First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction
Abstract
1. Introduction
2. Calculation and Experimental Methods
3. Results and Discussion
3.1. Modeling of ZrSiO4 Structure
3.2. Adsorption Behavior of C, CO and Cl2 on the (100) and (110) Planes
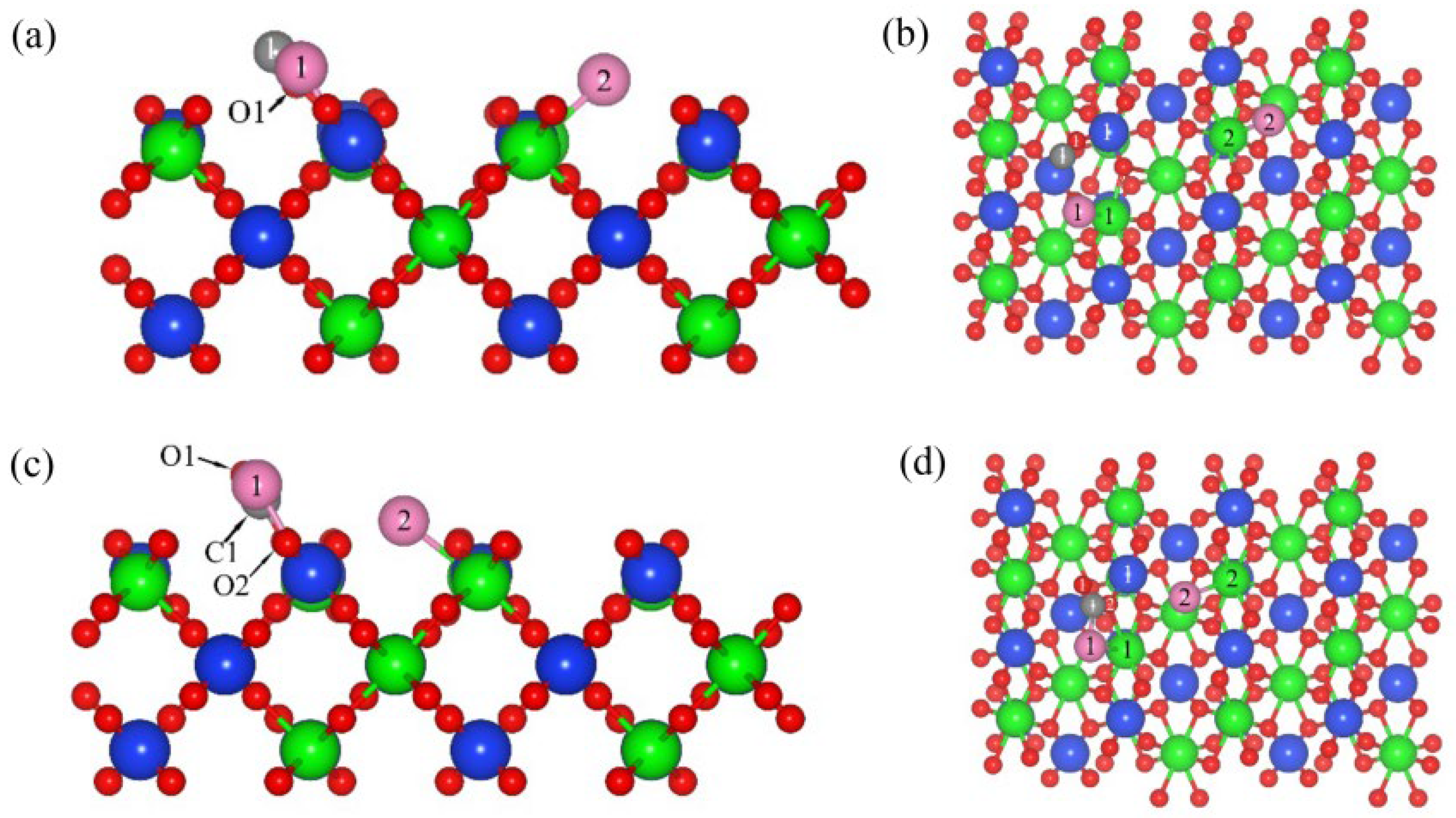
3.3. Co-Adsorption Behaviors of C, CO and Cl2 on the (100) Plane
3.4. Co-Adsorption Behavior of C, CO and Cl2 on the (110) Plane
3.4.1. Co-Adsorption on the (110) Plane with a Molar Ratio of C to Cl2 of 1:1
3.4.2. Co-Adsorption on the (110) Plane with a Molar Ratio of C to Cl2 of 1:2 and 2:2
3.5. Experimental Verification of Simulation Calculation Results
4. Conclusions
- (1)
- When C, CO and Cl2 co-adsorbed on the (100) and (110) surfaces, they interacted with the surface, the Cl2 molecules dissociated, and then the Zr-Cl and C=O bonds formed on the surface of ZrSiO4.
