Assessing the Impact of Sepiolite-Based Bio-Pigment Infused with Indigo Extract on Appearance and Durability of Water-Based White Primer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Production
2.3. Characterization
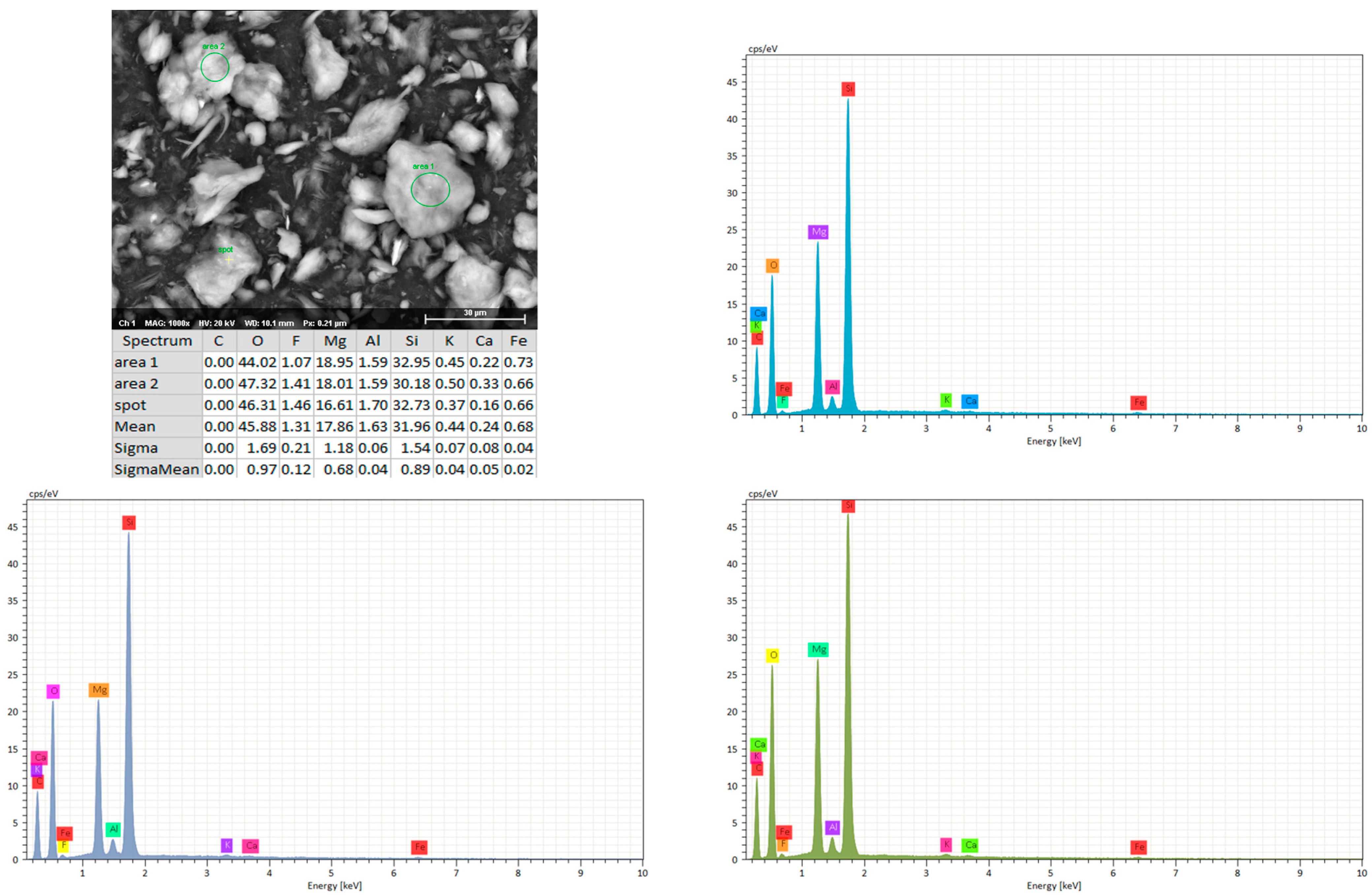
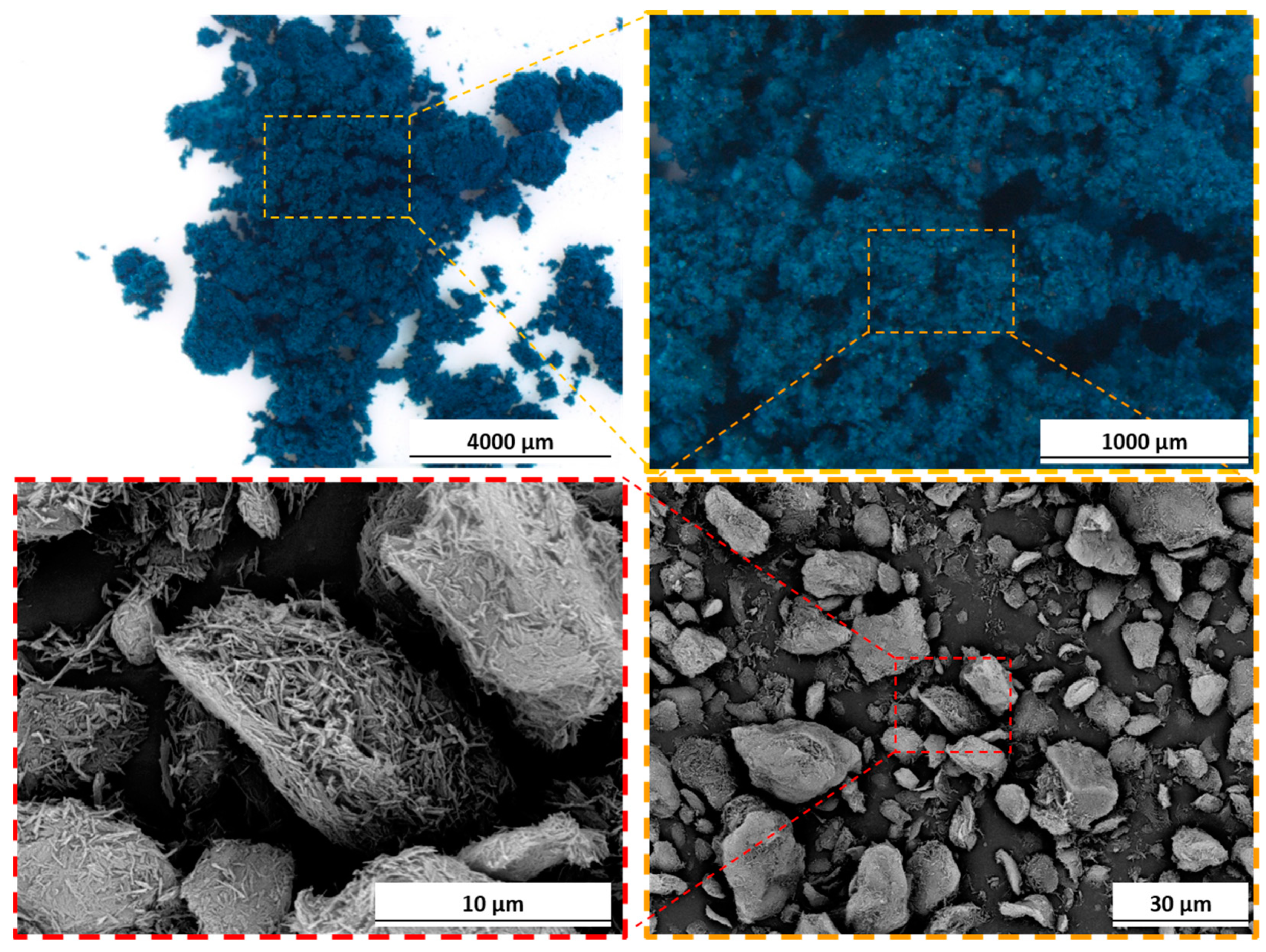
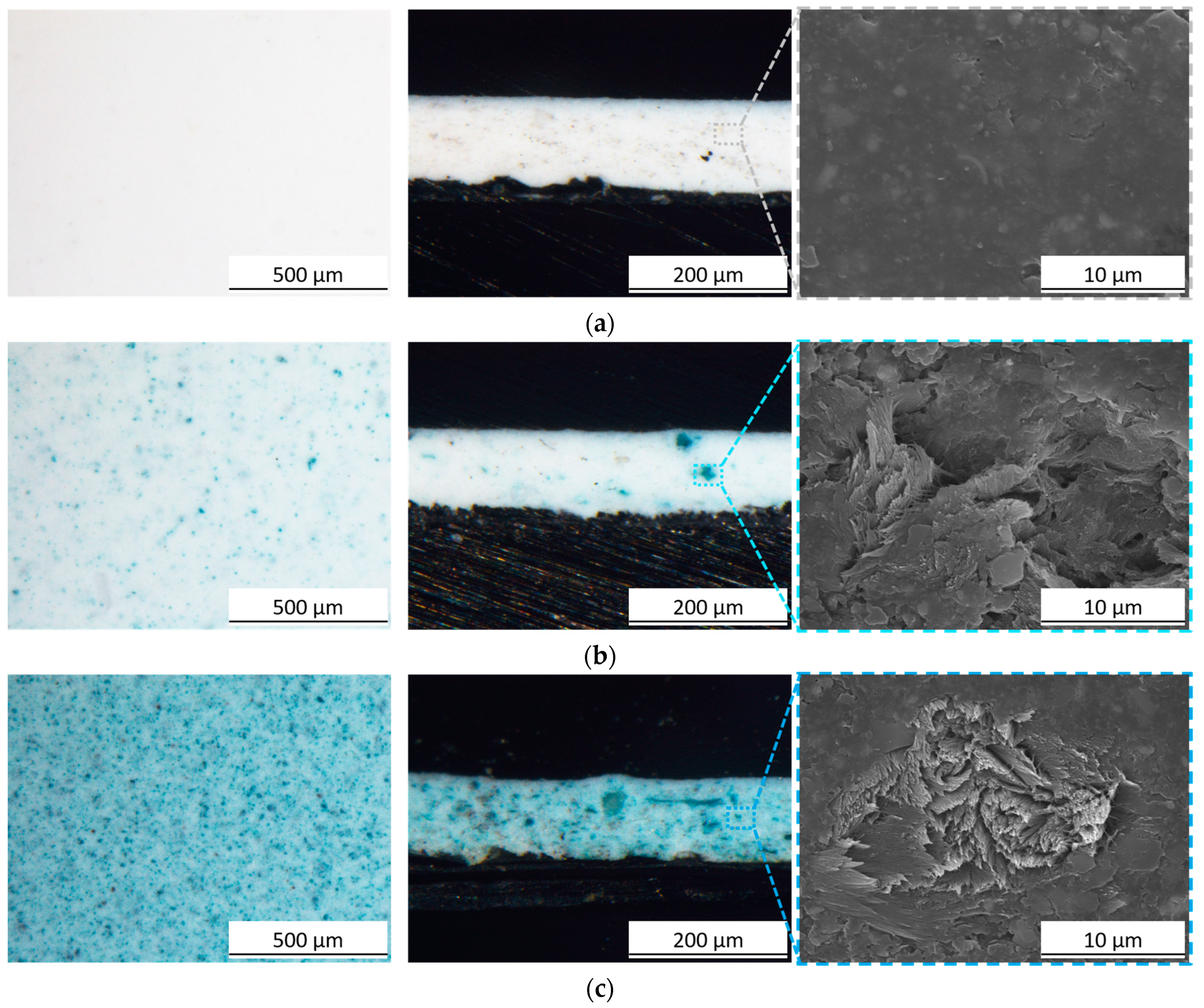
3. Results and Discussion
3.1. Pigment Appearance and Coatings Characterization
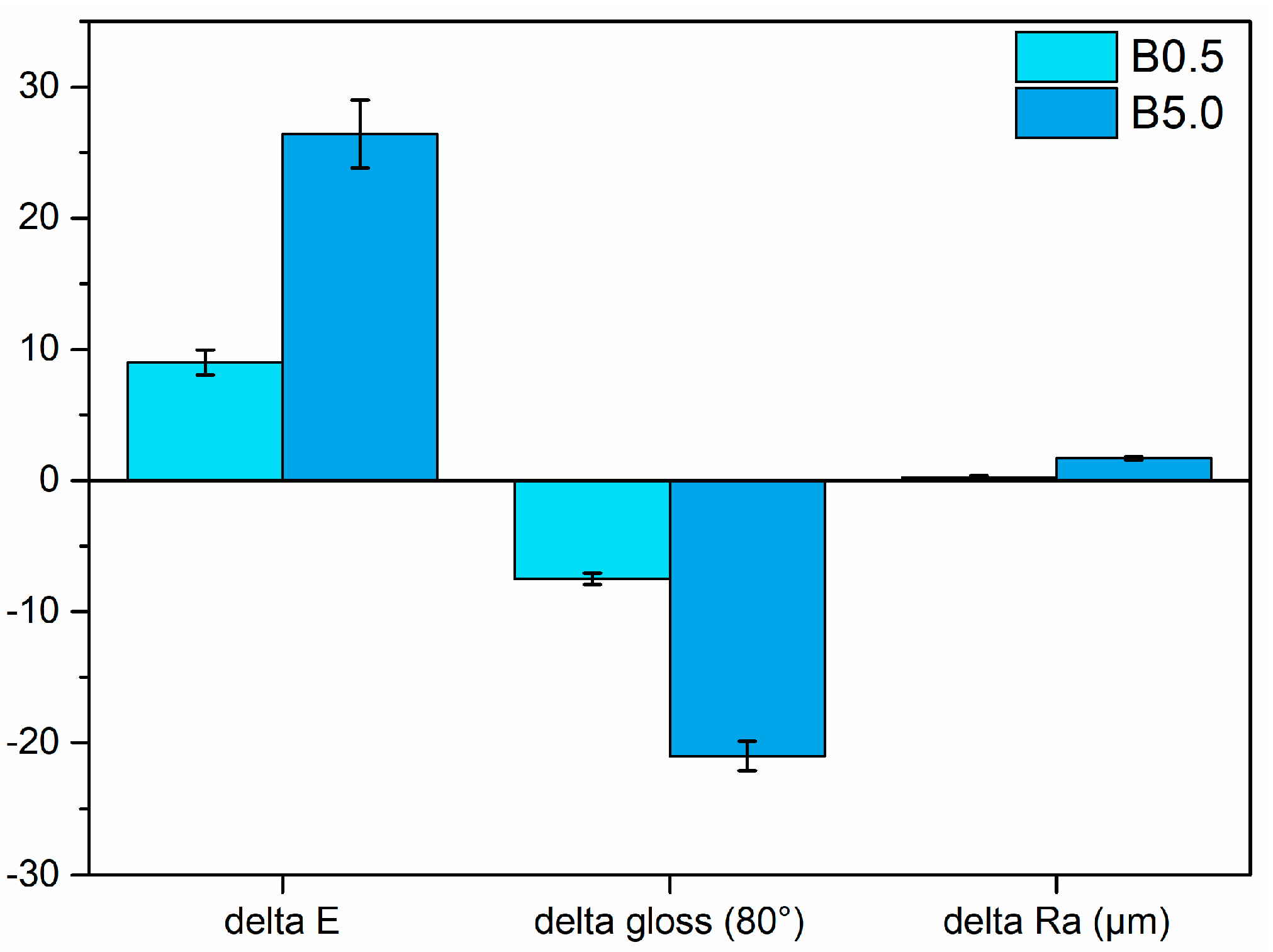
3.2. Coatings Durability
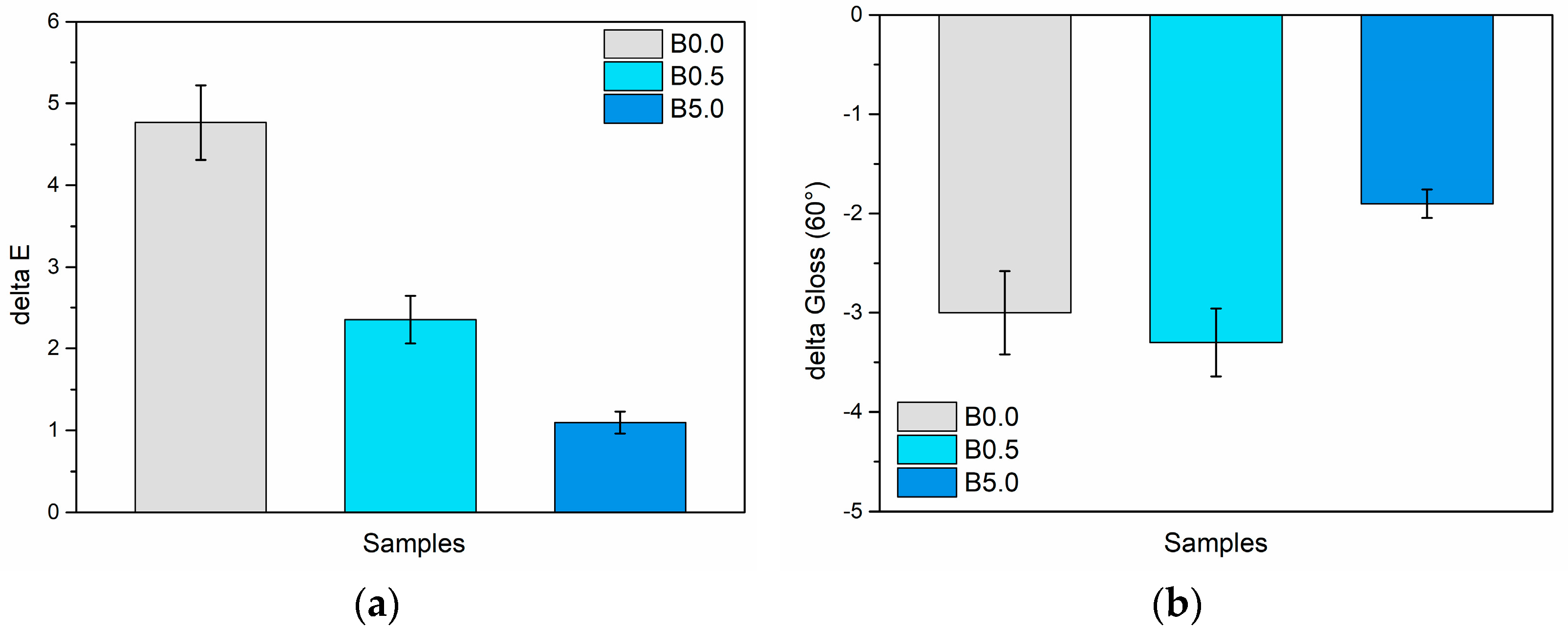
3.2.1. Salt Spray Chamber Exposure
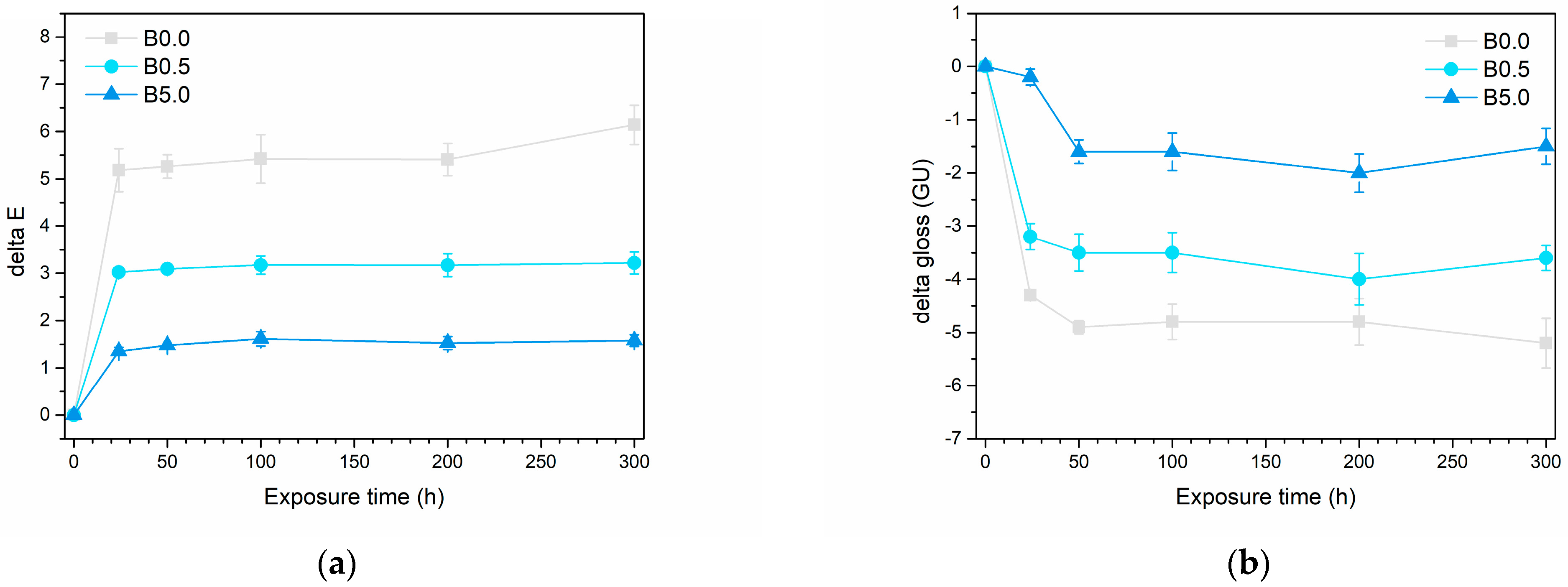
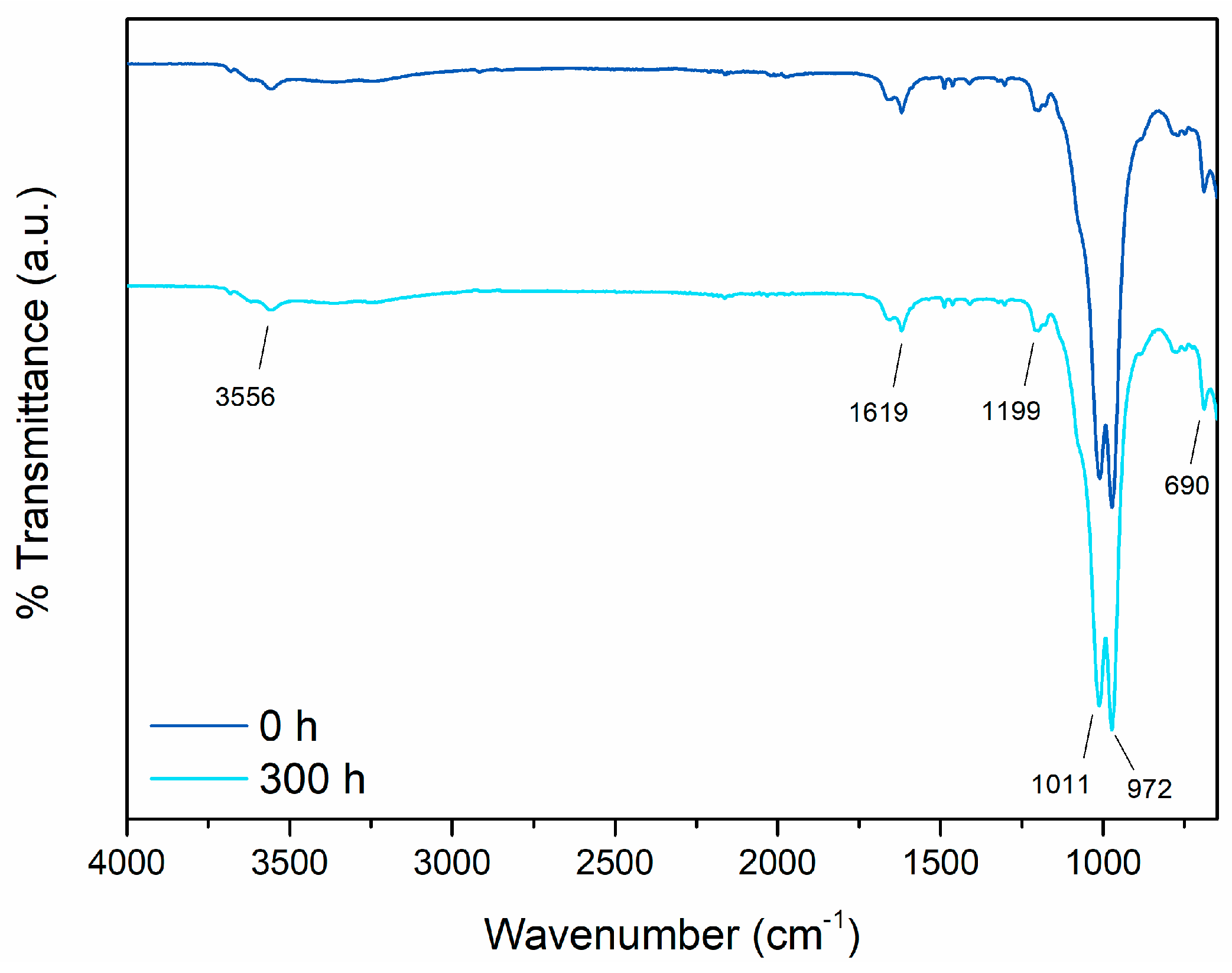
3.2.2. Outdoor Resistance
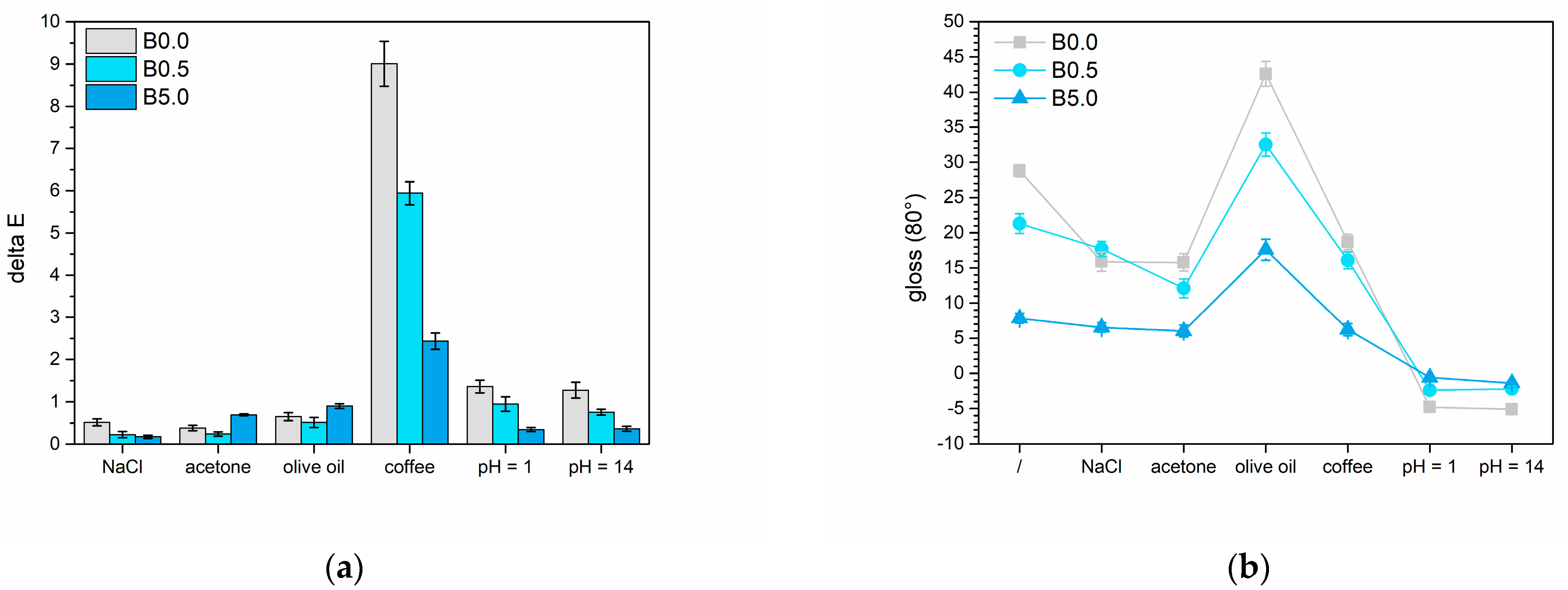
3.2.3. Liquids Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef]
- Razza, F.; Briani, C.; Breton, T.; Marazza, D. Metrics for quantifying the circularity of bioplastics: The case of bio-based and biodegradable mulch films. Resour. Conserv. Recycl. 2020, 159, 104753. [Google Scholar] [CrossRef]
- Richard, S.; Rajadurai, J.S.; Manikandan, V. Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate-reinforced polymer nanocomposites. Int. J. Polym. Anal. 2016, 21, 462–477. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Abdul Majid, R.; Mustapha, S.N.H. Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym.-Plast. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Binoj, J.; Raj, R.E.; Daniel, B. Comprehensive characterization of industrially discarded fruit fiber, Tamarindus indica L. as a potential eco-friendly bio-reinforcement for polymer composite. J. Clean. Prod. 2017, 142, 1321–1331. [Google Scholar] [CrossRef]
- Sanjay, M.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Arizaga, G.G.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Andrew, J.J.; Dhakal, H. Sustainable biobased composites for advanced applications: Recent trends and future opportunities–A critical review. Compos. C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Yan, L. Comparative Study of Fire Resistance and Char Formation of Intumescent Fire-Retardant Coatings Reinforced with Three Types of Shell Bio-Fillers. Polymers 2021, 13, 4333. [Google Scholar] [CrossRef]
- Yew, M.C.; Yew, M.K.; Saw, L.H.; Ng, T.C.; Durairaj, R.; Beh, J.H. Influences of nano bio-filler on the fire-resistive and mechanical properties of water-based intumescent coatings. Prog. Org. Coat. 2018, 124, 33–40. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Sahoo, P.K. Synthesis and study of thermal, mechanical and biodegradation properties of chitosan-g-PMMA with chicken egg shell (nano-CaO) as a novel bio-filler. Mater. Sci. Eng. C 2017, 80, 149–155. [Google Scholar] [CrossRef]
- Arias, J.; Fernandez, M. Biomimetic processes through the study of mineralized shells. Mater. Charact. 2003, 50, 189–195. [Google Scholar] [CrossRef]
