In Situ Preparation of rGO-Cement Using Thermal Reduction Method and Performance Study
Abstract
1. Introduction
2. Raw Materials and Experimental Methods
2.1. Raw Material
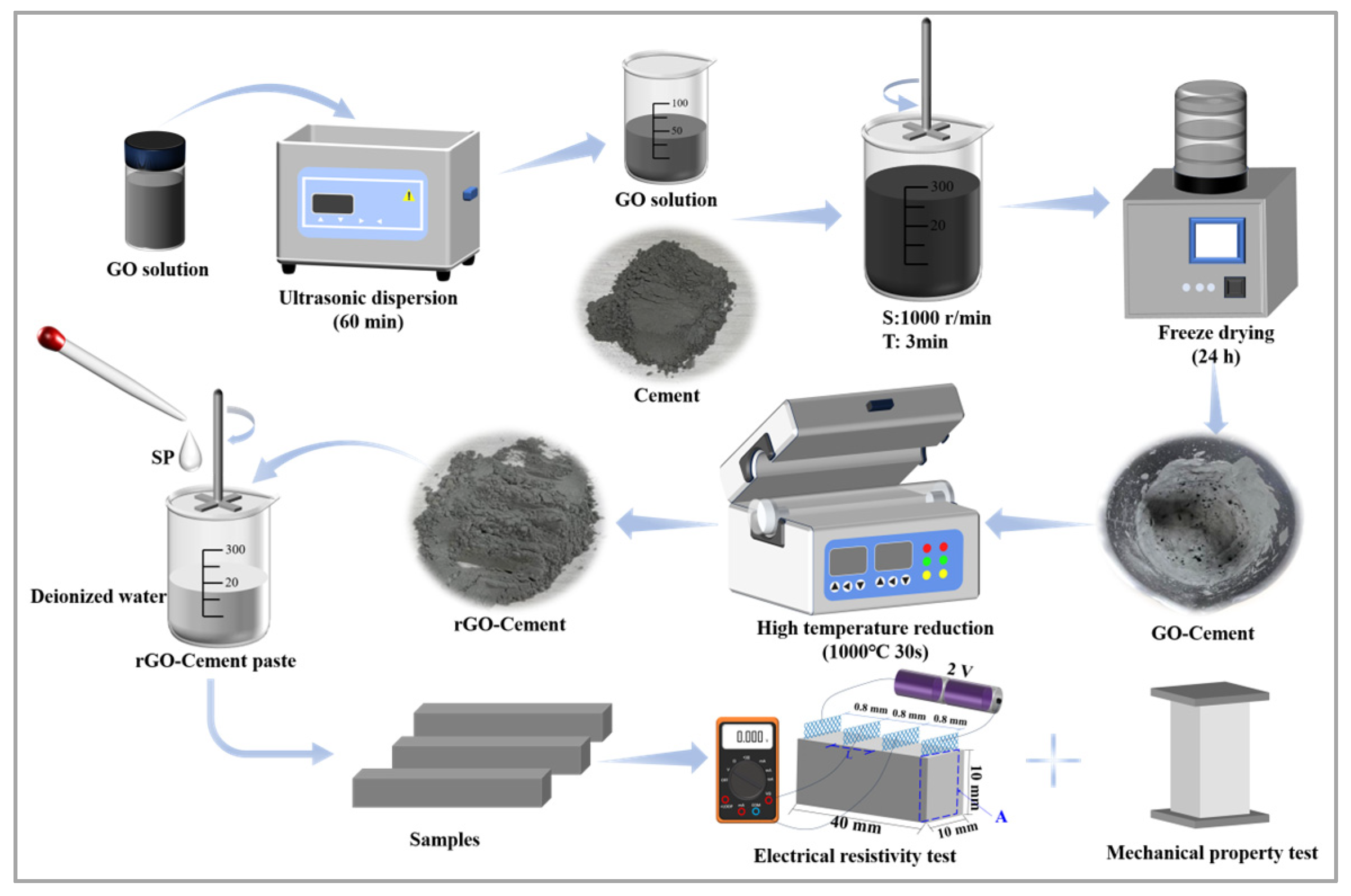
2.2. Sample Preparation
2.3. Performance Testing
2.3.1. X-ray Fluorescence (XRF) Characterization
2.3.2. Raman Spectra Analysis
2.3.3. X-ray Photoelectron Spectroscopy (XPS) Characterization
2.3.4. X-ray Diffraction (XRD) Characterization
2.3.5. Scanning Electron Microscope (SEM) Characterization
2.4. Electrical Resistivity Testing
2.5. Mechanical Property
3. Results and Discussion
3.1. Molecular Structure of GO-Cement and rGO-Cement
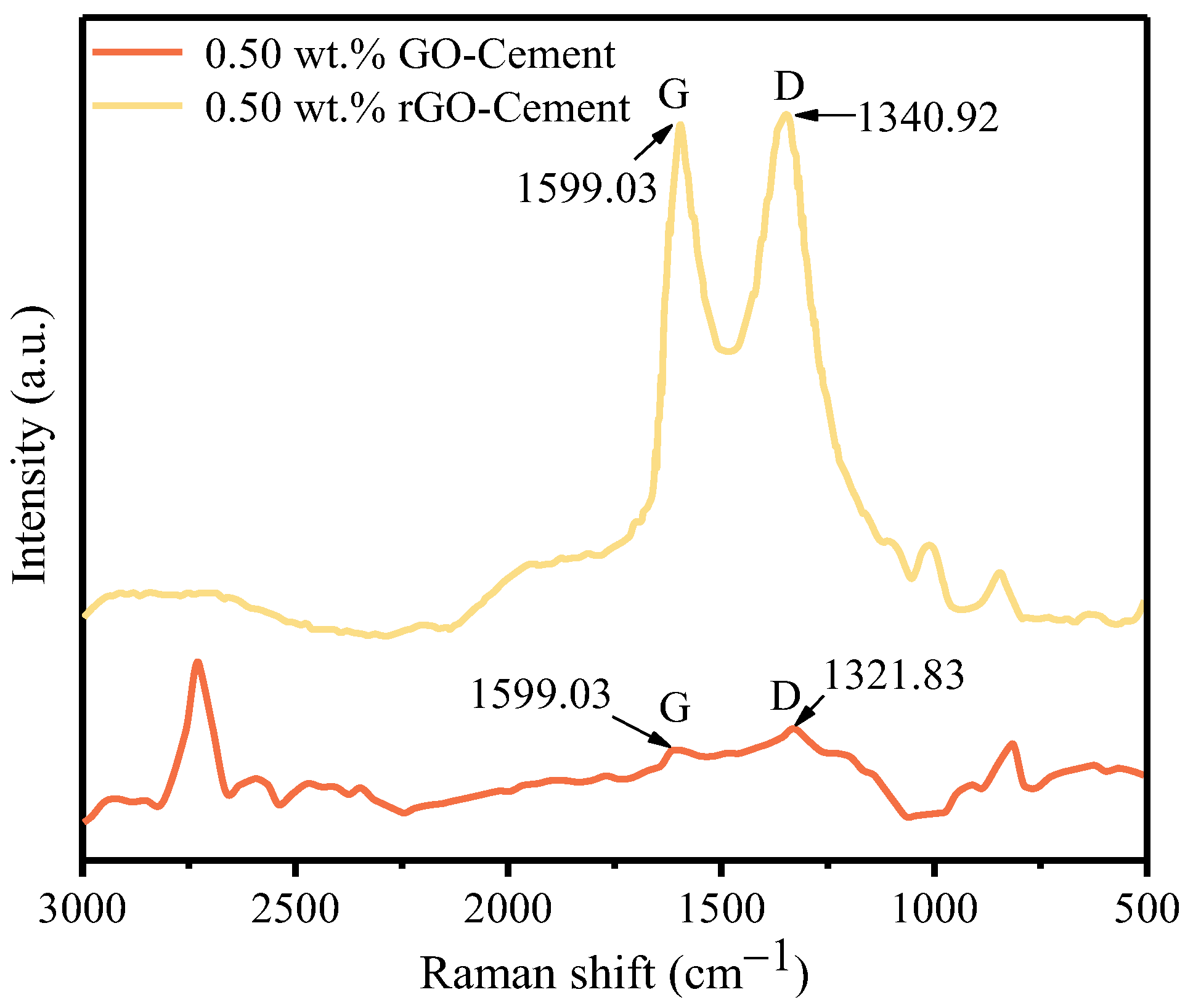
3.1.1. Raman Spectra Analysis
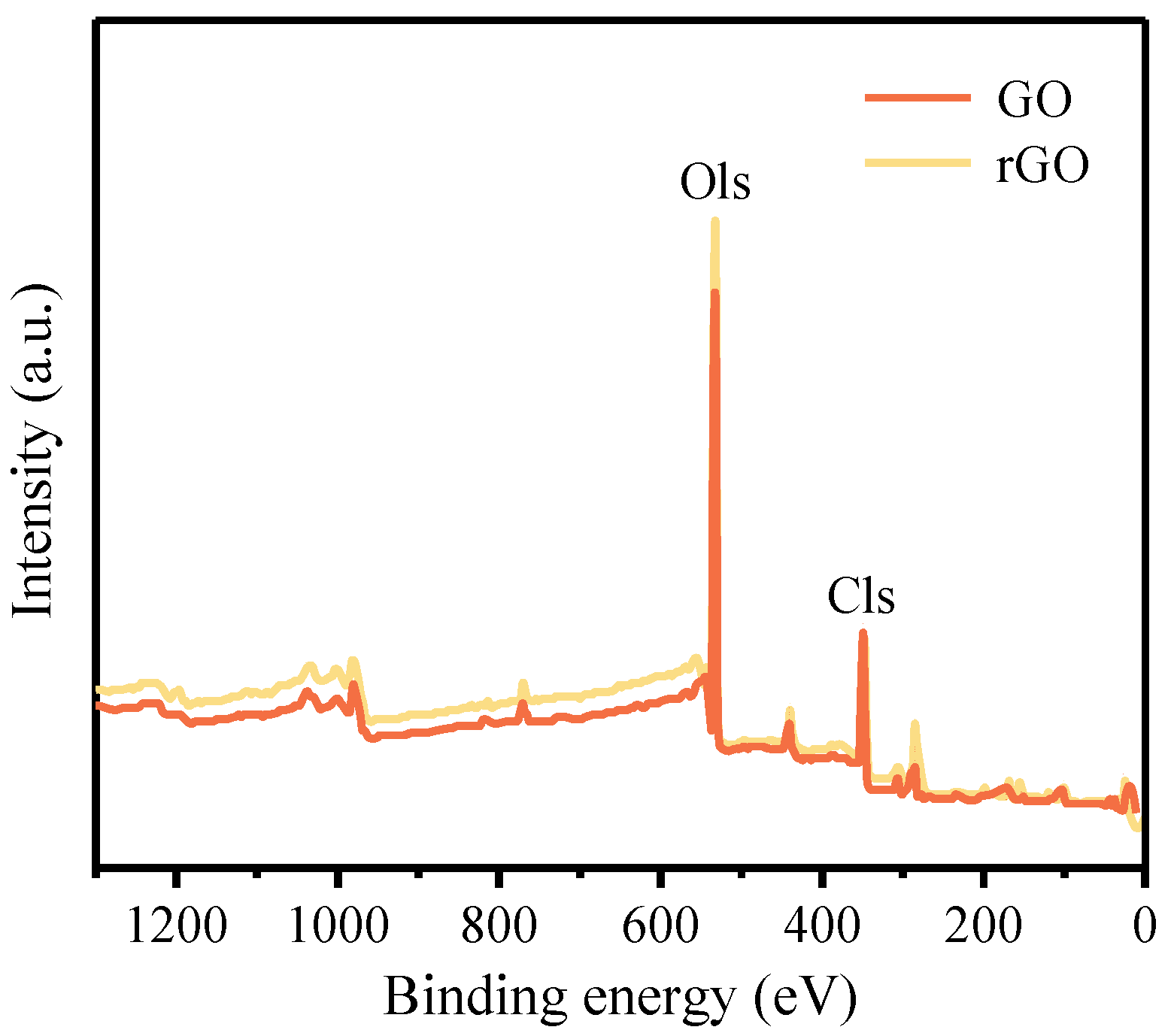
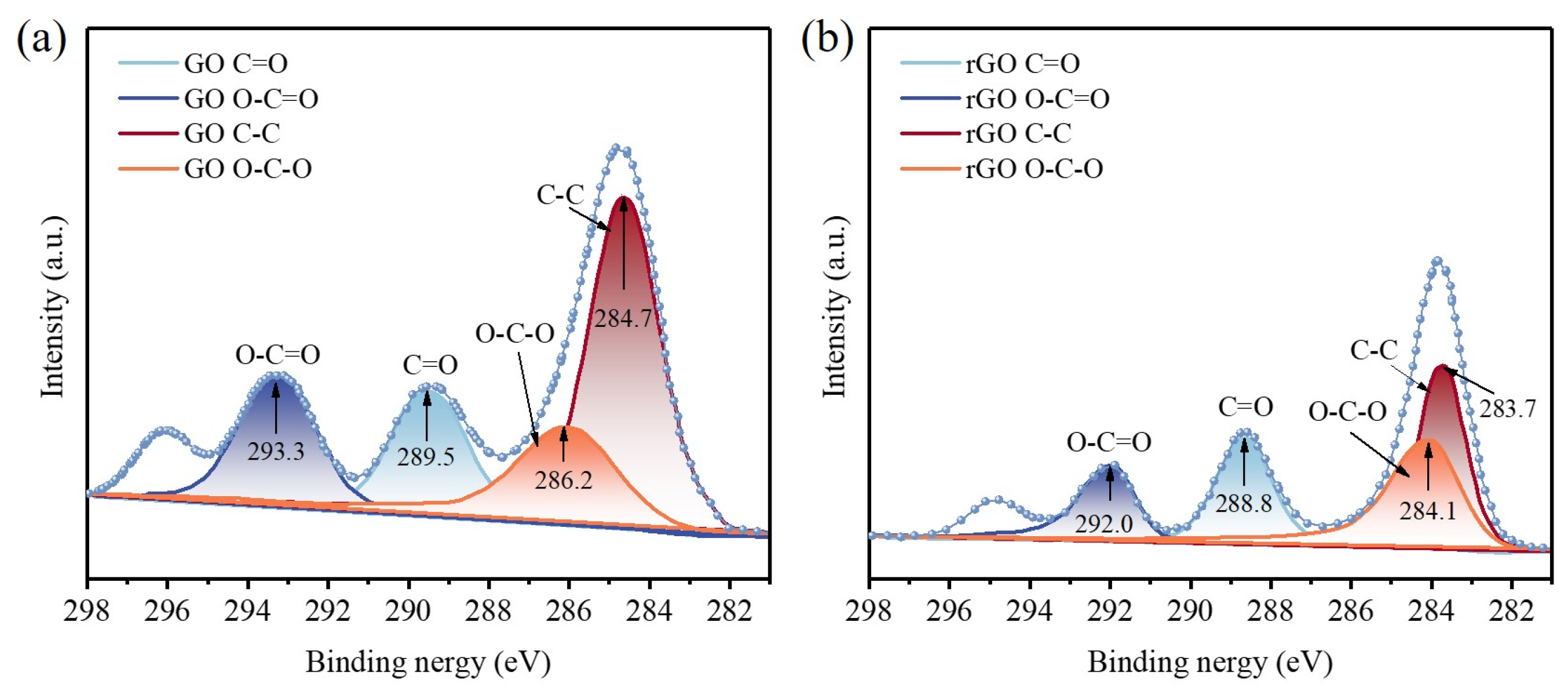
3.1.2. XPS Analysis
3.1.3. Microstructural Morphology of (GO/rGO)-Cement
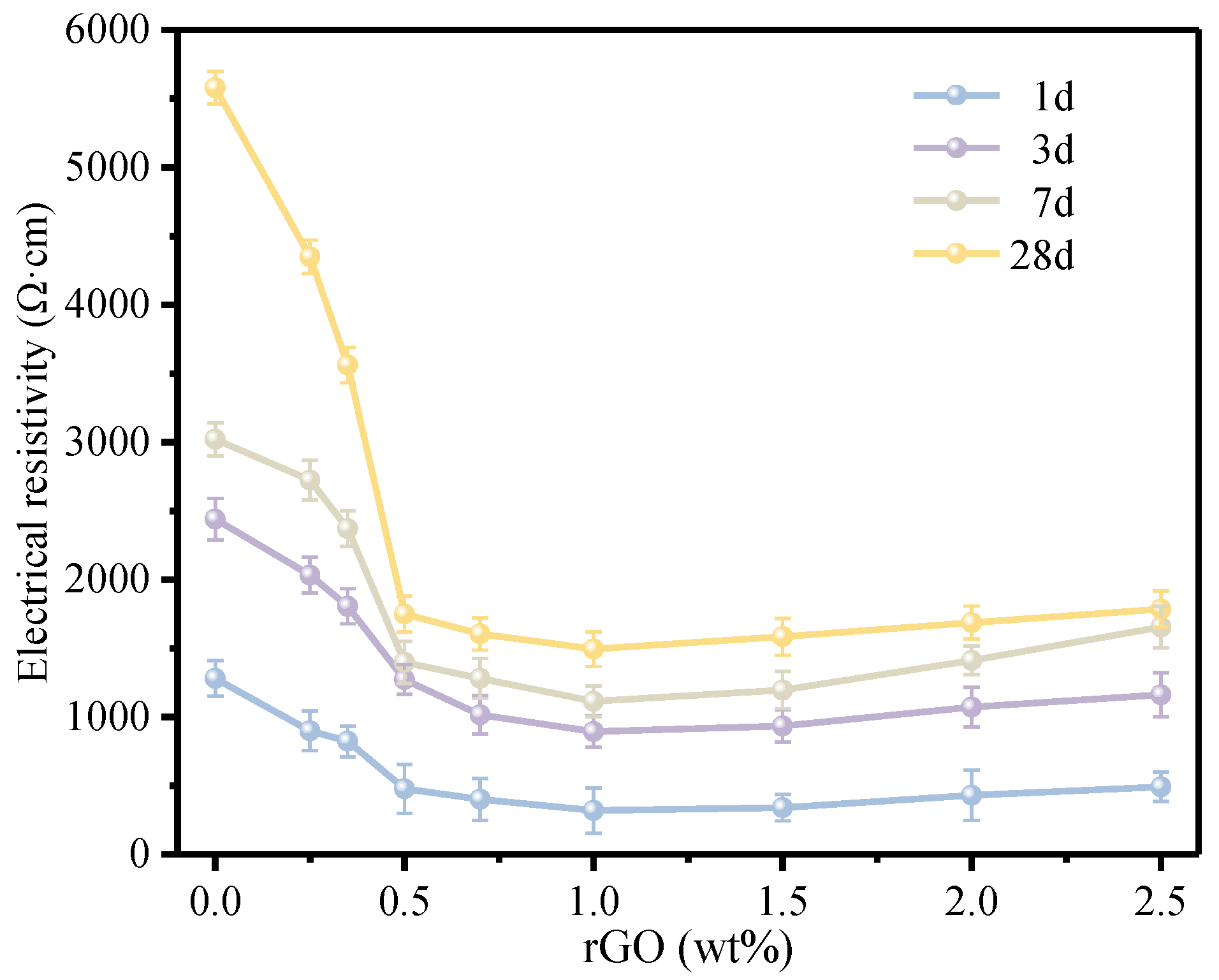
3.2. Electrical Conductivity of the rGO-Cement Paste
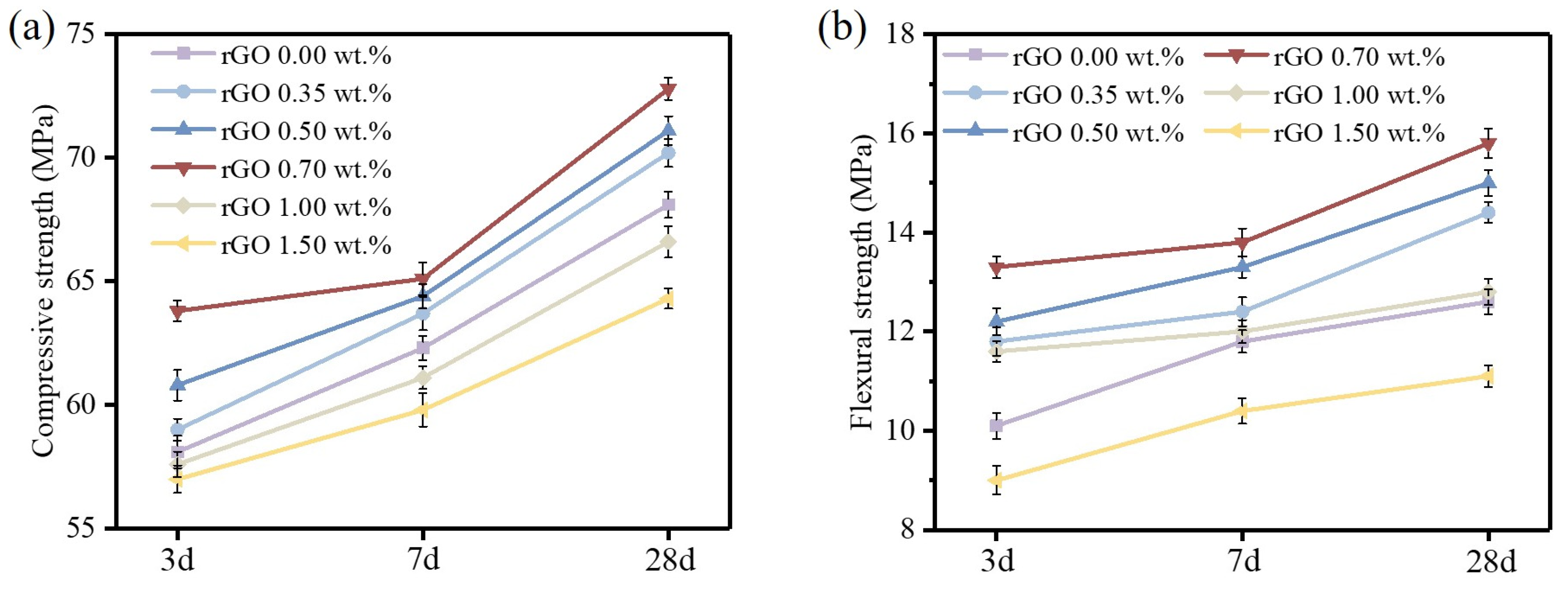
3.3. Mechanical Properties of rGO-Cement Paste
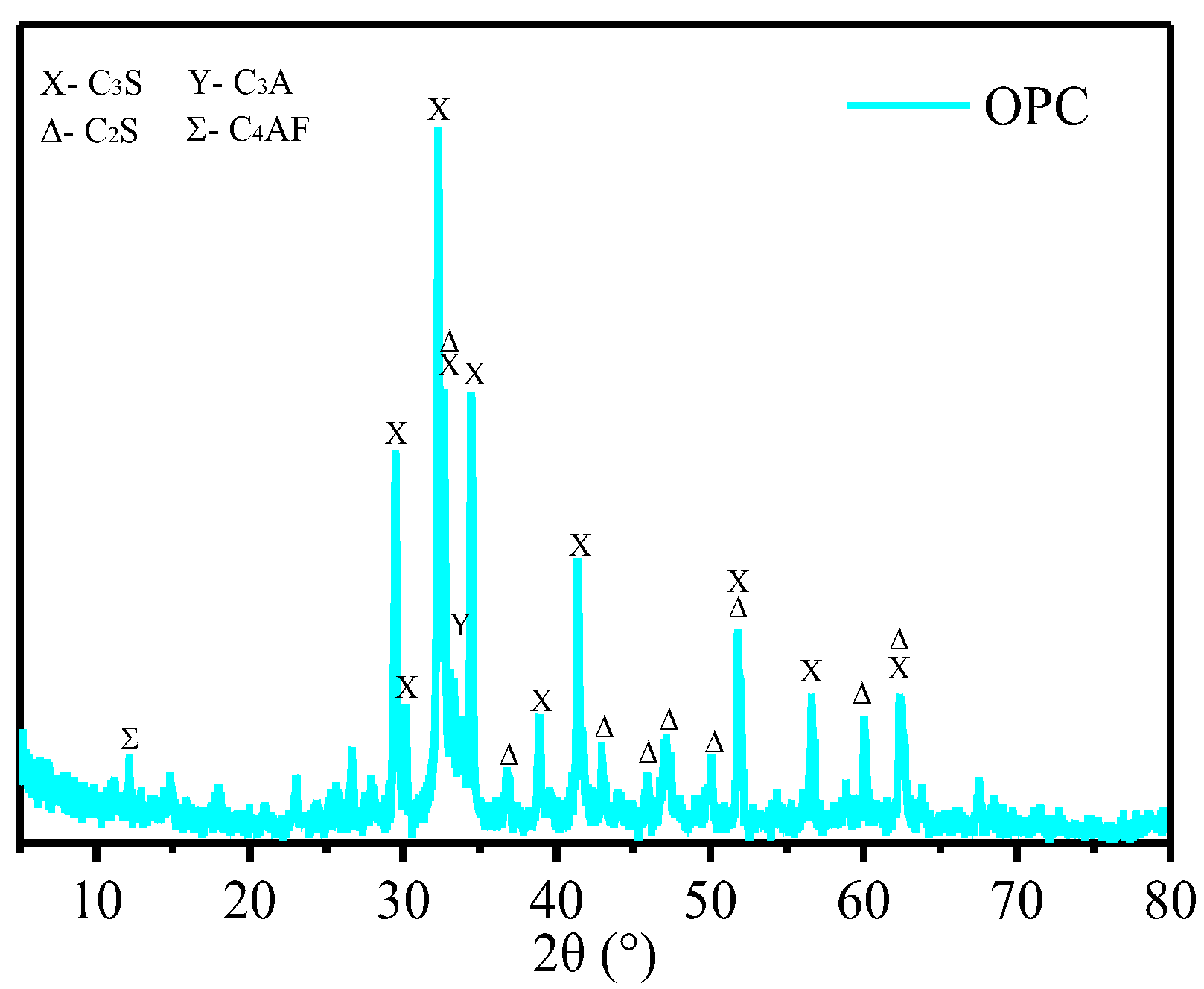
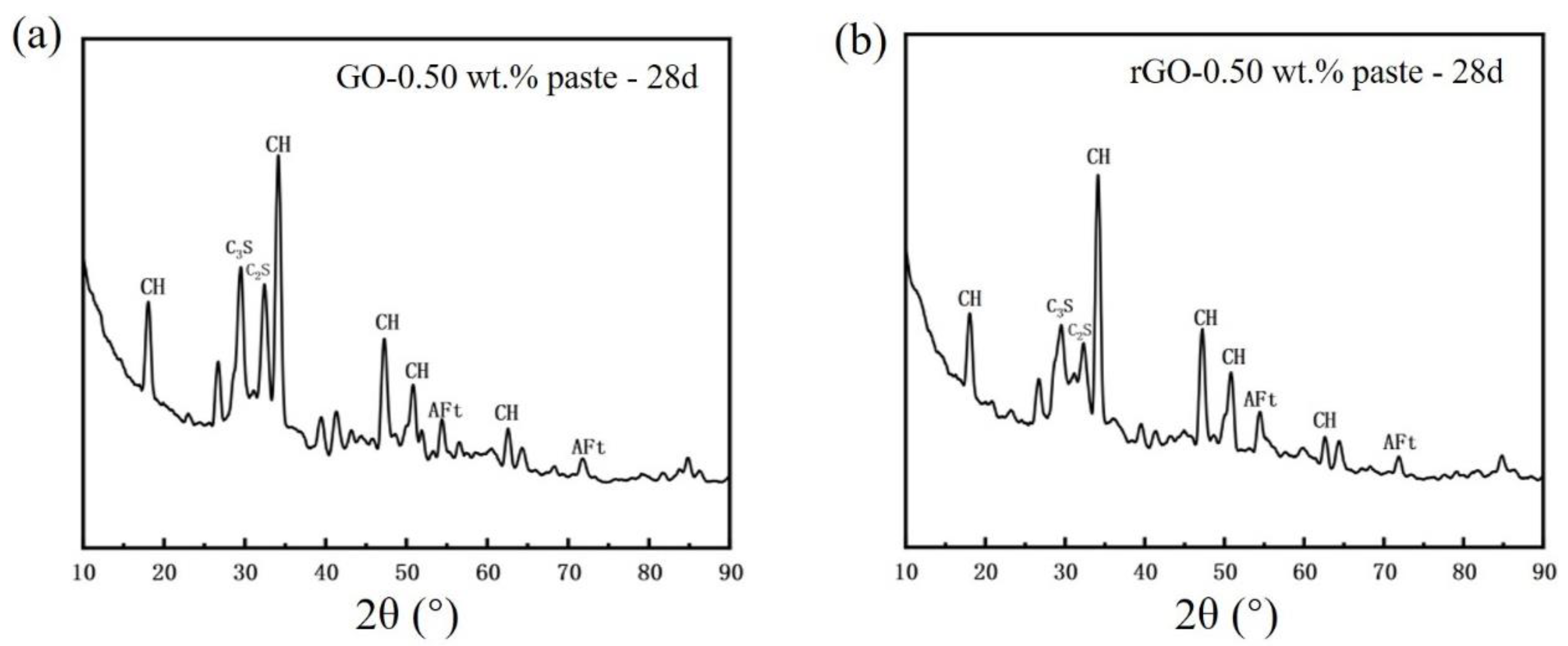
3.4. XRD Phase Analysis of (GO/rGO)-Cement Paste
4. Conclusions
- (1)
- After high-temperature thermal reduction, the Raman spectra exhibit the distinctive D and G characteristic peaks of graphene. The bonding energy of the O-C=O bond, O-C-O bond, and C=O bond weakens, and their quantity decreases, leading to the high-temperature decomposition of numerous oxygen-containing functional groups. This proves the conversion of GO on the cement surface to rGO. The reduction in the number of C-C bonds indicates the breakdown of large sheets of GO structure into smaller sheets of rGO. The microscopic morphology on the cement surface transitions