In-Situ Coating of Iron with a Conducting Polymer, Polypyrrole, as a Promise for Corrosion Protection
Abstract
1. Introduction
2. Experimental
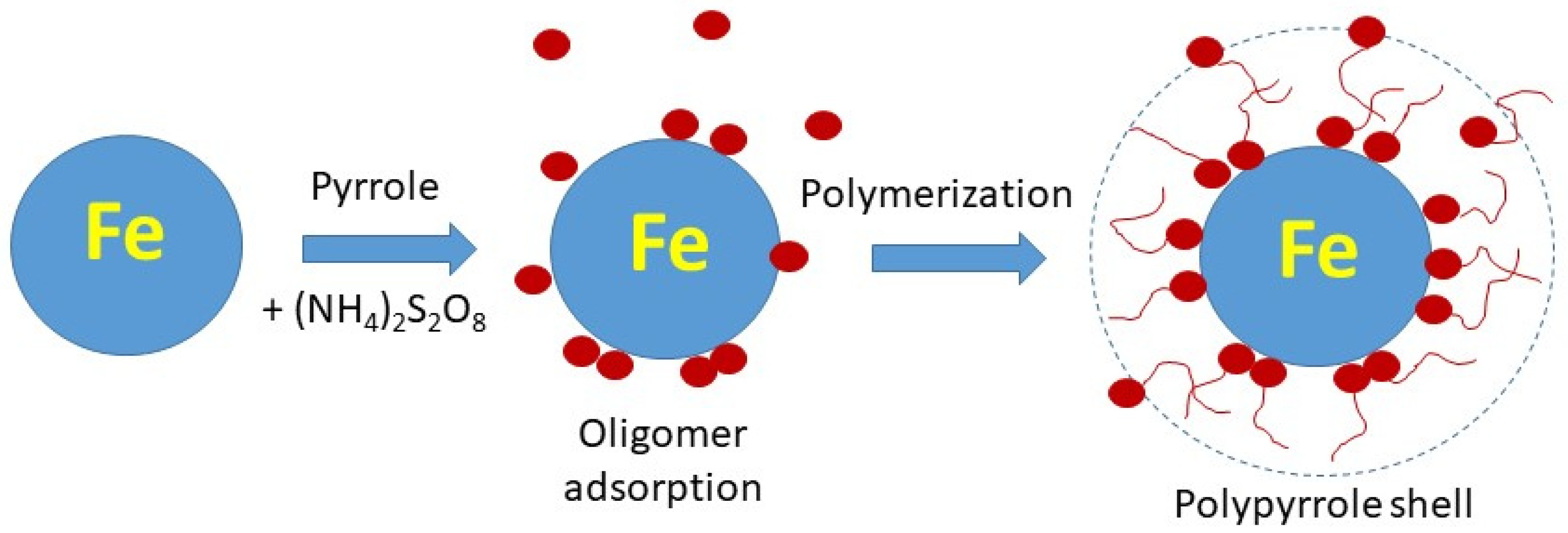
2.1. Preparation
2.2. Characterization
3. Results and Discussion
3.1. Composites
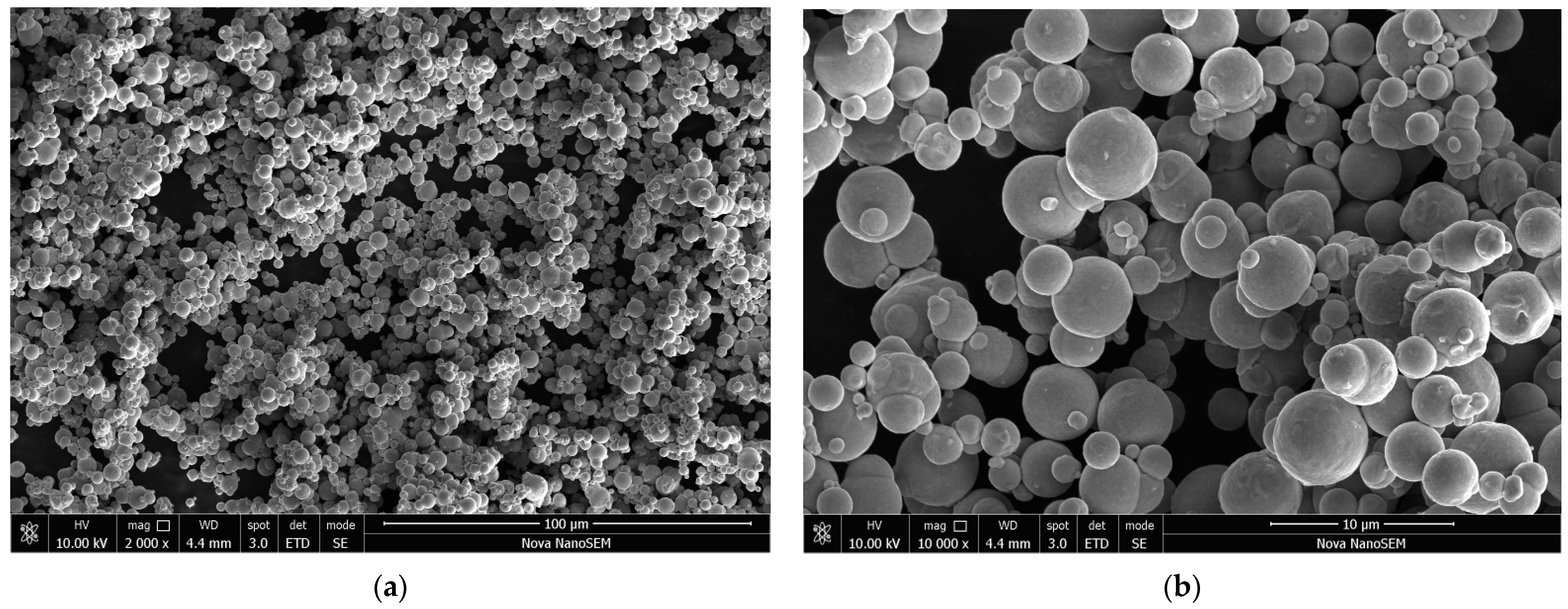
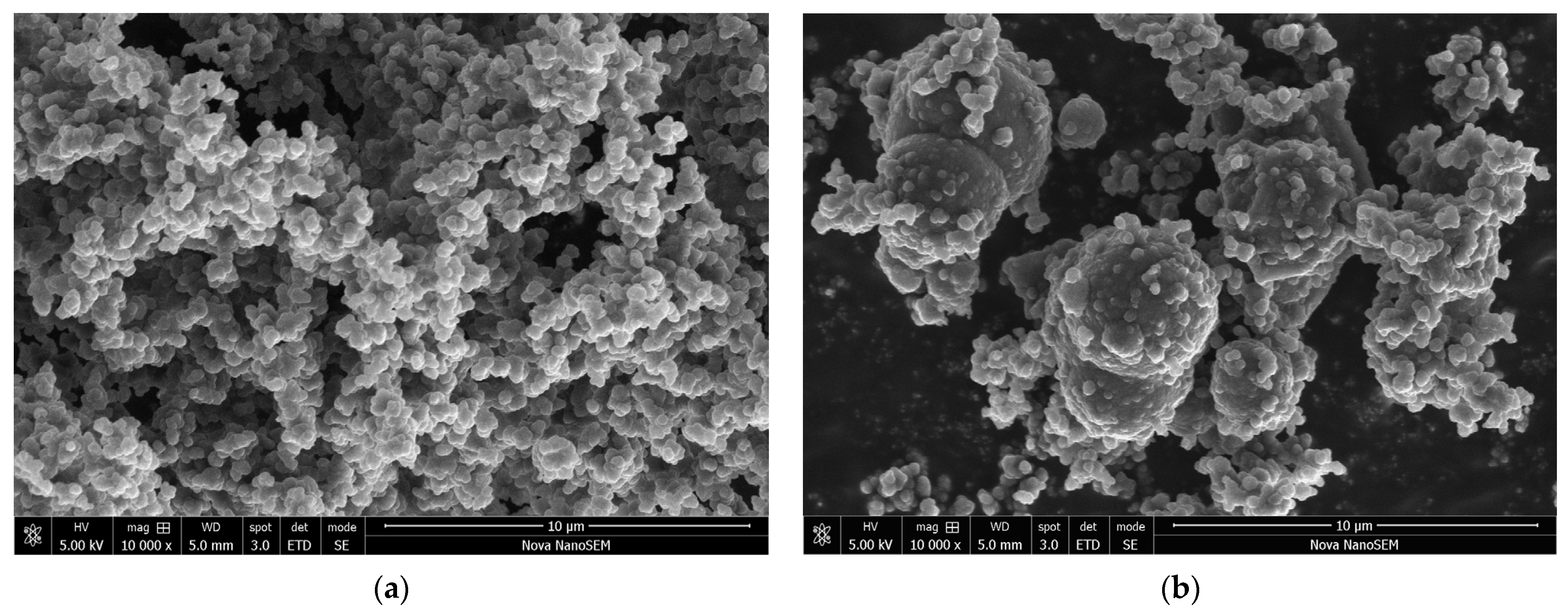
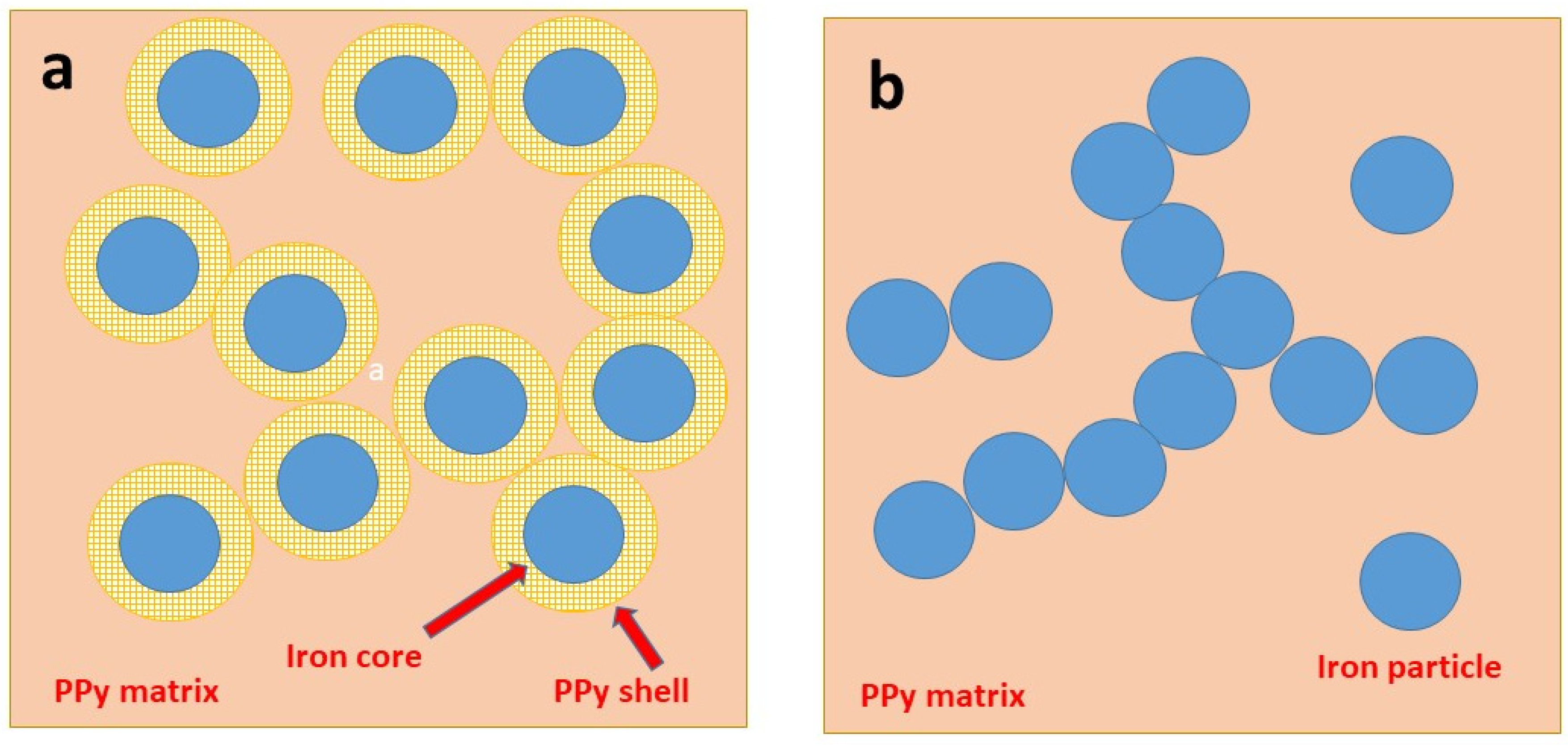
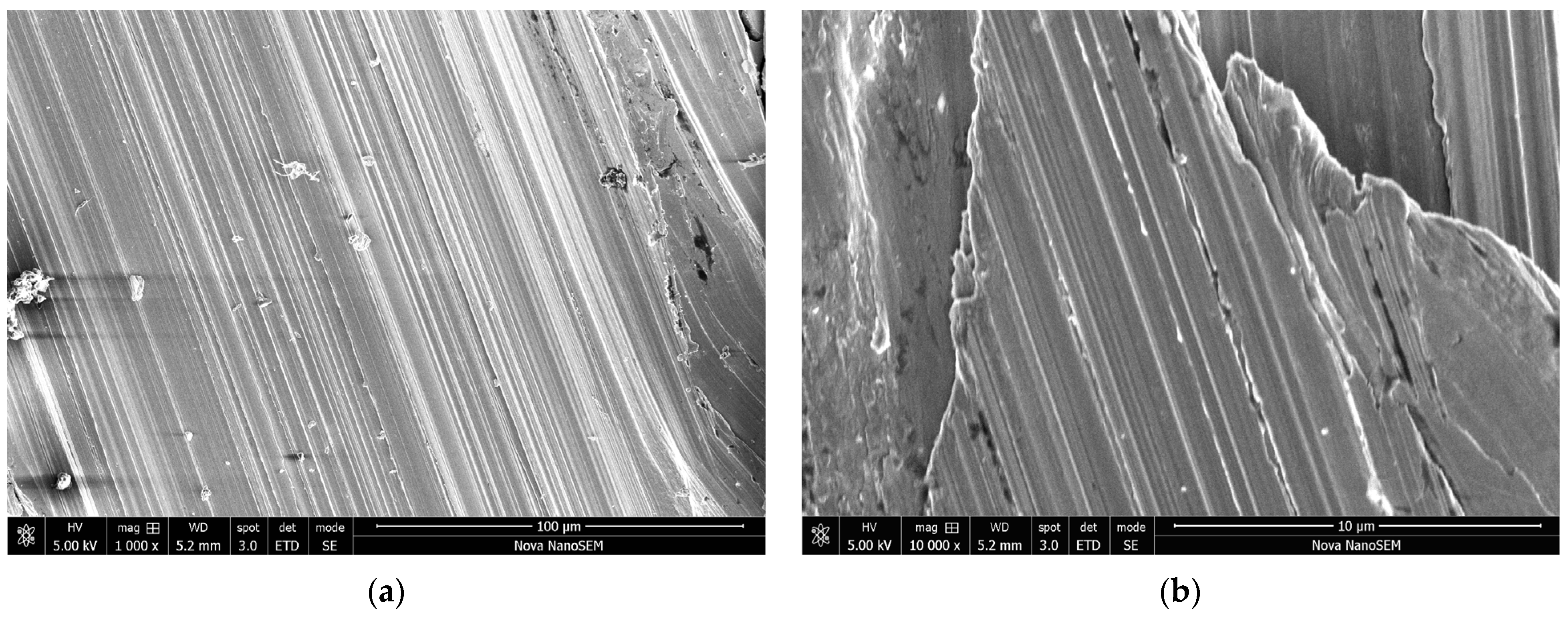
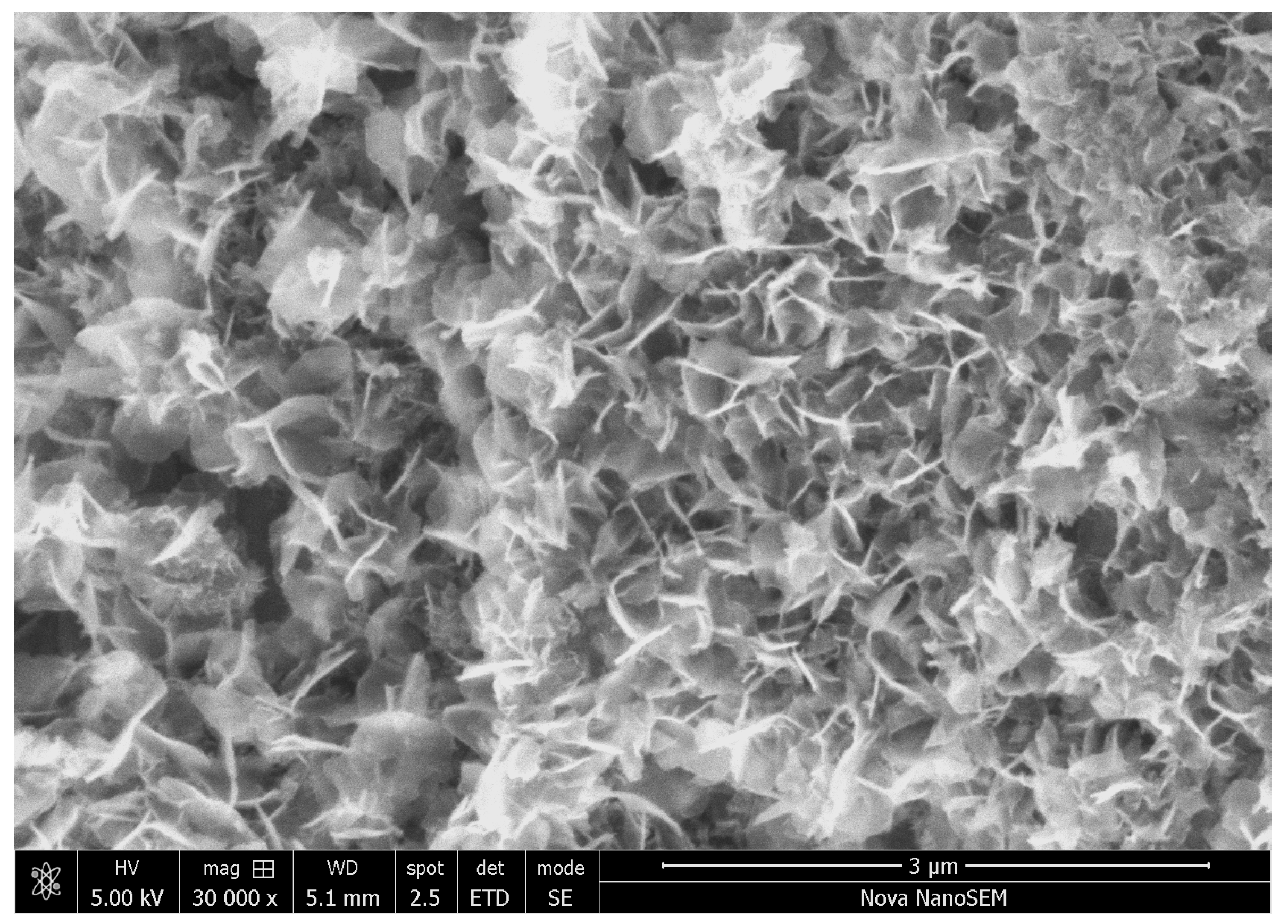
3.2. Morphology
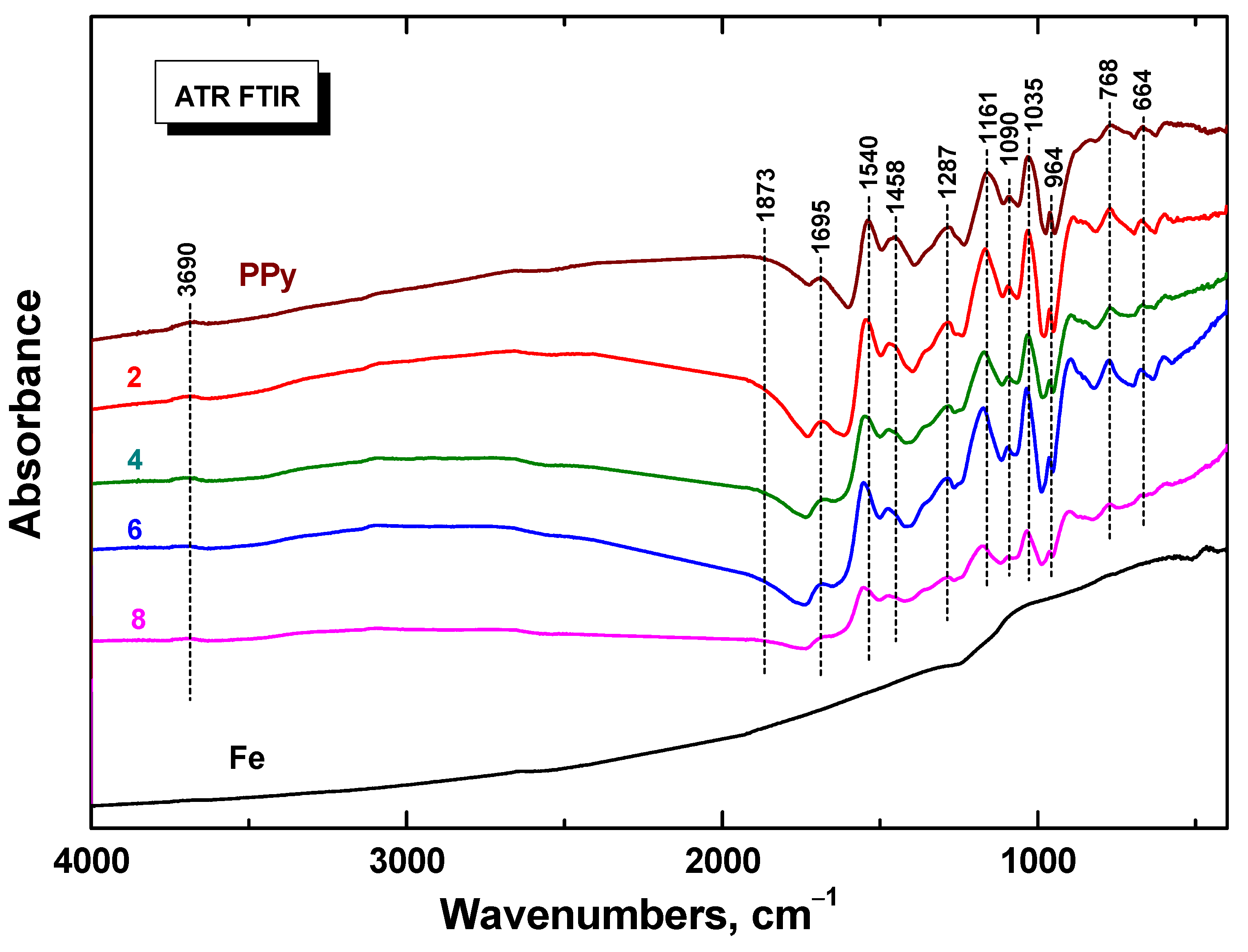
3.3. FTIR Spectra
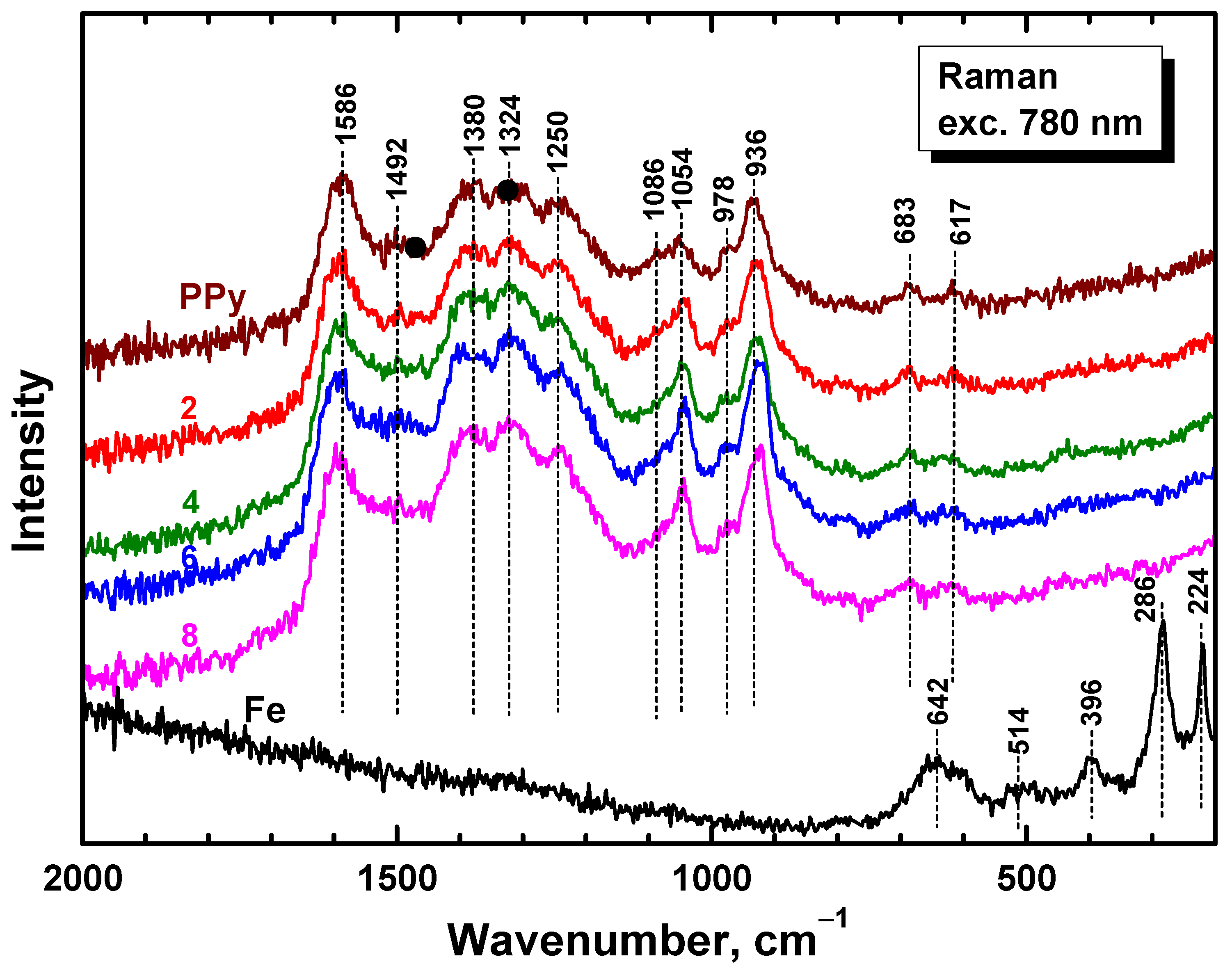
3.4. Raman Spectra
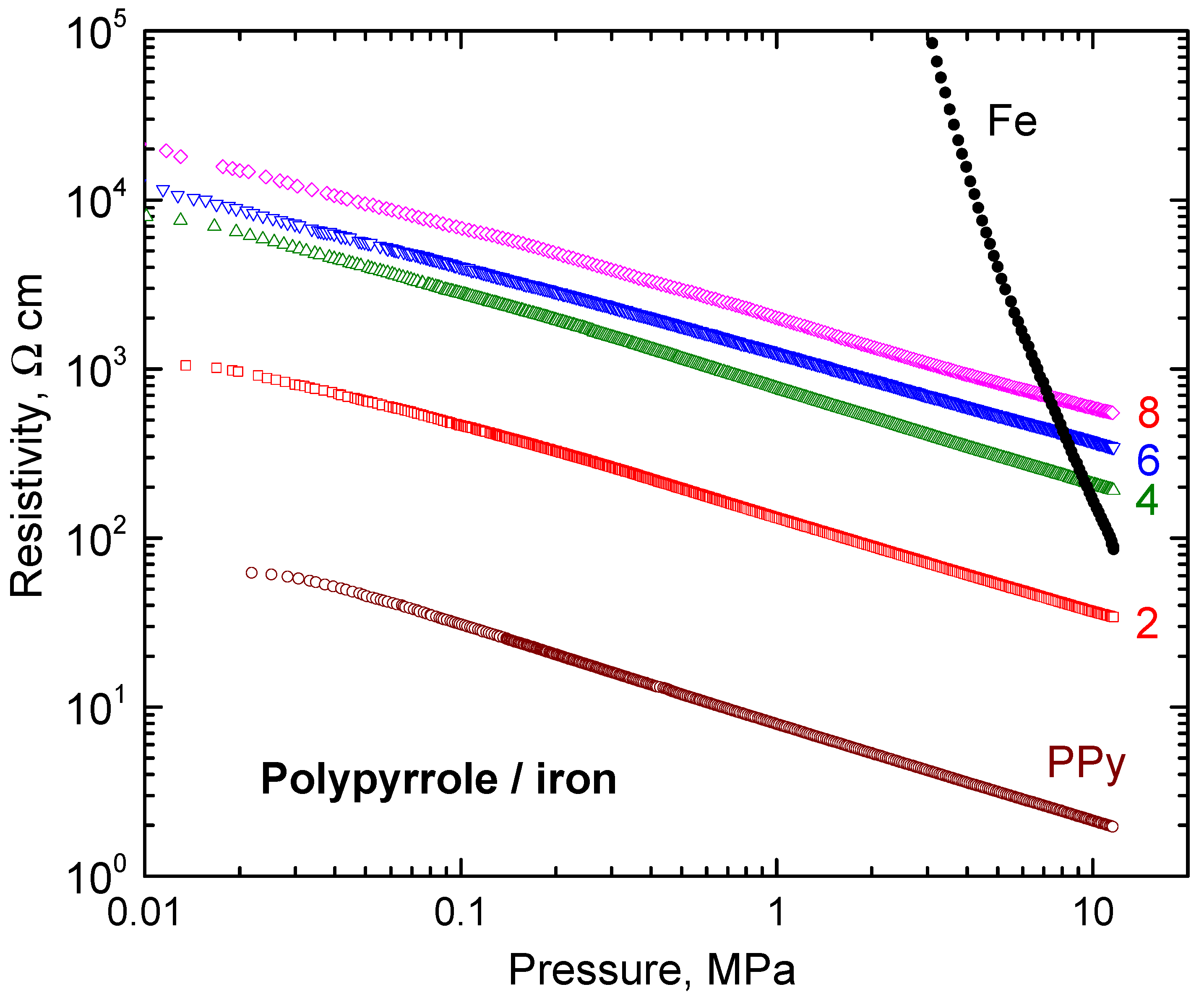
3.5. Electrical Properties
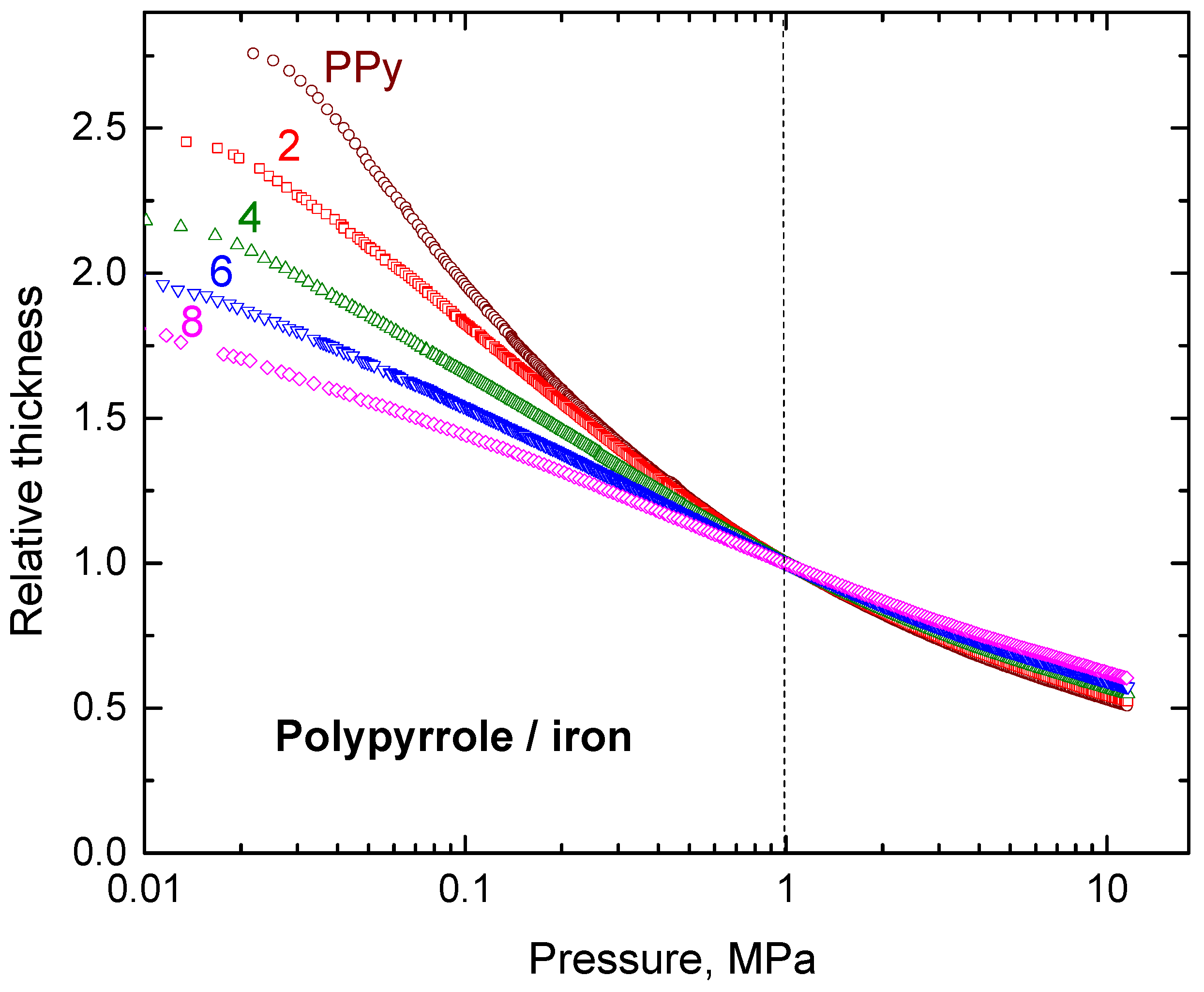
3.6. Mechanical Properties
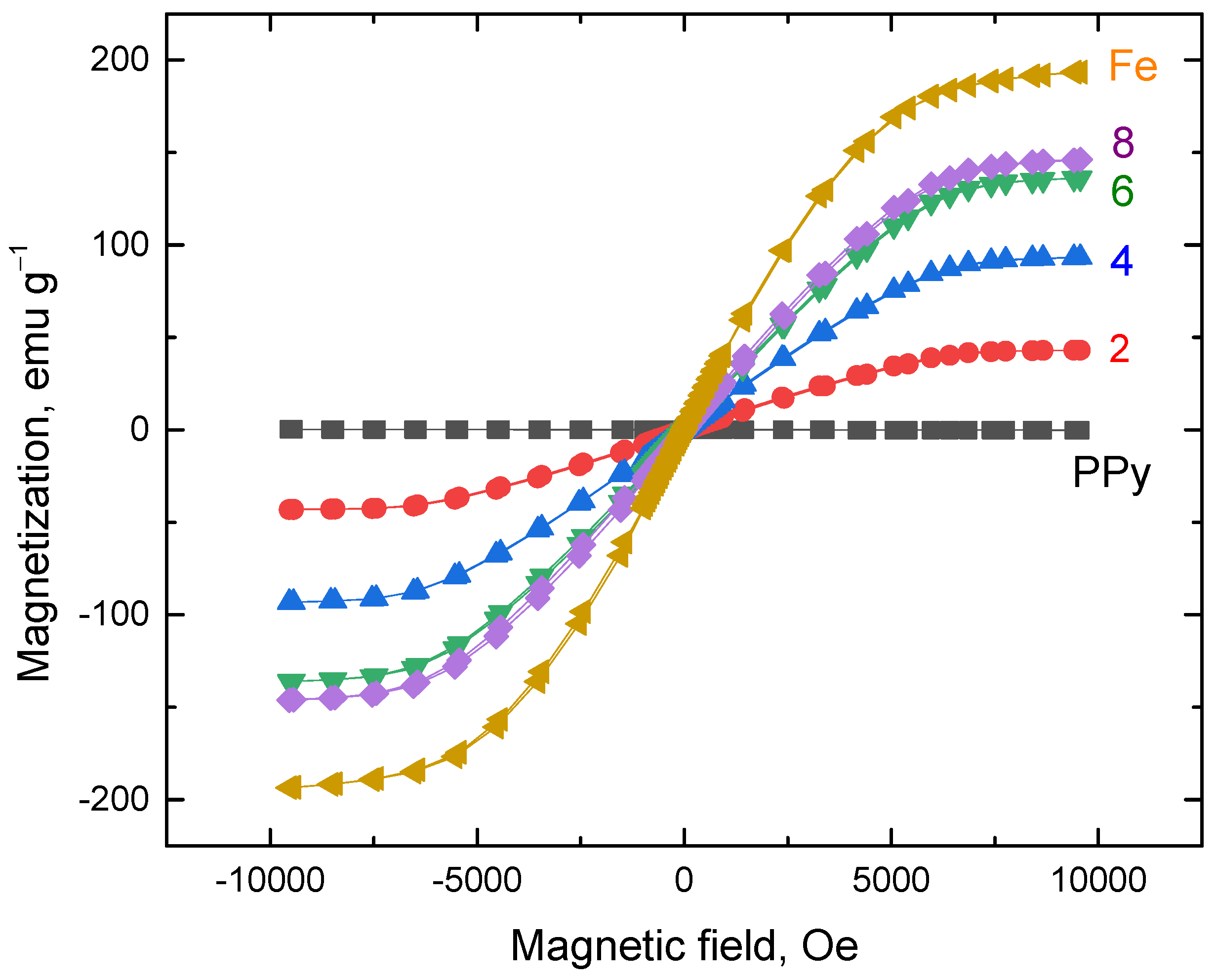
3.7. Magnetic Properties
3.8. Coating of Macroscopic Iron Object
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belyavskii, S.G.; Mingalyov, P.G.; Giulieri, F.; Combarrieau, R.; Lisichkin, G.V. Chemical modification of the surface of a carbonyl iron powder. Prot. Met. 2006, 42, 244–252. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlík, M.; Ilčíková, M.; Mosnáček, J.; Münster, L.; Pavlínek, V. Synthesis of silicone elastomers containing silyl-based polymer grafted carbonyl iron particles: An efficient way to improve magnetorheological, damping, and sensing performances. Macromolecules 2017, 50, 2189–2200. [Google Scholar] [CrossRef]
- Mrlík, M.; Sedlačík, M.; Pavlínek, V.; Bažant, P.; Sáha, P.; Peer, P.; Filip, P. Synthesis and magnetorheological characteristics of ribbon-like, polypyrrole-coated carbonyl iron suspensions under oscillatory shear. J. Appl. Polym. Sci. 2013, 128, 2977–2982. [Google Scholar] [CrossRef]
