Effect of Vanadium Addition on Solidification Microstructure and Mechanical Properties of Al–4Ni Alloy
Abstract
:1. Introduction
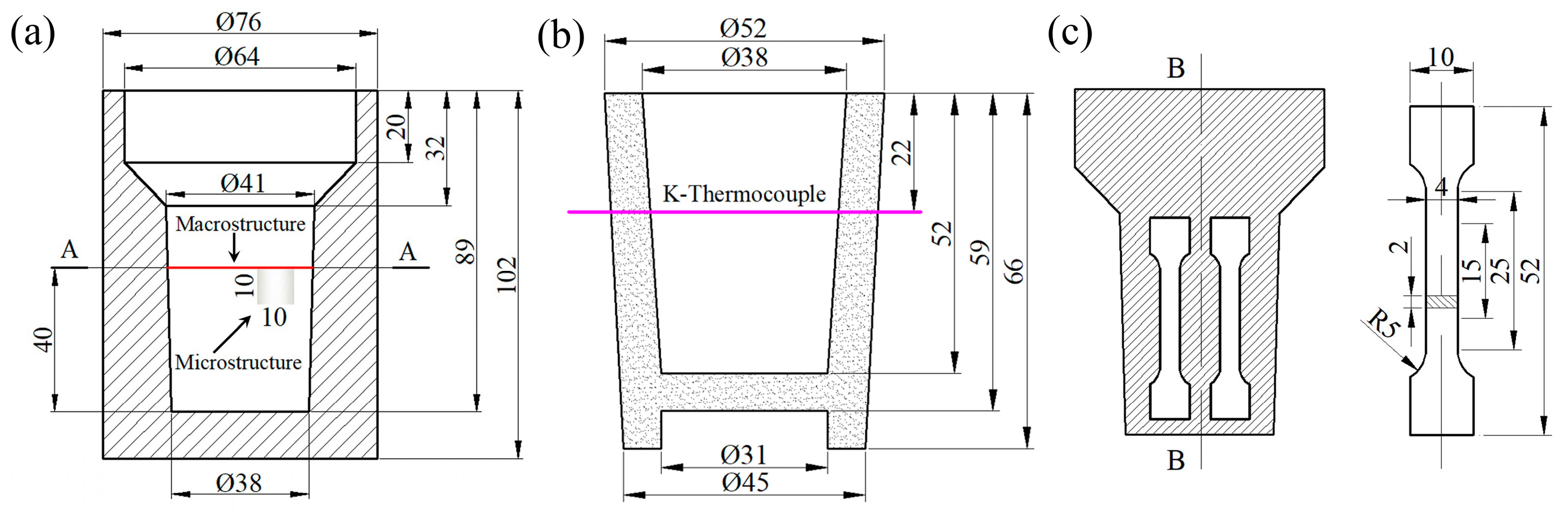
2. Experimental
3. Results and Discussion