from a dense coating layer to fragmented small sheets, and the folding is also a characteristic feature of graphene. The freeze-drying and high-temperature thermal reduction method for the in situ preparation of rGO-Cement offers the advantages of simplicity and efficiency, facilitating a better dispersion of rGO within cementitious materials.
- (2)
- The rGO coated on the surface of cement particles significantly enhances the electrical conductivity of rGO-Cement paste, reducing its electrical resistivity from 5.58 × 103 Ω·cm to 1.49 × 103 Ω·cm, a decrease of 73.3%. When 0.70 wt.% of rGO is coated on the surface of cement particles, the penetration threshold can be achieved. The best electrical conductivity is observed at a rGO content of 1.0 wt.%.
- (3)
- The presence of rGO on the surface of cement particles promotes the hydration of cement, contributing to the enhancement of compressive and flexural strength. However, an excessive coating of rGO around cement particles hinders the diffusion of ions, impeding the formation of hydration products and consequently reducing the mechanical properties. Therefore, with an increase in the rGO content, the mechanical performance initially increases and then decreases. The optimal mechanical properties were obtained when the content of rGO was 0.70 wt.%. At a hydration age of 28 days, the contents of C3S and C2S in the phase of rGO-Cement are lower than those in GO-Cement. The later-stage hydration of rGO-Cement is more thorough, leading to an improvement in the mechanical properties of the paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Ho, V.D.; Gholampour, A.; Losic, D.; Ozbakkaloglu, T. Enhancing the performance and environmental impact of alkali-activated binder-based composites containing graphene oxide and industrial by-products. Constr. Build. Mater. 2021, 284, 122811. [Google Scholar] [CrossRef]