- Mrlík, M.; Sedlačík, M.; Pavlínek, V.; Peer, P.; Filip, P.; Sáha, P. Magnetorheology of carbonyl iron particles coated with polypyrrole ribbons: The steady shear study. J. Phys. Conf. Ser. 2013, 412, 12016. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, J.; Zhang, B.; Yang, S.; Liu, S.J.; Chen, X. One-step preparation of sea urchin-like conducting polypyrrole-modified porous carbonyl iron powders with excellent microwave absorption properties. Mater. Res. Bull. 2024, 175, 112762. [Google Scholar] [CrossRef]
- Mrlík, M.; Kollár, J.; Borská, K.; Ilčíková, M.; Gorgol, D.; Osicka, J.; Sedlačík, M.; Ronzová, A.; Kasák, P.; Mosnáček, J. Atom transfer radical polymerization of pyrrole-bearing methacrylate for production of carbonyl iron particles with conducting shell for enhanced electromagnetic shielding. Int. J. Mol. Sci. 2022, 23, 8540. [Google Scholar] [CrossRef]
- Li, S.Y.; Tian, X.X.; Wang, J.F.; Ma, L.S.; Li, C.C.; Qin, Z.; Qu, S.B. Ternary heterogeneous core-shell structure CIP@PPy/MWCNTs composites for broadband microwave absorption. Diam. Relat. Mater. 2022, 130, 109420. [Google Scholar] [CrossRef]
- Sui, M.X.; Lu, X.L.; Xie, A.; Xu, W.D.; Rong, X.H.; Wu, G.J. The synthesis of three-dimensional (3D) polydopamine-functioned carbonyl iron powder@polypyrrole (CIP@PPy) aerogel composites for excellent microwave absorption. Synth. Met. 2015, 210, 156–164. [Google Scholar] [CrossRef]
- Xie, M.D.; Tian, X.X.; Qu, S.B.; Cheng, H.L. Synthesis and electromagnetic properties of porous carbonyl iron/SiO2/polypyrrole core-shell structure composites. Chin. J. Inorg. Chem. 2018, 34, 1261–1270. [Google Scholar] [CrossRef]
- Malinauskas, A.; Malinauskiene, J.; Ramanavicius, A. Conducting polymer-based nanostructurized materials: Electrochemical aspects. Nanotechnology 2005, 16, R51–R62. [Google Scholar] [CrossRef]
- Sedlačík, M.; Pavlínek, V.; Sáha, P.; Svrčinová, P.; Filip, P. Core-shell structured polypyrrole-coated magnetic carbonyl iron microparticles and their magnetorheology. AIP Conf. Proc. 2011, 1375, 284–291. [Google Scholar] [CrossRef]
