A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Mechanical Activation
2.3. Design of Experiments (DoE)
- i.
- Maximize the amorphous content (Y1), as it is significantly more reactive than crystalline phases. While the amorphous content is mainly from Kaol transformation, the other crystalline phases in K (illite, microcline, and quartz) may also contribute to the total amorphous content through partial or complete amorphization.
- ii.
- Minimize the impurity of ZrO2 (Y2) to avoid impurities in the material and prevent the deterioration of the milling media.
- iii.
- Achieve a minimum (≈0%; target = 0) of crystalline Kaol (Y3) to ensure complete amorphization of the initial Kaol.
3. Results
DoE Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winnefeld, F.; Leemann, A.; German, A.; Lothenbach, B. CO2 storage in cement and concrete by mineral carbonation. Curr. Opin. Green Sustain. Chem. 2022, 38, 100672. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental impact of cement production and Solutions: A review. Mater. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, S.; Chen, Y. Modeling of the Thermal Efficiency of a Whole Cement Clinker Calcination System and Its Application on a 5000 MT/D Production Line. Energies 2020, 13, 5257. [Google Scholar] [CrossRef]
- Hosten, C.; Fidan, B. An industrial comparative study of cement clinker grinding systems regarding the specific energy consumption and cement properties. Powder Technol. 2012, 221, 183–188. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Al-Dhamri, H.; Ram, G.; Chatterjee, V.P. An overview of alternative raw materials used in cement and clinker manufacturing. Int. J. Sustain. Eng. 2021, 14, 743–760. [Google Scholar] [CrossRef]
- Pisciotta, M.; Pilorgé, H.; Davids, J.; Psarras, P. Opportunities for cement decarbonization. Clean. Eng. Technol. 2023, 15, 100667. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sui, T. Alternative fuels—Effects on clinker process and properties. Cem. Concr. Res. 2019, 123, 105777. [Google Scholar] [CrossRef]
- Aguirre Castillo, J.; Broström, M.; Eriksson, M. Phase evolution and burnability of cement raw meal. Adv. Cem. Res. 2023, 35, 577–587. [Google Scholar] [CrossRef]
- Krour, H.; Trauchessec, R.; Lecomte, A.; Diliberto, C.; Barnes-Davin, L.; Bolze, B.; Delhay, A. Incorporation rate of recycled aggregates in cement raw meals. Constr. Build. Mater. 2020, 248, 118217. [Google Scholar] [CrossRef]
- Kriven, W.M.; Leonelli, C.; Provis, J.L.; Boccaccini, A.R.; Attwell, C.; Ducman, V.S.; Ferone, C.; Rossignol, S.; Luukkonen, T.; van Deventer, J.S.J.; et al. Why geopolymers and alkali-activated materials are key components of a sustainable world: A perspective contribution. J. Am. Ceram. Soc. 2024, 107, 5159–5177. [Google Scholar] [CrossRef]
- Shah, I.H.; Miller, S.A.; Jiang, D.; Myers, R.J. Cement substitution with secondary materials can reduce annual global CO2 emissions by up to 1.3 gigatons. Nat. Commun. 2022, 13, 5758. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Nehdi, M.L.; Marani, A.; Zhang, L. Is net-zero feasible: Systematic review of cement and concrete decarbonization technologies. Renew. Sustain. Energy Rev. 2024, 191, 114169. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Suraneni, P.; Hajibabaee, A.; Ramanathan, S.; Wang, Y.; Weiss, J. New insights from reactivity testing of supplementary cementitious materials. Cem. Concr. Compos. 2019, 103, 331–338. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Saillio, M.; Baroghel-Bouny, V.; Bertin, M.; Pradelle, S.; Vincent, J. Phase assemblage of cement pastes with SCM at different ages. Constr. Build. Mater. 2019, 224, 144–157. [Google Scholar] [CrossRef]
- Alvarez-Coscojuela, A.; Marco-Gibert, J.; Mañosa, J.; Formosa, J.; Chimenos, J.M. Thermal activation of kaolinite through potassium acetate intercalation: A structural and reactivity study. Appl. Clay Sci. 2024, 259, 107515. [Google Scholar] [CrossRef]
- Alujas Diaz, A.; Almenares Reyes, R.S.; Hanein, T.; Irassar, E.F.; Juenger, M.; Kanavaris, F.; Maier, M.; Marsh, A.T.; Sui, T.; Thienel, K.-C.; et al. Properties and occurrence of clay resources for use as supplementary cementitious materials: A paper of RILEM TC 282-CCL. Mater. Struct. 2022, 55, 139. [Google Scholar] [CrossRef]
- Weise, K.; Ukrainczyk, N.; Koenders, E. Pozzolanic Reactions of Metakaolin with Calcium Hydroxide: Review on Hydrate Phase Formations and Effect of Alkali Hydroxides, Carbonates and Sulfates. Mater. Des. 2023, 231, 112062. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Reactivity of kaolinitic clays calcined in the 650 °C–1050 °C temperature range: Towards a robust assessment of overcalcination. Cem. Concr. Compos. 2024, 146, 105380. [Google Scholar] [CrossRef]
- Daou, I.; Lecomte-Nana, G.L.; Tessier-Doyen, N.; Peyratout, C.; Gonon, M.F.; Guinebretiere, R. Probing the Dehydroxylation of Kaolinite and Halloysite by In Situ High Temperature X-ray Diffraction. Minerals 2020, 10, 480. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.-C.; Zunino, F.; Marsh, A.T.M.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.G.; Ben Haha, M.; Avet, F.; et al. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2021, 55, 3. [Google Scholar] [CrossRef]
- Fitos, M.; Badogiannis, E.G.; Tsivilis, S.G.; Perraki, M. Pozzolanic activity of thermally and mechanically treated kaolins of hydrothermal origin. Appl. Clay Sci. 2015, 116–117, 182–192. [Google Scholar] [CrossRef]
- Balczár, I.; Korim, T.; Kovács, A.; Makó, É. Mechanochemical and thermal activation of kaolin for manufacturing geopolymer mortars—Comparative study. Ceram. Int. 2016, 42, 15367–15375. [Google Scholar] [CrossRef]
- Souri, A.; Kazemi-Kamyab, H.; Snellings, R.; Naghizadeh, R.; Golestani-Fard, F.; Scrivener, K. Pozzolanic activity of mechanochemically and thermally activated kaolins in cement. Cem. Concr. Res. 2015, 77, 47–59. [Google Scholar] [CrossRef]
- Mañosa, J.; Gómez-Carrera, A.M.; Svobodova-Sedlackova, A.; Maldonado-Alameda, A.; Fernández-Jiménez, A.; Chimenos, J.M. Potential reactivity assessment of mechanically activated kaolin as alternative cement precursor. Appl. Clay Sci. 2022, 228, 106648. [Google Scholar] [CrossRef]
- Mañosa, J.; Calvo de la Rosa, J.; Silvello, A.; Maldonado, A.; Chimenos, J. Kaolinite structural modifications induced by mechanical activation. Appl. Clay Sci. 2023, 238, 106918. [Google Scholar] [CrossRef]
- Bizley, D. World Cement. 2023. Available online: https://www.worldcement.com/product-news/12122023/thyssenkrupp-polysius-presents-new-meca-clay-clinker-reduction-solution/ (accessed on 19 September 2024).
