Grain Structure Engineering in Screen-Printed Silver Flake-Based Inks for High-Temperature Printed Electronics Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ink Formulation
2.3. Sample Fabrication
2.4. Testing and Characterization
3. Results and Discussion
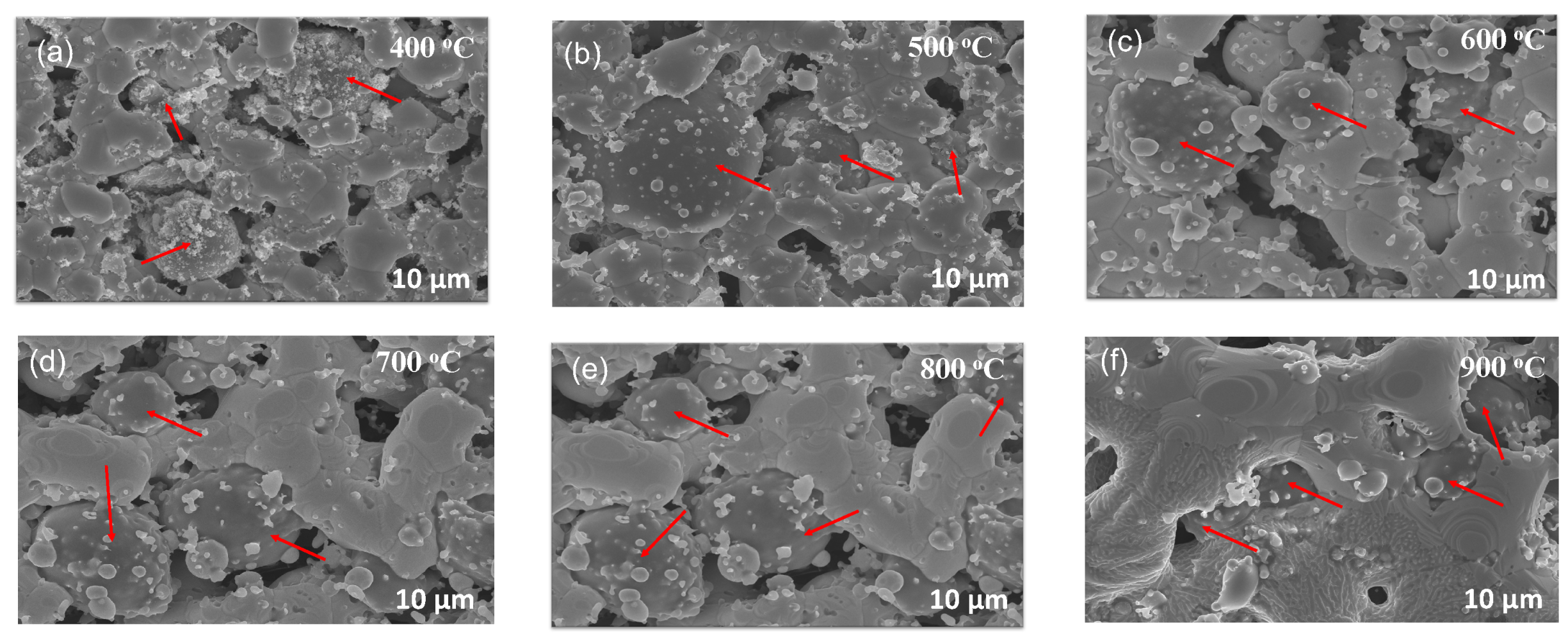