- Rashmi, H.M.; Revanasiddappa, M.; Manjunatha, S.; Surekha, M.; Ravikiran, Y.T. Low frequency alternating current response of PPy-PVA-Fe nanocomposite films. Chem. Pap. 2024, 78, 1435–1442. [Google Scholar] [CrossRef]
- Mamatha, G.M.; Dixit, P.; Krishna, H.R.; Kumar, G.S. Polymer based composites for electromagnetic interference (EMI) shielding: The role for magnetic fillers in effective attenuation of microwaves, a review. Hybrid Adv. 2024, 6, 100200. [Google Scholar] [CrossRef]
- Truong, V.T.; Riddell, S.Z.; Muscat, R.F. Polypyrrole based microwave absorbers. J. Mater. Sci. 1998, 33, 4971–4976. [Google Scholar] [CrossRef]
- Sun, K.; Yang, X.C.; Lei, Y.H.; Du, H.L.; Dudziak, T.; Fan, R.H. Core-shell structural design and microwave absorption enhancement of multi-dimensional graphene oxide@polypyrrole/carbonyl iron fiber nanocomposites. J. Compd. Alloys 2023, 390, 167446. [Google Scholar] [CrossRef]
- Rezazadeh, N.; Rezazadeh, J. Fabrication of ultra-thin, hydrophobic and flexible electromagnetic wave absorber sheets based on nano-carbon/carbonyl iron in a polypyrrole/silicone rubber matrix. J. Magn. Magn. Mater. 2019, 475, 201–204. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Choi, K.; Kwon, S.H.; Nam, J.D.; Choi, H.J. Nanoparticles functionalized by conducting polymers and their electrorheological and magnetorheological applications. Polymers 2020, 12, 204. [Google Scholar] [CrossRef]
- Munteanu, A.; Plachý, T.; Munteanu, L.; Ngwabebhoh, F.A.; Stejskal, J.; Trchová, M.; Kubík, M.; Sedlačík, M. Bidisperse magnetorheological fluids utilizing composite polypyrrole nanotubes /magnetite nanoparticles and carbonyl iron microspheres. Rheol. Acta 2023, 62, 462–472. [Google Scholar] [CrossRef]
- Bairagi, H.; Vashishth, P.; Ji, G.; Shukla, S.K.; Ebenso, E.E.; Mangla, B. Polymers and their composites for corrosion inhibition application: Development, advancement, and future scope—A critical review. Corros. Commun. 2024, 15, 79–94. [Google Scholar] [CrossRef]