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Ben Haha, M.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; De Weerdt, K.; Dhandapani, Y.; et al. Reactivity tests for supplementary cementitious materials: RILEM TC 267-TRM phase 1. Mater. Struct. 2018, 51, 151. [Google Scholar] [CrossRef]
- Ababneh, A.; Matalkah, F.; Matalkeh, B. Effects of kaolin characteristics on the mechanical properties of alkali-activated binders. Constr. Build. Mater. 2022, 318, 126020. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Farhanchi, M.; Neysari, M.; Vatankhah Barenji, R.; Heidarzadeh, A.; Taherzadeh Mousavian, R. Mechanical activation process for self-propagation high-temperature synthesis of ceramic-based composites. J. Therm. Anal. Calorim. 2015, 122, 123–133. [Google Scholar] [CrossRef]
- ASTM C1897-20; ASTM International Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water Measurements. ASTM: West Conshohocken, PA, USA, 2020.
- Chen, G.; Xiong, K.; Peng, J.; Chen, J. Optimization of combined mechanical activation-roasting parameters of titania slag using response surface methodology. Adv. Powder Technol. 2010, 21, 331–335. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 2011, 21, 2118–2125. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Frost, R.L. Vibrational Spectroscopy of Intercalated Kaolinites. Part I. Appl. Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef]
- Alex, T.C.; Kumar, R.; Roy, S.K.; Mehrotra, S.P. Mechanical Activation of Al-oxyhydroxide Minerals–A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 1–26. [Google Scholar] [CrossRef]
- Sun, D.; Li, B.; Li, Y.; Yu, C.; Zhang, B.; Fei, H. Characterization of exfoliated/delamination kaolinite. Mater. Res. Bull. 2011, 46, 101–104. [Google Scholar] [CrossRef]