- Uzun, G.; Aydemir, D. Biocomposites from polyhydroxybutyrate and bio-fillers by solvent casting method. Bull. Mater. Sci. 2017, 40, 383–393. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Impact of High Concentrations of Cellulose Fibers on the Morphology, Durability and Protective Properties of Wood Paint. Coatings 2023, 13, 721. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Duquesne, S.; Darwish, N.A. Characterization of bio-filler derived from seashell wastes and its effect on the mechanical, thermal, and flame retardant properties of ABS composites. Polym. Compos. 2017, 38, 2788–2797. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Yan, L.; Feng, Y. Comparative Study of Fire Resistance and Anti-Ageing Properties of Intumescent Fire-Retardant Coatings Reinforced with Conch Shell Bio-Filler. Polymers 2021, 13, 2620. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Xu, Z.; Yan, L.; Xie, X.; Wang, Z. Synergistic effect of clam shell bio-filler on the fire-resistance and char formation of intumescent fire-retardant coatings. J. Mater. Res. Technol. 2020, 9, 14718–14728. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Olive pit powder as multifunctional pigment for waterborne paint: Influence of the bio-based filler on the aesthetics, durability and mechanical features of the polymer matrix. Ind. Crops Prod. 2023, 194, 116326. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Functional Olive Pit Powders: The Role of the Bio-Based Filler in Reducing the Water Uptake Phenomena of the Waterborne Paint. Coatings 2023, 13, 442. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Veiravan, A.; Kaliyamoorthy, R.; Kumar, V.; Veeman, D. A spatial distribution effect of almond shell bio filler on physical, mechanical, thermal deflection and water absorption properties of vinyl ester polymer composite. Polym. Compos. 2022, 43, 3204–3218. [Google Scholar] [CrossRef]
- Balasundar, P.; Narayanasamy, P.; Senthil, S.; Al-Dhabi, N.A.; Prithivirajan, R.; Kumar, R.S.; Ramkumar, T.; Bhat, K.S. Physico-chemical study of pistachio (Pistacia vera) nutshell particles as a bio-filler for eco-friendly composites. Mater. Res. Express 2019, 6, 105339. [Google Scholar] [CrossRef]
- Qaiss, A.; Bouhfid, R.; Essabir, H. Characterization and use of coir, almond, apricot, argan, shells, and wood as reinforcement in the polymeric matrix in order to valorize these products. In Agricultural Biomass Based Potential Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 305–339. [Google Scholar]
- Muniyadi, M.; Ng, T.Y.S.; Munusamy, Y.; Ooi, Z.X. Mimusops elengi seed Shell powder as a new bio-filler for polypropylene-based bio-composites. BioResources 2018, 13, 272–289. [Google Scholar] [CrossRef]
- Prabhakar, M.; Shah, A.U.R.; Rao, K.C.; Song, J.-I. Mechanical and thermal properties of epoxy composites reinforced with waste peanut shell powder as a bio-filler. Fibers Polym. 2015, 16, 1119–1124. [Google Scholar] [CrossRef]
- Zuluaga-Parra, J.D.; Ramos-deValle, L.F.; Sánchez-Valdes, S.; Torres-Lubián, J.R.; Rodriguez-Fernadez, O.S.; Hernández-Hernández, E.; da Silva, L.; Rodríguez-Gonzalez, J.A.; Borjas-Ramos, J.J.; Vázquez-Rodríguez, S. Phosphorylated avocado seed: A renewable biomaterial for preparing a flame retardant biofiller. Fire Mater. 2022, 46, 968–980. [Google Scholar] [CrossRef]
