Effect of Graphene on the Performance of Silicon–Carbon Composite Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
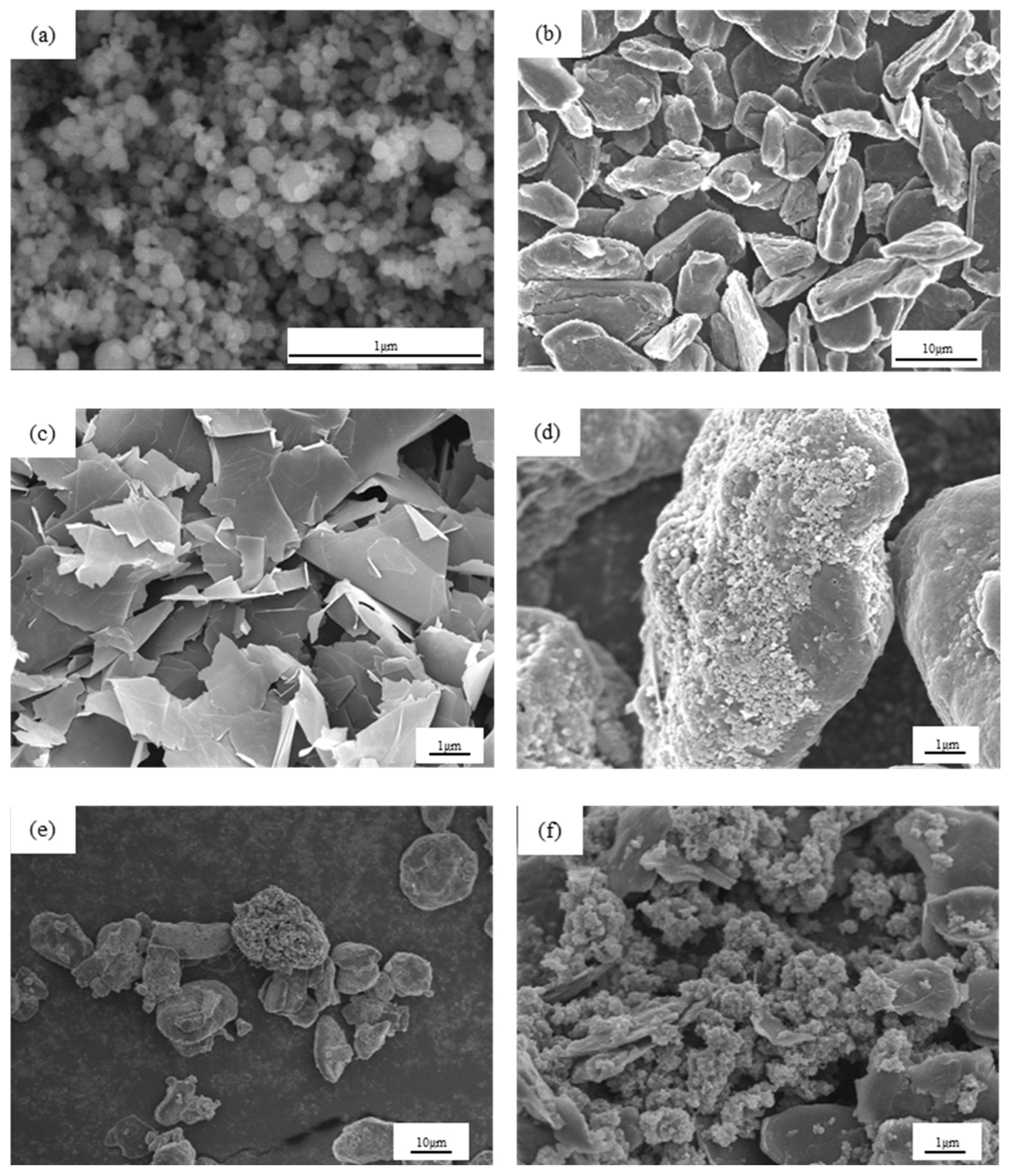
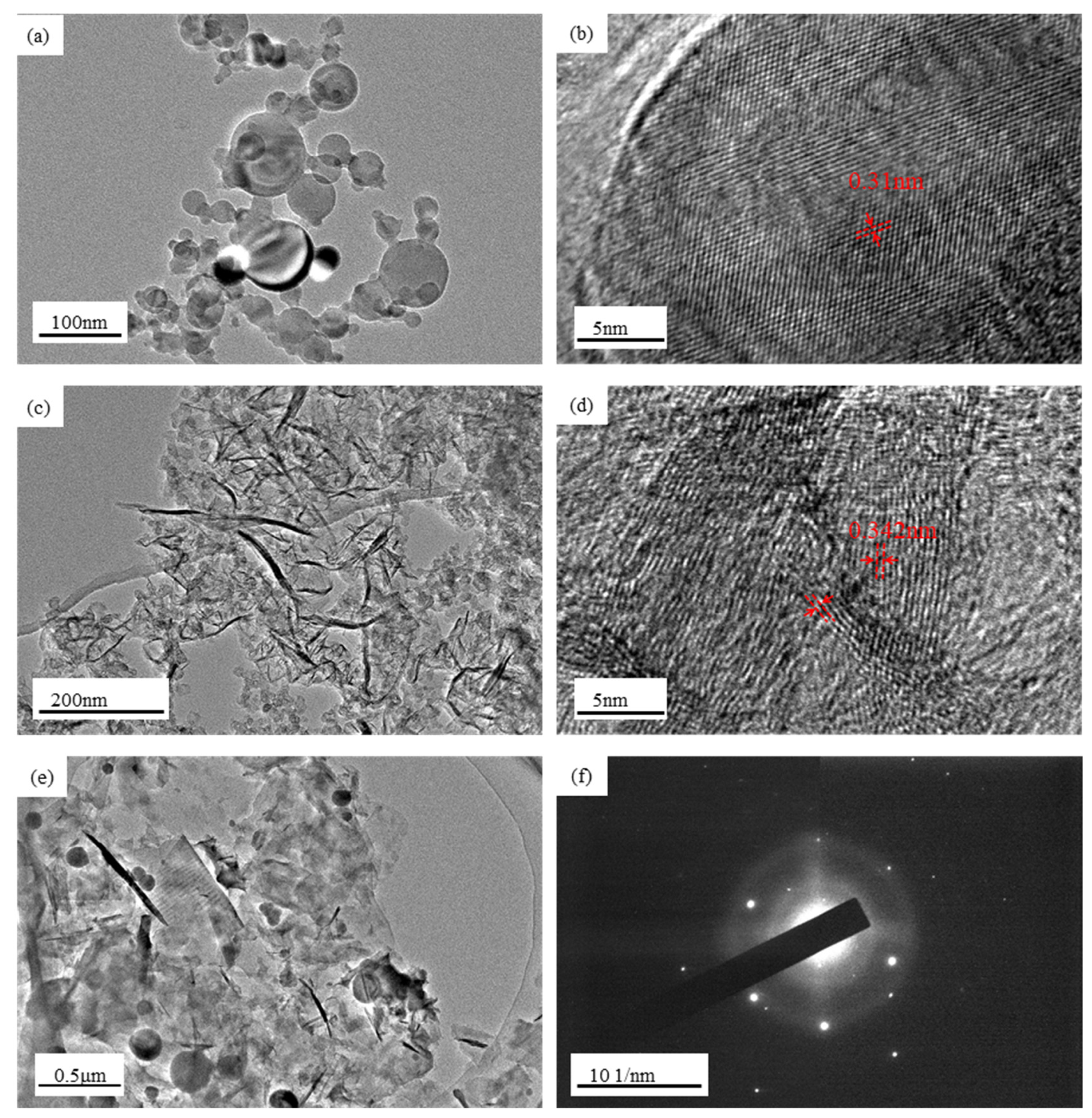
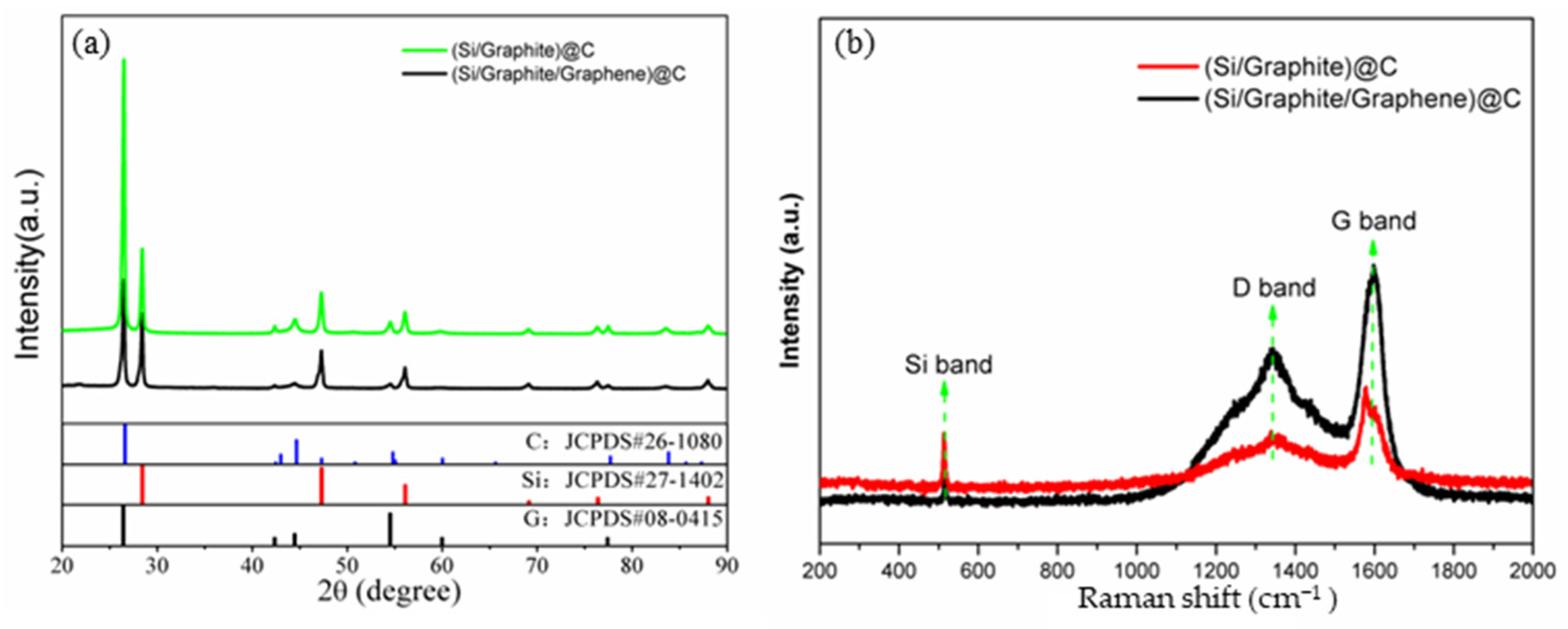
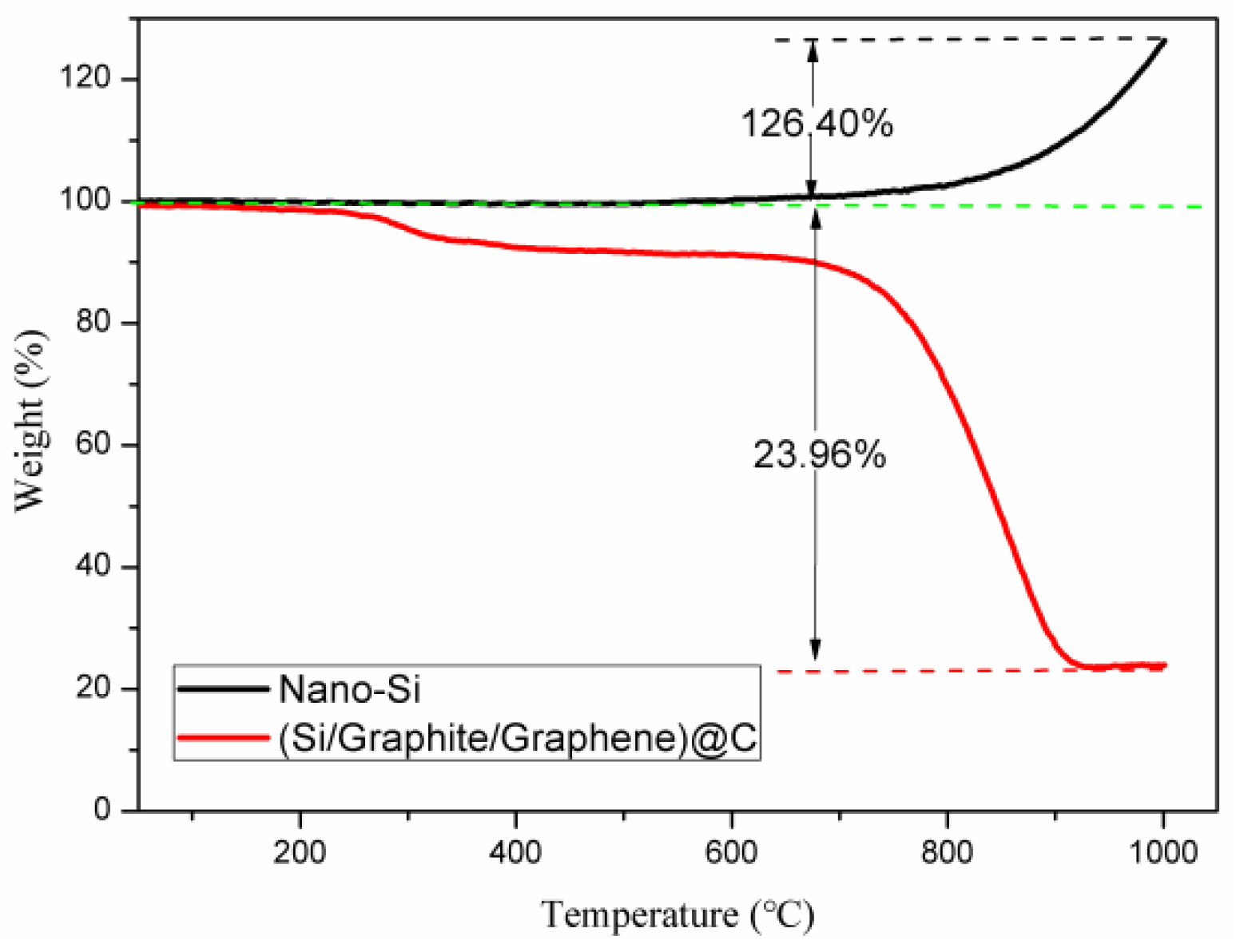
3.1. Materials Characterization
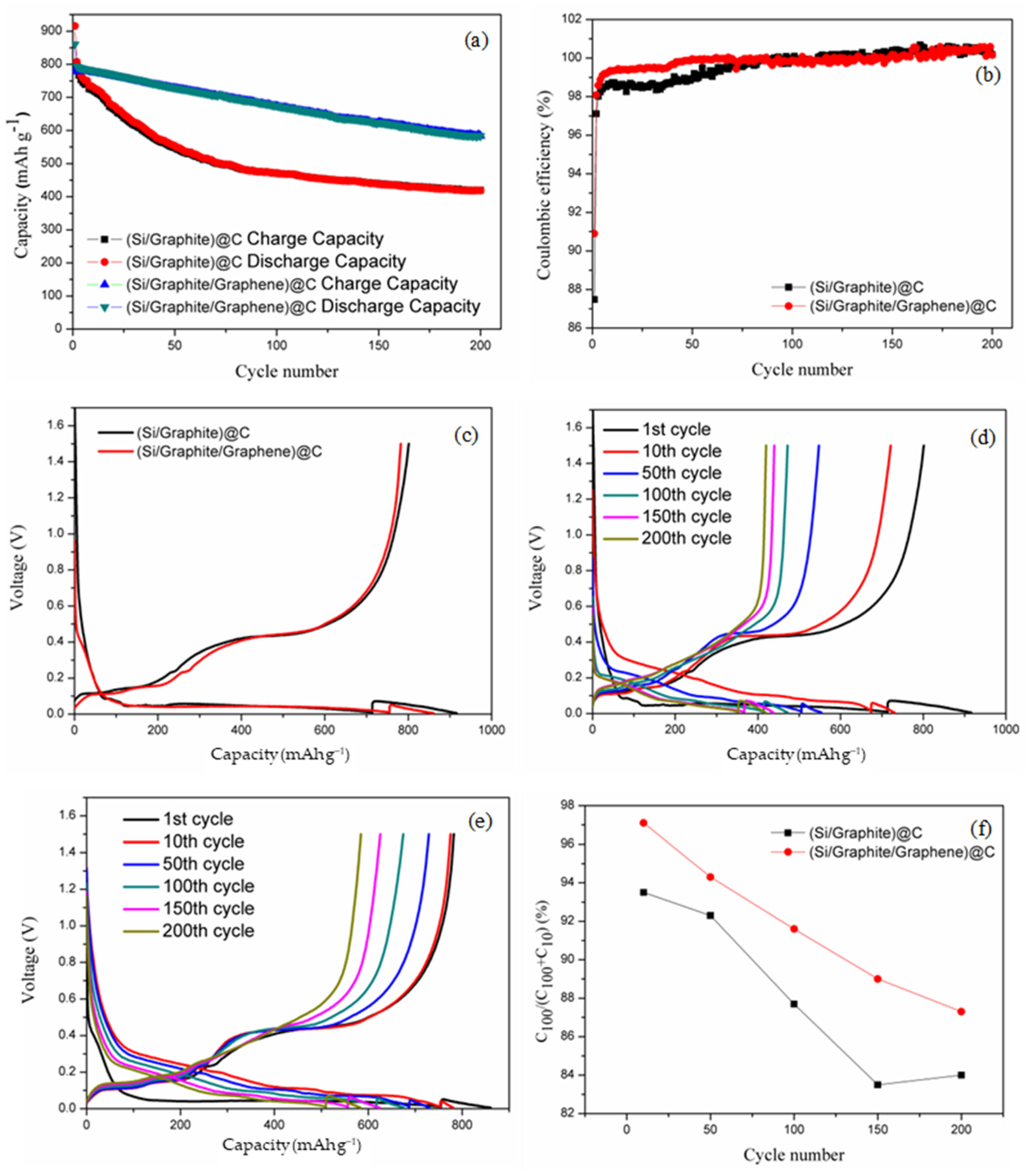
3.2. Electrochemical Properties
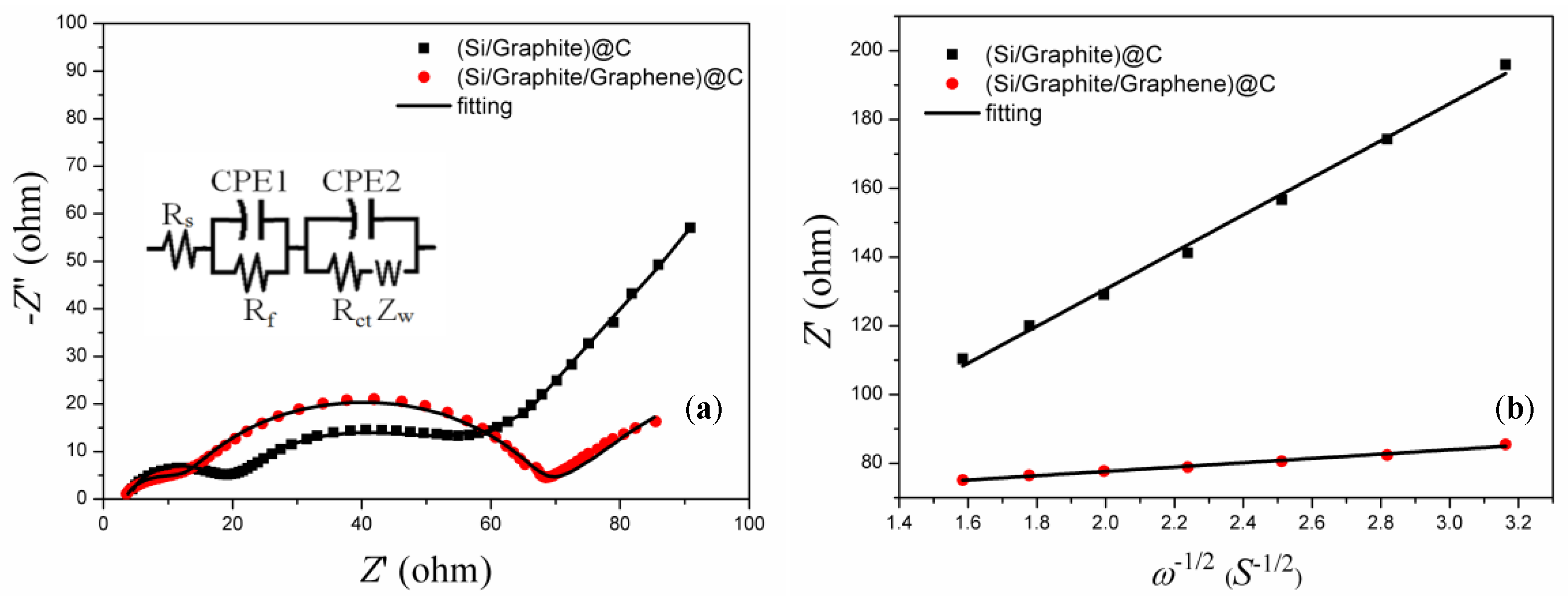
3.3. Electrochemical Kinetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Tan, S.; Shadike, Z.; Cai, X.Y.; Lin, R.Q.; Kludze, A.; Borodin, O.; Lucht, B.L.; Wang, C.S.; Hu, E.Y.; Xu, K.; et al. Review on Low-Temperature Electrolytes for Lithium-Ion and Lithium Metal Batteries. Electrochem. Energy Rev. 2023, 6, 35. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Tian, H.H.; Graczyk-Zajac, M.; Kessler, A.; Weidenkaff, A.; Riedel, R. Recycling and Reusing of Graphite from Retired Lithium-ion Batteries: A Review. Adv. Mater. 2023, 35, e2308494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Lehto, V.P. Challenges and prospects of nanosized silicon anodes in lithium-ion batteries. Nanotechnology 2021, 32, 042002. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Tomey, R.; Vilatela, J.J. Nanotextile 100% Si Anodes for the Next Generation Energy-Dense Li-ion Batteries. Adv. Energy Mater. 2024, 14, 2304018. [Google Scholar] [CrossRef]
