Bio-Based Phase Change Materials for Sustainable Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bio-PCM Preparation
2.2. Characterization of Bio-PCMs
3. Results and Discussion
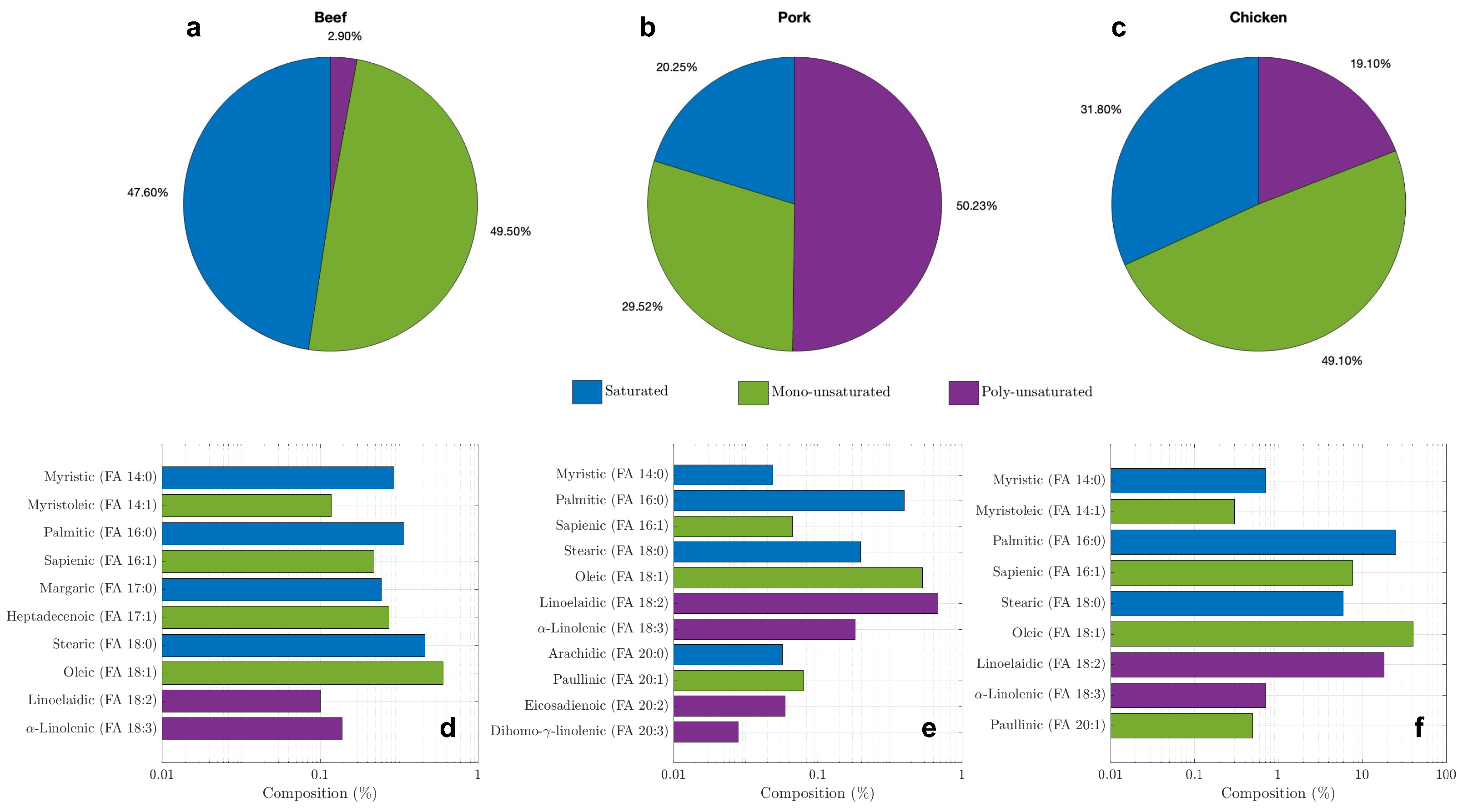
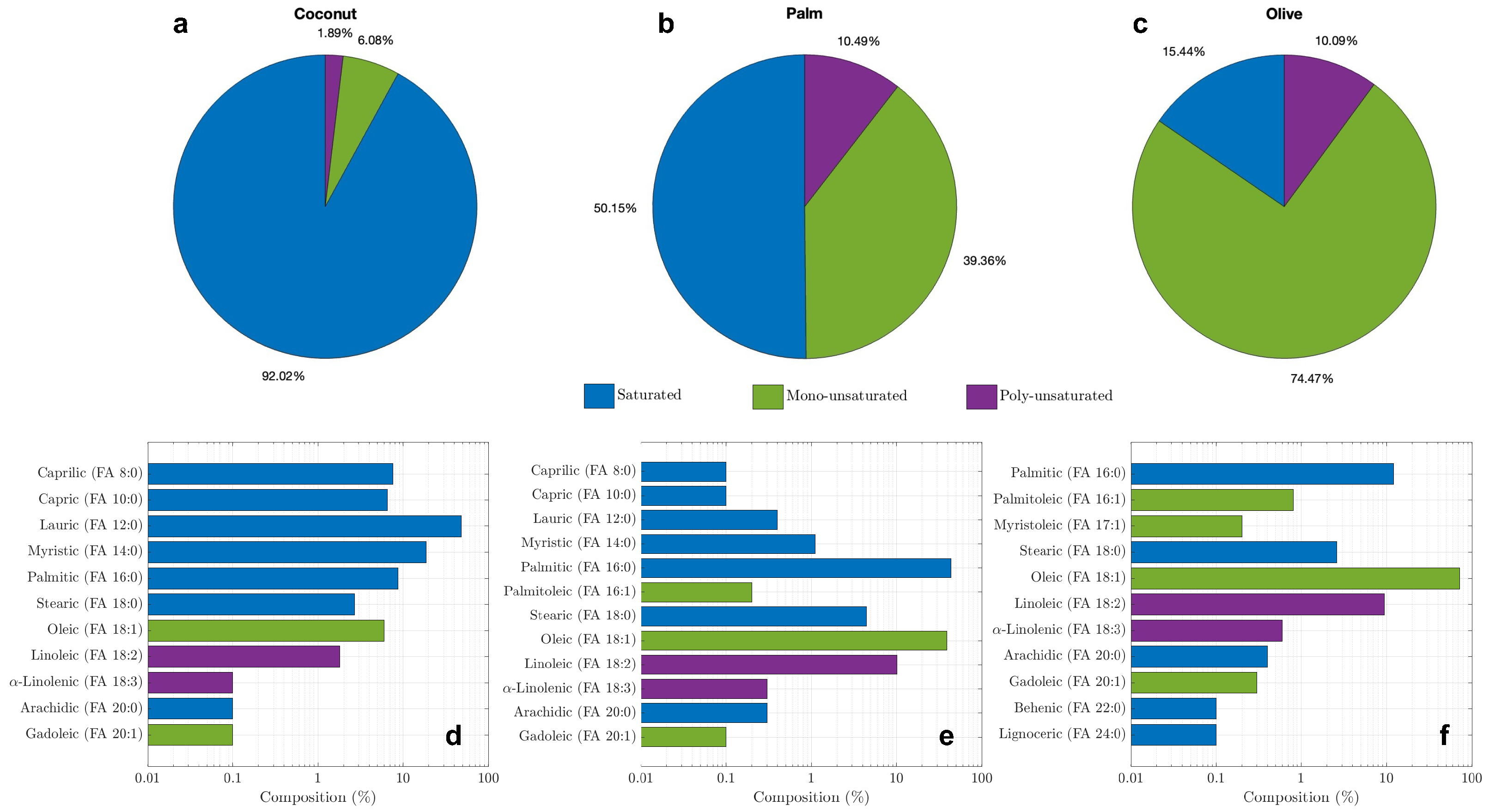
3.1. Molecular Analysis
3.2. Thermal Stability
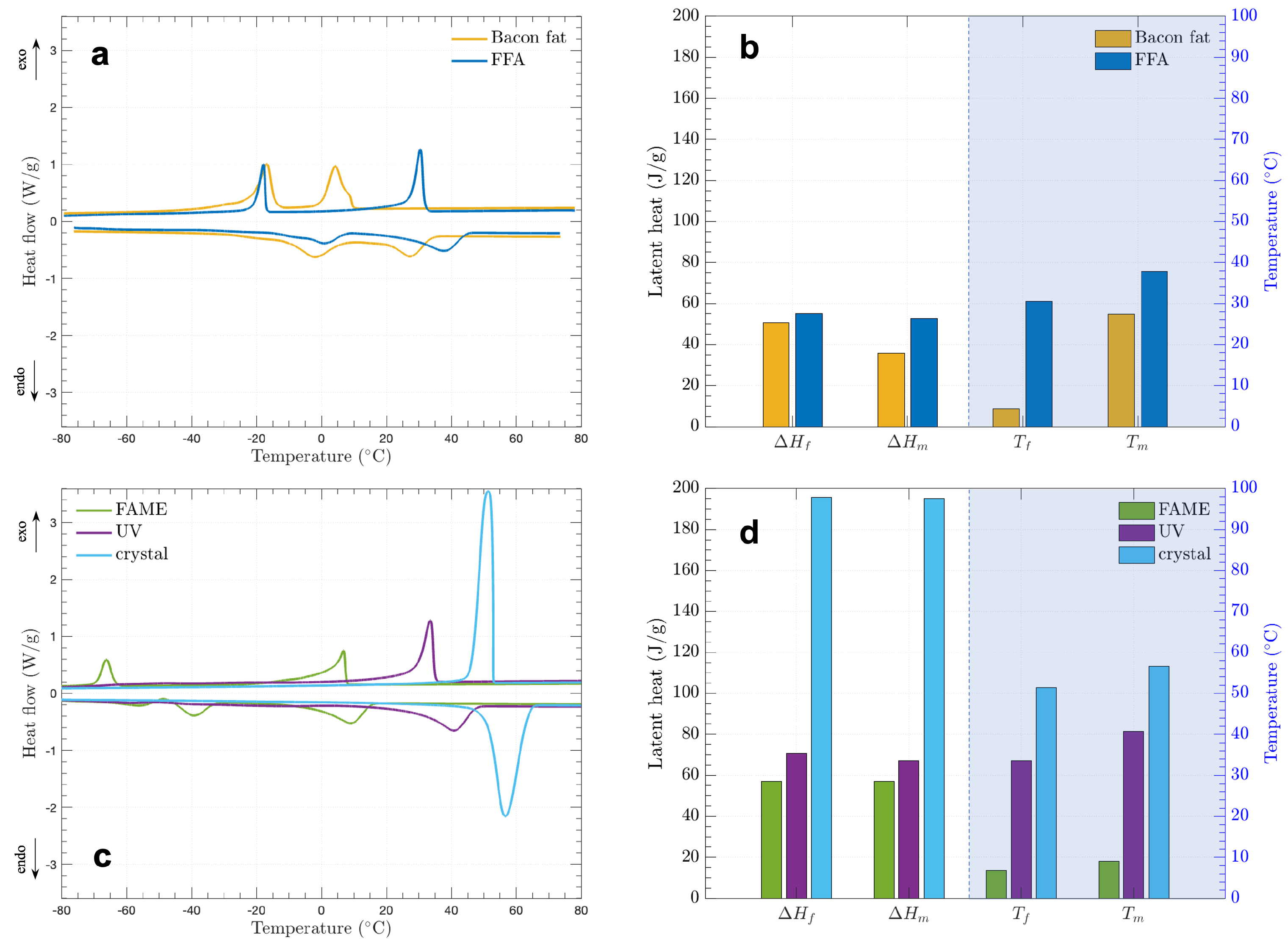
3.3. Thermophysical Properties of the Bio-PCMs
4. Future Work on Bio-PCM Development
- For large-scale manufacturing, the fatty acids can be separated into individual components in Table 3 which typically exhibit higher latent heats than the eutectic alternatives, and the cost-benefit analysis will be conducted to optimize the yield.
- The unsaturated fatty acids can be converted to saturated fatty acids by hydrogenation as the saturated ones commonly exhibit a higher latent heat. Different saturation levels provide different melting temperature and latent heat, which can be used to design bio-PCMs for specific applications.
- The saturated fatty acids can be crystallized into a highly structured solid, which may significantly increase the latent heat. The durability and lifetime of the bio-PCMs will be investigated to assure the long-term performance with environmental impact.
- Multiscale modeling and characterization of the molecular and atomic structure changing with temperature can predict and optimize the PCM synthesis process. Our recently developed singum model [72] can predict the stiffness changing with temperature. It can be extended to evaluate the latent heat with the nanostructure of the bio-PCM, and thus provide a guideline for material design.