- Suthan, R.; Jayakumar, V.; Bharathiraja, G. Wear analysis of bio-fillers reinforced epoxy composites. Mater. Today Proc. 2020, 22, 793–798. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Synergistic contribution of bio-based additives in wood paint: The combined effect of pigment deriving from spirulina and multifunctional filler based on carnauba wax. Prog. Org. Coat. 2023, 182, 107713. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Eco-Friendly Multilayer Coating Harnessing the Functional Features of Curcuma-Based Pigment and Rice Bran Wax as a Hydrophobic Filler. Materials 2023, 16, 7086. [Google Scholar] [CrossRef]
- Shome, A.; Das, A.; Rawat, N.; Rather, A.M.; Manna, U. Reduction of imine-based cross-linkages to achieve sustainable underwater superoleophobicity that performs under challenging conditions. J. Mater. Chem. A 2020, 8, 15148–15156. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Comparative analysis of the advantages and disadvantages of utilizing spirulina-derived pigment as a bio-based colorant for wood impregnator. Coatings 2023, 13, 1154. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Mitrea, L.; Teleky, B.E.; Szabo, K.; Martău, A.G.; Nemes, S.A.; Plamada, D.; Pascuta, M.S.; Barta, G.; Varvara, R.A. Fruit and vegetable waste and by-products for pigments and color. In Fruit and Vegetable Waste Utilization and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 77–100. [Google Scholar]
- Bezirhan Arikan, E.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using Aspergillus carbonarius. J. Fungi 2020, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Puttipan, R.; Khankaew, S. Novel bio-based, time-temperature dependent colorimetric ink and film containing colorants from the red pitaya (Hylocereus costaricensis). Prog. Org. Coat. 2023, 178, 107470. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. From wood waste to wood protection: New application of black bio renewable water-based dispersions as pigment for bio-based wood paint. Prog. Org. Coat. 2023, 180, 107577. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. On the nature of polyimide-clay hybrid composites. Chem. Mater. 1994, 6, 573–575. [Google Scholar] [CrossRef]
- Singer, A. Palygorskite and sepiolite. Soil Min. Environ. Appl. 2002, 7, 555–583. [Google Scholar]
- Feng, X.; Zhang, N.; Chen, X.; Gong, L.; Lv, C.; Guo, Y. Exploitation contradictions concerning multi-energy resources among coal, gas, oil, and uranium: A case study in the Ordos Basin (Western North China Craton and Southern Side of Yinshan Mountains). Energies 2016, 9, 119. [Google Scholar] [CrossRef]
- Xiong, H.; Qi, F.; Zhao, N.; Yuan, H.; Wan, P.; Liao, B.; Ouyang, X. Effect of organically modified sepiolite as inorganic nanofiller on the anti-corrosion resistance of epoxy coating. Mater. Lett. 2020, 260, 126941. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, X.; Jin, Z.; Liu, N.; Zhang, B.; Wang, L.; Duan, J.; Hou, B. Fabrication of an environment-friendly epoxy coating with flexible superhydrophobicity and anti-corrosion performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127545. [Google Scholar] [CrossRef]