- Koebbing, L.; Latz, A.; Horstmann, B. Voltage Hysteresis of Silicon Nanoparticles: Chemo-Mechanical Particle-SEI Model. Adv. Funct. Mater. 2023, 33, 2308818. [Google Scholar] [CrossRef]
- Sztymela, K.; Rossignol, F.; Bienia, M.; Zapp, N.; Nikolowski, K.; Cerbelaud, M. Fabrication of 3D silicon anode by inkjet printing: Opportunities and challenges. J. Energy Storage 2024, 75, 109567. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kong, J.; Zhuang, Q.; Liu, H.; Shao, Q.; Lu, N.; Feng, Y.N.; Wujcik, E.K.; Gao, Q.; et al. Polyborosilazane derived ceramics-Nitrogen sulfur dual doped graphene nanocomposite anode for enhanced lithium ion batteries. Electrochim. Acta 2019, 296, 925–937. [Google Scholar] [CrossRef]
- Maddipatla, R.; Loka, C.; Lee, K.S. Exploring the Potential of Carbonized Nano-Si within G@C@Si Anodes for Lithium-Ion Rechargeable Batteries. ACS Appl. Mater. Interfaces 2023, 15, 58437–58450. [Google Scholar] [CrossRef]
- Araño, K.G.; Yang, G.; Armstrong, B.L.; Aytug, T.; Chambers, M.S.; Self, E.C.; Meyer, H.M., III; Quinn, J.; Browning, J.F.; Wang, C.M.; et al. Carbon Coating Influence on the Formation of Percolating Electrode Networks for Silicon Anodes. Acs Appl. Energy Mater. 2023, 6, 11308–11321. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, A.R.; Park, J.M.; Park, J.S.; Behera, S.; Cho, E.M.; Park, Y.C.; Kim, N.Y.; Jung, J.W.; Lee, S.J.; et al. Analogous Design of a Microlayered Silicon Oxide-Based Electrode to the General Electrode Structure for Thin-Film Lithium-Ion Batteries. Adv. Mater. 2024, 36, 2309183. [Google Scholar] [CrossRef] [PubMed]