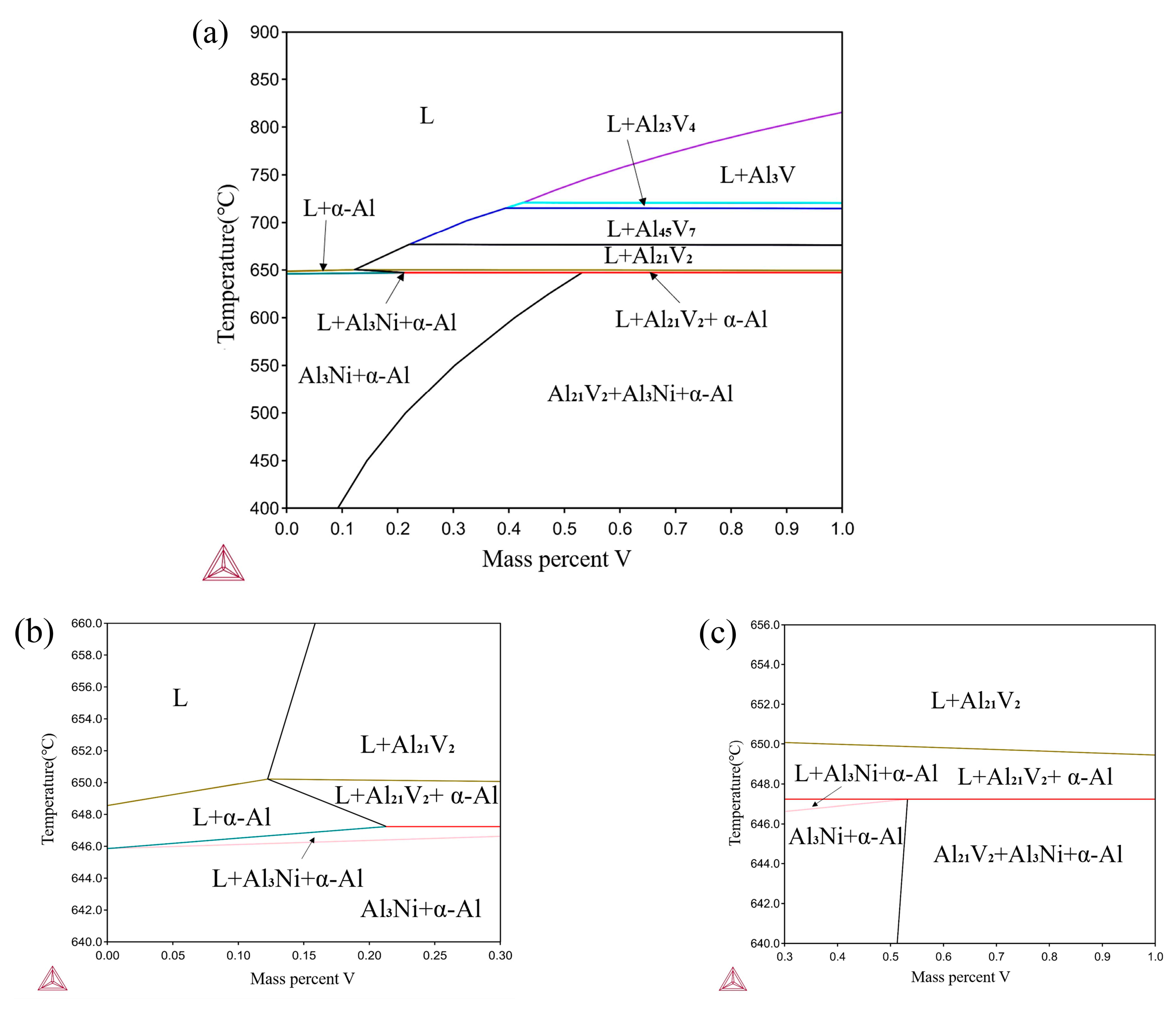
3.1. Thermodynamic Analyses of the Al–4Ni–V System
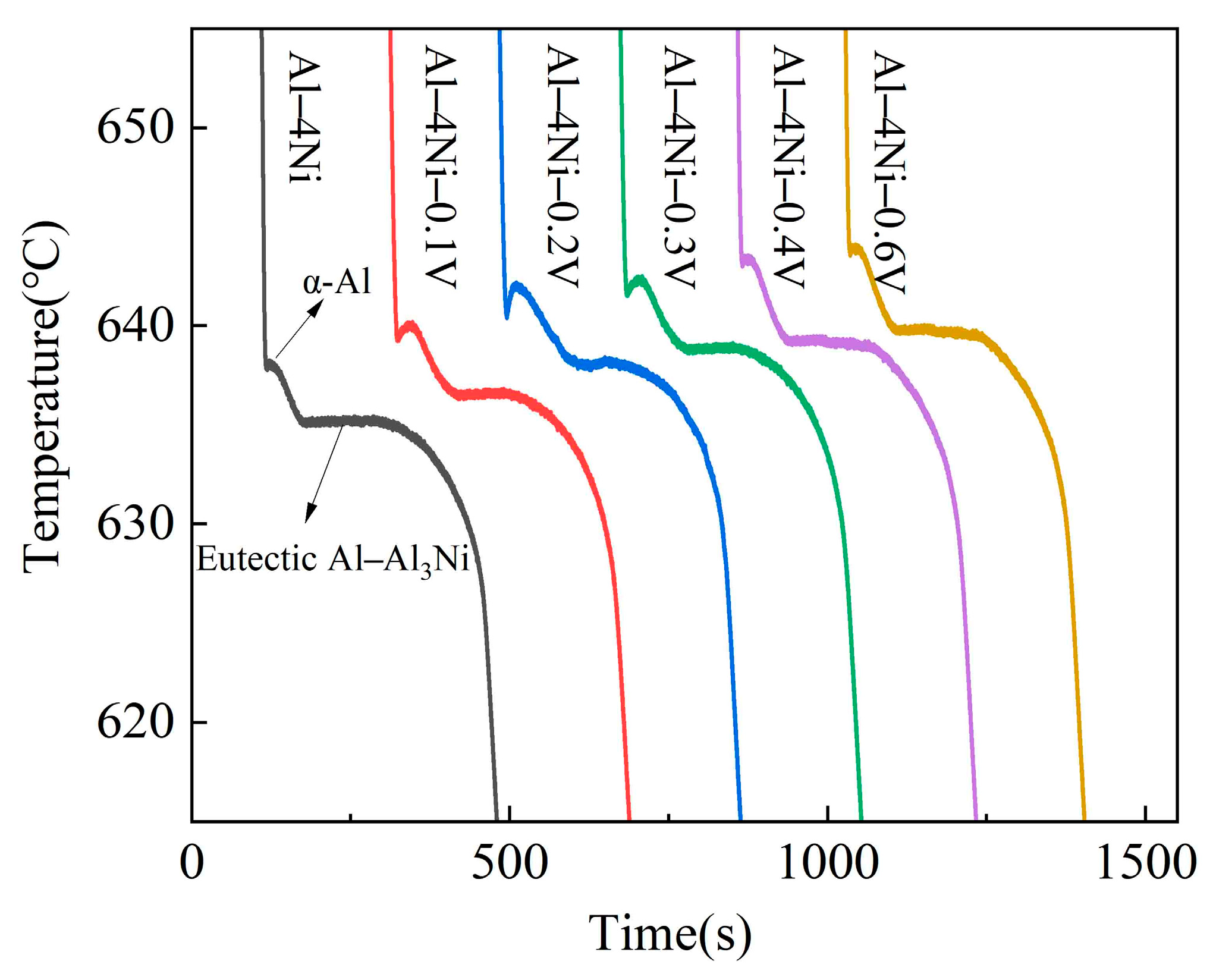
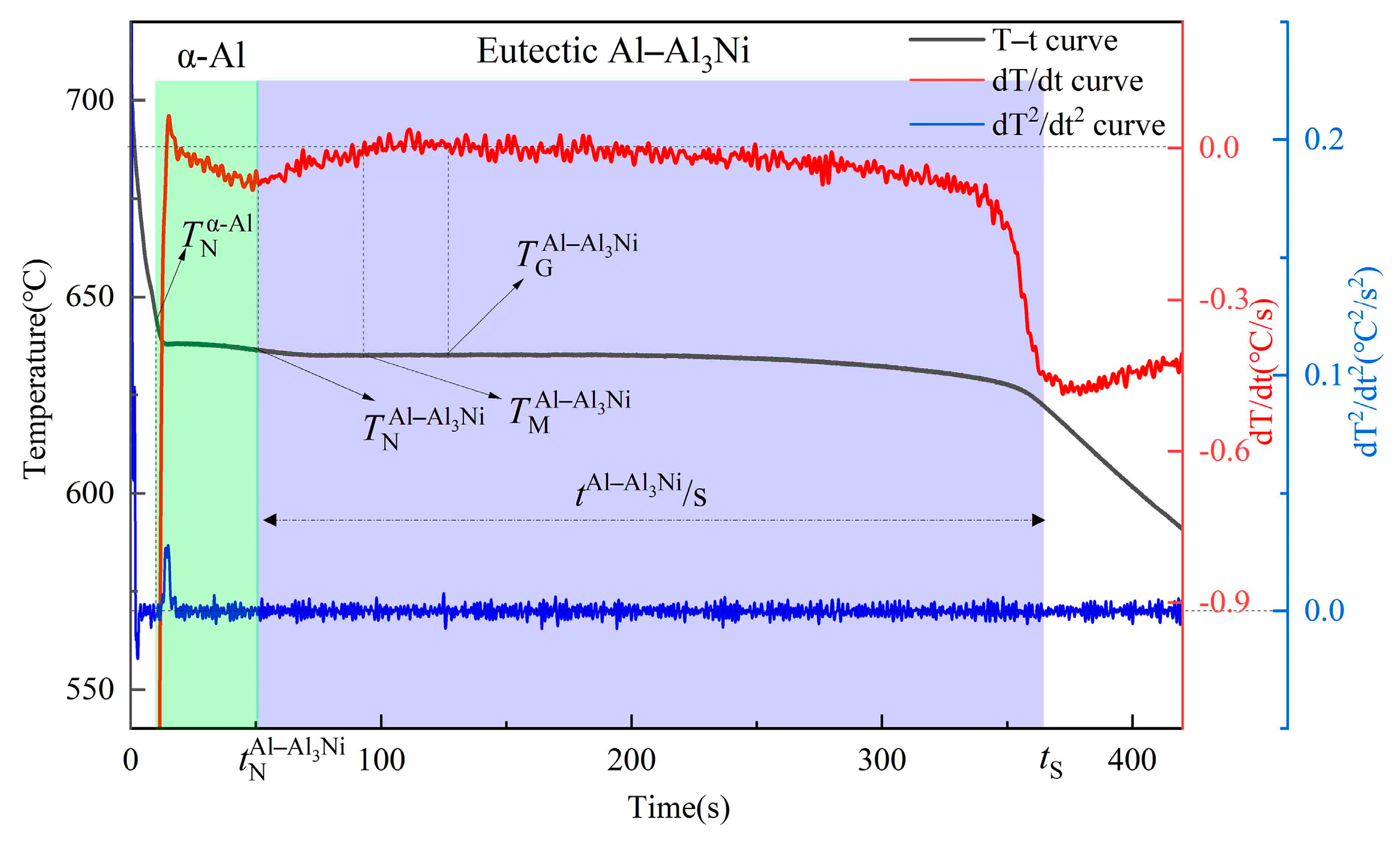
3.2. Solidification Process