- Chen, B.; Jia, Y.; Zhang, M.; Li, X.; Yang, J.; Zhang, X. Facile modification of sepiolite and its application in superhydrophobic coatings. Appl. Clay Sci. 2019, 174, 1–9. [Google Scholar] [CrossRef]
- Sangerano, M.; Pallaro, E.; Roppolo, I.; Rizza, G. UV-cured epoxy coating reinforced with sepiolite as inorganic filler. J. Mater. Sci. 2009, 44, 3165–3171. [Google Scholar] [CrossRef]
- Xu, T.; Xu, H.; Zhong, Y.; Zhang, L.; Qian, D.; Hu, Y.; Zhu, Y.; Mao, Z. Preparation and characteristics of sepiolite-waterborne polyurethane composites. J. Polym. Eng. 2022, 42, 66–74. [Google Scholar] [CrossRef]
- Wang, F.; Liang, J.S.; Tang, Q.G.; Wang, N.; Li, L.W. Preparation and properties of thermal insulation latex paint for exterior wall based on defibred sepiolite and hollow glass microspheres. Adv. Mater. Res. 2009, 58, 103–108. [Google Scholar] [CrossRef]
- Chen, C.; Wang, F.; Liang, J.S.; Tang, Q.G. Thermal insulation coatings containing sepiolite mineral fibers and their performance. Adv. Mat. Res. 2011, 178, 318–323. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Doménech-Carbó, M.T.; Vidal-Lorenzo, C.; de Agredos-Pascual, M.L.V.; Osete-Cortina, L.; Valle-Algarra, F.M. Discovery of indigoid-containing clay pellets from La Blanca: Significance with regard to the preparation and use of Maya Blue. J. Archaeol. Sci. 2014, 41, 147–155. [Google Scholar] [CrossRef]
- Buti, D.; Domenici, D.; Grazia, C.; Ostapkowicz, J.; Watts, S.; Romani, A.; Presciutti, F.; Brunetti, B.G.; Sgamellotti, A.; Miliani, C. Further Insight into Mesoamerican Paint Technology: Unveiling the Colour Palette of the Pre-Columbian Codex Fejérváry-Mayer by Means of Non-invasive Analysis. Archaeometry 2018, 60, 797–814. [Google Scholar] [CrossRef]
- Giulieri, F.; Ovarlez, S.; Chaze, A.M. Indigo/sepiolite nanohybrids: Stability of natural pigments inspired by Maya blue. Int. J. Nanotechnol. 2012, 9, 605–617. [Google Scholar]
- Ouellet-Plamondon, C.; Aranda, P.; Favier, A.; Habert, G.; Van Damme, H.; Ruiz-Hitzky, E. The Maya blue nanostructured material concept applied to colouring geopolymers. RSC Adv. 2015, 5, 98834–98841. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Li, H.; An, X.; Wang, A. Cobalt blue hybrid pigment doped with magnesium derived from sepiolite. Appl. Clay Sci. 2018, 157, 111–120. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, B.; Zhang, M.; Li, X.; Yang, J. A novel colorful sepiolite-based superhydrophobic coating with excellent mechanical and chemical stability and self-cleaning property. Mater. Lett. 2019, 254, 340–343. [Google Scholar] [CrossRef]
- Yin, S.; Zhai, J.; Zhang, Z.; Chen, H.; Yu, Y.; Zhang, T.; Yan, H.; Dong, X. Study on the effects of cationic dyes species on the structure and performance of sepiolite hybrid pigments. Mater. Res. Express 2022, 9, 035001. [Google Scholar] [CrossRef]
- Ovarlez, S.; Chaze, A.-M.; Giulieri, F.; Delamare, F. Indigo chemisorption in sepiolite. Application to Maya blue formation. Comptes Rendus Chim. 2006, 9, 1243–1248. [Google Scholar] [CrossRef]