- Since sources of animal fats and plant oils are still various and scattered at the same time [19], it is necessary to explore more research with consistent source from plants or animals.
- As effective thermal storage needs to have high thermal conductivity as well as high latent heat, novel materials or synthesis processes will be explored to overcome the trade-off relationship between those quantities for practical usage of sustainable bio-based PCMs.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef] [PubMed]
- IEA. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach. 2023. Available online: https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach (accessed on 26 September 2024).
- Grubler, A.; Wilson, C.; Bento, N.; Boza-Kiss, B.; Krey, V.; McCollum, D.L.; Rao, N.D.; Riahi, K.; Rogelj, J.; Stercke, S.D.; et al. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Clark, M.A.; Domingo, N.G.G.; Colgan, K.; Thakrar, S.K.; Tilman, D.; Lynch, J.; Azevedo, I.L.; Hill, J.D. Global food system emissions could preclude achieving the 1.5° and 2 °C climate change targets. Science 2020, 370, 705–708. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. 2019. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 26 September 2024).
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A low energy demand scenario for meeting the 1.5 °C target and sustainable development goals without negative emission technologies. Nat. Energy 2018, 3, 515–527. [Google Scholar] [CrossRef]
- Mckay, D.I.A.; Staal, A.; Abrams, J.F.; Winkelmann, R.; Sakschewski, B.; Loriani, S.; Fetzer, I.; Cornell, S.E.; Rockström, H.; Lenton, T.M. Exceeding 1.5 °C global warming could trigger multiple climate tipping points. Science 2022, 377, 1171. [Google Scholar] [CrossRef]
- Sachs1, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six Transformations to achieve the Sustainable Development Goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- Mastrucci, A.; Byers, E.; Pachauri, S.; Rao, N.D. Improving the SDG energy poverty targets: Residential cooling needs in the Global South. Energy Build. 2019, 186, 405–415. [Google Scholar] [CrossRef]
- Khosla, R.; Miranda, N.D.; Trotter, P.A.; Mazzone, A.; Renaldi, R.; McElroy, C.; Cohen, F.; Jani, A.; Perera-Salazar, R.; McCulloch, M. Cooling for sustainable development. Nat. Sustain. 2021, 4, 201–208. [Google Scholar] [CrossRef]
- Badshah, M.A.; Leung, E.M.; Liu, P.; Strzelecka1, A.A.; Gorodetsky, A.A. Scalable manufacturing of sustainable packaging materials with tunable thermoregulability. Nat. Sustain. 2022, 5, 434–443. [Google Scholar] [CrossRef]
- Devahastin, S.; Pitaksuriyarat, S. Use of latent heat storage to conserve energy during drying and its effect on drying kinetics of a food product. Appl. Therm. Eng. 2006, 26, 1705–1713. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Ji, J.; Zhang, C. Cold chain transportation energy conservation and emission reduction based on phase change materials under dual-carbon background: A review. J. Energy Storage 2024, 86, 111258. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Peña, J.; Rieradevall, J.; Montero, J.I. Analysis of the technical, environmental and economic potential of phase change materials (PCM) for root zone heating in Mediterranean greenhouses. Renew. Energy 2017, 103, 570–581. [Google Scholar] [CrossRef]
- Mu, M.; Zhang, S.; Yang, S.; Wang, Y. Phase change materials applied in agricultural greenhouses. J. Energy Storage 2022, 49, 104100. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Jia, F.R.; Jing, W.T.; Liu, G.X.; Yue, Q.; Wang, H.M.; Shi, L. Paraffin-based crude oil refining process unit-level energy consumption and CO2 emissions in China. J. Clean. Prod. 2020, 255, 120347. [Google Scholar] [CrossRef]