- D’Elia, A.; Pinto, D.; Eramo, G.; Giannossa, L.C.; Ventruti, G.; Laviano, R. Effects of processing on the mineralogy and solubility of carbonate-rich clays for alkaline activation purpose: Mechanical, thermal activation in red/ox atmosphere and their combination. Appl. Clay Sci. 2018, 152, 9–21. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Másilko, J.; Wasserbauer, J. Preparation of dehydroxylated and delaminated talc: Meta-talc. Ceram. Int. 2013, 39, 9055–9061. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; del Carmen Jiménez de Haro, M.; Pérez-Maqueda, L.A.; Varona, I.; Pérez-Rodríguez, J.L. Effects of Dry Grinding on the Structural Changes of Kaolinite Powders. J. Am. Ceram. Soc. 2000, 83, 1649–1657. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kamitani, M.; Nagai, T.; Kano, J.; Saito, F. Amorphization of kaolinite and media motion in grinding by a double rotating cylinders mill—A comparison with a tumbling ball mill. Adv. Powder Technol. 2000, 11, 235–244. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-642-09426-2. [Google Scholar]
- Horváth, E.; Frost, R.L.; Makó, É.; Kristóf, J.; Cseh, T. Thermal treatment of mechanochemically activated kaolinite. Thermochim. Acta 2003, 404, 227–234. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Zhang, J.; Wang, J.; Lyu, X. Effects of mechanical grinding on pozzolanic activity and hydration properties of quartz. Adv. Powder Technol. 2020, 31, 4500–4509. [Google Scholar] [CrossRef]
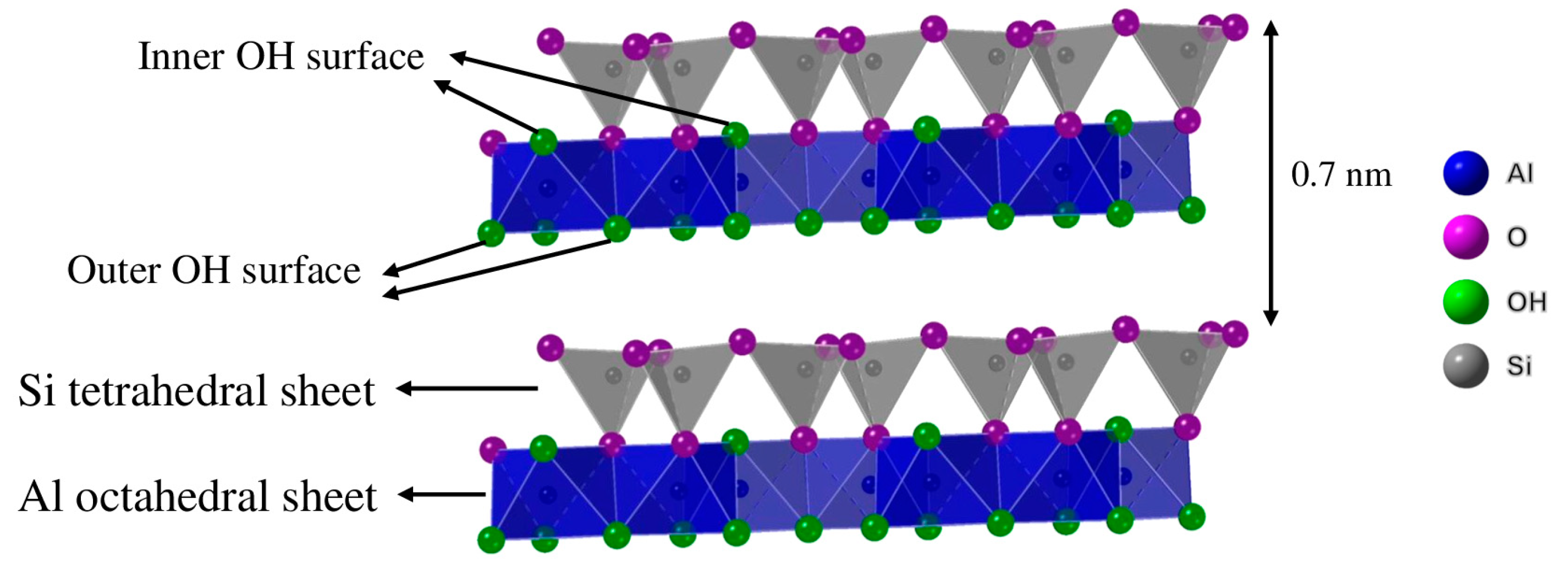
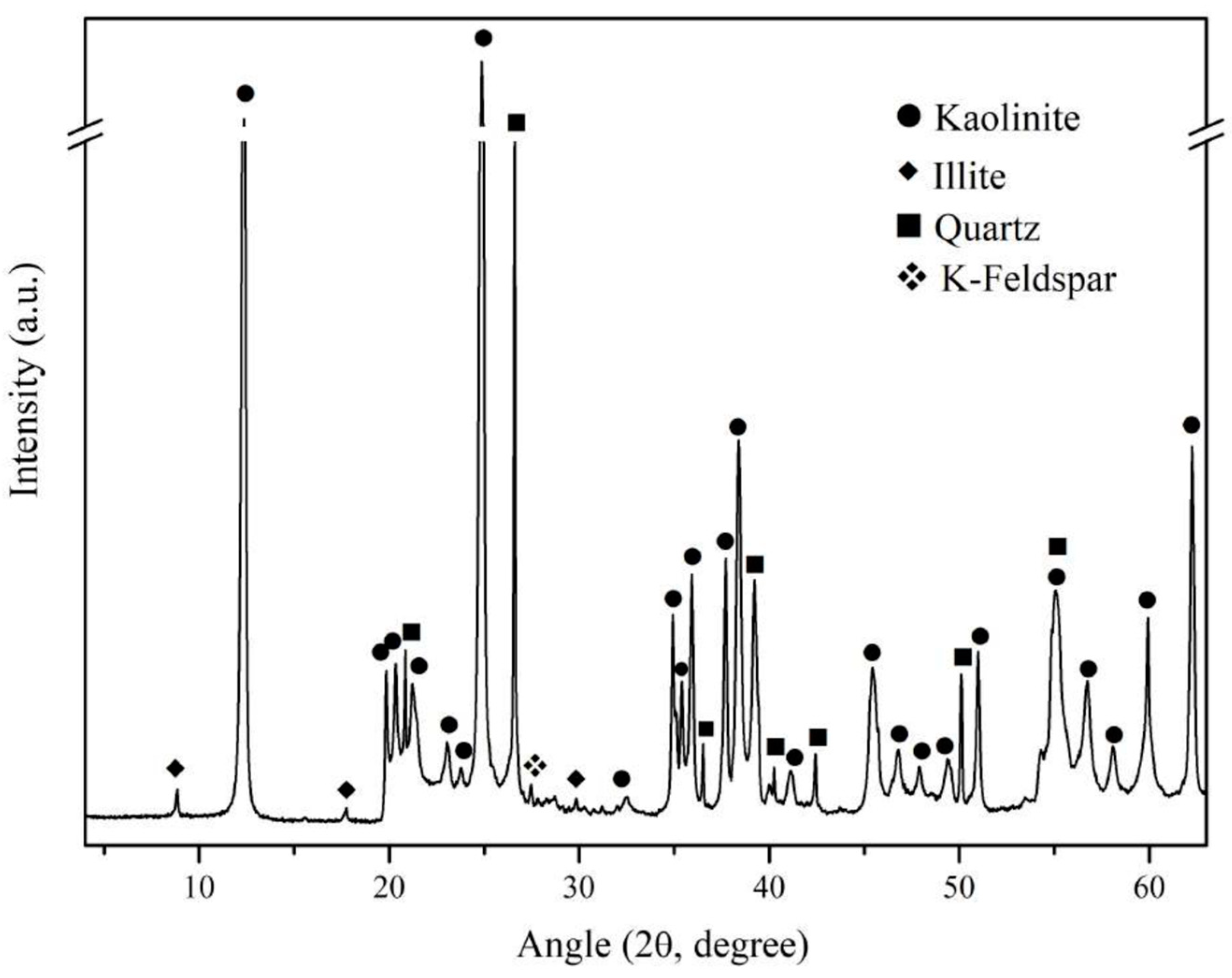


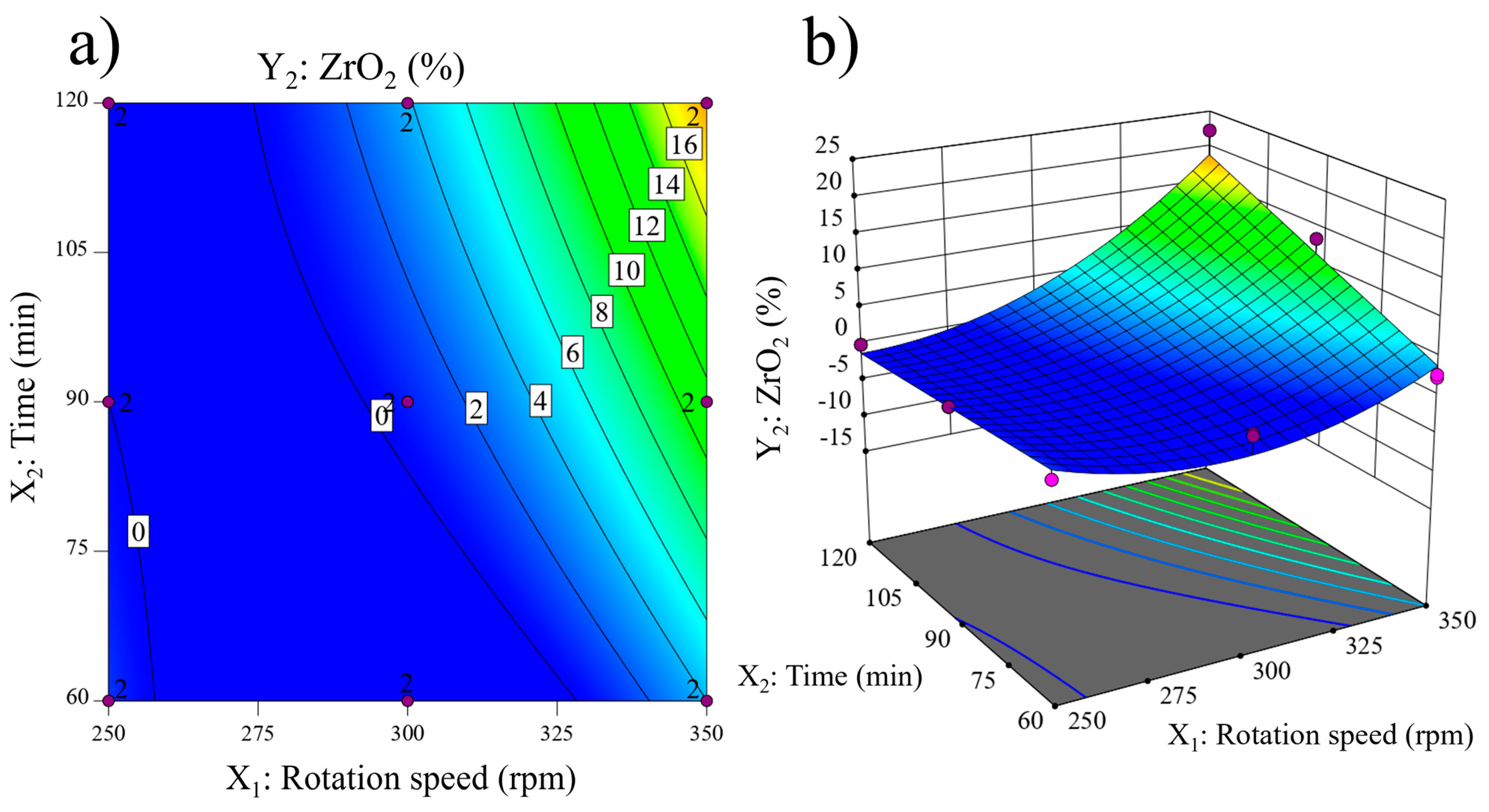
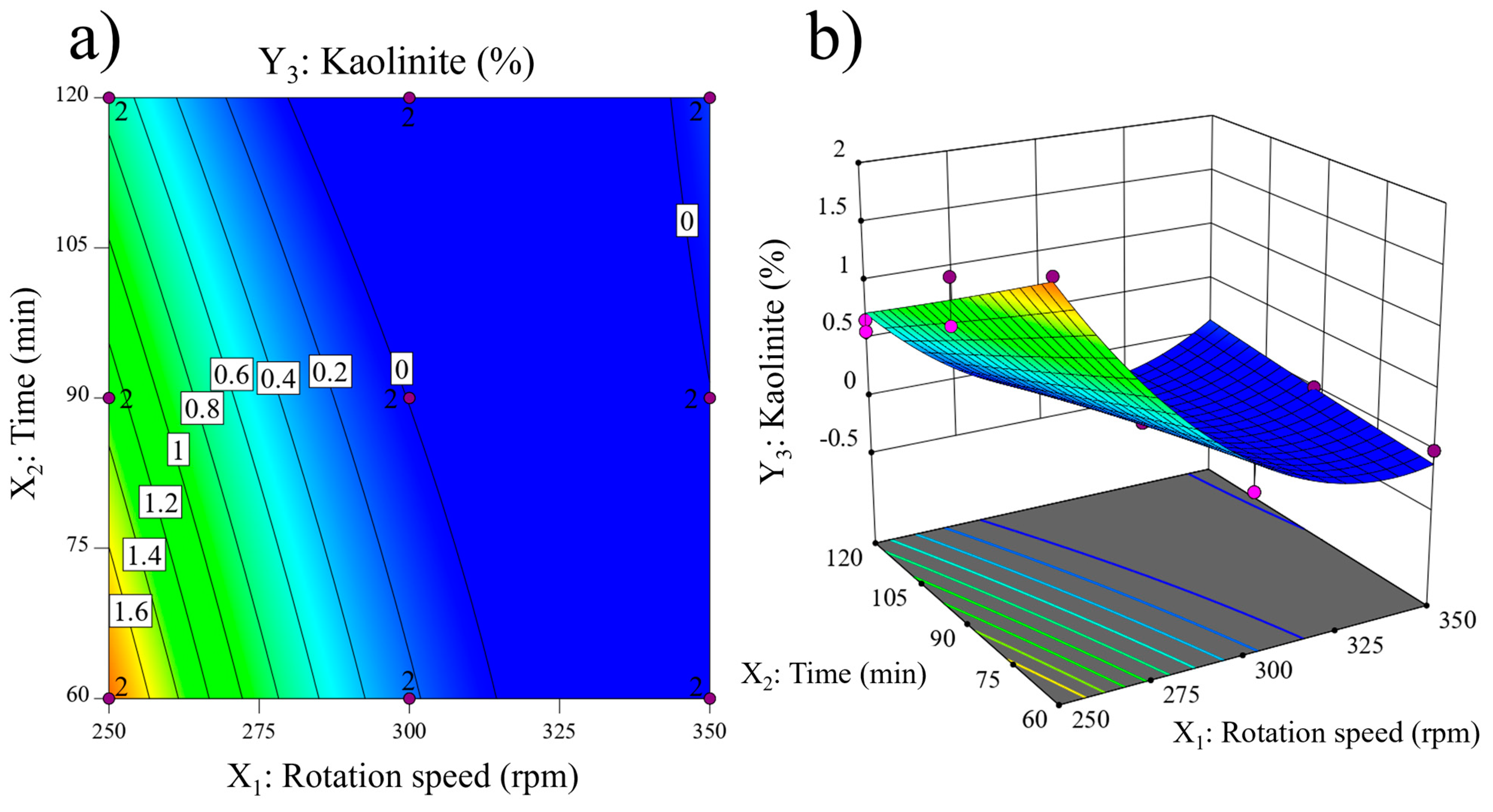
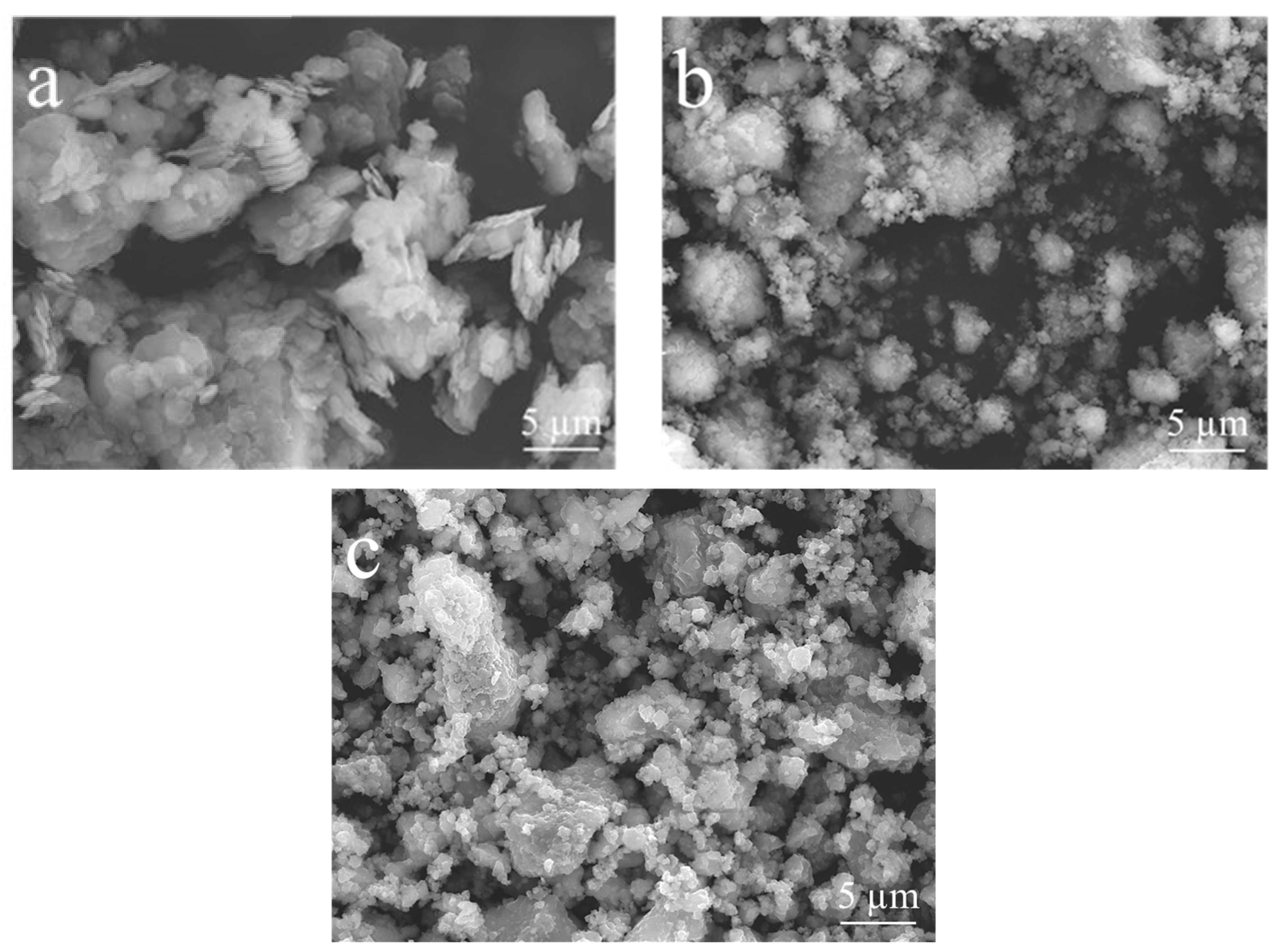
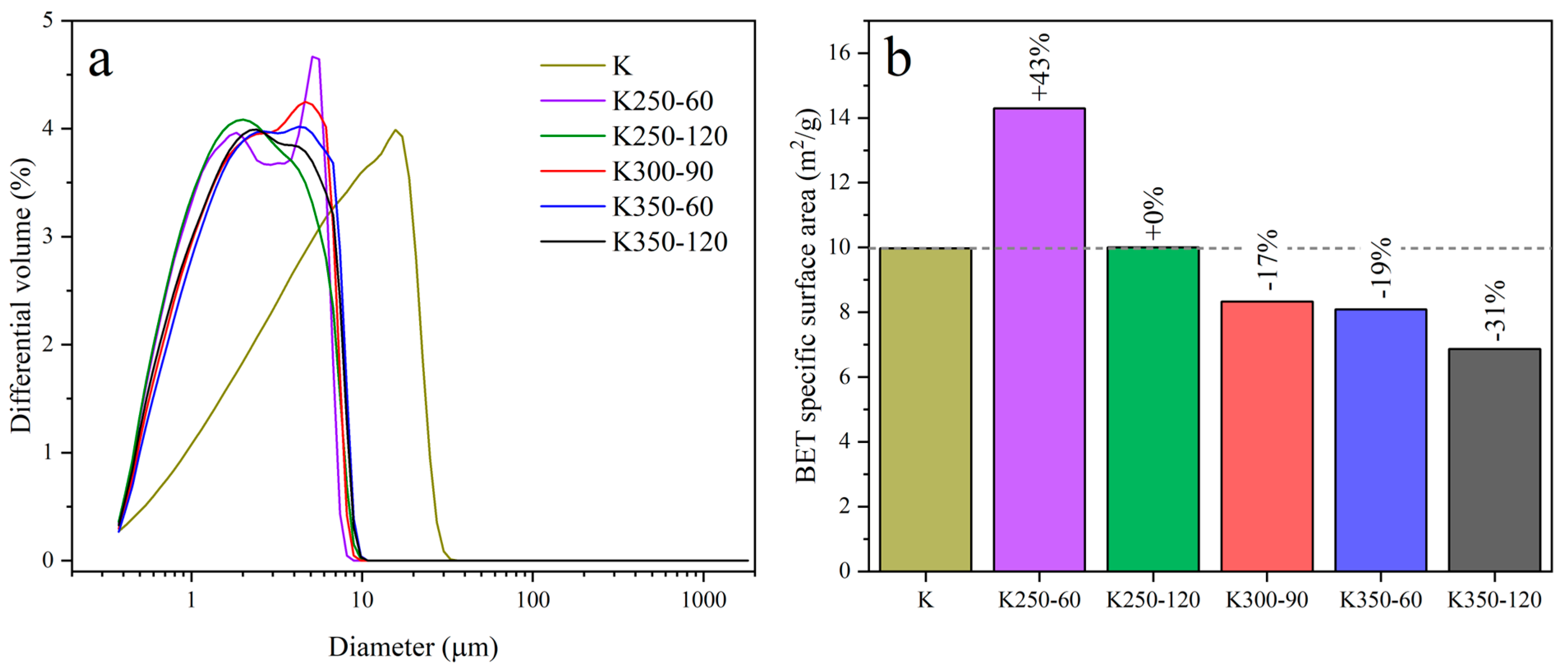
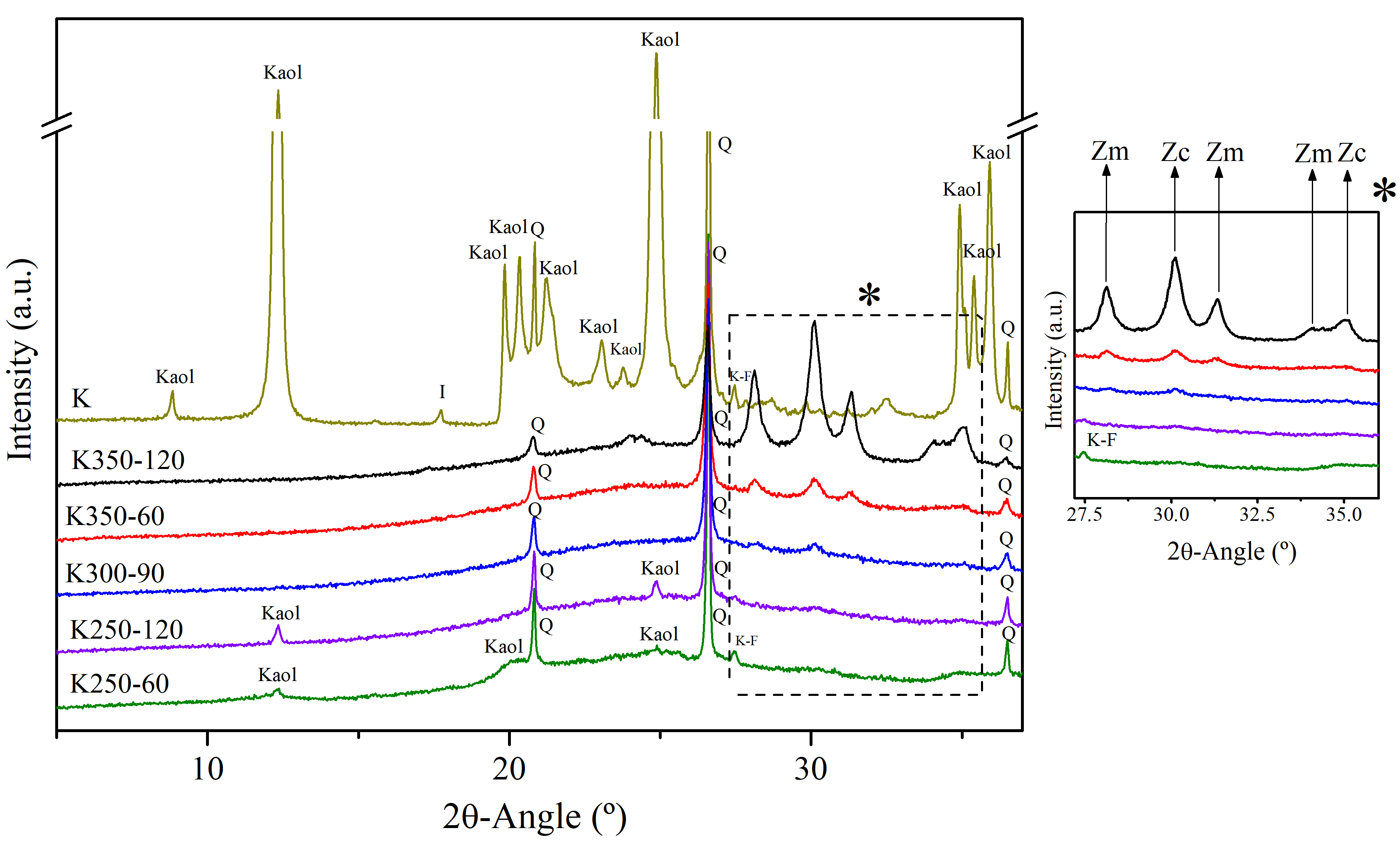
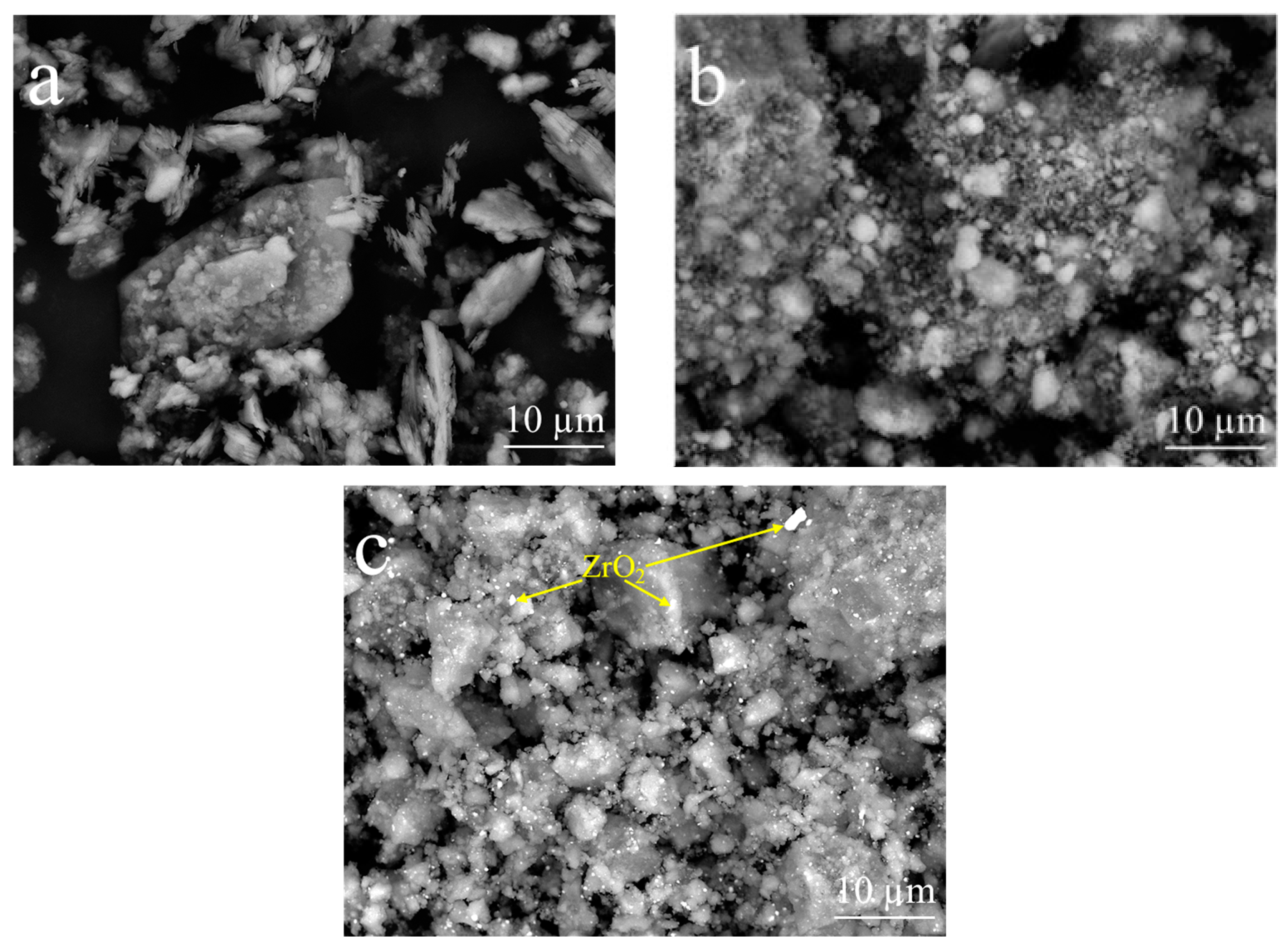
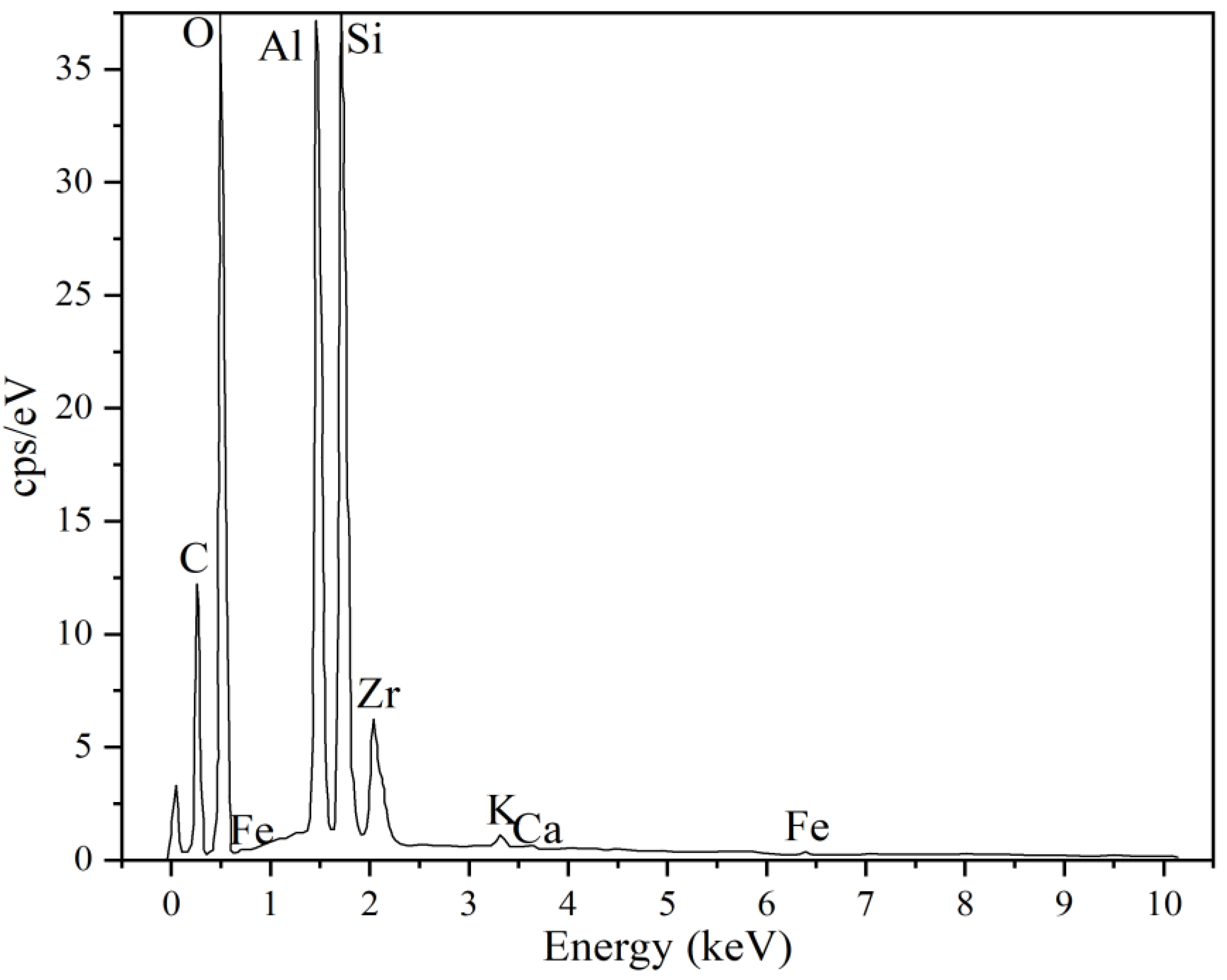
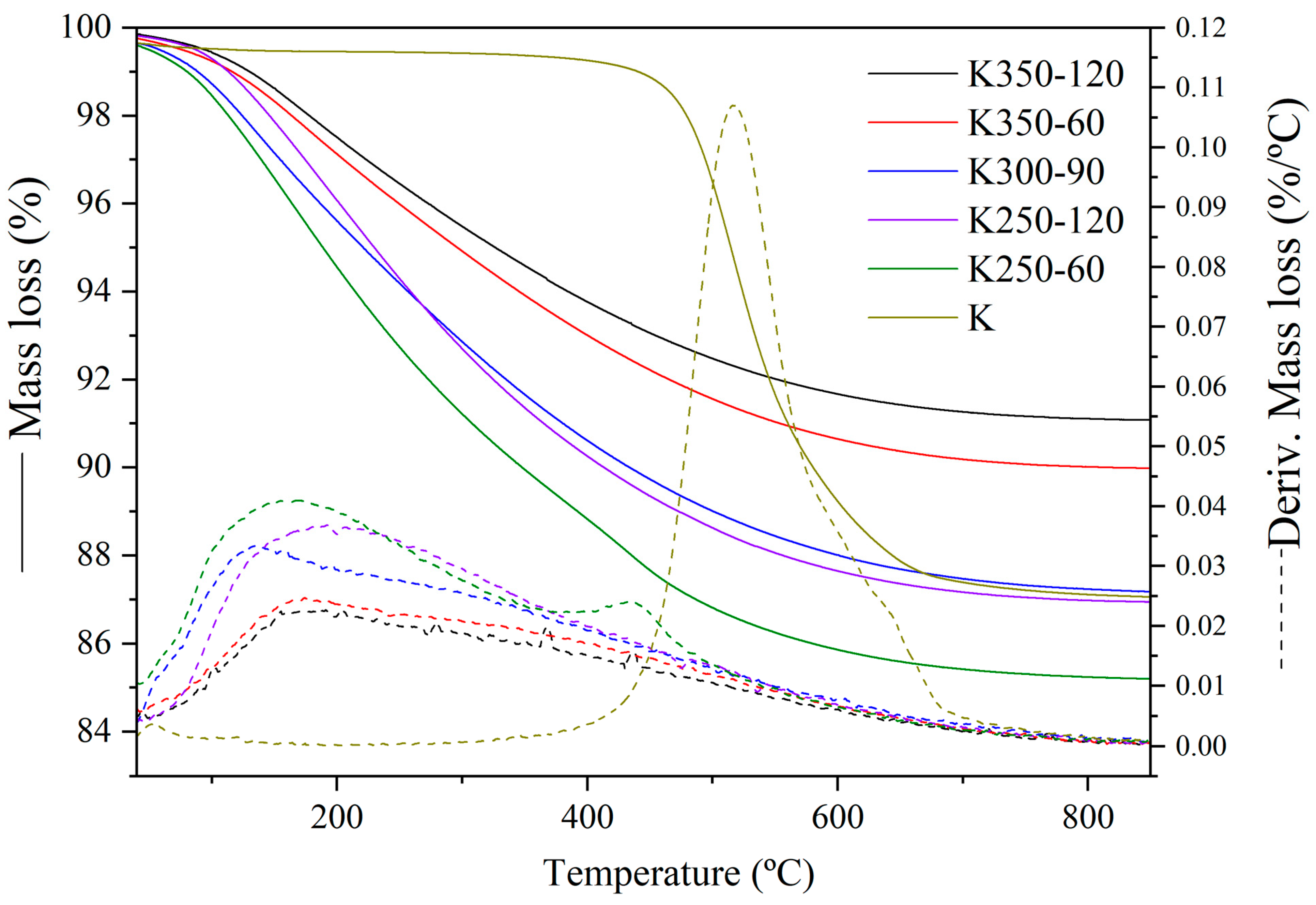

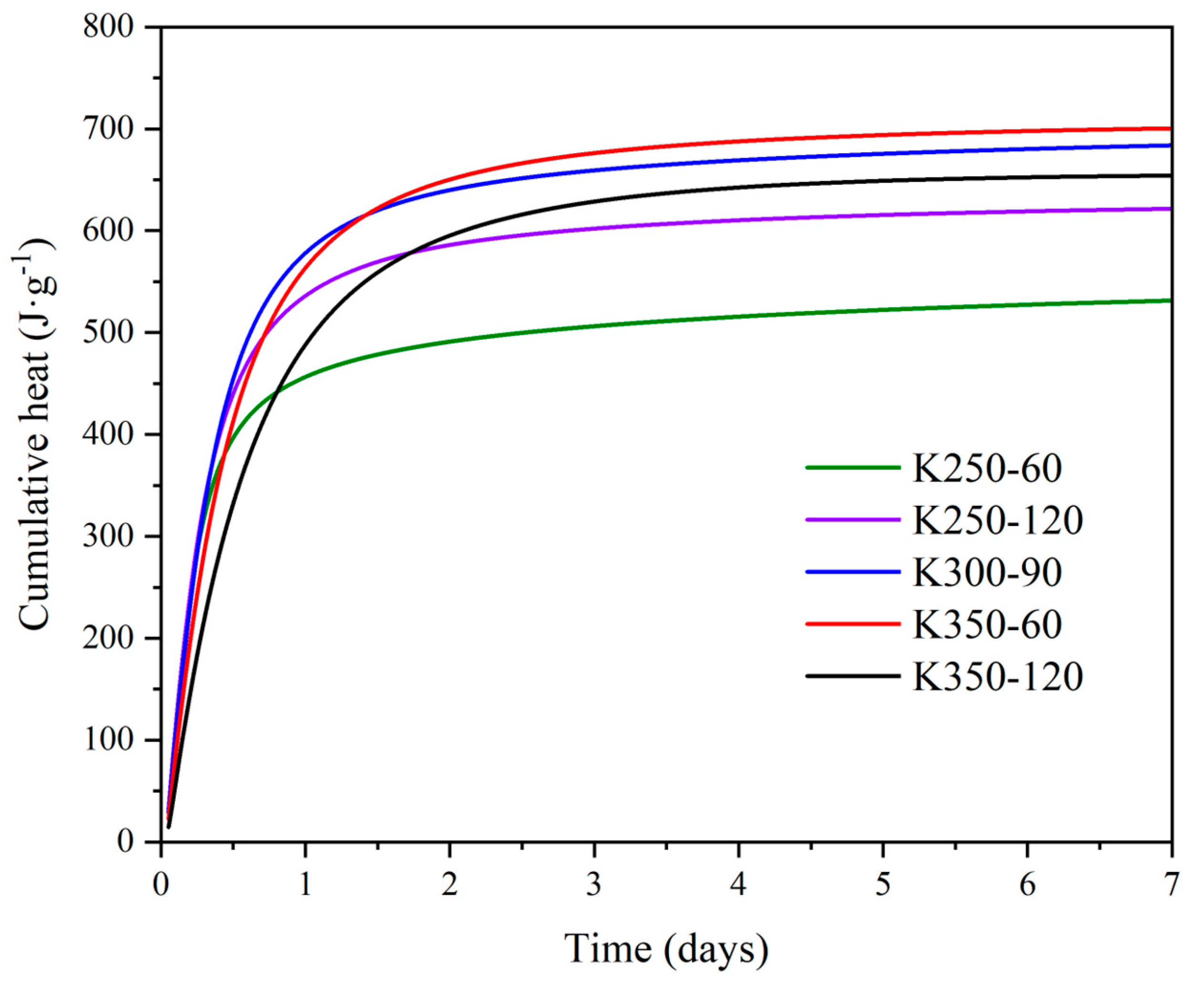