- (2)
- Compared with CO, the co-adsorption energy of C and Cl2 on the (100) and (110) planes of ZrSiO4 was lower, and the co-adsorption structure was more stable. Adding Cl2 molecules did not result in a significant change in co-adsorption energy, while increasing the C content resulted in a significant decrease in co-adsorption energy. The co-adsorption energy of the 110-2C-2Cl2 structure was the lowest (−13.45 eV), and the co-adsorption structure was the most stable.
- (3)
- When C, CO and Cl2 co-adsorbed on the (100) and (110) planes, C provided electrons, while O and Cl accepted electrons. The charge aggregation regions were formed around Si-O and C-O-Si bonds, while charge-depleted regions were formed around the Zr-Cl bond. C can promote the co-adsorption, charge transfer and reaction between Cl2 and the surface; increasing the C content was beneficial for promoting co-adsorption and reaction with Cl2 on the surface of ZrSiO4, removing surface oxygen atoms, which is consistent with the experimental results.
- (4)
- Compared with CO, the reaction energy barrier of C and Cl2 was lower. The higher the C content, the lower the adsorption energy barrier, which was beneficial for promoting the charge transfer and dissociation of Cl2.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Professional Editorial Board of Chemical Encyclopedia. Metallurgy and Metal Materials-Chemical Encyclopedia Professional Volume; Chemical Industry Press: Beijing, China, 2000. [Google Scholar]
- Northwood, D.O. The development and applications of zirconium alloys. Mater. Des. 1985, 6, 58–70. [Google Scholar] [CrossRef]
- Xiong, B.K.; Lin, Z.H.; Yang, X.M. Preparation and Application of Zirconia; Metallurgy Industry Press: Beijing, China, 2008. [Google Scholar]
- Mo, L.P.; Zhang, Z.H. Recent applications of zirconium compounds as catalysts or reagents in organic synthesis. Curr. Org. Chem. 2011, 15, 3800–3823. [Google Scholar] [CrossRef]
- Zhou, G.F.; Wang, Q.; Zeng, R.Q.; Fu, X.K.; Yang, X.B. Preparation and application of zirconium phosphate and its derivatives. Prog. Chem. 2014, 26, 87–99. [Google Scholar]
- Yin, Y.S.; Li, J. Zirconia Ceramics and Their Composite Materials, 1st ed.; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Jiang, D.M.; Wang, L.J.; Che, X.K. Preparation Process and Application of Zirconium Oxychloride, 1st ed.; Metallurgical Industry Press: Beijing, China, 2012. [Google Scholar]
- Ma, X.D.; Liu, F.L.; Zhang, H.; Xu, L.J.; Wei, S.Z.; Li, J.W.; Zhang, G.S. Preparation of zirconia nanoparticles in ionic liquid-water and characterization. Appl. Mech. Mater. 2011, 52–54, 630–632. [Google Scholar] [CrossRef]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, S.P.N. ZrOCl2•8H2O: An efficient and recyclable catalyst for the three-component synthesis of amidoalkyl naphthols under solvent-free conditions. J. Saudi Chem. Soc. 2016, 20, 138–150. [Google Scholar] [CrossRef]
- Liu, R.; Qu, J.K.; Song, J.; Qi, T.; Du, A.L. Analysis of water leaching and transition processes in zirconium oxychloride octahydrate production. Ceram. Int. 2014, 40, 1431–1438. [Google Scholar] [CrossRef]
- Wang, Z.R.; Xu, Q.; Xu, M.; Wang, S.; You, J.L. In situ spectroscopic studies of decomposition of ZrSiO4 during alkali fusion process using various hydroxides. RSC Adv. 2015, 5, 11658–11666. [Google Scholar] [CrossRef]
- Fang, Z.T.; Dixon, D.A. Hydrolysis of ZrCl4 and HfCl4: The initial steps in the high temperature oxidation of metal chlorides to produce ZrO2 and HfO2. J. Phys. Chem. C 2013, 117, 7459–7474. [Google Scholar] [CrossRef]