- Polverino, S.; Del Rio Castillo, A.E.; Brencich, A.; Marasco, L.; Bonaccorso, F.; Morbiducci, R. Few-Layers Graphene-Based Cement Mortars: Production Process and Mechanical Properties. Sustainability 2022, 14, 784. [Google Scholar] [CrossRef]
- Janowska-Renkas, E.; Zdrojek, M.; Kozioł, M.; Kaliciak-Kownacka, A. Effect of composition of geopolymer composites containing fly ash and waste glass powder on their durability and resistivity demonstrated in presence of a nanocarbon additive in a form of graphene. Measurement 2023, 211, 112616. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Pierpaoli, M.; Bastianelli, L.; Mazzoli, A.; Ruello, M.L.; Bellezze, T.; Tittarelli, F. Mechanical, durability, depolluting and electrical properties of multifunctional mortars prepared with commercial or waste carbon-based fillers. Constr. Build. Mater. 2021, 283, 122768. [Google Scholar] [CrossRef]
- Lambert, T.N.; Chavez, C.A.; Hernandez-Sanchez, B.; Lu, P.; Bell, N.S.; Ambrosini, A.; Friedman, T.; Boyle, T.J.; Wheeler, D.R.; Huber, D.L. Synthesis and Characterization of Titania-Graphene Nanocomposites. J. Phys. Chem. 2009, 113, 19812–19823. [Google Scholar] [CrossRef]
- Chintalapudi, K.; Pannem, R.M.R. The effects of Graphene Oxide addition on hydration process, crystal shapes, and microstructural transformation of Ordinary Portland Cement. J. Build. Eng. 2020, 32, 101551. [Google Scholar] [CrossRef]
- Karachevtsev, V.A.; Kurnosov, N.V. The temperature dependence of electron transport in a composite film of graphene oxide with single-wall carbon nanotubes: An analysis and comparison with a nanotube film. Low Temp. Phys. 2019, 45, 1109–1116. [Google Scholar] [CrossRef]