- Nizetic, S.; Arici, M.; Bilgin, F.; Grubisic-Cabo, F. Investigation of pork fat as potential novel phase change material for passive cooling application in photovoltaics. J. Clean. Prod. 2018, 170, 1006–1016. [Google Scholar] [CrossRef]
- Mehrizi, A.A.; Karimi-Maleh, H.; Naddafi, M.; Karimi, F. Application of bio-based phase change materials for effective heat management. J. Energy Storage 2023, 61, 106859. [Google Scholar] [CrossRef]
- Gatto, A.; Chepeliev, M. Global food loss and waste estimates show increasing nutritional and environmental pressures. Nat. Food 2024, 5, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Plytaria, M.T.; Tzivanidis, C.; Bellos, E.; Antonopoulos, K.A. Parametric analysis and optimization of an underfloor solar assisted heating system with phase change materials. Therm. Sci. Eng. Prog. 2019, 10, 59–72. [Google Scholar] [CrossRef]
- Faraj, K.; Faraj, J.; Hachem, F.; Bazzi, H.; Khaled, M.; Castelain, C. Analysis of underfloor electrical heating system integrated with coconut oil-PCM plates. Appl. Therm. Eng. 2019, 158, 113778. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Eames, P.; Wu, Y. A state-of-the-art review of the application of phase change materials (PCM) in Mobilized-Thermal Energy Storage (M-TES) for recovering low-temperature industrial waste heat (IWH) for distributed heat supply. Renew. Energy 2021, 168, 1040–1057. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Kumar, S.; Negi, S. Transformation of waste cooking oil into C-18 fatty acids using a novel lipase produced by Penicillium chrysogenum through solid state fermentation. Biotech 2015, 5, 847–851. [Google Scholar] [CrossRef]
- Bragalia, M.; Lamastra, F.R.; Berrocal, J.A.; Paleari, L.; Nanni, F. Sustainable phase change materials (PCMs): Waste fat from cooking pork meat confined in polypropylene fibrous mat from waste surgical mask and porous bio-silica. Mater. Today Sustain. 2023, 23, 100454. [Google Scholar] [CrossRef]
- Jurcevic, M.; Nizetic, S.; Coko, D.; Hoang, A.T.; Papadopoulos, A.M. Experimental investigation of novel hybrid phase change materials. Clean Technol. Environ. Policy 2022, 24, 201–212. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F.; Cotana, F. Assessin the potentiality of animal fat based-bio phase change materials (PCM) for building applications: An innovative multipurpose thermal investigation. Energies 2019, 12, 1111. [Google Scholar] [CrossRef]
- Gallart-Sirvent, P.; Martin, M.; Villorbina, G.; Balcells, M.; Sole, A.; Barrenche, C.; Cabeza, L.F.; Cenela-Garayoa, R. Fatty acid eutectic mixtures and derivatives from non-edible animal fat as phase change materials. Rsc Adv. 2017, 7, 24133. [Google Scholar] [CrossRef]
- Thaib, R.; Amin, M.; Umar, H. Thermal Properties of Beef Tallow/Coconut Oil Bio PCM Using T-History Method for Wall Building Applications. Eur. J. Eng. Technol. Res. 2019, 4, 38–40. [Google Scholar] [CrossRef]
- Németh, B.; Ágnes, S. Németh.; Ujhidy, A.; Tóth, J.; Trif, L.; Gyenis, J.; Feczkó, T. Fully bio-originated latent heat storing calcium alginate microcapsules with high coconut oil loading. Sol. Energy 2018, 170, 314–322. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Boussaba, L.; Makhlouf, S.; Foufa, A.; Lefebvre, G.; Royon, L. vegetable fat: A low-cost bio-based phase change material for thermal energy storage in buildings. J. Build. Eng. 2019, 21, 222–229. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132. [Google Scholar] [CrossRef]
- Vakhshouri, A.R. Paraffin as Phase Change Material. In Paraffin; Soliman, F.S., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 5. [Google Scholar] [CrossRef]