- Knizia, G.; Klein, J.E.M.N. Electron flow in reaction mechanisms-revealed from first principles. Angew. Chem. Int. Ed. Engl. 2015, 54, 5518–5522. [Google Scholar] [CrossRef]
- Lee, B.; Stowe, H.M.; Lee, K.H.; Hur, N.H.; Hwang, S.J.; Paek, E.; Hwang, G.S. Understanding CO2 capture mechanisms in aqueous hydrazine via combined NMR and first-principles studies. Phys. Chem. Chem. Phys. 2017, 19, 24067–24075. [Google Scholar] [CrossRef]
- Janesko, B.G. Replacing hybrid density functional theory: Motivation and recent advances. Chem. Soc. Rev. 2021, 50, 8470–8495. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.; Yamijala, S.S.; Wong, B.M. Improved band gaps and structural properties from Wannier-Fermi-Löwdin self-interaction corrections for periodic systems. J. Phys. Condens. Matter. 2021, 33, 115501. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.E. High temperature chlorination of titanium bearing minerals: Part IV. Metall. Trans. B 1979, 10, 271–277. [Google Scholar] [CrossRef]
- Vogtenhuber, D.; Podloucky, R.; Radinger, J. Ab initio study of atomic Cl adsorption on stoichiometric and reduced rutile TiO2 (110) surfaces. Surf. Sci. 2000, 454, 369–373. [Google Scholar] [CrossRef]
- Inderwildi, O.R.; Kraft, M. Adsorption, diffusion and desorption of chlorine on and from rutile TiO2 {110}: A theoretical investigation. ChemPhysChem 2007, 8, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wen, L.Y.; Peng, Q.; Zhao, Y.; Xu, J.; Hu, M.L.; Zhang, S.F.; Yang, Z.Q. Prediction of structural and electronic properties of Cl2 adsorbed on TiO2 (100) surface with C or CO in fluidized chlorination process: A first-principles study. J. Cent. South Univ. 2021, 28, 29–38. [Google Scholar] [CrossRef]
- Korkin, A.; Kamisaka, H.; Yamashita, K.; Safonov, A.; Bagatur’yants, A. Computational study of ZrSiO4 polymorphs. Appl. Phys. Lett. 2006, 88, 18913. [Google Scholar] [CrossRef]
- Askeland, D.R.; Phulé, P.P. Essentials of Materials Science and Engineering; Cengage Learning: Stamford, CT, USA, 2010; pp. 1–578. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]














| Adsorption Structure | Element | Bader Charge |
|---|---|---|
| 100-C-Cl2 | C | −1.06 |
| Cl | 0.44 | |
| Cl | 0.62 | |
| 100-CO-Cl2 | O | 1.05 |
| C | −1.78 | |
| Cl | 0.16 | |
| Cl | 0.64 |
| Adsorption Structure | Element | Bader Charge |
|---|---|---|
| 110-C-Cl2 | C | −1.13 |
| Cl | 0.54 | |
| Cl | 0.62 | |
| 110-CO-Cl2 | O | 1.05 |
| C | −1.76 | |
| Cl | 0.17 | |
| Cl | 0.67 |
| Adsorption Structure | Element | Bader Charge |
|---|---|---|
| 110-C-2Cl2 | C | −1.14 |
| Cl | 0.55 | |
| Cl | 0.60 | |
| Cl | −0.20 | |
| Cl | 0.64 | |
| 110-2C-2Cl2 | C | −1.13 |
| C | −1.17 | |
| Cl | 0.53 | |
| Cl | 0.61 | |
| Cl | 0.63 | |
| Cl | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, F.; Zhao, Y.; Azhati, A.; Wang, X.; Zhang, Z.; Lv, X. First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction. Materials 2024, 17, 1500. https://doi.org/10.3390/ma17071500
Liu X, Wang F, Zhao Y, Azhati A, Wang X, Zhang Z, Lv X. First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction. Materials. 2024; 17(7):1500. https://doi.org/10.3390/ma17071500
Chicago/Turabian StyleLiu, Xingping, Fumin Wang, Yalan Zhao, Arepati Azhati, Xingtao Wang, Zhengliang Zhang, and Xueqian Lv. 2024. "First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction" Materials 17, no. 7: 1500. https://doi.org/10.3390/ma17071500
APA StyleLiu, X., Wang, F., Zhao, Y., Azhati, A., Wang, X., Zhang, Z., & Lv, X. (2024). First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction. Materials, 17(7), 1500. https://doi.org/10.3390/ma17071500