- Spinks, G.M.; Dominis, A.J.; Wallace, G.G.; Tallman, D.E. Electroactive conducting polymers for corrosion control—Part 2. Ferrous metals. J. Solid State Electrochem. 2002, 6, 85–100. [Google Scholar] [CrossRef]
- Kraljić, M.; Mandić, Z.; Duić, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–185. [Google Scholar] [CrossRef]
- Rohwerder, M.; Michalik, A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim. Acta 2007, 53, 1300–1313. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Kausar, A. Conductive nanocomposite coatings-manufacturing, features, and technical revolution. Polym. Plast. Technol. Mater. 2024, 63, 2000–2020. [Google Scholar] [CrossRef]
- Sood, Y.; Singh, K.; Mudila, H.; Lokhande, P.E.; Singh, L.; Kumar, D.; Kumar, A.; Mubarak, N.M.; Dehghani, M.H. Insights into properties, synthesis and emerging applications of polypyrrole-based composites, and future prospective: A review. Heliyon 2024, 10, e33643. [Google Scholar] [CrossRef]
- Ye, W.; Zhu, J.; Liao, X.J.; Jiang, S.H.; Li, Y.H.; Fang, H.; Hou, H.Q. Hierarchical three-dimensional micro/nano-architecture of polyaniline nanowires wrapped-on polyimide nanofibers for high performance lithium-ion battery separators. J. Power Sources 2015, 299, 417–424. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Sapurina, I.; Stejskal, J. Anticorrosion efficiency of organic coatings depending on the pigment volume concentration of polyaniline phosphate. Prog. Org. Coat. 2008, 63, 228–237. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog. Polym. Sci. 2010, 35, 1420–1481. [Google Scholar] [CrossRef]
- Zhou, C.F.; Du, X.S.; Liu, Z.; Ringer, S.P.; Mai, Y.W. Solid phase mechanochemical synthesis of polyaniline branched nanofibers. Synth. Met. 2009, 159, 1302–1307. [Google Scholar] [CrossRef]