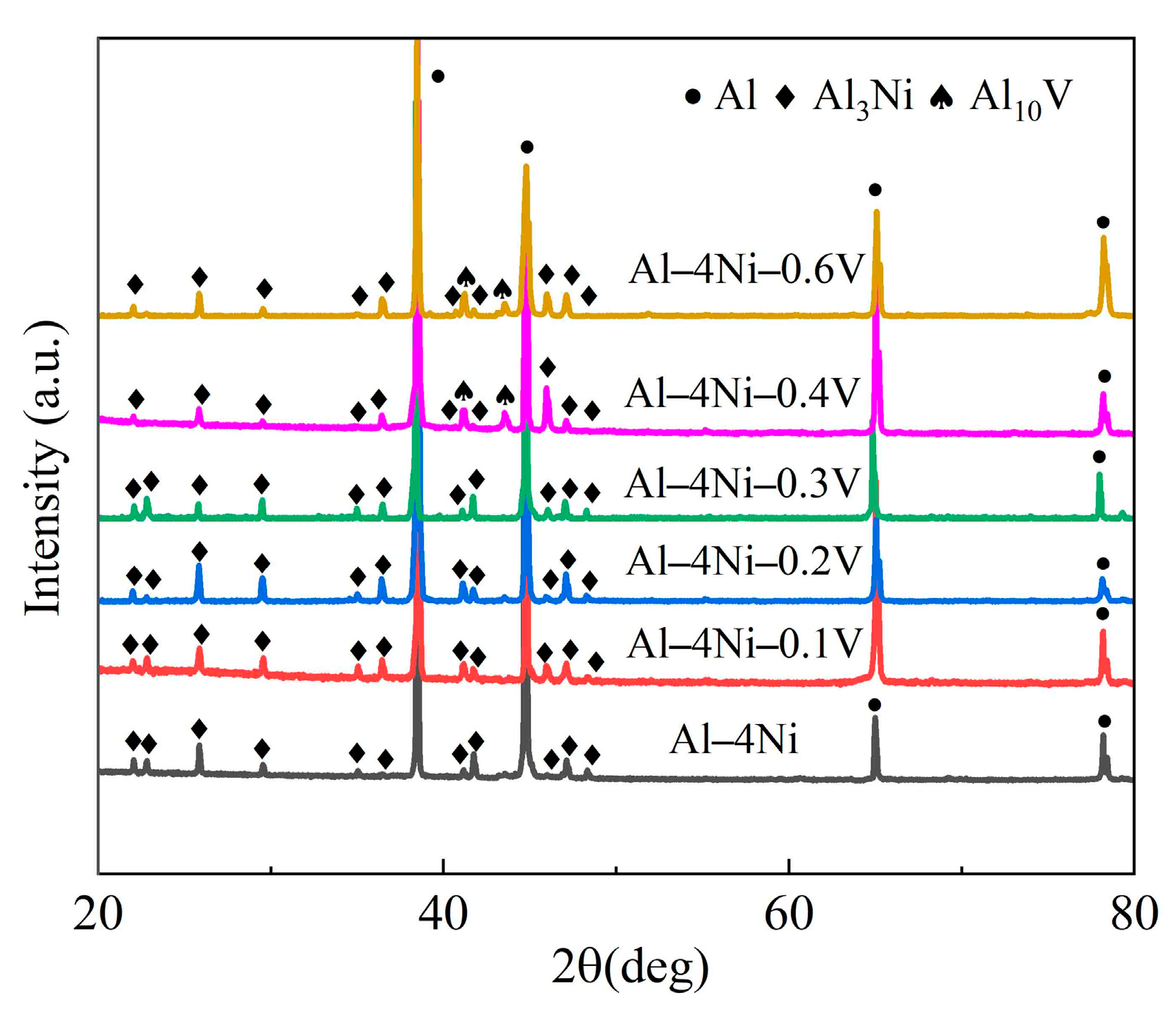
3.3. XRD Phase Analysis
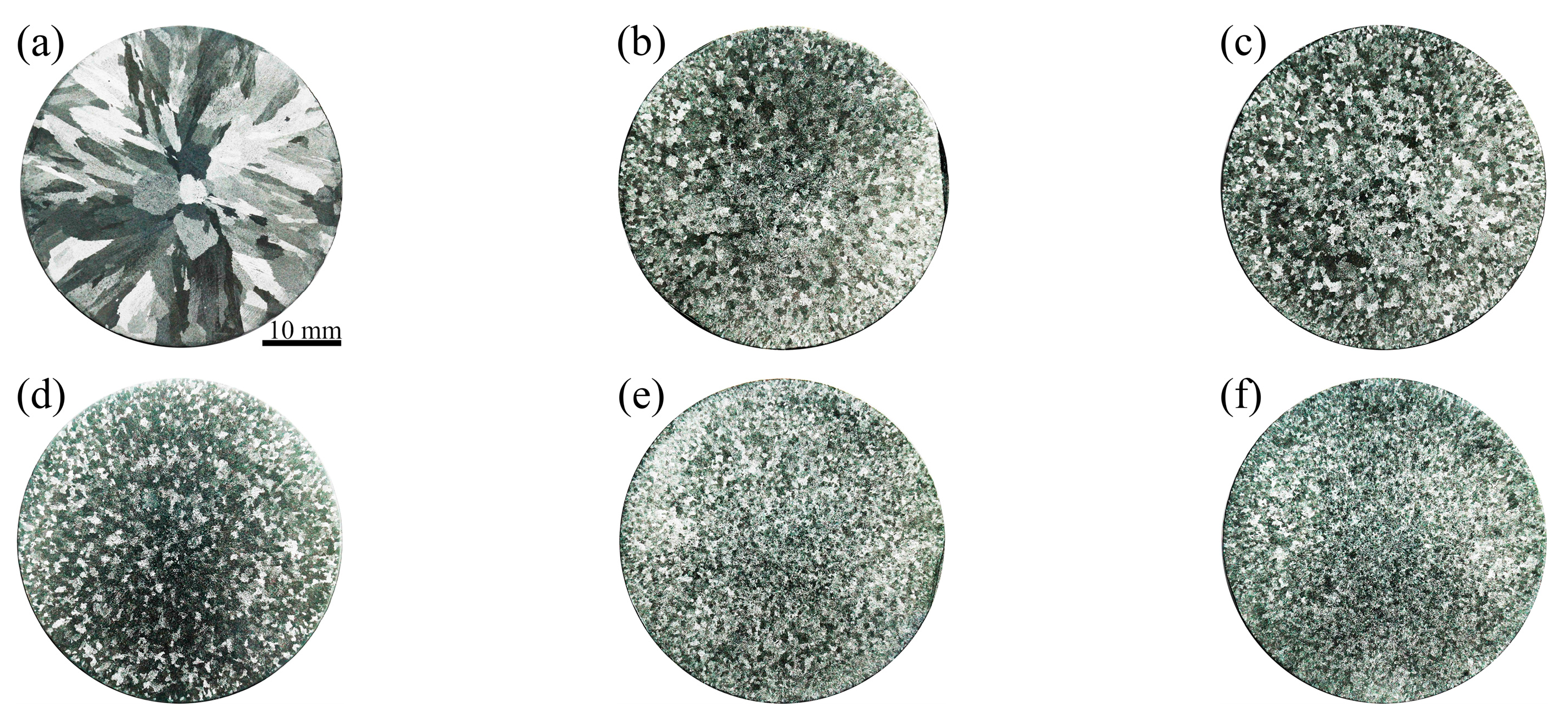
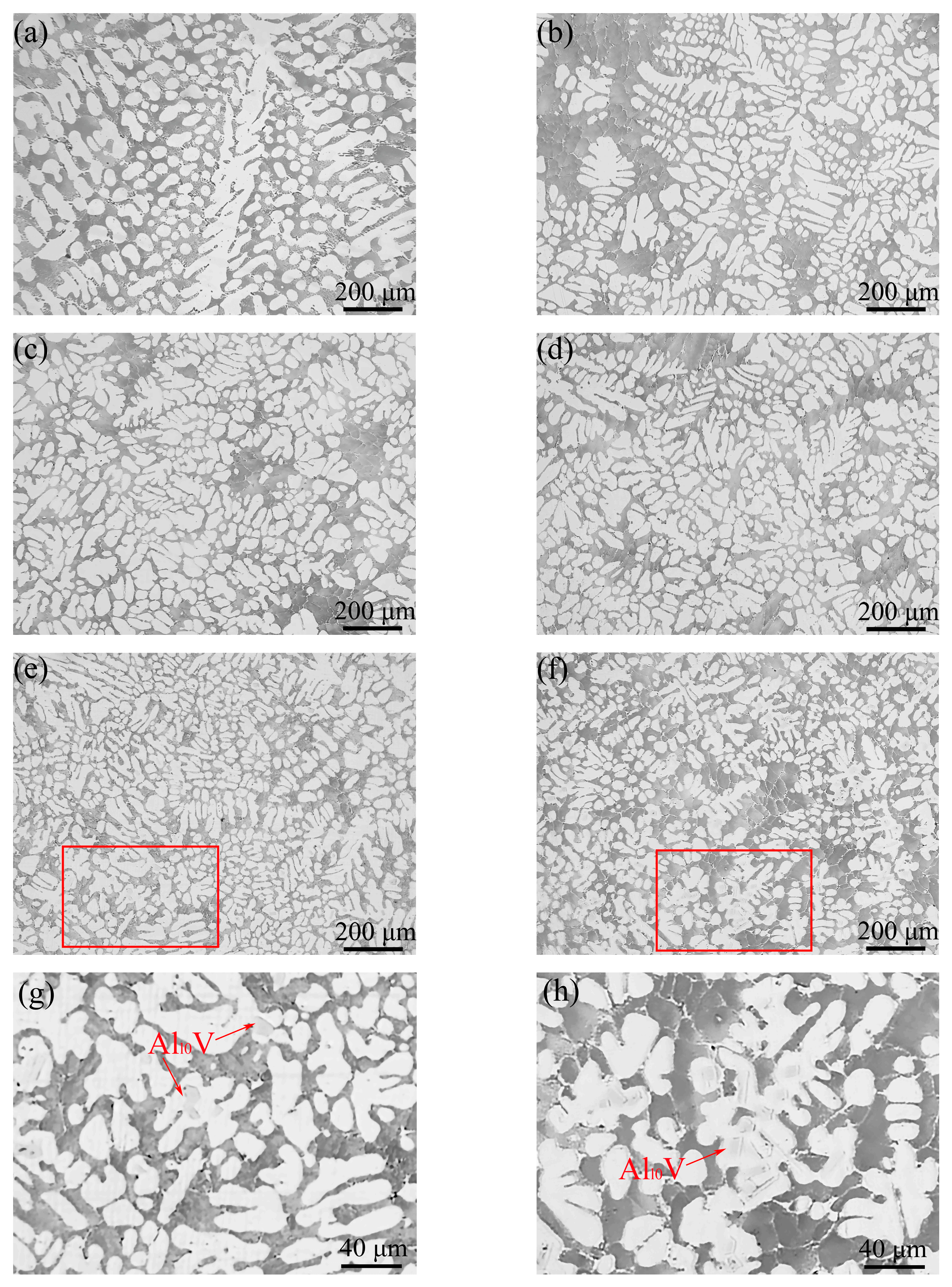
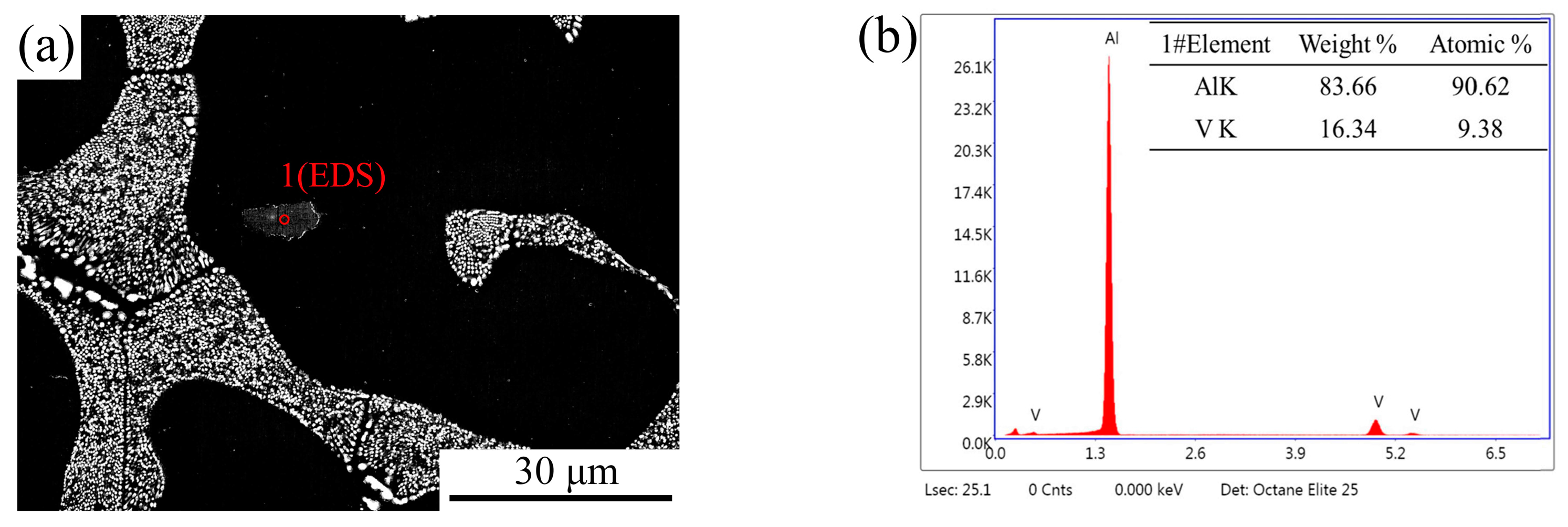