- Bitew, Z.; Tesemma, M.; Beyene, Y.; Amare, M. Nano-structured silicon and silicon based composites as anode materials for lithium ion batteries: Recent progress and perspectives. Sustain. Energy Fuels 2022, 6, 1014–1050. [Google Scholar] [CrossRef]
- Schulze, M.C.; Fink, K.; Palmer, J.; Carroll, G.M.; Dutta, N.S.; Zwiefel, C.; Engtrakul, C.; Han, S.D.; Neale, N.R.; de Villers, B.T. Pitch Carbon-coated Ultrasmall Si Nanoparticle Lithium-ion Battery Anodes Exhibiting Reduced Reactivity with Carbonate-based Electrolyte. Batter. Supercaps 2023, 6, e202300186. [Google Scholar] [CrossRef]
- Church, R.B.; Gao, H.N.; Gallant, B.M.; Hart, A.J. Thick Architected Silicon Composite Battery Electrodes Using Honeycomb Patterned Carbon Nanotube Forests. J. Electrochem. Soc. 2023, 170, 090543. [Google Scholar] [CrossRef]
- Vila, M.N.; Bernardez, E.M.; Li, W.Z.; Stackhouse, C.A.; Kern, C.J.; Head, A.R.; Tong, X.; Yan, S.; Wang, L.; Bock, D.C.; et al. Interfacial Reactivity of Silicon Electrodes: Impact of Electrolyte Solvent and Presence of Conductive Carbon. Acs Appl. Mater. Interfaces 2022, 14, 20404–20417. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Hou, X.H.; Qu, L.N.; Qin, H.Q.; Ru, Q.; Huang, Y.; Hu, S.J.; Lam, K.H. Electrochemical properties of core-shell nano-Si@carbon composites as superior anode materials for high-performance Li-ion batteries. J. Mater. Sci.-Mater. Electron. 2017, 28, 250–258. [Google Scholar] [CrossRef]