| Compound | SiO2 | Al2O3 | K2O | Fe2O3 | CaO | TiO2 | Na2O | MgO | P2O5 | LOI a |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 49.85 | 36.31 | 0.69 | 0.47 | 0.16 | 0.15 | 0.13 | 0.11 | 0.08 | 12.50 |
| Mineral Phases | (wt.%) |
|---|---|
| Kaolinite | 82.0 |
| Quartz | 5.7 |
| Illite | 5.3 |
| K-feldspar | 1.6 |
| Amorphous content | 5.4 |
| Independent Variables | Symbol | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Rotation speed (rpm) | X1 | 250 | 300 | 350 |
| Milling time (min) | X2 | 60 | 90 | 120 |
| Experiment | X1 (rpm) | X2 (Min) | Y1 (%) | Y2 (%) | Y3 (%) |
|---|---|---|---|---|---|
| 1 | 250 (−1) | 60 (−1) | 89.92 | - | 1.94 |
| 2 | 250 (−1) | 60 (−1) | 89.71 | - | 2.11 |
| 3 | 350 (+1) | 60 (−1) | 87.73 | 2.46 | - |
| 4 | 350 (+1) | 60 (−1) | 87.94 | 2.83 | - |
| 5 | 250 (−1) | 120 (+1) | 91.92 | - | 0.67 |
| 6 | 250 (−1) | 120 (+1) | 92.05 | - | 0.57 |
| 7 | 350 (+1) | 120 (+1) | 67.24 | 22.25 | - |