- Sim, H.J.; Li, Z.; Xiao, P.; Lu, H. The Influence of Lateral Size and Oxidation of Graphene Oxide on Its Chemical Reduction and Electrical Conductivity of Reduced Graphene Oxide. Molecules 2022, 27, 7840. [Google Scholar] [CrossRef]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. In Situ fabrication and characterization of graphene/geopolymer composites. Ceram. Int. 2015, 41, 11242–11250. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Panesar, D.K. Impact of graphene oxide and highly reduced graphene oxide on cement based composites. Constr. Build. Mater. 2019, 206, 71–83. [Google Scholar] [CrossRef]
- Yaseen, S.A.; Yiseen, G.A.; Li, Z. Elucidation of Calcite Structure of Calcium Carbonate Formation Based on Hydrated Cement Mixed with Graphene Oxide and Reduced Graphene Oxide. ACS Omega 2019, 4, 10160–10170. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, R.; Zhang, T.; Sun, R.; Fu, Y. Effects of graphene oxygen content on durability and microstructure of cement mortar composites. Constr. Build. Mater. 2022, 354, 129121. [Google Scholar] [CrossRef]
- Gholampour, A.; Valizadeh Kiamahalleh, M.; Tran, D.N.H.; Ozbakkaloglu, T.; Losic, D. From Graphene Oxide to Reduced Graphene Oxide: Impact on the Physiochemical and Mechanical Properties of Graphene–Cement Composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effects of treatment temperature on the reduction of GO under alkaline solution during the preparation of graphene/geopolymer composites. Ceram. Int. 2016, 42, 18181–18188. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Duan, X.; Yang, Z.; Wang, S.; Zhou, Y. In Situ Processing of Graphene/Leucite Nanocomposite Through Graphene Oxide/Geopolymer. J. Am. Ceram. Soc. 2016, 99, 1164–1173. [Google Scholar] [CrossRef]