- Abdiryim, T.; Xiao-Gang, Z.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymer hydrogels. Chem. Pap. 2017, 71, 269–291. [Google Scholar] [CrossRef]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Kalendová, A.; Sapurina, I.; Stejskal, J.; Veselý, D. Anticorrosion properties of polyaniline-coated pigments in organic coatings. Corros. Sci. 2008, 50, 3549–3560. [Google Scholar] [CrossRef]
- Boga, K.; Pothu, R.; Arukula, R.; Boddula, R.; Gaddam, S.K. The role of anticorrosive polymer coatings for the protection of metallic surface. Corros. Rev. 2021, 39, 547–559. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Chen, P.Z.; Chen, W.X. A rational route towards dual wave-transparent type of carbonyl iron@SiO2@heterogeneous state polypyrrole@paraffin composites for electromagnetic wave absorption application. J. Colloid Interface Sci. 2021, 581, 84–95. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of intrinsically conducting polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Hoque, M.I.U.; Holze, R. Intrinsically conducting polymer composites as active masses in supercapacitors. Polymers 2023, 15, 730. [Google Scholar] [CrossRef]
- Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chem. Pap. 2020, 74, 1–54. [Google Scholar] [CrossRef]
- Ravikumar, R.; Jagadeshvaran, P.L.; Biju, R.; Binoy, L.; Raghavan, J.R.V.; Krishnakumar, T.S.; Indulal, C.R. Role of polypyrrole-based SrO-CuO nanocomposite on flame retardancy and heat dissipation applications. Chem. Pap. 2023, 77, 3413–3426. [Google Scholar] [CrossRef]
- Le, H.N.T.; Bernard, M.C.; Garcia-Renaud, B.; Deslouis, C. Raman spectroscopy analysis of polypyrrole films as protective coatings on iron. Synth. Met. 2004, 140, 287–293. [Google Scholar] [CrossRef]
- Liao, Z.W.; Zoumhani, O.; Boutry, C.M. Recent advances in magnetic polymer composites for bioMEMS: A review. Materials 2023, 16, 3802. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Dutta, S.D.; Patil, T.V.; Ganguly, K.; Randhawa, A.; Lim, K.T. A review on electroactive polymer-metal composites: Development and applications for tissue regeneration. J. Funct. Biomater. 