- Hubbard, B.; Kuang, W.; Moser, A.; Facey, G.A.; Detellier, C. Structural study of Maya Blue: Textural, thermal and solid-state multinuclear magnetic resonance characterization of the palygorskite-indigo and sepiolite-indigo adducts. Clays Clay Miner. 2003, 51, 318–326. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Kozanecki, M.; Nowakowska, J.; Rogowski, J.; Maniukiewicz, W.; Marzec, A. Ecological hybrid pigments with improved thermal, light, and chemical stability based on purpurin dye and different minerals for applications in polymer materials. Dye. Pigment. 2023, 217, 111430. [Google Scholar] [CrossRef]
- Fei, X.; Li, S.; Wu, B.; Ren, F.; Zhu, S.; Cao, L.; Liu, L. Properties of a composite pigment of CI pigment red 177 prepared via a solid-state ball milling method. Dye. Pigment. 2022, 203, 110331. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Zhuang, G.; Jiang, R. A new method to prepare ‘Maya red’pigment from sepiolite and Basic red 46. Appl. Clay Sci. 2019, 174, 38–46. [Google Scholar] [CrossRef]
- Li, S.; Yan, P.; Mu, B.; Kang, Y.; Wang, A. Preparation of Hybrid Nanopigments with Excellent Environmental Stability, Antibacterial and Antioxidant Properties Based on Monascus Red and Sepiolite by One-Step Grinding Process. Nanomaterials 2023, 13, 1792. [Google Scholar] [CrossRef]
- Yuldasheva, N.; Ul’chenko, N.; Glushenkova, A.; Ergashev, A. Lipids from Seeds of Indigofera tinctoria. Chem. Nat. Compd. 2016, 52, 32–34. [Google Scholar] [CrossRef]
- de Fátima Silva Lopes, H.; Tu, Z.; Sumi, H.; Furukawa, H.; Yumoto, I. Indigofera tinctoria leaf powder as a promising additive to improve indigo fermentation prepared with sukumo (composted Polygonum tinctorium leaves). J. Microbiol. Biotechnol. 2021, 37, 179. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R. Indigo dye and reduction techniques. In Denim; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–67. [Google Scholar]
- ASTM D523-14; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–12.
- ASTM D3359-17; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–10.
- ASTM B117:2011; Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2011; pp. 1–12.
- ASTM D4587-11(2019)e1; Standard Practice for Fluorescent UV-Condensation Exposures of Paint and Related Coatings. ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–6.
- EN 12720-14; Assessment of Surface Resistance to Cold Liquids. European Committee for Standardization: Bruxelles, Belgium, 2014; pp. 1–4.
- ASTM D7334–08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–3.
- Giustetto, R.; Wahyudi, O.; Corazzari, I.; Turci, F. Chemical stability and dehydration behavior of a sepiolite/indigo Maya Blue pigment. Appl. Clay Sci. 2011, 52, 41–50. [Google Scholar] [CrossRef]
- ASTM-E308-18; Standard Practice for Computing the Colors of Objectives by Using the CIE System. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–45.
- Mokrzycki, W.; Tatol, M. Colour difference∆ E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- ISO 4628; Evaluation of Degradation of Coatings. ISO: Geneva, Switzerland, 2012.