- Ouikhalfan, M.; Sarı, A.; Hekimoğlu, G.; Gencel, O.; Tyagi, V. Thermal energy storage properties, thermal conductivity, chemical/and thermal reliability of three different organic phase change materials doped with hexagonal boron nitride. Surfaces Interfaces 2022, 32, 102176. [Google Scholar] [CrossRef]
- Inoue, T.; Hisatsugu, Y.; Suzuki, M.; Wang, Z.; Zheng, L. Solid–liquid phase behavior of binary fatty acid mixtures: 3. Mixtures of oleic acid with capric acid (decanoic acid) and caprylic acid (octanoic acid). Chem. Phys. Lipids 2004, 132, 225–234. [Google Scholar] [CrossRef]
- Zuo, J.; Li, W.; Weng, L. Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change material (PCM). Energy Build. 2011, 43, 207–210. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Sarı, A.; Mazumder, M.A.J.; Salhi, B.; Hekimoğlu, G.; Al-Sulaiman, F.A.; Inamuddin. Thermal energy storage and thermal conductivity properties of fatty acid/fatty acid-grafted-CNTs and fatty acid/CNTs as novel composite phase change materials. Sci. Rep. 2020, 10, 15388. [Google Scholar] [CrossRef]
- Ayaz, H.; Chinnasamy, V.; Jeon, Y.; Cho, H. Thermo-physical studies and corrosion analysis of caprylic acid–cetyl alcohol binary mixture as novel phase change material for refrigeration systems. Energy Rep. 2022, 8, 7143–7153. [Google Scholar] [CrossRef]
- Mei, D.; Zhang, B.; Liu, R.; Zhang, Y.; Liu, J. Preparation of capric acid/halloysite nanotube composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 2772–2777. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H. Study on preparation and thermal properties of binary fatty acid/diatomite shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 2412–2416. [Google Scholar] [CrossRef]
- Sarı, A. Eutectic mixtures of some fatty acids for low temperature solar heating applications: Thermal properties and thermal reliability. Appl. Therm. Eng. 2005, 25, 2100–2107. [Google Scholar] [CrossRef]
- Chen, Z.; Shan, F.; Cao, L.; Fang, G. Synthesis and thermal properties of shape-stabilized lauric acid/activated carbon composites as phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 102, 131–136. [Google Scholar] [CrossRef]
- Wu, S.; Fang, G. Dynamic performances of solar heat storage system with packed bed using myristic acid as phase change material. Energy Build. 2011, 43, 1091–1096. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Lin, L.; Fei, X.; Wei, Q.; Song, L.; Hu, Y.; Fong, H. Preparation, morphology and thermal properties of electrospun fatty acid eutectics/polyethylene terephthalate form-stable phase change ultrafine composite fibers for thermal energy storage. Energy Convers. Manag. 2012, 64, 245–255. [Google Scholar] [CrossRef]
- Mjallal, I.; Feghali, E.; Hammoud, M.; Habchi, C.; Lemenand, T. Exploring the colligative properties of Arachidic acid for potential use as PCM. Sol. Energy 2021, 214, 19–25. [Google Scholar] [CrossRef]
- Şahan, I.; Paksoy, H. Designing behenic acid microcapsules as novel phase change material for thermal energy storage applications at medium temperature. Int. J. Energy Res. 2020, 44, 3922–3933. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Inoue, T.; Sato, T.; Suzuki, M.; Sato, K. Pressure effect on transformation of cis-unsaturated fatty acid polymorphs. 3. Erucic acid (cis-ω9-docosenoic acid) and asclepic acid (cis-ω7-octadecenoic acid). Chem. Phys. Lipids 1992, 61, 283–291. [Google Scholar] [CrossRef]
- Ueno, S.; Miyazaki, A.; Yano, J.; Furukawa, Y.; Suzuki, M.; Sato, K. Polymorphism of linoleic acid (cis-9, cis-12-Octadecadienoic acid) and α-linolenic acid (cis-9, cis-12, cis-15-Octadecatrienoic acid). Chem. Phys. Lipids 2000, 107, 169–178. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Fan, J.; Lu, W.; White, E.; Rabinowitz, J.D. Liquid Chromatography–High Resolution Mass Spectrometry Analysis of Fatty Acid Metabolism. Anal. Chem. 2011, 83, 9114–9122. [Google Scholar] [CrossRef] [PubMed]
