Effects of Superfine Tricalcium Silicate Powder on the Physicochemical and Mechanical Properties of Its Premixed Cement as a Root Canal Filling Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of C3S Powder
2.2. Characterization of Tricalcium Silicate Powder
2.3. Preparation of Premixed Tricalcium Silicate Root Canal Sealer
2.4. Evaluation of Injectability and Flowability
2.5. Setting Time
2.6. Compressive Strength
2.7. Washout Resistance
2.8. Alkalizing Activity
2.9. Statistical Analysis
3. Results and Discussion
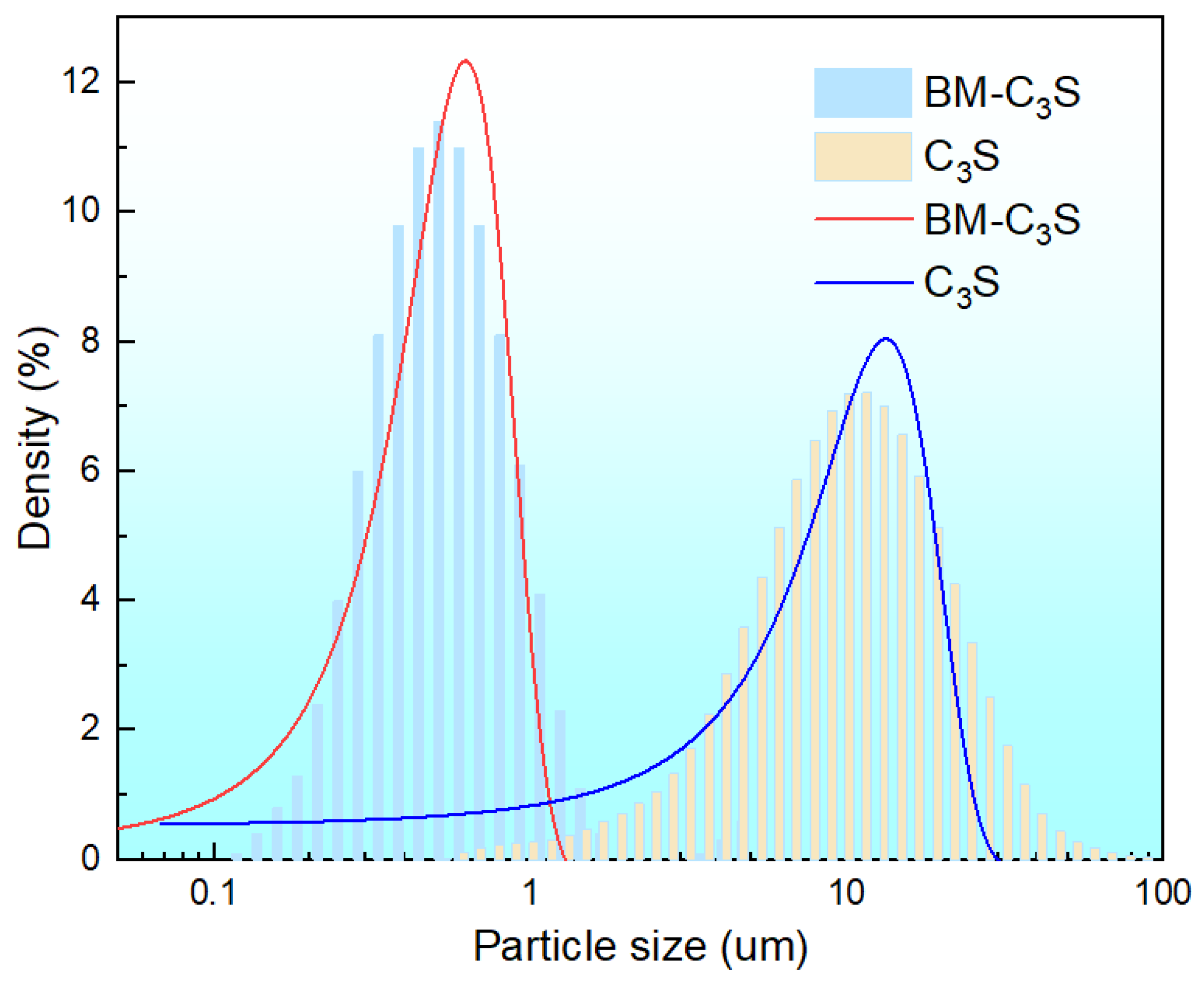
3.1. Properties of Tricalcium Silicate Powder before and after Ball Milling
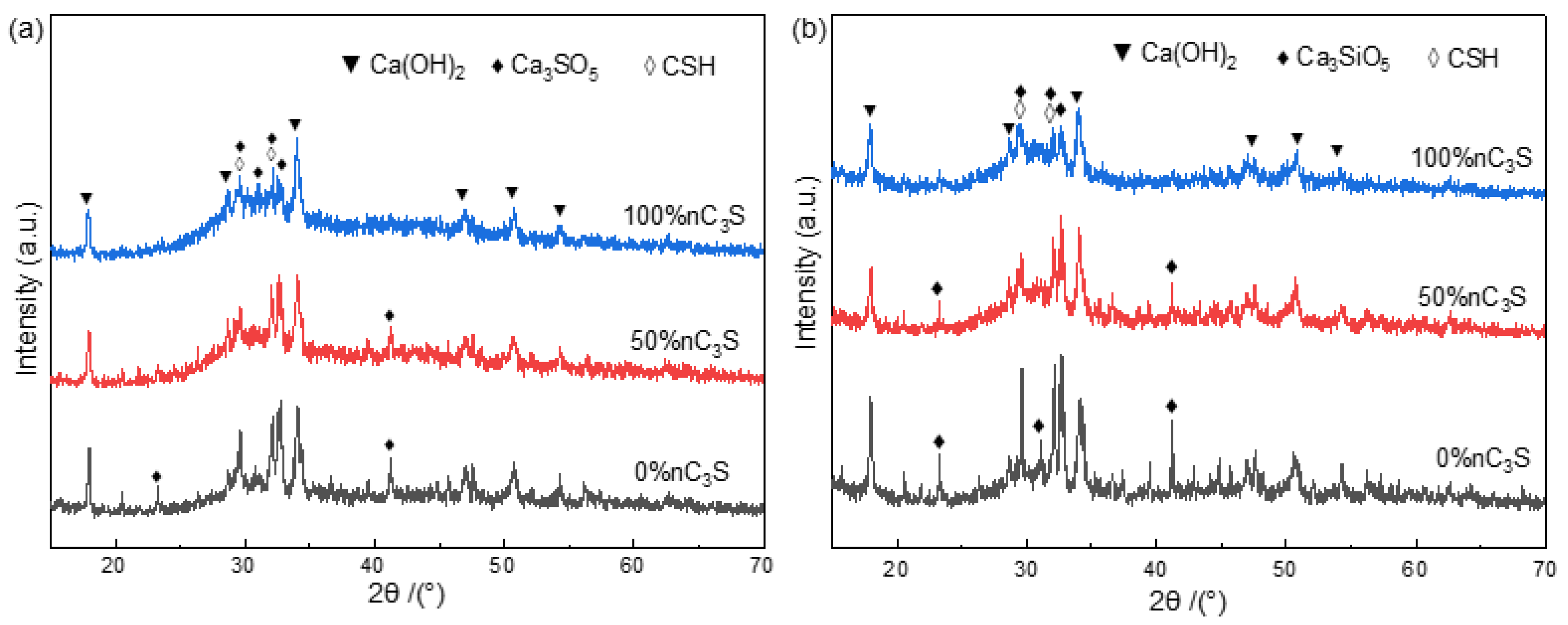
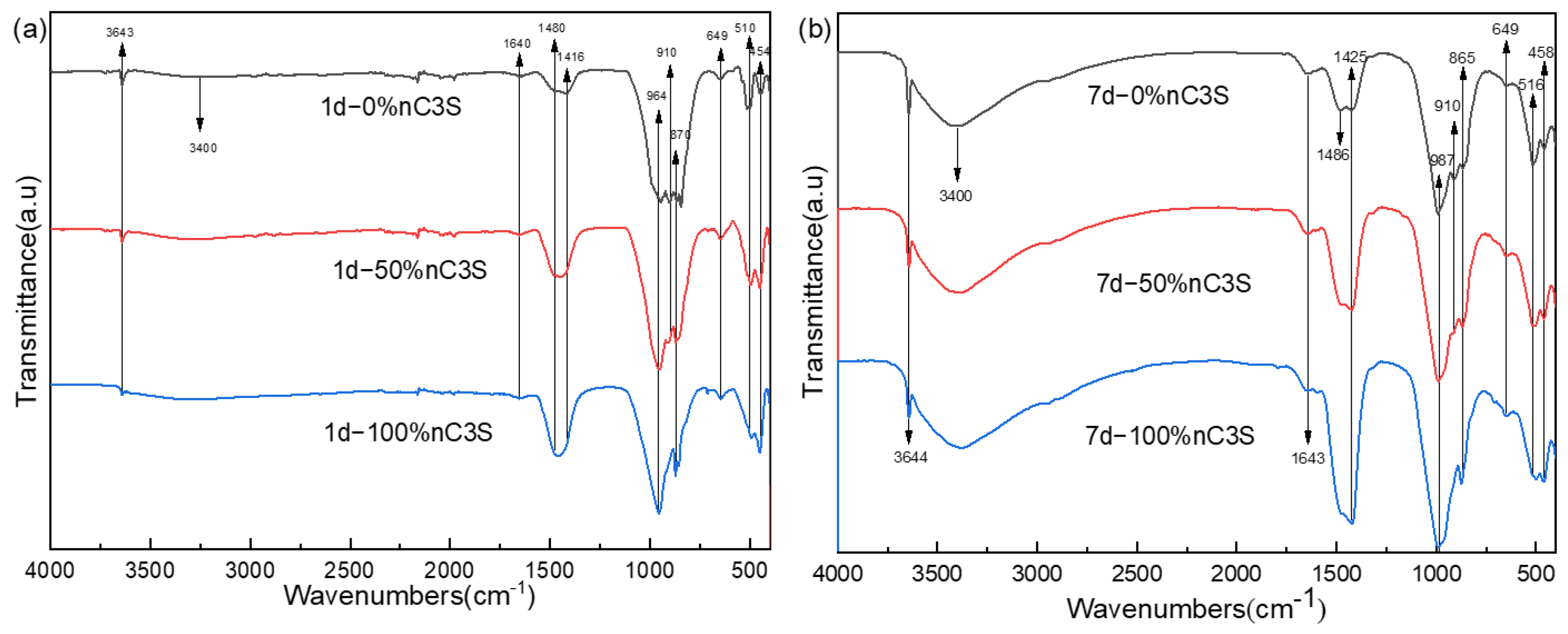
3.2. Hydration Phase Components of Premixed Cement Slurry
3.3. The Injectability, Flow Property, Setting Time, and Compressive Strength of Premixed Cement
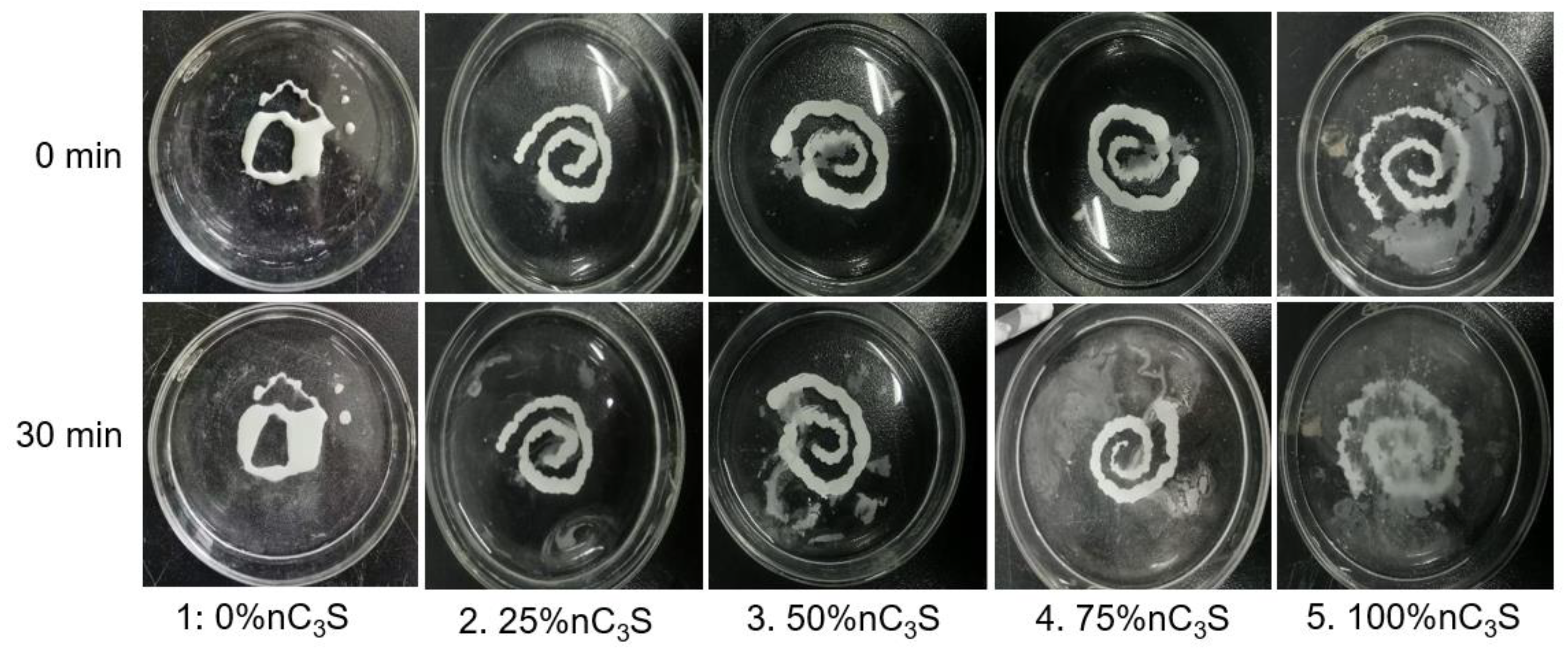
3.4. Washout Resistance of Premixed Cement
3.5. Alkalizing Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, P.N.R. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Siqueira Junior, J.F.; Rocas, I.D.N.; Marceliano-Alves, M.F.; Perez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32, 2–19. [Google Scholar] [CrossRef]