- Hamid, F.H.; Karunawan, J.; Irmawati, Y.; Laksono, B.T.; Peiner, E.; Wasisto, H.S.; Iskandar, F.; Sumboja, A. Cationic surfactant assisted electrostatic self-assembly of nitrogen-rich carbon enwrapped silicon anode: Unlocking high lithium storage performance. Electrochim. Acta 2023, 471, 143384. [Google Scholar] [CrossRef]
- Lim, G.H.; Song, D.Y.; O, M.J.; Kang, Y.C.; Lee, J.W.; Roh, K.C. Utilizing Hydrolysate Derived from Biorefinery as a Carbon Coating Source for Silicon-Carbon Anodes in Lithium-Ion Capacitors. Acs Appl. Energy Mater. 2023, 6, 11100–11107. [Google Scholar] [CrossRef]
- Wang, W.C.; Du, J.T.; Xu, Z.P.; Liu, Z.T.; Jia, H.A.; Li, T.J.; Nie, Y.; Song, K.D. Porous dual carbon framework coated silicon nanoparticles for high-performance lithium-ion batteries. J. Mater. Sci.-Mater. Electron. 2023, 34, 809. [Google Scholar] [CrossRef]
- Liu, W.P.; Xu, H.R.; Qin, H.Q.; Lv, Y.L.; Wang, F.; Zhu, G.S.; Lin, F.; Wang, L.H.; Ni, C.Y. The effect of carbon coating on graphite@nano-Si composite as anode materials for Li-ion batteries. J. Solid State Electrochem. 2019, 23, 3363–3372. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.W.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.B.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, e2301045. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.; Park, D.; Kim, M.; Choi, M.; Choi, W. Hydrophobic dispersion-derived Si/rGO nanocomposites in SiOC ceramic matrix as anode materials for high performance lithium-ion batteries. J. Mater. Chem. A 2023, 11, 15277–15285. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, S.G.; Chung, K.Y.; Lee, G.W.; Choi, Y.G.; Kim, K.B. Roll-Pressed Silicon Anodes with High Reversible Volumetric Capacity Achieved by Interfacial Stabilization and Mechanical Strengthening of a Silicon/Graphene Hybrid Assembly. Small 2023, 19, e2301744. [Google Scholar] [CrossRef] [PubMed]
- Su, M.R.; Wang, Z.X.; Guo, H.J.; Li, X.H.; Huang, S.L.; Xiao, W.; Gan, L. Enhancement of the Cyclability of a Si/Graphite@Graphene composite as anode for Lithium-ion batteries. Electrochim. Acta 2014, 116, 230–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Z.Y.; Fan, R.J.; Zhao, S.R.; Zhou, R.; Guo, H.J.; Wang, Z.X. Scalable synthesis of Si/SiO2@C composite from micro-silica particles for high performance lithium battery anodes. Powder Technol. 2015, 284, 365–370. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, H.J.; Wang, Z.X.; Li, X.H.; Zhou, R.; Peng, W.J. Improved electrochemical performance of Si/C material based on the interface stability. J. Alloys Compd. 2017, 725, 1304–1312. [Google Scholar] [CrossRef]