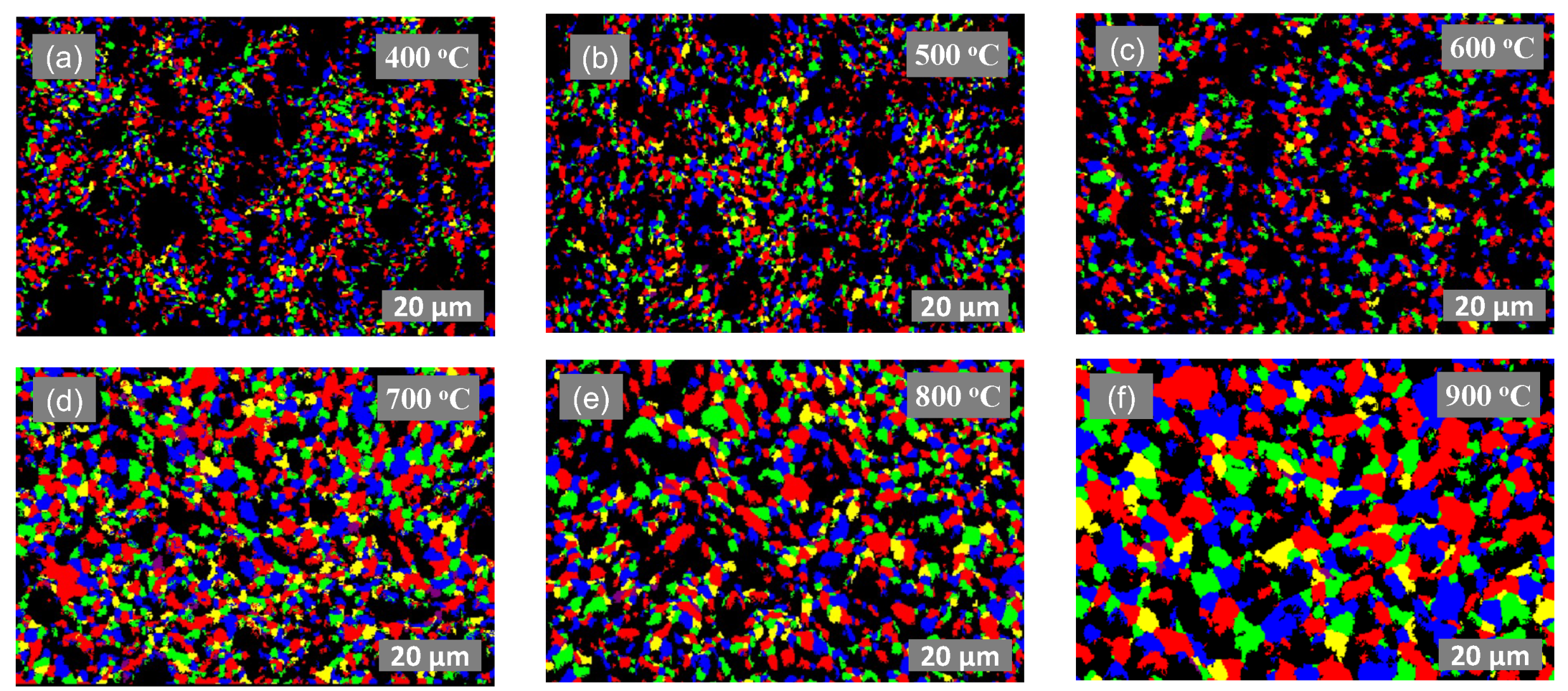
3.1. Material Characterization
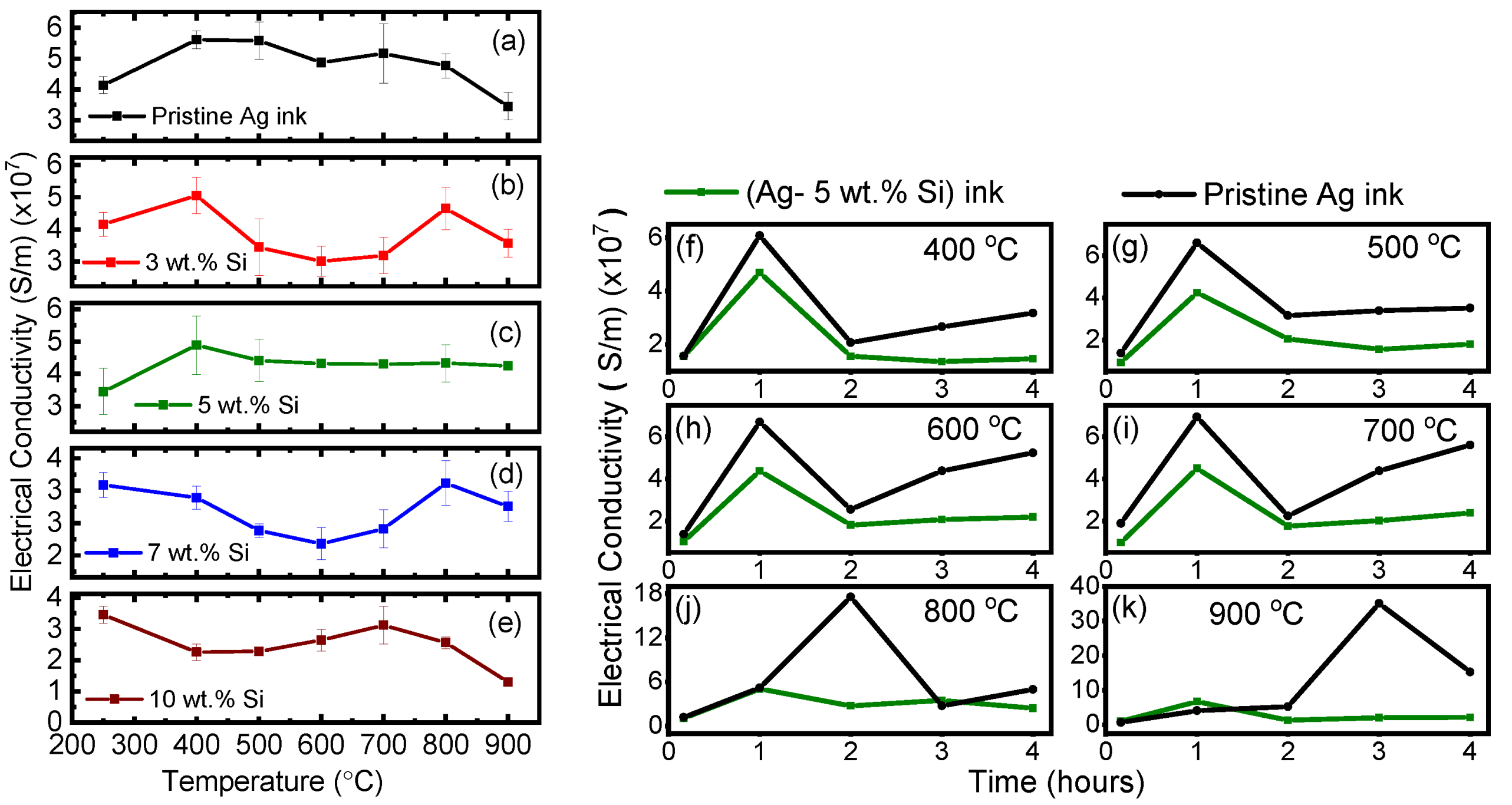
3.2. Evolution of Electrical Conductivity
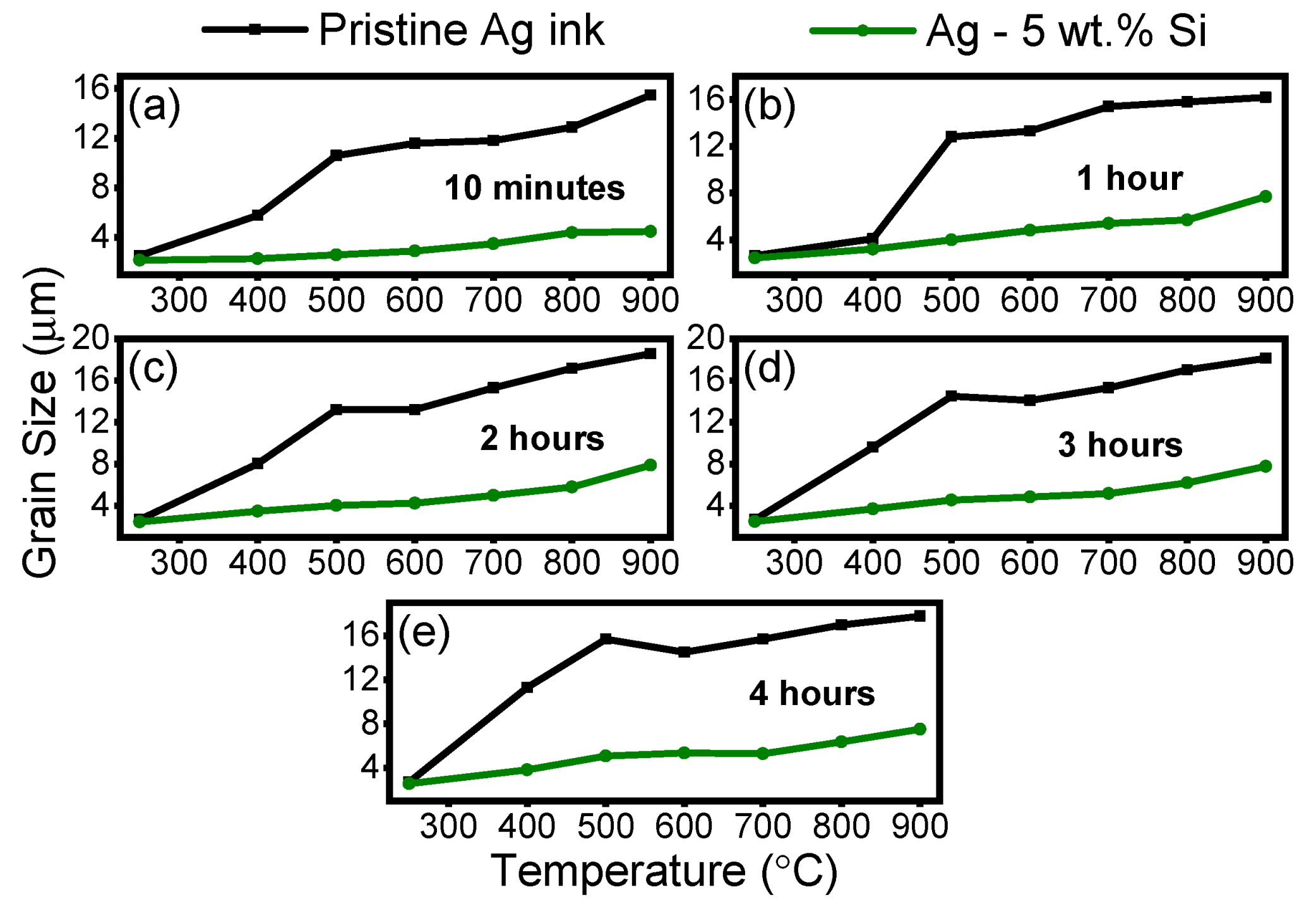
3.3. Evolution of Grain Size
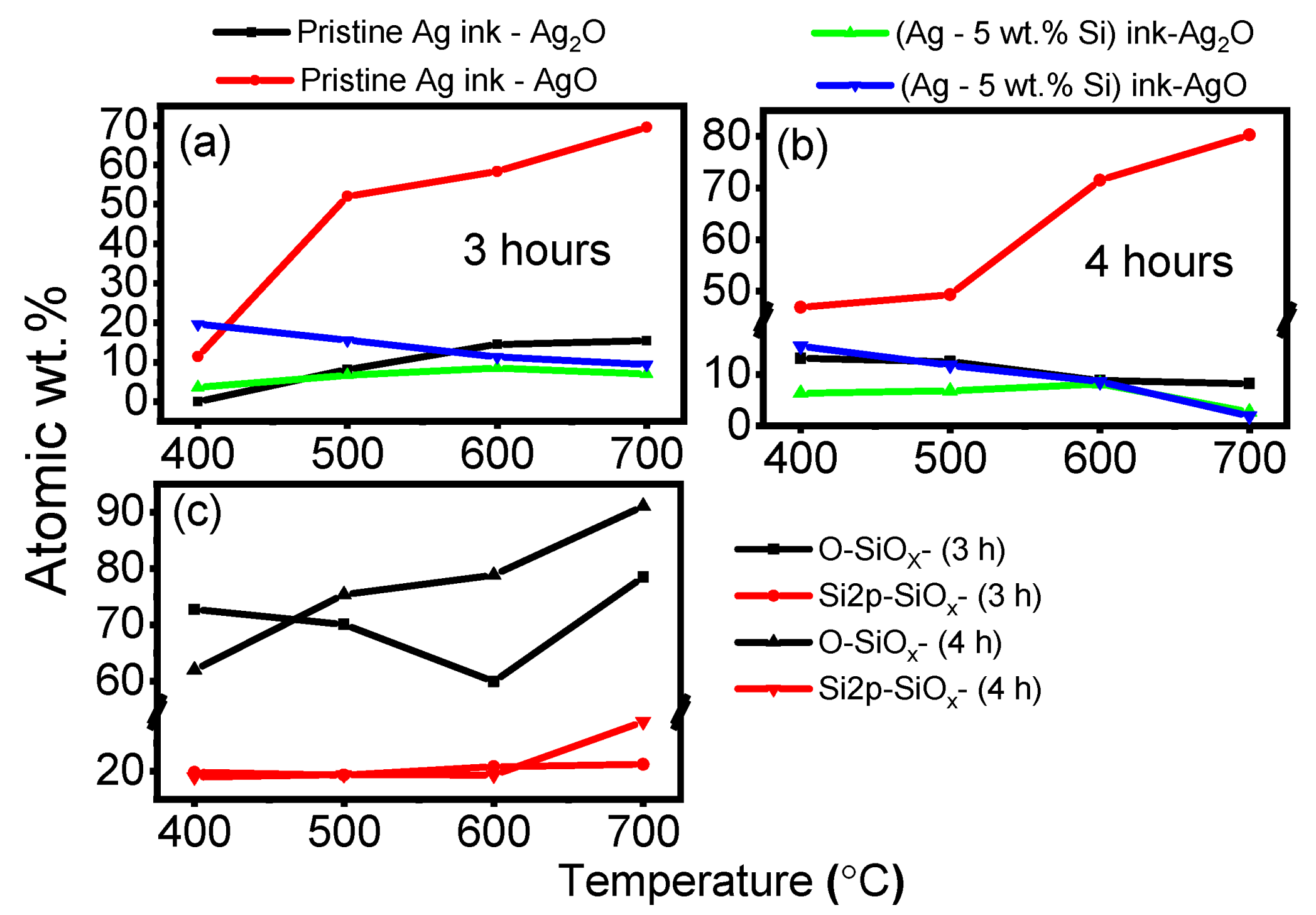
3.4. Oxidation States