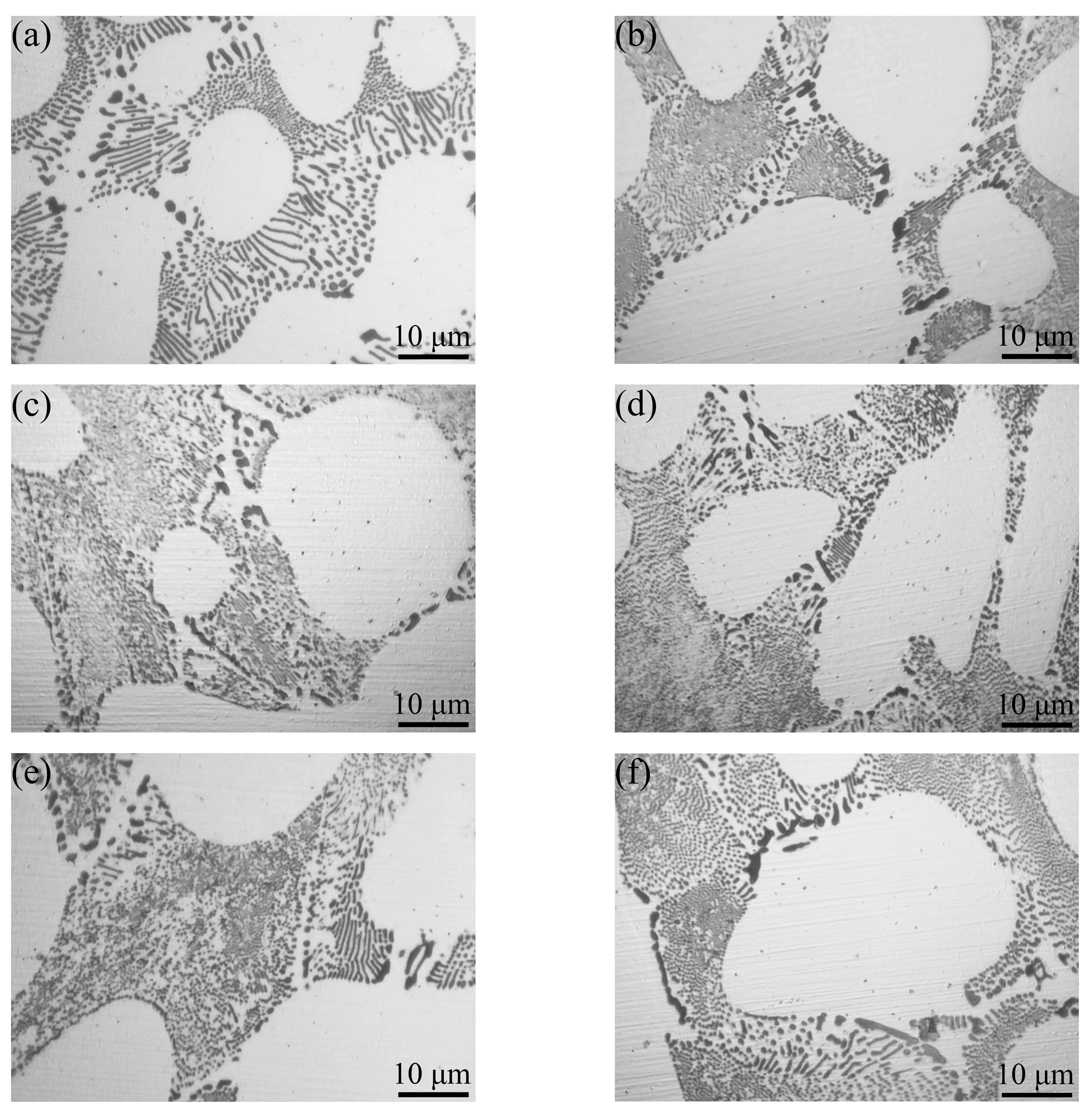
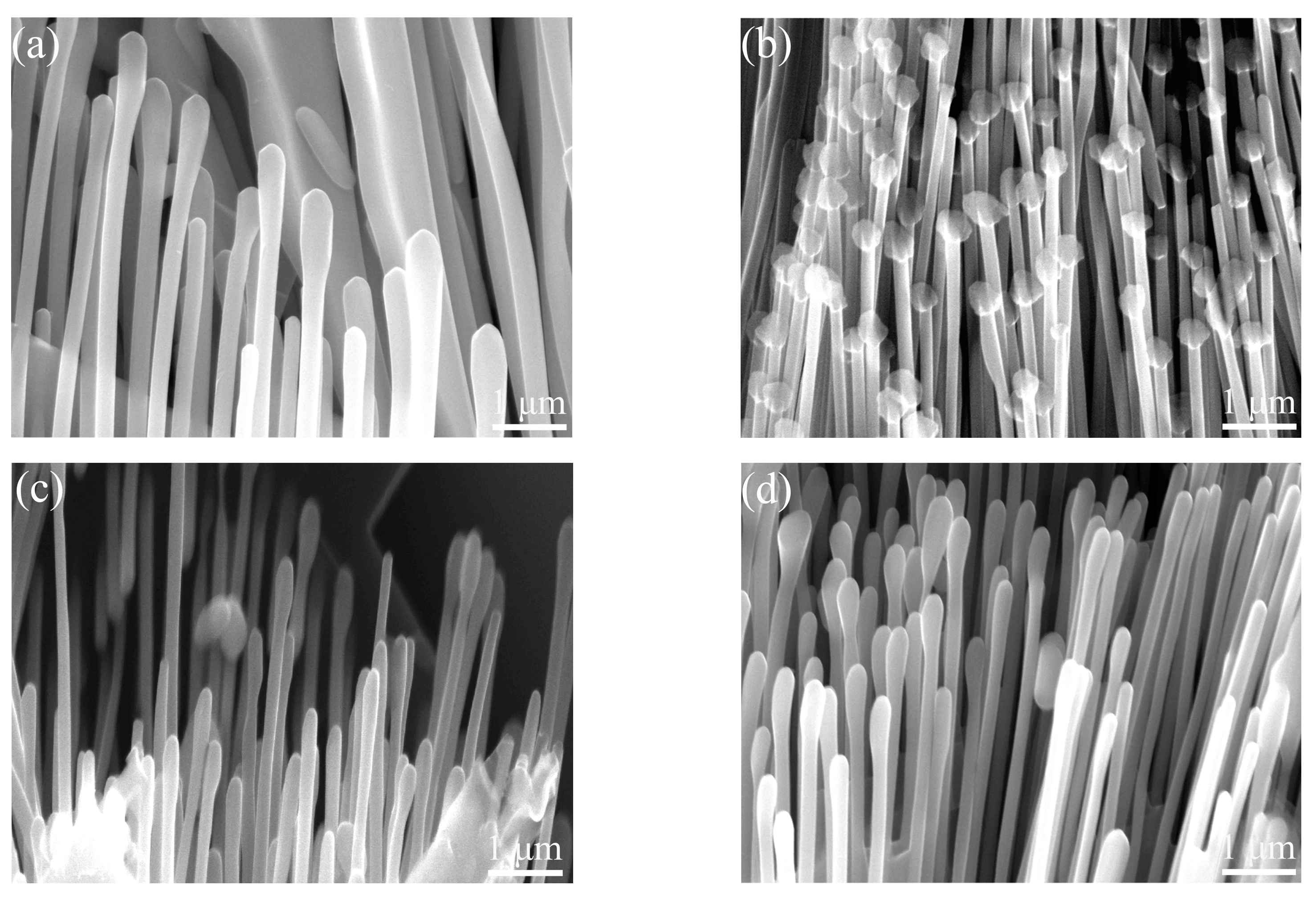
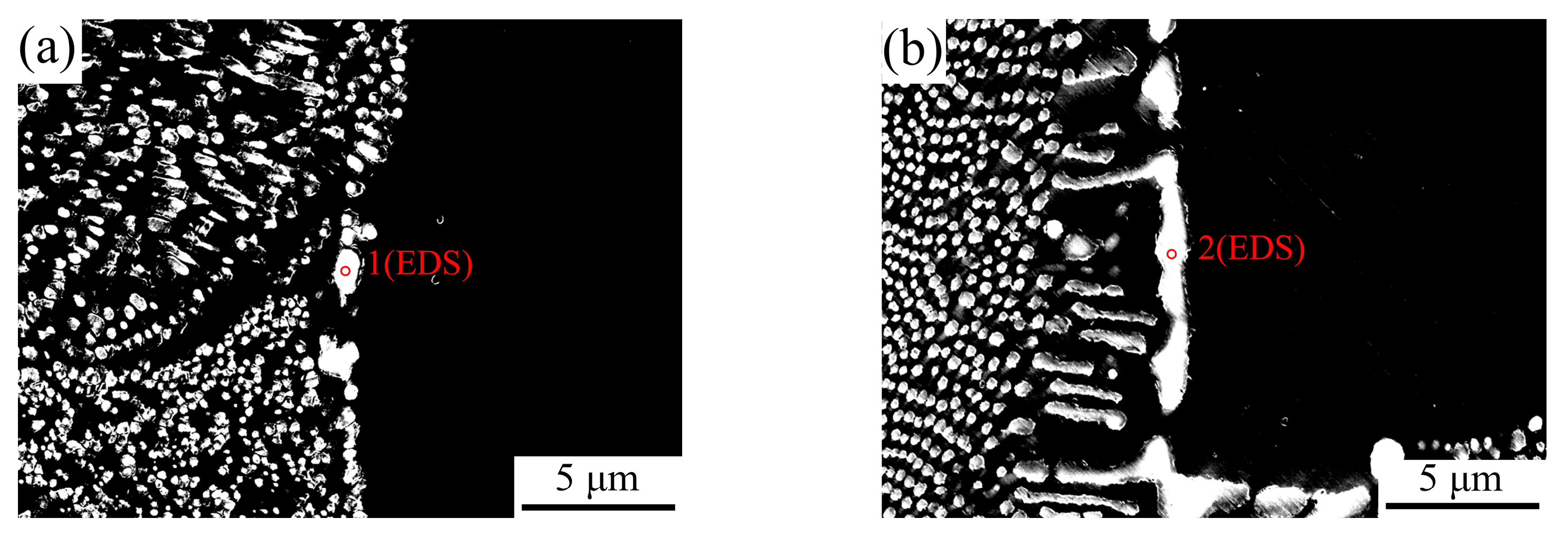
3.4. Microstructural Evolution
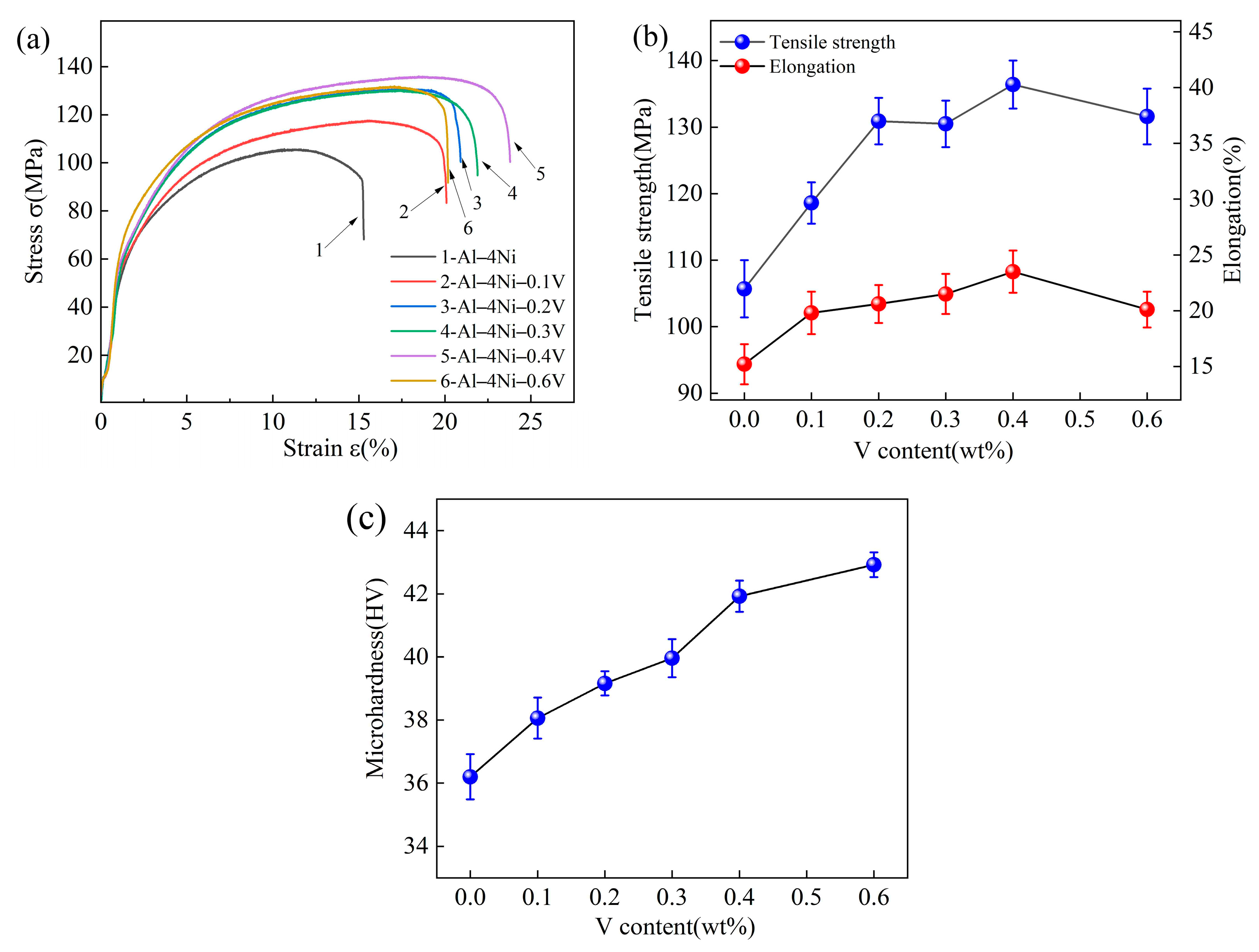
3.5. Mechanical Properties
4. Conclusions
- (1)
- With increased vanadium addition (0–0.6 wt%), the increased. When the vanadium addition was 0.6 wt%, the increased from 642.5 °C for the Al–4Ni alloy to 646.5 °C.