- Naveenkumar, P.; Maniyazagan, M.; Yang, H.W.; Kang, W.S.; Kim, S.J. Nitrogen-doped graphene/silicon-oxycarbide nanosphere as composite anode for high-performance lithium-ion batteries. J. Energy Storage 2023, 59, 106572. [Google Scholar] [CrossRef]
- Tseng, W.S.; Chen, Y.C.; Hsu, C.C.; Lu, C.H.; Wu, C.I.; Yeh, N.C. Direct large-area growth of graphene on silicon for potential ultra-low-friction applications and silicon-based technologies. Nanotechnology 2020, 31, 335602. [Google Scholar] [CrossRef]
- Chen, H.D.; Shen, K.X.; Hou, X.H.; Zhang, G.Z.; Wang, S.F.; Chen, F.M.; Fu, L.J.; Qin, H.Q.; Xia, Y.C.; Zhou, G.F. Si-based anode with hierarchical protective function and hollow ring-like carbon matrix for high performance lithium ion batteries. Appl. Surf. Sci. 2019, 470, 496–506. [Google Scholar] [CrossRef]
- Ni, C.Y.; Xia, C.D.; Liu, W.P.; Shan, Z.Q.; Lei, X.X.; Qin, H.Q.; Xu, W. Effect of combination methods for nanosilicon and graphite composites on the anode performance of lithium batteries. J. Mater. Sci.-Mater. Electron. 2023, 34, 1432. [Google Scholar] [CrossRef]
- Xu, W.; Ni, C.Y.; Xia, C.D.; Piao, Z.Y.; Liu, W.P. Effect of silicon/carbon composite on properties of Graphite anode materials in Li-ion batteries. Int. J. Electrochem. Sci. 2022, 17, 221117. [Google Scholar] [CrossRef]
- Kasper, M.; Moertelmaier, M.; Ragulskis, M.; R-Smith, N.A.; Angerer, J.; Aufreiter, M.; Romero, A.; Krummacher, J.; Xu, J.J.; Root, D.E.; et al. Calibrated Electrochemical Impedance Spectroscopy and Time-Domain Measurements of a 7 kWh Automotive Lithium-Ion Battery Module with 396 Cylindrical Cells. Batter. Supercaps 2023, 6, e202200415. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G.H. Lithium Batteries and the Solid Electrolyte Interphase (SEI)-Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Morris, L.V.; Ortíz-Ledón, C.A.; Hamers, R.J. Adapting Simultaneous in Operando Electrochemical Quartz Crystal Microbalance and Electrochemical Impedance Spectroscopy to Studies of Solid Electrolyte Interface Layer Formation on Amorphous Silicon Anodes. J. Electrochem. Soc. 2023, 170, 050503. [Google Scholar] [CrossRef]
- Yesilbas, G.; Chou, C.Y.; Bandarenka, A.S. A Physical Impedance Model of Lithium Intercalation into Graphite Electrodes for a Coin-Cell Assembly. Chemelectrochem 2023, 10, e202300270. [Google Scholar] [CrossRef]
- Yang, C.F.; Zhang, X.S.; Huang, M.Y.; Huang, J.J.; Fang, Z.B. Preparation and Rate Capability of Carbon Coated LiNi1/3Co1/3Mn1/3O2 as Cathode Material in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415. [Google Scholar] [CrossRef]
- Wang, X.Y.; Hao, H.; Liu, J.L.; Huang, T.; Yu, A.S. A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries. Electrochim. Acta 2011, 56, 4065–4069. [Google Scholar] [CrossRef]
| Material Structure | First Discharge Capacity | Initial Columbic Efficiency | Reference |
|---|---|---|---|
| Graphite-loaded carbon-coated nanosilicon and carbon-coated graphite | 460 mAh/g | 89.8% | [31] |