- The physical grain-pinning effect due to silicon inclusion ions present along silver grain boundaries, preventing silver domains from recombining (Zener pinning).
- The scavenger effect of the silicon particles to preferentially oxidize as opposed to silver particles, redirecting incoming thermal energy to the formation of silicon-oxygen bonds.
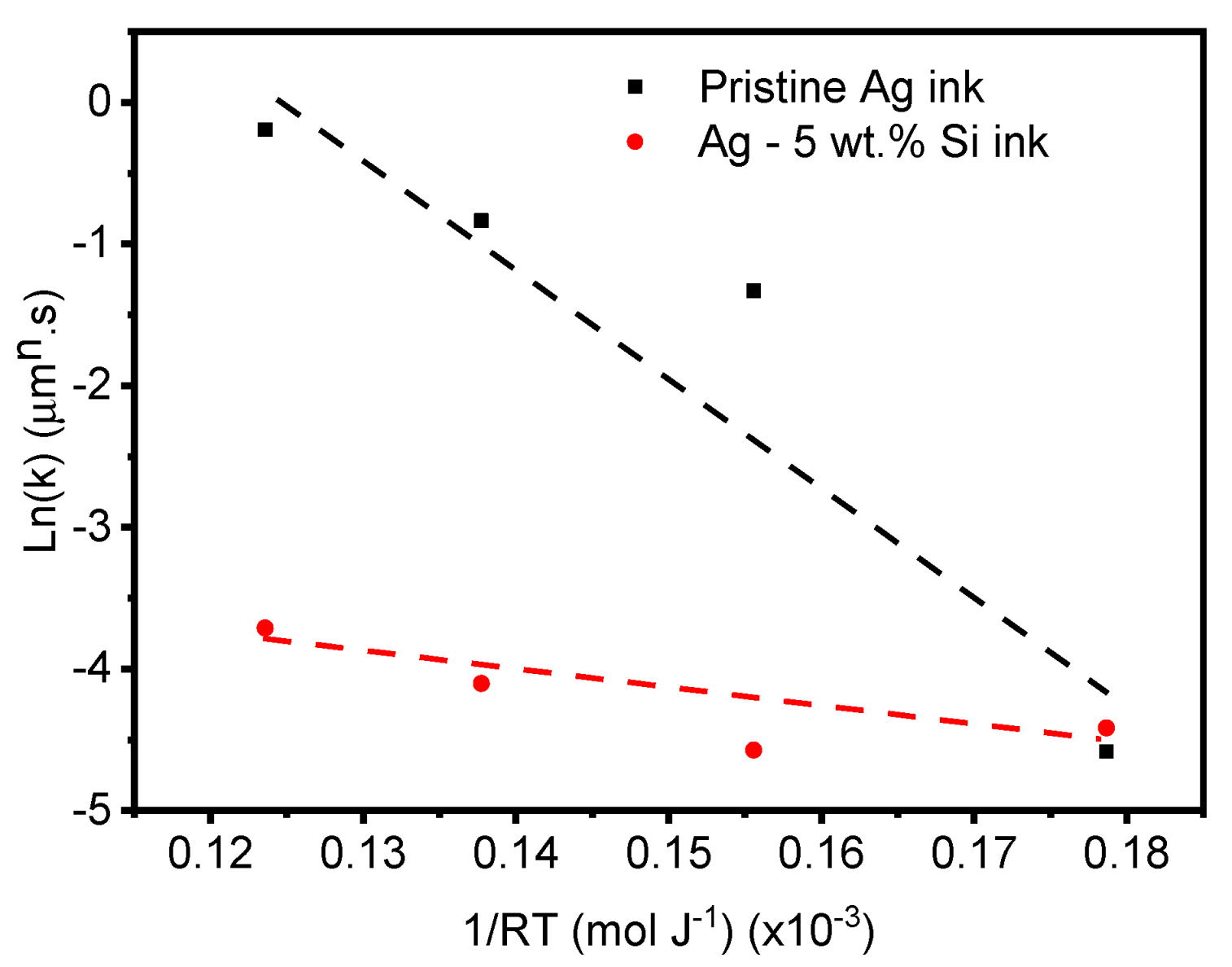
3.5. Grain Growth Kinetics
4. Conclusions
- Between 400 °C and 700 °C, the modified (Ag–Si) undergoes a 2× increase in overall grain size, exhibiting normal grain growth, whereas the pristine silver ink undergoes a 7× increase in grain size and exhibits abnormal grain growth.