- (2)
- When the vanadium addition was 0.2 wt%, the and increased from 636.2 °C and 0.2 °C for the Al–4Ni alloy to 640.5 °C and 0.7 °C, respectively. The decreased from 310 s to 282 s. As the vanadium addition continued to increase, the did not change significantly, and the began to increase.
- (3)
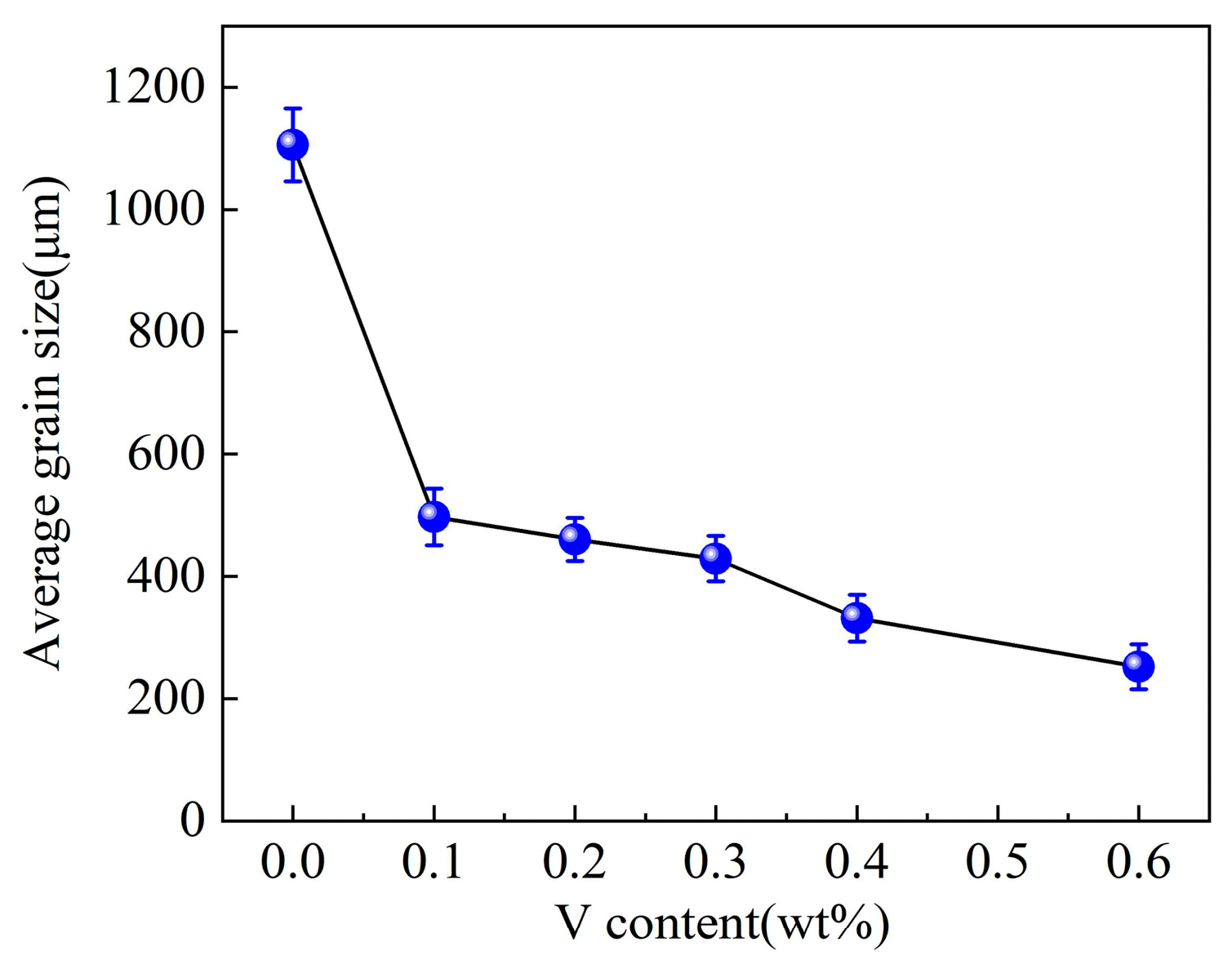
- The columnar-to-equiaxed transition of primary α-Al took place when adding vanadium to the Al–4Ni alloy. The average grain size of the primary α-Al reduced from 1105 μm to 252 μm when the vanadium addition was increased from 0 to 0.6 wt%.
- (4)
- When the vanadium addition was less than 0.2 wt%, the eutectic structure was refined. The average diameter of the eutectic Al3Ni phases in the Al–4Ni–0.2V alloy was 0.14 μm, which was 46% lower than 0.26 μm for the Al–4Ni alloy. As the vanadium additions became higher than 0.2 wt%, the eutectic Al3Ni phases began to coarsen again.
- (5)
- The mechanical properties of Al–4Ni alloys can be improved with the addition of vanadium. The Al–4Ni–0.4V alloy obtained the highest tensile strength and elongation of 136.4 MPa and 23.5%, which were 29.1% and 54.6% higher than that of the Al–4Ni alloy, respectively. When the vanadium addition was 0.6 wt%, the tensile strength and elongation decreased. The microhardness of the Al–4Ni alloys increased gradually with increased additions of vanadium.