- Siqueira, J.F. Aetiology of root canal treatment failure: Why well-treated teeth can bail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Ren, H.; Wang, B.; Li, X.; Peng, S.; Zhang, Q.; Yan, Y. Effects of ATP on the Physicochemical Properties and Cytocompatibility of Calcium Sulfate/Calcium Citrate Composite Cement. Materials 2023, 16, 3947. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endodont. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R.P. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Espir, C.G.; Guerreiro-Tanomaru, J.M.; Spin-Neto, R.; Chavez-Andrade, G.M.; Camargo Villela Berbert, F.L.; Tanomaru-Filho, M. Solubility and bacterial sealing ability of MTA and root-end filling materials. J. Appl. Oral. Sci. 2016, 24, 121–125. [Google Scholar] [CrossRef]
- Solanki, N.P.; Venkappa, K.K.; Shah, N.C. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. JCD 2018, 21, 10–15. [Google Scholar]
- Camilleri, J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent. Mater. 2011, 27, 836–844. [Google Scholar] [CrossRef]
- Grech, L.; Mallia, B.; Camilleri, J. Characterization of set Intermediate Restorative Material, Biodentine, Bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int. Endod. J. 2013, 46, 632–641. [Google Scholar] [CrossRef]
- Luo, J.; Engqvist, H.; Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 2018, 81, 304–314. [Google Scholar] [CrossRef]