- Liu, R.; Guo, W.; Sun, B.; Pang, J.; Pei, M.; Zhou, G. Composites of rutile TiO2 nanorods loaded on graphene oxide nanosheet with enhanced electrochemical performance. Electrochim. Acta 2015, 156, 274–282. [Google Scholar] [CrossRef]
- Alaie, M.M.; Jahangiri, M.; Rashidi, A.M.; Asl, A.H.; Izadi, N. A novel selective H2S sensor using dodecylamine and ethylenediamine functionalized graphene oxide. J. Ind. Eng. Chem. 2015, 29, 97–103. [Google Scholar] [CrossRef]
- Abujabal, M.; Abunahla, H.; Mohammad, B.; Alazzam, A. Tunable Switching Behavior of GO-Based Memristors Using Thermal Reduction. Nanomaterials 2022, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Grilli, F.; Gohari, P.H.; Zou, S. Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6802. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Kumar, S.S.S.; Pal, S.K.; Chakraborty, S. Characterization of structural transformation of graphene oxide to reduced graphene oxide during thermal annealing. J. Mater. Res. 2020, 35, 1197–1204. [Google Scholar] [CrossRef]
- Jung, H. Multiple dispensing and photo-thermal reduction of graphene oxide solution for line patterning. Chem. Phys. Lett. 2022, 802, 139757. [Google Scholar] [CrossRef]
- Klemeyer, L.; Park, H.; Huang, J. Geometry-Dependent Thermal Reduction of Graphene Oxide Solid. ACS Mater. Lett. 2021, 3, 511–515. [Google Scholar] [CrossRef]
- Ruttanapun, C.; Phrompet, C.; Tuichai, W.; Karaphun, A.; Daengsakul, S.; Sriwong, C. Influence of free electron charge and free extra framework anions in calcium aluminate@ rGO (CA@ rGO) cement composites with enhanced dielectric and electrochemical properties. J. Taiwan Inst. Chem. Eng. 2021, 127, 334–348. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Tunstall, L.E.; Scherer, G.W.; Prud’Homme, R.K. A new hypothesis for air loss in cement systems containing fly ash. Cem. Concr. Res. 2021, 142, 106352. [Google Scholar] [CrossRef]