- (6)
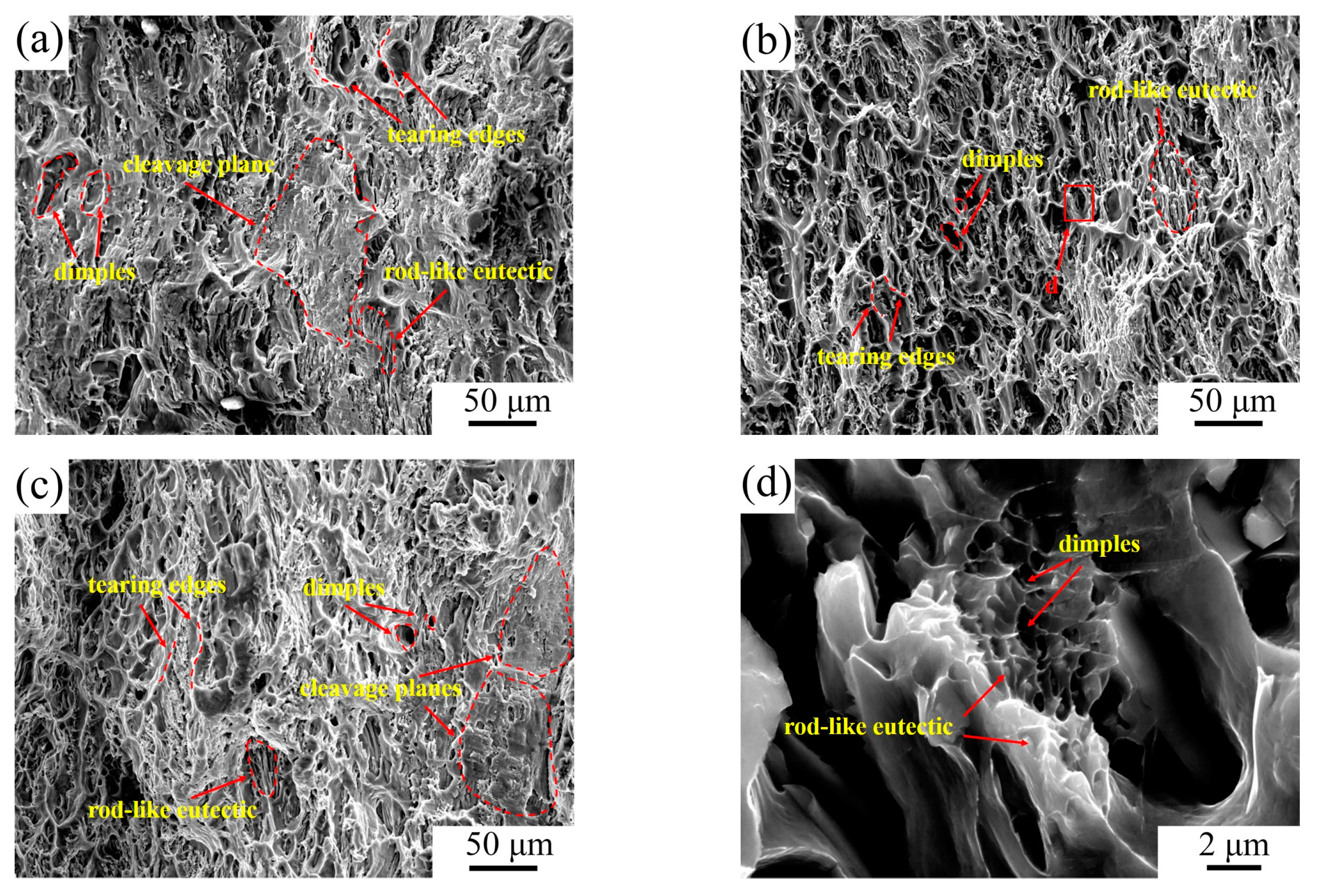
- The fracture of the Al–4Ni–0.4V alloy was composed of dimples and tear edges, demonstrating ductile fracture. Meanwhile, the fracture of the Al–4Ni–0.6V alloy was composed of dimples, tear edges, and cleavage planes, showing mixed ductile–brittle fracture. The cleavage planes were caused by the primary Al10V and coarse Al3Ni phases at the boundary of the eutectic cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Tian, Y.; Zheng, X.; Zhang, Y.; Chen, L.; Wang, J. Research Progress on Multi-Component Alloying and Heat Treatment of High Strength and Toughness Al-Si-Cu-Mg Cast Aluminum Alloys. Materials 2023, 16, 1065. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, C.S.; Pandey, P.; Sarkar, S.; Das, R.; Samal, S.; Biswas, K.; Chattopadhyay, K. Five decades of research on the development of eutectic as engineering materials. Prog. Mater. Sci. 2022, 123, 100793. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Wang, J.; Chen, C.; Li, X.; Wang, L.; Wang, M. Effect of Alloying Elements on the Short-Range Orders and Atomic Diffusion Behavior of Liquid Al-9Si Cast Alloys. Materials 2023, 16, 6768. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Liao, H.C.; Tang, Y.Y. Enhanced high-cycle fatigue strength of Al-12Si-4Cu-1.2Mn-T6 cast aluminum alloy at room temperature and 350 C. Mater. Sci. Eng. A 2021, 825, 141917. [Google Scholar] [CrossRef]
- Hu, X.; Song, D.; Wang, H.; Jia, Y.; Zou, H.; Chen, M. Effect of Ultrasonic-Assisted Modification Treatment on the Microstructure and Properties of A356 Alloy. Materials 2022, 15, 3714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, M.L.; Fan, L.Y.; Bai, J.B.; Chen, X.L.; Zhang, J.; Li, Z.; Guan, R. Towards high mechanical performance Al-Cu-Mg-Fe-Ni alloy: Influence of composition, solution treatment and aged process on microstructural evolution and mechanical properties. J. Mater. Res. Technol. 2023, 23, 2054–2064. [Google Scholar] [CrossRef]
- Shah, A.W.; Ha, S.-H.; Siddique, J.A.; Kim, B.-H.; Yoon, Y.-O.; Lim, H.-K.; Kim, S.K. Microstructure Evolution and Mechanical Properties of Al-Cu-Mg Alloys with Si Addition. Materials 2023, 16, 2783. [Google Scholar] [CrossRef]
- Bogdanoff, T.; Dahle, A.K.; Seifeddine, S. Effect of Co and Ni Addition on the Microstructure and Mechanical Properties at Room and Elevated Temperature of an Al-7%Si Alloy. Int. J. Cast Met. Res. 2018, 12, 434–440. [Google Scholar] [CrossRef]
- Li, D.; Liu, K.; Rakhmonov, J.; Chen, X.G. Enhanced thermal stability of precipitates and elevated-temperature properties via microalloying with transition metals (Zr, V and Sc) in Al-Cu 224 cast alloys. Mater. Sci. Eng. A 2021, 827, 142090. [Google Scholar] [CrossRef]
- Fan, Y.; Makhlouf, M.M. The Al-Al3Ni Eutectic Reaction: Crystallography and Mechanism of Formation. Metall. Mater. Trans. A 2015, 46, 3808–3812. [Google Scholar] [CrossRef]
- Cooke, A.R.; Martin, J.W. Tensile yielding of Al-Al3Ni eutectic crystals. J. Mater. Sci. 1976, 11, 665. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Pandee, P.; Patakham, U.; Limmaneevichitra, C. New generation of eutectic Al-Ni casting alloys for elevated temperature services. Mater. Sci. Eng. A 2018, 709, 46. [Google Scholar] [CrossRef]
- Carrara, A.P.; Kakitani, R.; Garcia, A.; Cheung, N. Effect of cooling rate on microstructure and micro–hardness of hypereutectic Al-Ni alloy. Archi. Civ. Mech. Eng. 2021, 21, 14. [Google Scholar] [CrossRef]
- Pandey, P.; Kashyap, S.; Tiwary, C.S.; Chattopadhyayet, K. Development of high-strength high-temperature cast Al-Ni-Cr alloys through evolution of a novel composite eutectic structure. Metall. Mater. Trans. 2017, 48, 5940–5950. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, H.; Sun, Y.; Su, Y.; Wang, Y.; Wu, C.; Liu, J. Influence of Co Contents and Super-Gravity Field on Refinement of In-Situ Ultra-Fined Fibers in Al-2.5Ni Eutectic Alloys. J. Alloys Compd. 2020, 822, 153607. [Google Scholar] [CrossRef]
- Sankanit, P.; Uthaisangsuk, V.; Pandee, P. Thermal stability of Al-4Ni-1Mn alloy with Sc and Zr addition. Mater. Charact. 2022, 192, 112227. [Google Scholar] [CrossRef]
- Wang, K.; Hu, S.; Zhong, Y.; Jin, S.; Zhou, Z.; Wang, Z.; Chen, J.; Wan, B.; Li, W. Effects of Trace Ytterbium Addition on Microstructure, Mechanical and Thermal Properties of Hypoeutectic Al-5Ni Alloy. J. Rare Earths 2022, 40, 1305–1315. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Toinin, J.P.; Michi, R.A.; Pandee, P.; Dunand, D.C.; Limmaneevichitr, C. Strengthening mechanisms in Al-Ni-Sc alloys containing Al3Ni microfibers and Al3Sc nanoprecipitates. Acta. Mater. 2019, 64, 334–346. [Google Scholar] [CrossRef]
- Song, Q.; Li, C.; Deng, Z.; Zhang, L.; Liu, L. Experimental Investigation and Thermodynamic Assessment of the Ternary Al-Ni-Er System. Processes 2023, 11, 1061. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. The Effect of Transition Elements on High-Temperature Mechanical Properties of Al-Si Foundry Alloys—A Review. Adv. Eng. Mater. 2016, 18, 1096–1105. [Google Scholar] [CrossRef]
- Kilinc, E.; Kiremitci, D.; Birol, Y.; Dokumacit, E. Effect of vanadium and zirconium additions on mechanical properties and microstructure of gravity die-cast AlSi9Cu2 alloy cylinder heads. Int. J. Cast Met. Res. 2019, 13, 137–145. [Google Scholar] [CrossRef]