- Salem Milani, A.; Radmand, F.; Rahbani, B.; Hadilou, M.; Haji Abbas Oghli, F.; Salehnia, F.; Baseri, M. Effect of Different Mixing Methods on Physicochemical Properties of Mineral Trioxide Aggregate: A Systematic Review. Int. J. Dent. 2023, 2023, 5226095. [Google Scholar] [CrossRef]
- Persson, C.; Engqvist, H. Premixed calcium silicate cement for endodontic applications: Injectability, setting time and radiopacity. Biomatter 2011, 1, 76–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, C.; Wang, X.; Dou, Y.; Huan, Z.; Chang, J. Fast setting tricalcium silicate/magnesium phosphate premixed cement for root canal filling. Ceram. Int. 2018, 44, 3015–3023. [Google Scholar] [CrossRef]
- Formosa, L.M.; Mallia, B.; Camilleri, J. A quantitative method for determining the antiwashout characteristics of cement-based dental materials including mineral trioxide aggregate. Int. Endod. J. 2013, 46, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wen, Y.; Zhou, Y.; Zhu, Y.; Dou, Y.; Huan, Z.; Chang, J. In vitro self-setting properties, bioactivity, and antibacterial ability of a silicate-based premixed bone cement. Int. J. Appl. Ceram. Technol. 2018, 15, 460–471. [Google Scholar] [CrossRef]
- Wiltbank, K.B.; Schwartz, S.A.; Schindler, W.G. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J. Endodont. 2007, 33, 1235–1238. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, D.; Huan, Z.; Wu, C.; Chang, J. Novel tricalcium silicate/magnesium phosphate composite bone cement having high compressive strength, in vitro bioactivity and cytocompatibility. Acta Biomater. 