- The electrical conductivity of both inks reaches a maximum at 1 h of isothermal exposure to each temperature point, indicating a transition point from sintering to grain growth. Between 1 and 2 h of exposure, the electrical conductivity reduces, indicating grain growth.
- Beyond 3 h, the pristine silver ink shows an erratic rise in electrical conductivity, indicating grain growth transitioning to the bulk material phase. This phenomenon is significant at higher exposure temperatures (800–900 °C).
- On the other hand, beyond 2 h, the electrical conductivity of the modified (Ag–Si) ink remains stable due to the Zener pinning effect.
- XPS data confirm a stark rise in silver oxide species in pristine silver ink with an increase in exposure temperatures, while the silicon particles in the modified (Ag–Si) ink preferentially bond with the oxygen, behaving like scavengers, thereby retarding the oxidation of the silver ink.
- The calculated activation energy for the modified (Ag–Si) inks is between 38 and 43 kJ mol−1, which is significantly lower than that for the pristine ink.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Khuje, S.; Chivate, A.; Huang, Y.; Hu, Y.; An, L.; Shao, Z.; Wang, J.; Chang, S.; Ren, S. Printable Copper Sensor Electronics for High Temperature. ACS Appl. Electron. Mater. 2020, 2, 1867–1873. [Google Scholar] [CrossRef]
- Alhendi, M.; Alshatnawi, F.; Abbara, E.M.; Sivasubramony, R.; Khinda, G.; Umar, A.I.; Borgesen, P.; Poliks, M.D.; Shaddock, D.; Hoel, C.; et al. Printed electronics for extreme high temperature environments. Addit. Manuf. 2022, 54, 102709. [Google Scholar] [CrossRef]
- Lim, D.I.; Cha, J.R.; Gong, M.S. Preparation of flexible resistive micro-humidity sensors and their humidity-sensing properties. Sens. Actuators B Chem. 2013, 183, 574–582. [Google Scholar] [CrossRef]
- Sheng, A.; Khuje, S.; Yu, J.; Petit, D.; Parker, T.; Zhuang, C.G.; Kester, L.; Ren, S. Ultrahigh Temperature Copper-Ceramic Flexible Hybrid Electronics. Nano Lett. 2021, 21, 9279–9284. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, Q.; Norton, M. Thick Film Platinum Temperature Sensors. Microelectron. Int. 1986, 3, 33–35. [Google Scholar] [CrossRef]
- Rebenklau, L.; Gierth, P.; Paproth, A.; Irrgang, K.; Lippmann, L.; Wodtke, A.; Niedermeyer, L.; Augsburg, K.; Bechtold, F. Temperature Sensors Based on Thermoelectric Effect. In Proceedings of the 2015 European Microelectronics Packaging Conference (EMPC), Friedrichshafen, Germany, 14–16 September 2015; p. 5. [Google Scholar]
- Arsenov, P.V.; Vlasov, I.S.; Efimov, A.A.; Minkov, K.N.; Ivanov, V.V. Aerosol Jet Printing of Platinum Microheaters for the Application in Gas Sensors. IOP Conf. Ser. Mater. Sci. Eng. 2019, 473, 012042. [Google Scholar] [CrossRef]
- Arsenov, P.V.; Efimov, A.A.; Ivanov, V.V. Optimizing Aerosol Jet Printing Process of Platinum Ink for High-Resolution Conductive Microstructures on Ceramic and Polymer Substrates. Polymers 2021, 13, 918. [Google Scholar] [CrossRef]
- Lee, D.; Vu, N.B. Metal Oxide Nanoparticle Ink Composition, Method of Producing Same, and Method of Forming Conductive Layer Pattern Using Same. U.S. Patent US10999934B2, 4 May 2021. [Google Scholar]
- Li, Z.; Scheers, S.; An, L.; Chivate, A.; Khuje, S.; Xu, K.; Hu, Y.; Huang, Y.; Chang, S.; Olenick, K.; et al. All-Printed Conformal High-Temperature Electronics on Flexible Ceramics. ACS Appl. Electron. Mater. 2020, 2, 556–562. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Fish, B.M.; Li, Z.R.; Lewittes, M.; Soper, P.D.; Pepin, J.G.; Carroll, A.F. Screen-Printable Silver Pastes with Metallic Nano-Zinc and Nano-Zinc Alloys for Crystalline Silicon Photovoltaic Cells. ACS Appl. Mater. Interfaces 2011, 3, 606–611. [Google Scholar] [CrossRef]
- Cruz, M.A.; Ye, S.; Kim, M.J.; Reyes, C.; Yang, F.; Flowers, P.F.; Wiley, B.J. Multigram Synthesis of Cu-Ag Core–Shell Nanowires Enables the Production of a Highly Conductive Polymer Filament for 3D Printing Electronics. Part. Part. Syst. Charact. 2018, 35, 1700385. [Google Scholar] [CrossRef]
- Yim, C.; Kockerbeck, Z.A.; Jo, S.B.; Park, S.S. Hybrid Copper-Silver-Graphene Nanoplatelet Conductive Inks on PDMS for Oxidation Resistance Under Intensive Pulsed Light. ACS Appl. Mater. Interfaces 2017, 9, 37160–37165. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Ge, Y.; Wang, Y.; Lu, S.; Zhao, Y.; Tang, Y.; Soomro, A.M.; Hong, Q.; Yang, X.; et al. Cu Nanowires Passivated with Hexagonal Boron Nitride: An Ultrastable, Selectively Transparent Conductor. ACS Nano 2020, 14, 6761–6773. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Hsu, H.C.; Tuan, W.H. Oxidation Behavior of Copper at a Temperature below 300 °C and the Methodology for Passivation. Mater. Res. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- Global Conductive Ink Market by Product (Dielectric Ink and Conductive Silver Ink), by Application (Membrane Switches, Photovoltaic, Automotive, and Displays), by Region and Companies. Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2023–2033 Conductive Ink Market Size, Share|CAGR of 6.3%; Market US: New York, NY, USA, 2023; Volume 67457, p. 374.