2023, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Jurča, M.; Vilčáková, J.; Kazantseva, N.E.; Munteanu, A.; Munteanu, L.; Sedlačík, M.; Stejskal, J.; Trchová, M.; Prokeš, J. Conducting and magnetic hybrid polypyrrole/nickel composites and their application in magnetorheology. Materials 2024, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Abshinova, M.A.; Kazantseva, N.E.; Sáha, P.; Sapurina, I.; Kovářová, J.; Stejskal, J. The enhancement of the oxidation resistance of carbonyl iron by polyaniline coating and consequent changes in electromagnetic properties. Polym. Degrad. Stab. 2008, 93, 1826–1831. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin films and colloidal dispersions—(IUPAC technical report). Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Breslin, C.B.; Fenelon, A.M.; Conroy, K.G. Surface engineering: Corrosion protection using conducting polymers. Mater. Des. 2005, 26, 233–237. [Google Scholar] [CrossRef][Green Version]
- Rammelt, U.; Duc, L.M.; Plieth, W. Improvement of protection performance of polypyrrole by dopant anions. J. Appl. Electrochem. 2005, 35, 1225–1230. [Google Scholar] [CrossRef]
- Sun, Y.H.; Hu, C.Q.; Cui, J.C.; Shen, S.L.; Qiu, H.X.; Li, J. Electrodeposition of polypyrrole coatings doped by benzenesulfonic acid-modified graphene oxide on metallic bipolar plates. Prog. Org. Coat. 2022, 170, 106995. [Google Scholar] [CrossRef]
- Trung, V.Q.; Hung, H.M.; Khoe, L.V.; Duc, L.M.; Viet, N.T.B.; Linh, D.K.; Huong, V.T.; Dat, N.D.; Oanh, D.T.Y.; Luong, N.X.; et al. Synthesis and characterization of polypyrrole film doped with both molybdate and salicylate and its application in the corrosion protection for low carbon steel. ACS Omega 2022, 7, 19842–19852. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, V.; Cedron, S.; Azambuja, D.S.; Castagno, K.R.L. Polypyrrole film doped with corrosion-inhibitors electropolymerized on AA 1100. Mater. Res. 2019, 22, e20180919. [Google Scholar] [CrossRef]