- Lavagna, L.; Bartoli, M.; Musso, S.; Suarez-Riera, D.; Tagliaferro, A.; Pavese, M. A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition. Nanomaterials 2022, 12, 2346. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Her, S.; Nezhad, E.Z.; Lim, S.; Bae, S. Synthesis of Highly-Dispersed Graphene Oxide Nanoribbons–Functionalized Carbon Nanotubes–Graphene Oxide (GNFG) Complex and Its Application in Enhancing the Mechanical Properties of Cementitious Composites. Nanomaterials 2021, 11, 1669. [Google Scholar] [CrossRef]
- Darsanasiri, A.G.N.D.; Matalkah, F.; Ramli, S.; Al-Jalode, K.; Balachandra, A.; Soroushian, P. Ternary alkali aluminosilicate cement based on rice husk ash, slag and coal fly ash. J. Build. Eng. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Collier, N.C.; Sharp, J.H.; Milestone, N.B.; Hill, J.; Godfrey, I. The influence of water removal techniques on the composition and microstructure of hardened cement pastes. Cem. Concr. Res. 2008, 38, 737–744. [Google Scholar] [CrossRef]
- GB/T 17617-2007; Test Method for Strength of Cement Paste (ISO Method). The Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- Ding, F.; Ji, H.; Chen, Y.; Herklotz, A.; Dörr, K.; Mei, Y.; Rastelli, A.; Schmidt, O.G. Stretchable Graphene: A Close Look at Fundamental Parameters through Biaxial Straining. Nano Lett. 2010, 10, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, L.; Cutroneo, M.; Manno, D.; Serra, A.; Torrisi, A.; Silipigni, L. Proton beam dosimetry based on the graphene oxide reduction and Raman spectroscopy. Vacuum 2022, 201, 111113. [Google Scholar] [CrossRef]