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef]
- Rajan, K.; Roppolo, I.; Chiappone, A.; Bocchini, S.; Perrone, D.; Chiolerio, A. Silver nanoparticle ink technology: State of the art. Nanotechnol. Sci. Appl. 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Denchev, Z.Z.; Cruz, S.F.; Viana, J.C. Chemistry of solid metal-based inks and pastes for printed electronics—A review. Appl. Mater. Today 2019, 15, 416–430. [Google Scholar] [CrossRef]
- Wiklund, J.; Karakoç, A.; Palko, T.; Yiğitler, H.; Ruttik, K.; Jäntti, R.; Paltakari, J. A Review on Printed Electronics: Fabrication Methods, Inks, Substrates, Applications and Environmental Impacts. J. Manuf. Mater. Process. 2021, 5, 89. [Google Scholar] [CrossRef]
- Yan, J. A Review of Sintering-Bonding Technology Using Ag Nanoparticles for Electronic Packaging. Nanomaterials 2021, 11, 927. [Google Scholar] [CrossRef]
- Oliber, E.A.; Cugno, C.; Moreno, M.; Esquivel, M.; Haberkorn, N.; Fiscina, J.E. Sintering of porous silver compacts at controlled heating rates in oxygen or argon. Matéria 2003, 8, 350–357. [Google Scholar]
- Chen, C.; Suganuma, K. Microstructure and mechanical properties of sintered Ag particles with flake and spherical shape from nano to micro size. Mater. Des. 2019, 162, 311–321. [Google Scholar] [CrossRef]
- Volkman, S.K.; Yin, S.; Bakhishev, T.; Puntambekar, K.; Subramanian, V.; Toney, M.F. Mechanistic Studies on Sintering of Silver Nanoparticles. Chem. Mater. 2011, 23, 4634–4640. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of Silver Nanomaterials at Low Temperatures: Processes, Properties, and Applications. Acs Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z. (Ed.) Sintering of Advanced Materials: Fundamentals and Processes; Woodhead Publisher: Oxford, UK, 2010. [Google Scholar]
- Long, X.; Du, C.; Hu, B.; Li, M. Comparison of sintered silver micro and nano particles: From microstructure to property. In Proceedings of the 20th International Conference on Electronic Materials and Packaging (EMAP), Clear Water Bay, Hong Kong, China, 17–20 December 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Roberson, D.A.; Wicker, R.B.; Murr, L.E.; Church, K.; MacDonald, E. Microstructural and Process Characterization of Conductive Traces Printed from Ag Particulate Inks. Materials 2011, 4, 963–979. [Google Scholar] [CrossRef]
- Choo, D.C.; Kim, T.W. Degradation mechanisms of silver nanowire electrodes under ultraviolet irradiation and heat treatment. Sci. Rep. 2017, 7, 1696. [Google Scholar] [CrossRef]
- Thompson, C.V.; Carel, R. Stress and grain growth in thin films. J. Mech. Phys. Solids 1996, 44, 657–673. [Google Scholar] [CrossRef]
- Frazier, W.E.; Hu, S.; Overman, N.; Lavender, C.; Joshi, V.V. Short communication on Kinetics of grain growth and particle pinning in U-10 wt.% Mo. J. Nucl. Mater. 2018, 498, 254–258. [Google Scholar] [CrossRef]
- Akbarpour, M.; Farvizi, M.; Kim, H. Microstructural and kinetic investigation on the suppression of grain growth in nanocrystalline copper by the dispersion of silicon carbide nanoparticles. Mater. Des. 2017, 119, 311–318. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.H.; Ahn, S.J.; Ha, T.H.; Kim, H.S. Grain-size effect on the electrical properties of nanocrystalline indium tin oxide thin films. Mater. Sci. Eng. B 2015, 199, 37–41. [Google Scholar] [CrossRef]
- Kim, I.; Lee, T.M.; Kim, J. A study on the electrical and mechanical properties of printed Ag thin films for flexible device application. J. Alloys Compd. 2014, 596, 158–163. [Google Scholar] [CrossRef]
- Shahandeh, S.; Militzer, M. Grain boundary curvature and grain growth kinetics with particle pinning. Philos. Mag. 2013, 93, 3231–3247. [Google Scholar] [CrossRef]
- Lohmiller, J.; Woo, N.C.; Spolenak, R. Microstructure–property relationship in highly ductile Au–Cu thin films for flexible electronics. Mater. Sci. Eng. A 2010, 527, 7731–7740. [Google Scholar] [CrossRef]
- Yu, J.; Khuje, S.; Sheng, A.; Kilczewski, S.; Parker, T.; Ren, S. High-Temperature Copper–Graphene Conductors via Aerosol Jetting. Adv. Eng. Mater. 2022, 24, 2200284. [Google Scholar] [CrossRef]