2015, 21, 217–227. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Milanesi, A.; Calucci, L.; Geppi, M.; Borsacchi, S.; Ridi, F. Effect of phosphate additives on the hydration process of magnesium silicate cements: Thermal and spectroscopic characterization. J. Therm. Anal. Calorim. 2019, 138, 3311–3321. [Google Scholar] [CrossRef]
- Wang, D.; Xiong, C.; Li, W.; Chang, J. Growth of Calcium Carbonate Induced by Accelerated Carbonation of Tricalcium Silicate. ACS Sustain. Chem. Eng. 2020, 8, 14718–14731. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Novel. bioactive composite bone cements based on the beta-tricalcium phosphate-monocalcium phosphate monohydrate composite cement system. Acta Biomater. 2009, 5, 1253–1264. [Google Scholar] [CrossRef]
- Lin, Q.; Lan, X.; Li, Y.; Yu, Y.; Ni, Y.; Lu, C.; Xu, Z. Anti-washout carboxymethyl chitosan modified tricalcium silicate bone cement: Preparation, mechanical properties and in vitro bioactivity. J. Mater. Sci.-Mater. Med. 2010, 21, 3065–3076. [Google Scholar] [CrossRef]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhou, J.; Huan, Z.; Chang, J. Bioactive tricalcium silicate/alginate composite bone cements with enhanced physicochemical properties. J. Biomed. Mater. Res. B 2018, 106, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Ding, Z.; Chen, H.; Peng, H.; Yan, Y. Design of novel organic-inorganic composite bone cements with high compressive strength, in vitro bioactivity and cytocompatibility. J. Biomed. Mater. Res. B 2019, 107, 2365–2377. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Liu, S.; Xu, Y.; Bao, S.; Wang, Y.; Liu, Y.; Zhang, F.; Gou, Z. Ball Milling Medium May Tune the Self-Curing Property and Root Canal Microleakage of beta-Dicalcium Silicate-Based Cement. Materials 2022, 15, 5043. [Google Scholar] [CrossRef]
- Majeed, R.; Elnawawy, H.M.; Kutty, M.G.; Yahya, N.A.; Azami, N.H.; Kasim, N.H.A.; Nabhan, M.S.; Cooper, P.R.; Camilleri, J.; Ahmed, H.M.A. Physicochemical, mechanical and biological properties of nano-calcium silicate-based cements: A systematic review. Odontology 2023, 111, 759–776. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; She, W.; Li, J. Molecular dynamics study of solvated aniline and ethylene glycol monomers confined in calcium silicate nanochannels: A case study of tobermorite. Phys. Chem. Chem. Phys. 2017, 19, 15145–15159. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Wang, Y.; Li, F.; Zhou, M.; Wu, X. A novel and facile route for synthesis of fine tricalcium silicate powders. Mater. Lett. 2018, 227, 187–190. [Google Scholar] [CrossRef]
- ISO 6876:2012; Dentistry-Root Canal Sealing Materials. International Standards Organization: Geneva, Switzerland, 2012.