- Xiao, N.L. Investigation on corrosion inhibition performance of polypyrrole coating on Q235 steel in civil structure. Alex. Eng. J. 2023, 70, 547–551. [Google Scholar] [CrossRef]
- Liu, A.S.; Almeida, L.N.; Evangelista, L.M.; Santos, D.M.L.; Cho, L.Y. Electrosynthesis of polypyrrole-bilayer doped with phosphoric and dodecylbenzenesulfonic acids on 2024 aluminum alloy. Mater. Rio de Janeiro 2024, 29, e20240034. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Tomšík, E.; Wang, J.X.; Morávková, Z.; Zhigunov, A.; Stejskal, J.; Trchová, M. Self-assembly of aniline oligomers. Chem. Asian J. 2013, 8, 129–137. [Google Scholar] [CrossRef]
- Tao, J.Z.; Yang, M.; Gao, H.Y.; Yu, J.; Wang, G. Synthesis and assembly of oligoaniline for hierarchical structures within stable and mild acid system. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 117–124. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Li, X.X.; Gong, X.X.; Han, J.; Guo, R. Ethanol-guided synthesis of (flower-on-leaf)-like aniline oligomers with excellent adsorption properties. New J. Chem. 2015, 49, 9257–9264. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 61, 135–176. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Sabet-Bokati, Z.; Sabet-Bokati, K.; Russell, Z.; Morshed-Behbahani, K.; Ouanani, S. Anticorrosion shape memory-assisted self-healing coatings: A review. Prog. Org. Coat. 2024, 188, 108193. [Google Scholar] [CrossRef]
- Dua, S.; Arora, N.; Prakashaiah, B.G.; Saxena, R.C.; Ganguly, S.K.; Senthilkumar, T. Conjugated polymer-based composites for anti-corrosion applications. Prog. Org. Coat. 2024, 188, 108231. [Google Scholar] [CrossRef]