- Villada, Y.; Inciarte, H.; Gomez, C.; Cardona, S.; Orozco, L.M.; Estenoz, D.; Rios, L. Alkyd-urethane resins based on castor oil: Synthesis, characterization and coating properties. Prog. Org. Coat. 2023, 180, 107556. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Uysal, R. Electrokinetic and rheological behaviors of sepiolite suspensions in the presence of poly (acrylic acid sodium salt) s, polyacrylamides, and poly (ethylene glycol) s of different molecular weights. J. Appl. Polym. Sci. 2008, 109, 1850–1860. [Google Scholar] [CrossRef]
- Lazarević, S.; Janković-Častvan, I.; Jovanović, D.; Milonjić, S.; Janaćković, D.; Petrović, R. Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl. Clay Sci. 2007, 37, 47–57. [Google Scholar] [CrossRef]
- Ongen, A.; Ozcan, H.K.; Ozbas, E.E.; Balkaya, N. Adsorption of Astrazon Blue FGRL onto sepiolite from aqueous solutions. Desalin. Water Treat. 2012, 40, 129–136. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Fiedler, A.; Schulz, H.; Baranska, M. In situ Raman and IR spectroscopic analysis of indigo dye. Anal. Methods 2010, 2, 1372–1376. [Google Scholar] [CrossRef]
- Hu, X.E.; Feng, X.; Fei, M.; Tian, M.; Zhang, R.; Zhou, Y.; Liu, Y.; Zeng, Z. Preparation of Acid Red73 adsorbed on chitosan-modified sepiolite with SiO2 coating as a highly stable hybrid pigment. Dye. Pigment. 2021, 185, 108938. [Google Scholar] [CrossRef]
- Chen, H.; Geng, J.; Zhang, Z.; Jiang, R.; Zhai, J.; Zhang, J. The structure and properties of sepiolite with partial lattice ions substituted by aluminum ions. Front. Chem. 2021, 9, 721225. [Google Scholar] [CrossRef]
- Reningtyas, R.; Rahayuningsih, E.; Kusumastuti, Y.; Kartini, I. Photofading of Natural Indigo Dye in Cotton Coated with Zinc Oxide Nanoparticles Synthesized by Precipitation Method. Int. J. Technol. 2022, 13, 553–564. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Shi, L.; Han, Q.; Lin, C.; Zhang, L.; Wu, M.; Zhang, W. Degradation pathways and mechanisms insight of indigo and shikonin with experiments and quantum chemical calculations. Dye. Pigment. 2023, 111455. [Google Scholar] [CrossRef]
- Iuga, C.; Ortiz, E.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Molecular description of indigo oxidation mechanisms initiated by OH and OOH radicals. J. Phys. Chem. A 2012, 116, 3643–3651. [Google Scholar] [CrossRef]
- Sarıkaya, Y.; Önal, M.; Pekdemir, A.D. Thermal degradation kinetics of sepiolite. Clay Miner. 2020, 55, 96–100. [Google Scholar] [CrossRef]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Rahmat, A.R.B.; Abdullah, N.A.S.B. Mechanical and thermal properties of sepiolite strengthened thermoplastic polymer nanocomposites: A comprehensive review. Alex. Eng. J. 2022, 61, 975–990. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters that affect the photodegradation of dyes and pigments in solution and on substrate–An overview. Dye. Pigment. 2023, 210, 110999. [Google Scholar] [CrossRef]
- GB/T11186.3-90; Method of Measurement of Coating Color. Part III: Calculation of Chromatic Aberration. Standardization Administration of the People’s Republic of China: Beijing, China, 1990; pp. 1–12.