- Akbari, E.; Akbari, I.; Ebrahimi, M.R. sp2/sp3 bonding ratio dependence of the band-gap in graphene oxide. Eur. Phys. J. B 2019, 92, 71–77. [Google Scholar] [CrossRef]
- Sharma, N.; Tomar, S.; Shkir, M.; Choubey, R.K.; Singh, A. Study of Optical and Electrical Properties of Graphene Oxide. Mater. Today Proc. 2021, 36, 730–735. [Google Scholar] [CrossRef]
- Hu, L.; Wang, A.; Wang, W. Structural Evolution of Graphene Oxide and Its Thermal Stability During High Temperature Sintering. J. Wuhan Univ. Technol. Sci. Ed. 2022, 37, 342–349. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Vasilets, V.N.; Michtchenko, A.; Shulga, Y.M. Influence of treatment with hydrazine and subsequent annealing on the composition and thermophysical properties of polytetrafluoroethylene–graphene oxide composite aerogel. Appl. Phys. A 2021, 127, 464. [Google Scholar] [CrossRef]
- Compton, O.C.; Jain, B.; Dikin, D.A.; Abouimrane, A.; Amine, K.; Nguyen, S.T. Chemically Active Reduced Graphene Oxide with Tunable C/O Ratios. ACS Nano 2011, 5, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Park, K.H.; Dhayal, M. Two-step process for programmable removal of oxygen functionalities of graphene oxide: Functional, structural and electrical characteristics. RSC Adv. 2015, 5, 95657–95665. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Yan, J.; Yu, W.; Zhao, R.; Du, S.; Niu, K. Effect of long-term ageing on graphene oxide: Structure and thermal decomposition. R. Soc. Open Sci. 2021, 8, 202309. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jia, Z.; Wang, Y.; Jiang, Y.; Miao, Z.; Zhou, Y.; Zhang, H. Enhanced thermoelectric performance of low carbon cement-based composites by reduced graphene oxide. Energy Build. 2021, 250, 111279. [Google Scholar] [CrossRef]
