 87, 622–632. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wang, Q.; Liu, Y.; Bai, Y.; He, T.; Yuan, G. Microstructural refinement by the formation of acicular ferrite on Ti-Mg oxide inclusion in low-carbon steel. Mater. Sci. Eng. A 2021, 824, 141795. [Google Scholar] [CrossRef]
- Curry, D.A. Grain-size dependence of cleavage fracture toughness in mild steel. Nature 1978, 276, 50–51. [Google Scholar] [CrossRef]
- Barritte, G.S.; Edmonds, D.V. Conference Record of Advances in the Physical Metallurgy and Applications of Steel; Metals Society: London, UK, 1982; p. 132. [Google Scholar]
- Zhang, S.; Hattori, N.; Enomoto, M.; Tarui, T. Ferrite nucleation at ceramic/austenite interfaces. ISIJ Int. 1996, 36, 1301–1309. [Google Scholar] [CrossRef]
- Zhang, Z.; Farrar, R.A. Role of non-metallic inclusions in formation of acicular ferrite in low alloy weld metals. Mater. Sci. Technol. 1996, 12, 237–260. [Google Scholar] [CrossRef]
- Ricks, R.A.; Howell, P.R.; Barritte, G.S. The nature of acicular ferrite in HSLA steel weld metals. J. Mater. Sci. 1982, 17, 732–740. [Google Scholar] [CrossRef]
- Yamada, T.; Terasaki, H.; Komizo, Y. Microscopic observation of inclusions contributing to formation of acicular ferrite in steel weld metal. Sci. Technol. Weld. Join. 2008, 13, 118–125. [Google Scholar] [CrossRef]
- Koseki, T.; Thewlis, G. Overview Inclusion assisted microstructure control in C-Mn and low alloy steel welds. Mater. Sci. Technol. 2005, 21, 867–879. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Capdevila, C.; Caballero, F.G.; Andrés, C.G.D. Influence of V precipitates on acicular ferrite transformation part 1: The role of nitrogen. ISIJ Int. 2008, 48, 1270–1275. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metal. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Shigesato, G.; Sugiyama, M.; Aihara, S.; Uemori, R.; Tomita, Y. Effect of Mn depletion on intra-granular ferrite transformation in heat affected zone of welding in low alloy steel. Tetsu Hagané 2001, 87, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, H.; Uemori, R.; Fujioka, M. The role of Mn depletion in intra-granular ferrite transformation in the heat affected zone of welded joints with large heat input in structural steels. ISIJ Int. 1996, 36, 1406–1412. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Nune, K.C.; Li, Y.; Cheng, L. In situ observation of acicular ferrite formation and grain refinement in simulated heat affected zone of high strength low alloy steel. Sci. Technol. Weld. Join. 2012, 20, 254–263. [Google Scholar] [CrossRef]
- Wan, X.L.; Wang, H.H.; Cheng, L.; Wu, K.M. The formation mechanisms of interlocked microstructures in low-carbon high-strength steel weld metals. Mater. Charact. 2012, 67, 41–51. [Google Scholar] [CrossRef]


| Steel | C | Si | Mn | P | S | Cr | Al | Ti |
|---|---|---|---|---|---|---|---|---|
| MD | 0.12 | 0.01 | 1.66 | 0.015 | 0.006 | 0.31 | 0.003 | - |
| AD | 0.13 | 0.03 | 1.66 | 0.016 | 0.005 | 0.32 | 0.02 | - |
| TD | 0.12 | 0.01 | 1.55 | 0.015 | 0.006 | 0.30 | 0.002 | 0.01 |
| Steel | Yield Strength [MPa] | Ultimate Tensile Strength [MPa] | Impact Toughness [J] |
|---|---|---|---|
| MD | 293 ± 1 | 465 ± 2 | 18 ± 2 |
| AD | 408 ± 0 | 587 ± 2 | 9 ± 2 |
| TD | 425 ± 3 | 584 ± 1 | 144 ± 16 |
| Phase | Lattice Parameter [Å] | Parallelisms | Misfit with Ferrite [%] |
|---|---|---|---|
| TiO | 4.293 | B-N | 3 |
| (111)TiO||(110)ferrite [−110]TiO||[010]ferrite | 18.2 | ||
| (111)TiO||(110)ferrite [−101]TiO||[−111]ferrite | 17 | ||
| (111)TiO||(110)ferrite [−110]TiO||[−110]ferrite | 42 | ||
| Ferrite | 2.886 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Wang, C.; Duan, H.; Hao, J.; Yuan, G. Microstructure Refinement via Nucleation of Intragranular Acicular Ferrite Stimulated by Ti-Containing Core–Shell Structured Particles in Low-Carbon Steel. Materials 2024, 17, 4644. https://doi.org/10.3390/ma17184644
Yan Z, Wang C, Duan H, Hao J, Yuan G. Microstructure Refinement via Nucleation of Intragranular Acicular Ferrite Stimulated by Ti-Containing Core–Shell Structured Particles in Low-Carbon Steel. Materials. 2024; 17(18):4644. https://doi.org/10.3390/ma17184644
Chicago/Turabian StyleYan, Zhu, Chao Wang, Hua Duan, Junjie Hao, and Guo Yuan. 2024. "Microstructure Refinement via Nucleation of Intragranular Acicular Ferrite Stimulated by Ti-Containing Core–Shell Structured Particles in Low-Carbon Steel" Materials 17, no. 18: 4644. https://doi.org/10.3390/ma17184644
APA StyleYan, Z., Wang, C., Duan, H., Hao, J., & Yuan, G. (2024). Microstructure Refinement via Nucleation of Intragranular Acicular Ferrite Stimulated by Ti-Containing Core–Shell Structured Particles in Low-Carbon Steel. Materials, 17(18), 4644. https://doi.org/10.3390/ma17184644