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, Y.; Wang, Q.; Dong, M.; Yang, M.; Chen, W.; Wang, S.; Zhang, H.; Zheng, S.; Cao, C.Y.; et al. A strontium and amorphous calcium phosphate dipped premixed injectable calcium silicate-based ceramic for dental root canal sealing. Ceram. Int. 2021, 47, 33738–33750. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Chang, J. Two-step precipitation preparation and self-setting properties of tricalcium silicate. Mat. Sci. Eng. C 2008, 28, 289–293. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.-C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Zhang, Y. Premixed tricalcium silicate/sodium phosphate dibasic cements for root canal filling. Mater. Chem. Phys. 2021, 257, 123682. [Google Scholar] [CrossRef]
- Zhang, L.; Yamauchi, K.; Li, Z.; Zhang, X.; Ma, H.; Ge, S. Novel understanding of calcium silicate hydrate from dilute hydration. Cem. Concr. Res. 2017, 99, 95–105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.M.; Jiang, Y.L.; Wang, Y.; Yang, W.S.; Cheng, T.X.; Zhou, G.D. Effect of sodium dodecyl benzene sulfonate on morphology and structure of calcium silicate hydrate prepared via precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 249–255. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Zhu, Z.; Chen, Y.; Zhou, L.; Xu, L.; Wu, K. Time-varying structure evolution and mechanism analysis of alite particles hydrated in restricted space. Constr. Build. Mater. 2022, 341, 127829. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; Mcmillan, P.F.; Cong, X.D. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, W.; Ye, J. FTIR study on the polymorphic structure of tricalcium silicate. Cem. Concr. Res. 2017, 99, 129–136. [Google Scholar] [CrossRef]
- Formosa, L.M.; Mallia, B.; Bull, T.; Camilleri, J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dent. Mater. 2012, 28, 584–595. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Navi, P.; Pignat, C. Effects of cement size distribution on capillary pore structure of the simulated cement paste. Comput. Mater. Sci. 1999, 16, 285–293. [Google Scholar] [CrossRef]
- Wu, M.; Tao, B.; Wang, T.; Zhang, Y.; Wei, W.; Wang, C. Fast-setting and anti-washout tricalcium silicate/disodium hydrogen phosphate composite cement for dental application. Ceram. Int. 2019, 45, 24182–24192. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.; Hirayama, S.; Sugawara, A. Premixed calcium-phosphate cement pastes. J. Biomed. Mater. Res. B 2003, 67B, 689–696. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]





| Group | nC3S (wt.%) | C3S (wt.%) |
|---|---|---|
| 0% nC3S | 0 | 100 |
| 25% nC3S | 25 | 75 |
| 50% nC3S | 50 | 50 |
| 75% nC3S | 75 | 25 |
| 100% nC3S | 100 | 0 |
| 5 h | 1 day | 3 days | 5 days | 7 days | |
|---|---|---|---|---|---|
| 0% nC3S | 10.689 ± 0.257 a | 10.879 ± 0.205 a | 10.763 ± 0.231 a | 10.730 ± 0.189 a | 10.255 ± 0.144 bcd |
| 25% nC3S | 10.602 ± 0.210 abc | 10.743 ± 0.177 a | 10.804 ± 0.207 a | 10.634 ± 0.238 ab | 10.128 ± 0.086 de |
| 50% nC3S | 10.617 ± 0.278 abc | 10.940 ± 0.237 a | 10.616 ± 0.297 abc | 10.709 ± 0.134 a | 10.176 ± 0.129 d |
| 75% nC3S | 10.238 ± 0.190 cd | 10.736 ± 0.225 a | 10.737 ± 0.220 a | 10.574 ± 0.234 abc | 9.772 ± 0.208 ef |
| 100% nC3S | 10.033 ± 0.201 de | 10.727 ± 0.242 a | 10.784 ± 0.157 a | 10.656 ± 0.243 a | 9.479 ± 0.180 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Tan, Y.; Zhang, D.; Wu, H. Effects of Superfine Tricalcium Silicate Powder on the Physicochemical and Mechanical Properties of Its Premixed Cement as a Root Canal Filling Material. Materials 2024, 17, 347. https://doi.org/10.3390/ma17020347
Duan X, Tan Y, Zhang D, Wu H. Effects of Superfine Tricalcium Silicate Powder on the Physicochemical and Mechanical Properties of Its Premixed Cement as a Root Canal Filling Material. Materials. 2024; 17(2):347. https://doi.org/10.3390/ma17020347
Chicago/Turabian StyleDuan, Xin, Yanni Tan, Dechang Zhang, and Hong Wu. 2024. "Effects of Superfine Tricalcium Silicate Powder on the Physicochemical and Mechanical Properties of Its Premixed Cement as a Root Canal Filling Material" Materials 17, no. 2: 347. https://doi.org/10.3390/ma17020347
APA StyleDuan, X., Tan, Y., Zhang, D., & Wu, H. (2024). Effects of Superfine Tricalcium Silicate Powder on the Physicochemical and Mechanical Properties of Its Premixed Cement as a Root Canal Filling Material. Materials, 17(2), 347. https://doi.org/10.3390/ma17020347