| Si/G composites (graphite mixed with silicon nanoparticles) | 703.8 mAh/g | 84.95% | [32] |
| (Si/G)@C composites (graphite mixed with silicon nanoparticles and coated with sucrose-cracked carbon) | 644.3 mAh/g | 88.93% | [32] |
| (Si/graphite/graphene)@C | 860.4 mAh/g | 90.9% | This work |
| (Si/graphite)@C | 915.9 mAh/g | 87.5% | This work |
| Samples | Rs (Ω) | Rf (Ω) | Rct (Ω) | Slope | DLi+ (cm2 s−1) |
|---|---|---|---|---|---|
| (Si/graphite)@C | 4.13 | 13.87 | 30.96 | 53.94 | 9.36 × 10−15 |
| (Si/graphite/graphene)@C | 3.39 | 8.52 | 55.74 | 6.30 | 6.86 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, C.; Xia, C.; Liu, W.; Xu, W.; Shan, Z.; Lei, X.; Qin, H.; Tao, Z. Effect of Graphene on the Performance of Silicon–Carbon Composite Anode Materials for Lithium-Ion Batteries. Materials 2024, 17, 754. https://doi.org/10.3390/ma17030754
Ni C, Xia C, Liu W, Xu W, Shan Z, Lei X, Qin H, Tao Z. Effect of Graphene on the Performance of Silicon–Carbon Composite Anode Materials for Lithium-Ion Batteries. Materials. 2024; 17(3):754. https://doi.org/10.3390/ma17030754
Chicago/Turabian StyleNi, Chengyuan, Chengdong Xia, Wenping Liu, Wei Xu, Zhiqiang Shan, Xiaoxu Lei, Haiqing Qin, and Zhendong Tao. 2024. "Effect of Graphene on the Performance of Silicon–Carbon Composite Anode Materials for Lithium-Ion Batteries" Materials 17, no. 3: 754. https://doi.org/10.3390/ma17030754