- Wang, F.; Chiu, Y.L.; Eskin, D.; Du, W.; Shearing, P.R. A Grain Refinement Mechanism of Cast Commercial Purity Aluminum by Vanadium. Mater. Charact. 2021, 181, 111468. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. Characterization of the Solidification Path and Microstructure of Secondary Al-7Si-3Cu-0.3Mg Alloy with Zr, V and Ni Additions. Mater. Charact. 2017, 128, 100–108. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ni (aluminum-nickel). J. Phase Equilib. Diffus. 2004, 25, 394. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Y.; Cen, L.; Cao, L.; Zhao, Z.; Zhu, Q.; Cui, J. Evolution of V-Containing Phases during Preparation of Al-Based Al−V Master Alloys. Trans. Nonferrous Met. Soc. China 2022, 32, 2110–2124. [Google Scholar] [CrossRef]
- Hong, Y.M.; Mishima, Y.; Suzuki, T. Accurate determination of γ’ solvus in Ni-Al-X ternary Systems. Mater. Res. Symp. Proc. 1988, 133, 429–440. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Ni-V (Aluminum-Nickel-Vanadium). J. Phase Equilib. Diffus. 2005, 26, 273–275. [Google Scholar] [CrossRef]
- Murray, J.L. Al-V (aluminum-vanadium). Bull. Alloy Phase Diagr. 1989, 10, 351–357. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhou, Y. Solidification of undercooled eutectic alloys containing a third element. Acta. Mater. 2009, 57, 1536–1545. [Google Scholar] [CrossRef]
- Farahany, S.; Ourdjini, A.; Idrsi, M.H.; Shabestari, S.G. Evaluation of the effect of Bi, Sb, Sr and cooling condition on eutectic phases in an Al-Si-Cu alloy (ADC12) by in situ thermal analysis. Thermochim. Acta 2013, 559, 59–68. [Google Scholar] [CrossRef]
- Xu, H.; Xu, L.; Zhang, S.; Han, Q. Effect of the alloy composition on the grain refinement of aluminum alloys. Scripta. Mater. 2006, 54, 2191–2196. [Google Scholar] [CrossRef]
- Okamoto, H. Al-V (Aluminum-Vanadium). J. Phase Equilib. Diffus. 2012, 33, 491. [Google Scholar] [CrossRef]
- Jahnátek, M.; Krajcí, M.; Hafner, J. Electronic structure and interatomic bonding in Al10V. J. Phys. Condens. Matterr. 2003, 15, 5675–5688. [Google Scholar] [CrossRef]
- Yu, W.; Hao, Q.; Fan, L.; Li, J.H. Eutectic solidification microstructure of an Al-4Ni-2Mn alloy. J. Alloys Compd. 2016, 688, 798–803. [Google Scholar] [CrossRef]
- Kakitani, R.; Reyes, R.V.; Garcia, A.; Spinelli, J.E.; Cheung, N. Relationship between Spacing of Eutectic Colonies and Tensile Properties of Transient Directionally Solidified Al-Ni Eutectic Alloy. J. Alloys Compd. 2018, 733, 59–68. [Google Scholar] [CrossRef]
- Tantardini, C.; Oganov, A.R. Thermochemical electronegativities of the elements. Nat. Commun. 2021, 12, 2087. [Google Scholar] [CrossRef]
- Yousefi, F.; Taghiabadi, R.; Baghshahi, S. Improving mechanical properties of Mn-added hypoeutectic Al-4Ni alloy by friction stir processing. Trans. Nonferrous Met. Soc. China 2019, 29, 460–472. [Google Scholar] [CrossRef]
- Zhu, X.P.; Wang, Y.S.; Wu, H.L.; Xie, L.J.; Liu, X.W.; Fan, Z.T.; Lü, S.L.; Wang, K.; Wang, Z.D. Grain refinement and modification of an Al-Si-Mg-Ti-Be cast alloy: Microstructure and tensile property. Ferroelectrics 2022, 601, 194–204. [Google Scholar] [CrossRef]
- Lei, W.; Liu, X.; Wang, W.; Sun, Q.; Xu, Y.; Cui, J. On the influences of Li on the microstructure and properties of hypoeutectic Al-7Si alloy. J. Alloys Compd. 2017, 729, 703–709. [Google Scholar] [CrossRef]
- Lei, Q.; Ramakrishnan, B.P.; Wang, S.J.; Wang, Y.C.; Mazumder, J.; Misra, A. Structural refinement and nanomechanical response of laser remelted Al-Al2Cu lamellar eutectic. Mater. Sci. Eng. A 2017, 706, 115–125. [Google Scholar] [CrossRef]

| V (wt%) | (°C) | (°C) | (°C) | (°C) | (°C) | (s) |
|---|---|---|---|---|---|---|
| 0 | 642.5 | 636.2 | 635.1 | 634.9 | 0.2 | 310 |
| 0.1 | 643.4 | 638.3 | 636.8 | 636.3 | 0.5 | 293 |
| 0.2 | 644.1 | 640.5 | 638.5 | 637.8 | 0.7 | 282 |
| 0.3 | 645.2 | 640.6 | 639.1 | 638.7 | 0.4 | 293 |
| 0.4 | 646.4 | 640.8 | 639.4 | 639.1 | 0.3 | 298 |
| 0.6 | 646.5 | 641 | 639.9 | 639.6 | 0.3 | 302 |
| Point | Al (at%) | Ni (at%) | V (at%) | Fe (at%) |
|---|---|---|---|---|
| 1 | 79.14 | 20.36 | 0.15 | 0.35 |
| 2 | 77.29 | 21.53 | 0.45 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, J.; Xi, W.; Cai, Q.; Cheng, J.; Jiang, W. Effect of Vanadium Addition on Solidification Microstructure and Mechanical Properties of Al–4Ni Alloy. Materials 2024, 17, 332. https://doi.org/10.3390/ma17020332
Chen X, Chen J, Xi W, Cai Q, Cheng J, Jiang W. Effect of Vanadium Addition on Solidification Microstructure and Mechanical Properties of Al–4Ni Alloy. Materials. 2024; 17(2):332. https://doi.org/10.3390/ma17020332
Chicago/Turabian StyleChen, Xu, Ji Chen, Weiguo Xi, Qizhou Cai, Jingfan Cheng, and Wenming Jiang. 2024. "Effect of Vanadium Addition on Solidification Microstructure and Mechanical Properties of Al–4Ni Alloy" Materials 17, no. 2: 332. https://doi.org/10.3390/ma17020332
APA StyleChen, X., Chen, J., Xi, W., Cai, Q., Cheng, J., & Jiang, W. (2024). Effect of Vanadium Addition on Solidification Microstructure and Mechanical Properties of Al–4Ni Alloy. Materials, 17(2), 332. https://doi.org/10.3390/ma17020332