- Ding, H.; Hussein, A.M.; Ahmad, I.; Latef, R.; Abbas, J.K.; Ali, A.T.A.; Saeed, S.M.; Abdulwahid, A.S.; Ramadan, M.F.; Rasool, H.A.; et al. Conducting polymers in industry: A comprehensive review on the characterization, synthesis and application. Alex. Eng. J. 2024, 88, 253–267. [Google Scholar] [CrossRef]



| Fe | Yield, g | Yield, % | w, wt% Fe | φ, vol% Fe |
|---|---|---|---|---|
| 0 (PPy) | 1.40 | 100 | 0 | 0 |
| 2 | 2.03 | 53.7 | 30.4 | 7.7 |
| 4 | 3.29 | 60.8 | 56.7 | 20.0 |
| 6 | 4.62 | 62.3 | 70.6 | 31.5 |
| 8 | 5.93 | 63.1 | 76.0 | 37.7 |
| Fe | σ*, S cm−1 | σ10, S cm−1 | −B | Coercitivity, Oe | Remanence, emu g−1 | Saturation Magnetization, emu g−1 |
|---|---|---|---|---|---|---|
| 0 | 1.35 | 0.470 | 0.580 | – | – | – |
| 2 | 0.120 | 2.72 × 10−2 | 0.550 | 6.72 | 0.061 | 43.1 |
| 4 | 2.67 × 10−2 | 4.83 × 10−3 | 0.566 | 6.02 | 0.102 | 93.3 |
| 6 | 1.77 × 10−2 | 2.67 × 10−3 | 0.514 | 5.94 | 0.147 | 136 |
| 8 | 9.63 × 10−3 | 1.70 × 10−3 | 0.536 | 5.87 | 0.160 | 146 |
| Fe | – | 5.78 × 10−3 | 4.89 | 4.92 | 0.216 | 193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejskal, J.; Jurča, M.; Trchová, M.; Prokeš, J.; Křivka, I. In-Situ Coating of Iron with a Conducting Polymer, Polypyrrole, as a Promise for Corrosion Protection. Materials 2024, 17, 4783. https://doi.org/10.3390/ma17194783
Stejskal J, Jurča M, Trchová M, Prokeš J, Křivka I. In-Situ Coating of Iron with a Conducting Polymer, Polypyrrole, as a Promise for Corrosion Protection. Materials. 2024; 17(19):4783. https://doi.org/10.3390/ma17194783
Chicago/Turabian StyleStejskal, Jaroslav, Marek Jurča, Miroslava Trchová, Jan Prokeš, and Ivo Křivka. 2024. "In-Situ Coating of Iron with a Conducting Polymer, Polypyrrole, as a Promise for Corrosion Protection" Materials 17, no. 19: 4783. https://doi.org/10.3390/ma17194783
APA StyleStejskal, J., Jurča, M., Trchová, M., Prokeš, J., & Křivka, I. (2024). In-Situ Coating of Iron with a Conducting Polymer, Polypyrrole, as a Promise for Corrosion Protection. Materials, 17(19), 4783. https://doi.org/10.3390/ma17194783