- Fratello, V.; Nino, G.; Wadhwa, A.; Kent, L. Refractory Metal Inks and Related Systemis for and Methods of Making High-Melting-Point Articles. U.S. Patent Application No. 15/429,080, 9 February 2017. [Google Scholar]
- Rahman, M.T.; McCloy, J.; Ramana, C.V.; Panat, R. Structure, electrical characteristics, and high-temperature stability of aerosol jet printed silver nanoparticle films. J. Appl. Phys. 2016, 120, 075305. [Google Scholar] [CrossRef]
- Salemi, A. Silicon Carbide Technology for High- and Ultra-High-Voltage Bipolar Junction Transistors and PiN Diodes. Ph.D. Thesis, Information and Communication Technology, KTH Royal Institute of Technology, Stockholm, Sweden, 2017. [Google Scholar]
- Shyjumon, I.; Gopinadhan, M.; Ivanova, O.; Quaas, M.; Wulff, H.; Helm, C.A.; Hippler, R. Structural deformation, melting point and lattice parameter studies of size selected silver clusters. Eur. Phys. J. D At. Mol. Opt. Plasma Phys. 2006, 37, 409–415. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Synthesis of Silver Nanostructures with Controlled Shapes and Properties. Accounts Chem. Res. 2007, 40, 1067–1076. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Kobayashi, H.; Chewchinda, P.; Ohtani, H.; Odawara, O.; Wada, H. Effects of Laser Energy Density on Silicon Nanoparticles Produced Using Laser Ablation in Liquid. J. Phys. Conf. Ser. 2013, 441, 012035. [Google Scholar] [CrossRef]
- Lee, W.J.; Chang, Y.H. Growth without Postannealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor. Coatings 2018, 8, 431. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Dinh Le, T.S.; An, J.; Peh, Z.K.; Yeong, W.Y.; Kim, Y.J. Wearable Bandage-Based Strain Sensor for Home Healthcare: Combining 3D Aerosol Jet Printing and Laser Sintering. ACS Sens. 2019, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Jewell, E.; Hamblyn, S.; Claypole, T.; Gethin, D. Deposition of High Conductivity Low Silver Content Materials by Screen Printing. Coatings 2015, 5, 172–185. [Google Scholar] [CrossRef]
- Kim, M.; Koo, J.B.; Baeg, K.J.; Noh, Y.Y.; Yang, Y.S.; Jung, S.W.; Ju, B.K.; You, I.K. Effect of Curing Temperature on Nano-Silver Paste Ink for Organic Thin-Film Transistors. J. Nanosci. Nanotechnol. 2012, 12, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Ryu, J.; Kim, H.S.; Hahn, H.T. Sintering of Inkjet-Printed Silver Nanoparticles at Room Temperature Using Intense Pulsed Light. J. Electron. Mater. 2011, 40, 2268–2277. [Google Scholar] [CrossRef]
- Aqida, S.N.; Ghazali, M.I.; Hashim, J. Effects of Porosity on Mechanical Properties of Metal Matrix Composite: An Overview. J. Teknol. A 2004, 40, 17–32. [Google Scholar] [CrossRef]
- Baker, S.P.; Saha, K.; Shu, J.B. Effect of thickness and Ti interlayers on stresses and texture transformations in thin Ag films during thermal cycling. Appl. Phys. Lett. 2013, 103, 191905. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Chen, Z.H.; Zou, J.P.; Zhang, Z.J. Grain Growth and Microstructure of Silver Film Doped Finite Glass on Ferrite Substrates at High Annealing Temperature. Russ. J. Phys. Chem. A 2022, 96, 1519–1524. [Google Scholar] [CrossRef]
- Chaparro, D.; Goudeli, E. Oxidation Rate and Crystallinity Dynamics of Silver Nanoparticles at High Temperatures. J. Phys. Chem. C 2023, 127, 13389–13397. [Google Scholar] [CrossRef]
- Kim, D.U.; Kim, K.S.; Jung, S.B. Effects of oxidation on reliability of screen-printed silver circuits for radio frequency applications. Microelectron. Reliab. 2016, 63, 120–124. [Google Scholar] [CrossRef]
- Na, W.K.; Lim, H.M.; Huh, S.H.; Park, S.E.; Lee, Y.S.; Lee, S.H. Effect of the average particle size and the surface oxidation layer of silicon on the colloidal silica particle through direct oxidation. Mater. Sci. Eng. B 2009, 163, 82–87. [Google Scholar] [CrossRef]
- Okada, R.; Iijima, S. Oxidation property of silicon small particles. Appl. Phys. Lett. 1991, 58, 1662–1663. [Google Scholar] [CrossRef]
- L’vov, B.V. Kinetics and mechanism of thermal decomposition of silver oxide. Thermochim. Acta 1999, 333, 13–19. [Google Scholar] [CrossRef]