- Mottram, H.R.; Crossman, Z.M.; Evershed, R.P. Regiospecific characterisation of the triacylglycerols in animal fats using high performance liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry. Analyst 2001, 126, 1018–1024. [Google Scholar] [CrossRef]
- Lee, K.T.; Foglia, T.A. Synthesis, purification, and characterization of structured lipids produced from chicken fat. J. Am. Oil Chem. Soc. 2000, 77, 1027–1034. [Google Scholar] [CrossRef]
- Raj, S.; Skiba, G.; Weremko, D.; Fandrejewski, H.; Migdał, W.; Borowiec, F.; Poławska, E. The relationship between the chemical composition of the carcass and the fatty acid composition of intramuscular fat and backfat of several pig breeds slaughtered at different weights. Meat Sci. 2010, 86, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Himawan, C.; Starov, V.; Stapley, A. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Khatkar, B. Physicochemical, rheological and functional properties of fats and oils in relation to cookie quality: A review. J. Food Sci. Technol. 2016, 53, 3633–3641. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonca, C.B. Fatty acid composition of vegetable oils and fats. Bol. Cent. Pesqui. Process. Aliment. 2007, 25, 111–120. [Google Scholar] [CrossRef]
- Okogeri, O.; Stathopoulos, V.N. What about greener phase change materials? A review on biobased phase change materials for thermal energy storage applications. Int. J. Thermofluids 2021, 10, 100081. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Japir, A.W.; Salimon, J.; Derawi, D.; Yahaya, B.; Bahadi, M.; Al-Shuja’a, S.; Yusop, M. A highly efficient separation and physicochemical characteristics of saturated fatty acids from crude palm oil fatty acids mixture using methanol crystallisation method. Ocl -Oilseeds Fats Crop. Lipids 2018, 25, A203. [Google Scholar] [CrossRef]
- Cermak, S.C.; Evangelista, R.L.; Kenar, J.A. Distillation of Natural Fatty Acids and Their Chemical Derivatives. In Distillation; Zereshki, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 5. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Karaipekli, A. Synthesis, characterization, thermal properties of a series of stearic acid esters as novel solid–liquid phase change materials. Mater. Lett. 2009, 63, 1213–1216. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Karaipekli, A.; Alkan, C.; Karadag, A. Synthesis, thermal energy storage properties and thermal reliability of some fatty acid esters with glycerol as novel solid–liquid phase change materials. Sol. Energy Mater. Sol. Cells 2010, 94, 1711–1715. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Fatty acid esters-based composite phase change materials for thermal energy storage in buildings. Appl. Therm. Eng. 2012, 37, 208–216. [Google Scholar] [CrossRef]
- Basturk, E.; Kahraman, M.V. Photocrosslinked biobased phase change material for thermal energy storage. J. Appl. Polym. Sci. 2016, 133, 43757. [Google Scholar] [CrossRef]
- Rogers, M.A. Chapter 18—Crystallization of Fats and Fatty Acids in Edible Oils and Structure Determination. In Fatty Acids; AOCS Press: Champaign, IL, USA, 2017; pp. 541–559. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Acevedo, N.; Maleky, F.; Co, E.; Peyronel, F.; Mazzanti, G.; Quinn, B.; Pink, D. Structure and functionality of edible fats. Soft Matter 2012, 8, 1275–1300. [Google Scholar] [CrossRef]
- Kim, B.W.; Liu, C.; Yin, H. Thermoelastic modeling of cubic lattices from granular materials to atomic crystals. J. Appl. Phys. 2024, 135, 075105. [Google Scholar] [CrossRef]



| SDG | Contribution of PCMs | Ref |
|---|---|---|
| 2. Zero Hunger | Improving crop quality and productivity | [15] |