| 8 | 350 (+1) | 120 (+1) | 71.81 | 17.48 | - |
| 9 | 250 (−1) | 90 (0) | 91.26 | - | 1.10 |
| 10 | 250 (−1) | 90 (0) | 91.09 | - | 1.49 |
| 11 | 350 (+1) | 90 (0) | 74.54 | 13.38 | - |
| 12 | 350 (+1) | 90 (0) | 79.40 | 10.00 | - |
| 13 | 300 (0) | 60 (−1) | 91.72 | 0.31 | - |
| 14 | 300 (0) | 60 (−1) | 92.82 | 0.03 | - |
| 15 | 300 (0) | 120 (+1) | 88.51 | 2.00 | - |
| 16 | 300 (0) | 120 (+1) | 90.88 | 1.17 | - |
| 17 | 300 (0) | 90 (0) | 91.94 | 0.57 | - |
| 18 | 300 (0) | 90 (0) | 92.85 | 0.30 | - |
| Coefficient | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|
| Value | p-Value | Value | p-Value | Value | p-Value | |
| b0 | 91.45 | 0.73 | −0.01 | |||
| b1 | −6.44 | <0.0001 | 5.70 | <0.0001 | −0.66 | <0.0001 |
| b2 | −3.12 | 0.0002 | 3.11 | 0.0002 | −0.23 | 0.0010 |
| b12 | −5.12 | <0.0001 | 4.31 | <0.0001 | 0.35 | 0.0002 |
| b11 | −6.90 | <0.0001 | 4.97 | 0.0005 | 0.66 | <0.0001 |
| b22 | 0.01 | 0.9944 * | 0.00 | 0.9981 * | 0.01 | 0.9240 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañosa, J.; Alvarez-Coscojuela, A.; Maldonado-Alameda, A.; Chimenos, J.M. A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments. Materials 2024, 17, 4651. https://doi.org/10.3390/ma17184651
Mañosa J, Alvarez-Coscojuela A, Maldonado-Alameda A, Chimenos JM. A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments. Materials. 2024; 17(18):4651. https://doi.org/10.3390/ma17184651
Chicago/Turabian StyleMañosa, Jofre, Adrian Alvarez-Coscojuela, Alex Maldonado-Alameda, and Josep Maria Chimenos. 2024. "A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments" Materials 17, no. 18: 4651. https://doi.org/10.3390/ma17184651
APA StyleMañosa, J., Alvarez-Coscojuela, A., Maldonado-Alameda, A., & Chimenos, J. M. (2024). A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments. Materials, 17(18), 4651. https://doi.org/10.3390/ma17184651