- Matsuda, T.; Inami, K.; Motoyama, K.; Sano, T.; Hirose, A. Silver oxide decomposition mediated direct bonding of silicon-based materials. Sci. Rep. 2018, 8, 10472. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Turnbull, D. Recrystallization and grain growth. Prog. Met. Phys. 1952, 3, 220–244. [Google Scholar] [CrossRef]
- Dannenberg, R.; Stach, E.A.; Groza, J.R.; Dresser, B.J. In-situ TEM observations of abnormal grain growth, coarsening, and substrate de-wetting in nanocrystalline Ag thin films. Thin Solid Film. 2000, 370, 54–62. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Cui, E.; Sun, Z.; Yu, H. Grain growth kinetics and grain refinement mechanism in Al2O3/WC/TiC/graphene ceramic composite. J. Eur. Ceram. Soc. 2021, 41, 1391–1398. [Google Scholar] [CrossRef]
- Rollett, A.D.; Gottstein, G.; Shvindlerman, L.S.; Molodov, D.A. Grain boundary mobility—A brief review. Z. Met. 2004, 95, 226–229. [Google Scholar] [CrossRef]
- Thompson, C. Grain Growth in Polycrystalline Thin Films. MRS Proc. 2011, 343. [Google Scholar] [CrossRef]
- Schlenker, T.; Valero, M.; Schock, H.; Werner, J. Grain growth studies of thin Cu(In, Ga)Se2 films. J. Cryst. Growth 2004, 264, 178–183. [Google Scholar] [CrossRef]
- Moravec, J. Determination of the Grain Growth Kinetics as a Base Parameter for Numerical Simulation Demand; EBSCOhost: Ipswich, MA, USA, 2015; p. 649. ISSN 1803-1269. [Google Scholar] [CrossRef]
- Gwalani, B.; Salloom, R.; Alam, T.; Valentin, S.G.; Zhou, X.; Thompson, G.; Srinivasan, S.G.; Banerjee, R. Composition-dependent apparent activation-energy and sluggish grain-growth in high entropy alloys. Mater. Res. Lett. 2019, 7, 267–274. [Google Scholar] [CrossRef]
- Mičian, M.; Frátrik, M.; Moravec, J.; Švec, M. Determination of Grain Growth Kinetics of S960MC Steel. Materials 2022, 15, 8539. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Hesari, F.A. Characterization and hardness of TiCu—Ti2 Cu3 intermetallic material fabricated by mechanical alloying and subsequent annealing. Mater. Res. Express 2016, 3, 045004. [Google Scholar] [CrossRef]
- Zhu, K.N.; Godfrey, A.; Hansen, N.; Zhang, X.D. Microstructure and mechanical strength of near- and sub-micrometre grain size copper prepared by spark plasma sintering. Mater. Des. 2017, 117, 95–103. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Enayati, M.H.; Karimzadeh, F. Nanoscale Grain Growth Behaviour of CoAl Intermetallic Synthesized by Mechanical Alloying. Bull. Mater. Sci. 2014, 37, 383–387. [Google Scholar] [CrossRef]
- Hillert, M. On the theory of normal and abnormal grain growth. Acta Metall. 1965, 13, 227–238. [Google Scholar] [CrossRef]
- Koo, J.B.; Yoon, D.Y. The dependence of normal and abnormal grain growth in silver on annealing temperature and atmosphere. Metall. Mater. Trans. A 2001, 32, 469–475. [Google Scholar] [CrossRef]
- Wu, S.P.; Zhao, Q.Y.; Zheng, L.Q.; Ding, X.H. Behaviors of ZnO-doped silver thick film and silver grain growth mechanism. Solid State Sci. 2011, 13, 548–552. [Google Scholar] [CrossRef]






| Temperature | Ag Only Ink | (Ag-5 wt.% Si) Ink | ||
|---|---|---|---|---|
| n | k | n | k | |
| 400 °C | 1.58 | 0.010 | 2.41 | 0.012 |
| 500 °C | 3.01 | 0.264 | 2.01 | 0.010 |
| 600 °C | 3.57 | 0.433 | 2.20 | 0.016 |
| 700 °C | 4.15 | 0.826 | 2.36 | 0.024 |
| Growth rate | Abnormal | Normal | ||
| Q (kJ mol−1) | 80.2 ± 19.15 | 38.52 ± 7.345 | ||
| R2 | 0.9 | 0.93 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadhwa, A.; Saadati, M.; Benavides-Guerrero, J.; Bolduc, M.; Cloutier, S.G. Grain Structure Engineering in Screen-Printed Silver Flake-Based Inks for High-Temperature Printed Electronics Applications. Materials 2024, 17, 4966. https://doi.org/10.3390/ma17204966
Wadhwa A, Saadati M, Benavides-Guerrero J, Bolduc M, Cloutier SG. Grain Structure Engineering in Screen-Printed Silver Flake-Based Inks for High-Temperature Printed Electronics Applications. Materials. 2024; 17(20):4966. https://doi.org/10.3390/ma17204966
Chicago/Turabian StyleWadhwa, Arjun, Mohammad Saadati, Jaime Benavides-Guerrero, Martin Bolduc, and Sylvain G. Cloutier. 2024. "Grain Structure Engineering in Screen-Printed Silver Flake-Based Inks for High-Temperature Printed Electronics Applications" Materials 17, no. 20: 4966. https://doi.org/10.3390/ma17204966