- Paradiso, V.M.; Giarnetti, M.; Summo, C.; Pasqualone, A.; Minervini, F.; Caponio, F. Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015, 45, 30–40. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Wang, X.; Pei, J.; Zhao, L. Directional preparation of indigo or indirubin from indican by an alkali-resistant glucosidase under specific pH and temperature. Process Biochem. 2023, 125, 239–247. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Lai, T.; Ren, G.; Wang, W. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Source 2022, 525, 231121. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Ren, G. Collective enhancement in hydrophobicity and electrical conductivity of gas diffusion layer and the electrochemical performance of PEMFCs. J. Power Source 2023, 575, 233077. [Google Scholar] [CrossRef]


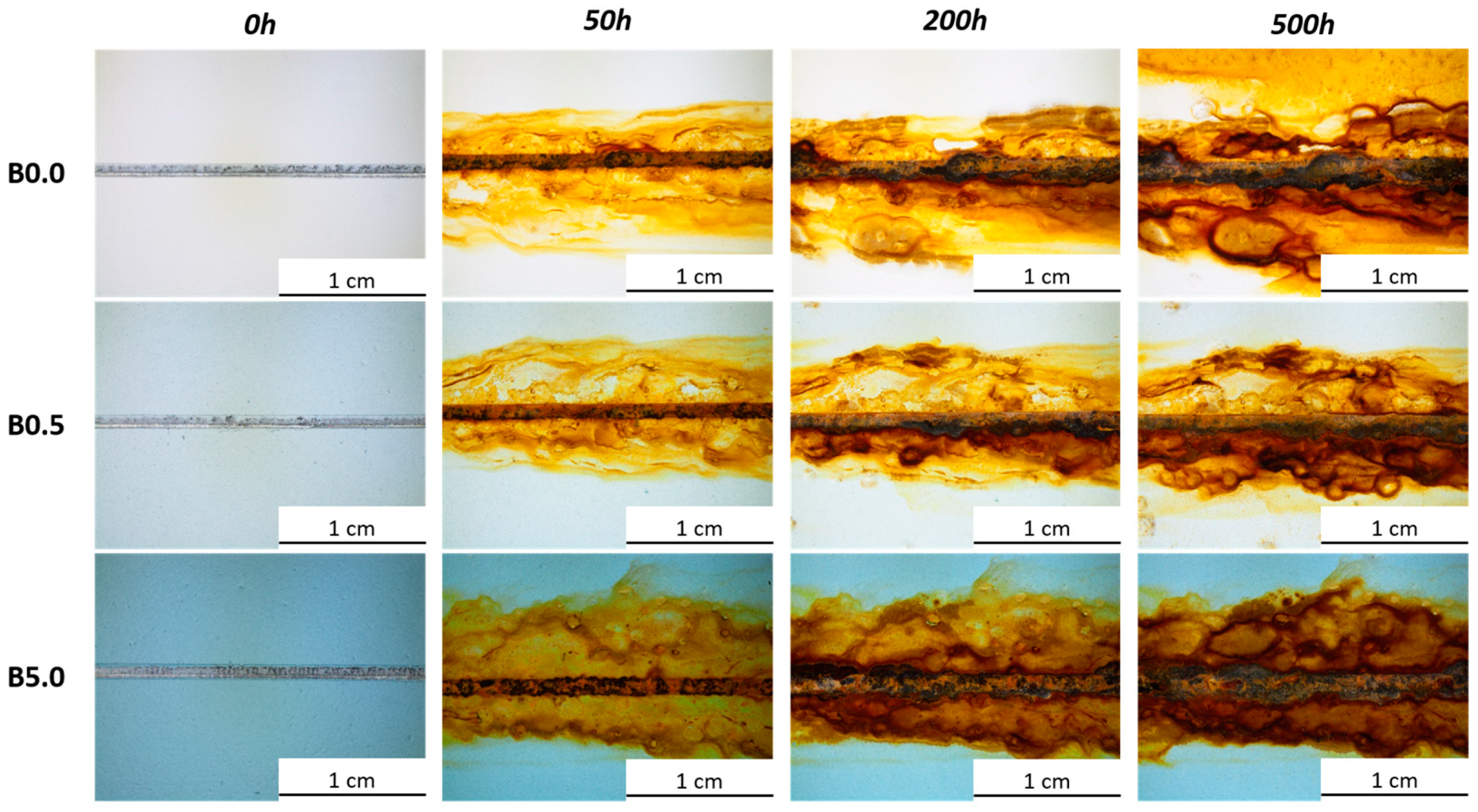
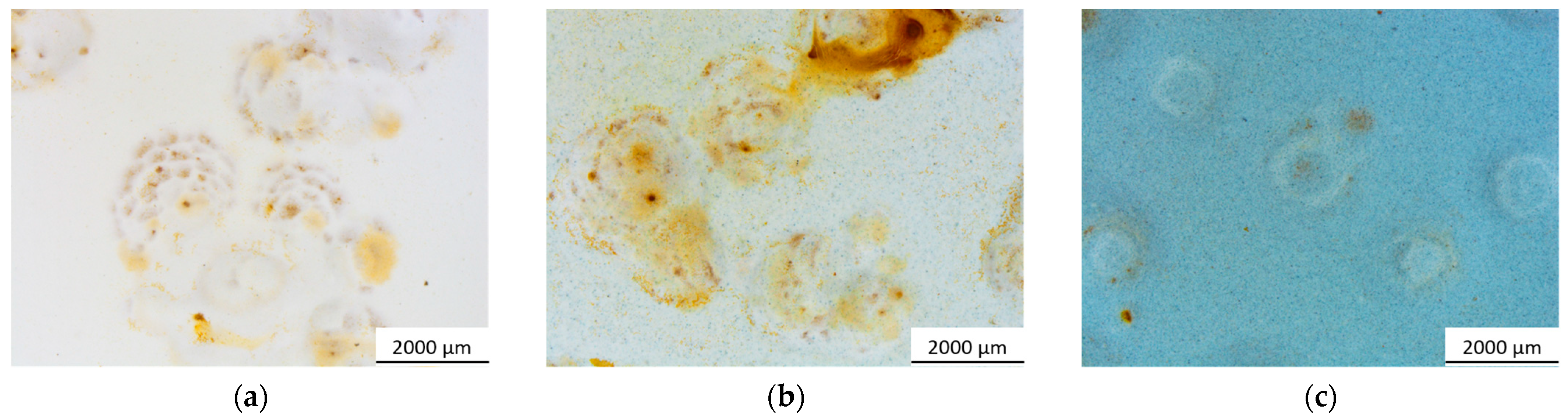
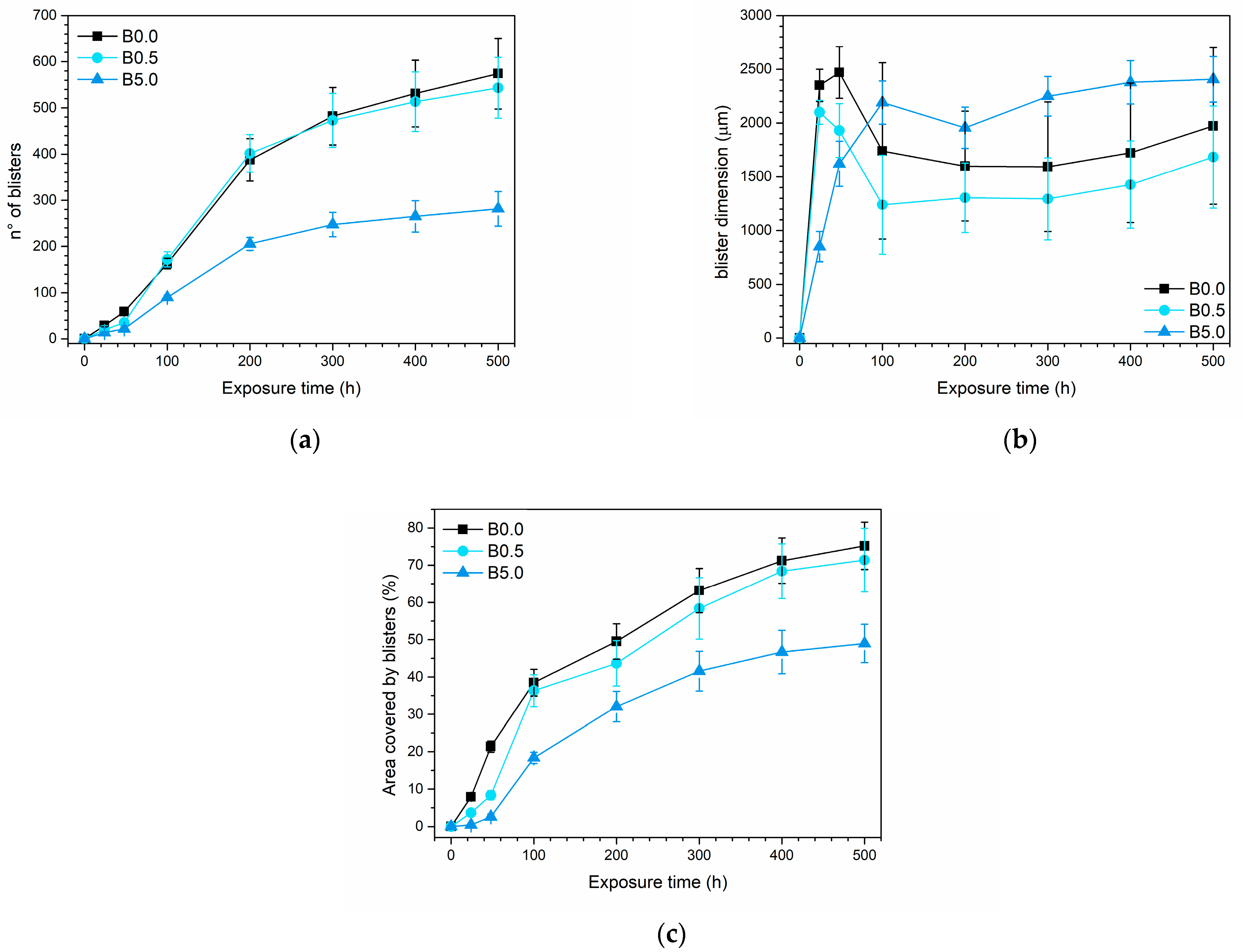
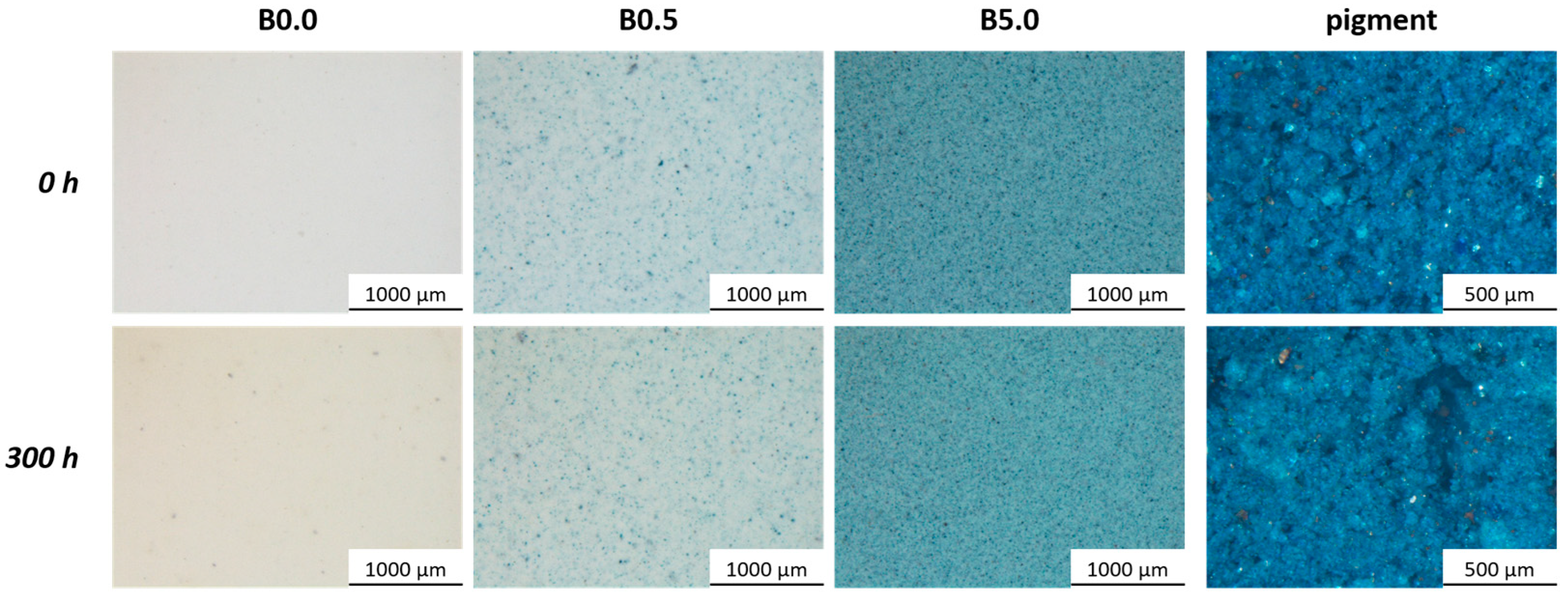

| Sample Nomenclature | Bio-Based Pigment Concentration (wt.%) |
|---|---|
| B0.0 | 0.0 |
| B0.5 | 0.5 |
| B5.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calovi, M.; Rossi, S. Assessing the Impact of Sepiolite-Based Bio-Pigment Infused with Indigo Extract on Appearance and Durability of Water-Based White Primer. Materials 2024, 17, 941. https://doi.org/10.3390/ma17040941
Calovi M, Rossi S. Assessing the Impact of Sepiolite-Based Bio-Pigment Infused with Indigo Extract on Appearance and Durability of Water-Based White Primer. Materials. 2024; 17(4):941. https://doi.org/10.3390/ma17040941
Chicago/Turabian StyleCalovi, Massimo, and Stefano Rossi. 2024. "Assessing the Impact of Sepiolite-Based Bio-Pigment Infused with Indigo Extract on Appearance and Durability of Water-Based White Primer" Materials 17, no. 4: 941. https://doi.org/10.3390/ma17040941
APA StyleCalovi, M., & Rossi, S. (2024). Assessing the Impact of Sepiolite-Based Bio-Pigment Infused with Indigo Extract on Appearance and Durability of Water-Based White Primer. Materials, 17(4), 941. https://doi.org/10.3390/ma17040941