- Zhai, S.; Pang, B.; Liu, G.; Zhang, Y.; Xu, K.; She, W.; Zhang, Y. Investigation on preparation and multifunctionality of reduced graphene oxide cement mortar. Constr. Build. Mater. 2021, 275, 122119. [Google Scholar] [CrossRef]
- Xing-Wen, J.; Xin, Z.; Jun-Meng, L.; Ping, W. Conductivity and conducting stability of copper-coated carbon-fiber-reinforced cement-based composite. Mater. Res. Express 2018, 5, 095705. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Li, X.; Bian, C.; Xiong, Z.; Sun, Y.; Chen, H.; Shen, L. Tunable rGO network in polymer coating for enhancing barrier property. Mater. Res. Bull. 2020, 122, 110648. [Google Scholar] [CrossRef]
- Zhang, N.; She, W.; Du, F.; Xu, K. Experimental Study on Mechanical and Functional Properties of Reduced Graphene Oxide/Cement Composites. Materials 2020, 13, 3015. [Google Scholar] [CrossRef]


| CaO | SiO2 | Al2O3 | SO3 | MgO | Fe2O3 | K2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| 49.7% | 24.1% | 9.5% | 5.7% | 3.4% | 2.7% | 0.9% | 0.3% | 3.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Guan, A.; Ruan, W.; Ma, Y. In Situ Preparation of rGO-Cement Using Thermal Reduction Method and Performance Study. Materials 2024, 17, 1209. https://doi.org/10.3390/ma17051209
Yao J, Guan A, Ruan W, Ma Y. In Situ Preparation of rGO-Cement Using Thermal Reduction Method and Performance Study. Materials. 2024; 17(5):1209. https://doi.org/10.3390/ma17051209
Chicago/Turabian StyleYao, Jie, Ao Guan, Wenqiang Ruan, and Ying Ma. 2024. "In Situ Preparation of rGO-Cement Using Thermal Reduction Method and Performance Study" Materials 17, no. 5: 1209. https://doi.org/10.3390/ma17051209
APA StyleYao, J., Guan, A., Ruan, W., & Ma, Y. (2024). In Situ Preparation of rGO-Cement Using Thermal Reduction Method and Performance Study. Materials, 17(5), 1209. https://doi.org/10.3390/ma17051209