| 7. Affordable and Clean Energy | Incorporating passive into active thermal systems for space cooling | [11] |
| Lowering the cost of heat storage and transportation | [17] | |
| 65% reduced auxiliary heater load | [22] | |
| 53.7% longer heat transfer duration and 58.9% reduced annual energy cost for electrical heater | [23] | |
| 12. Responsible Consumption and Production | Reducing food wastes for the passive thermal method | [21] |
| 13. Climate Action | Reducing 8.03–10.95 t CO2 equivalent per year per hectare by decreasing operation of root zone heating systems | [16] |
| Decreasing CO2 emissions up to 93% by mobilized thermal energy storage | [24] |
| Source | PCM | °C) | (J/g) | (W/mK) | Ref |
|---|---|---|---|---|---|
| Italian sausage | Filtered pork fat | 0 | 43.3 | 0.21 | [27] |
| 32 | 21.3 | ||||
| Local market and restaurant | Mixture of pork fat with burnt oil | - | - | 0.19 | [28] |
| Animal parts from a slaughterhouse | Mostly pig and chicken paste | 2 | 5.67 | - | [29] |
| 25 | 23.27 | ||||
| Non-edible fatty pig and chicken parts | PA-SA mixture | 53.4–55.2 | 157–183 | - | [30] |
| DHSA | 93.8 | 161 | |||
| DHSA salts | 160–231.6 | 76–236 | |||
| Beef tallow from market | Filtered beef fat | 37.4 | 101.05 | - | [31] |
| Soya Group | Coconut oil | 24 | 115.31 | – | [32] |
| Bakery | Palm oil | 3.29 | 33.03 | - | [33] |
| 33.82 | 13.68 | ||||
| Pacific Interlink SDNBHD | Hydrogenated palm kernel fat | 26.53 | 74.35 | 0.20 | [34] |
| Saturated | Formula | (°C) | (J/g) | (W/mK) | Ref |
|---|---|---|---|---|---|
| (8:0) Caprylic | CH3(CH2)6COOH | 15.4–16.1 | 142.6–158.4 | – | [38,39,40,41] |
| (10:0) Capric | CH3(CH2)8COOH | 29.6–31.5 | 139.8–155.5 | 0.15 | [42,43] |
| (12:0) Lauric | CH3(CH2)10COOH | 42.6–44.3 | 176.6–179.9 | 0.15 | [44,45] |
| (14:0) Myristic | CH3(CH2)12COOH | 49.0–63.3 | 178.1–210.7 | 0.15 | [44,46] |
| (16:0) Palmitic | CH3(CH2)14COOH | 58.9–69.4 | 164.8–420.0 | 0.16 | [44,47] |
| (18:0) Stearic | CH3(CH2)16COOH | 55.0–77.6 | 185.4–259.0 | 0.17 | [44,47] |
| (20:0) Arachidic | CH3(CH2)18COOH | 75.2 | 257.4 | – | [48] |
| (22:0) Behenic | CH3(CH2)20COOH | 69–88 | 232 | – | [49] |
| Unsaturated | Formula | (°C) | (J/g) | (W/mK) | Ref |
| (16:1) Palmitoleic | CH3(CH2)5CH= CH(CH2)7COOH | 2 | 125.2 | - | [50] |
| (18:1) Oleic | CH3(CH2)7= CH(CH2)7COOH | 13.6 | 138.1 | 0.10 | [51] |
| (18:2) Linoleic | CH3(CH2)4CH= CHCH2CH= CH(CH2)7COOH | 33.6 | 180.5 | - | [51] |
| (18:3) -Linolenic | CH3(CH2CH=CH)3 (CH2)7COOH | 27.8 | 164.8 | - | [51] |
| C:D Ratio | Name | (°C) | (°C/10 mmHg) | ||
|---|---|---|---|---|---|
| FFA | FAME | FFA | FAME | ||
| FA 16:0 | Palmitic | 62.5–64.0 | 28.5–30.5 | 212 | 184 |
| FA 18:0 | Steric | 68.8–71.2 | 37.7–39.1 | 227 | 205 |
| FA 18:1 | Oleic | 13.2–16.3 | −20.2–19.6 | 223 | 201 |
| PCM | Freezing Properties | Melting Properties | |||
|---|---|---|---|---|---|
| (°C) | (J/g) | (°C) | (J/g) | ||
| Bacon fat | 4.23 | 50.61 | 27.35 | 35.63 | |
| −16.48 | 62.97 | −2.14 | 42.11 | ||
| FFA | 30.45 | 55.05 | 37.78 | 52.73 | |
| −17.82 | 27.08 | 0.79 | 25.89 | ||
| FAME | 6.79 | 57.08 | 9.00 | 57.05 | |
| −66.19 | 22.40 | −39.40 | 26.54 | ||
| UV | 33.53 | 70.94 | 40.80 | 67.22 | |
| crystal | 51.42 | 195.55 | 56.61 | 195.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadshir, M.; Kim, B.-W.; Yin, H. Bio-Based Phase Change Materials for Sustainable Development. Materials 2024, 17, 4816. https://doi.org/10.3390/ma17194816
Zadshir M, Kim B-W, Yin H. Bio-Based Phase Change Materials for Sustainable Development. Materials. 2024; 17(19):4816. https://doi.org/10.3390/ma17194816
Chicago/Turabian StyleZadshir, Mehdi, Byung-Wook Kim, and Huiming Yin. 2024. "Bio-Based Phase Change Materials for Sustainable Development" Materials 17, no. 19: 4816. https://doi.org/10.3390/ma17194816








