Preparation of RDX/F2311/Fe2O3/Al Composite Hollow Microspheres by Electrospray and Synergistic Energy Release during Combustion between Components
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Precursor Preparation
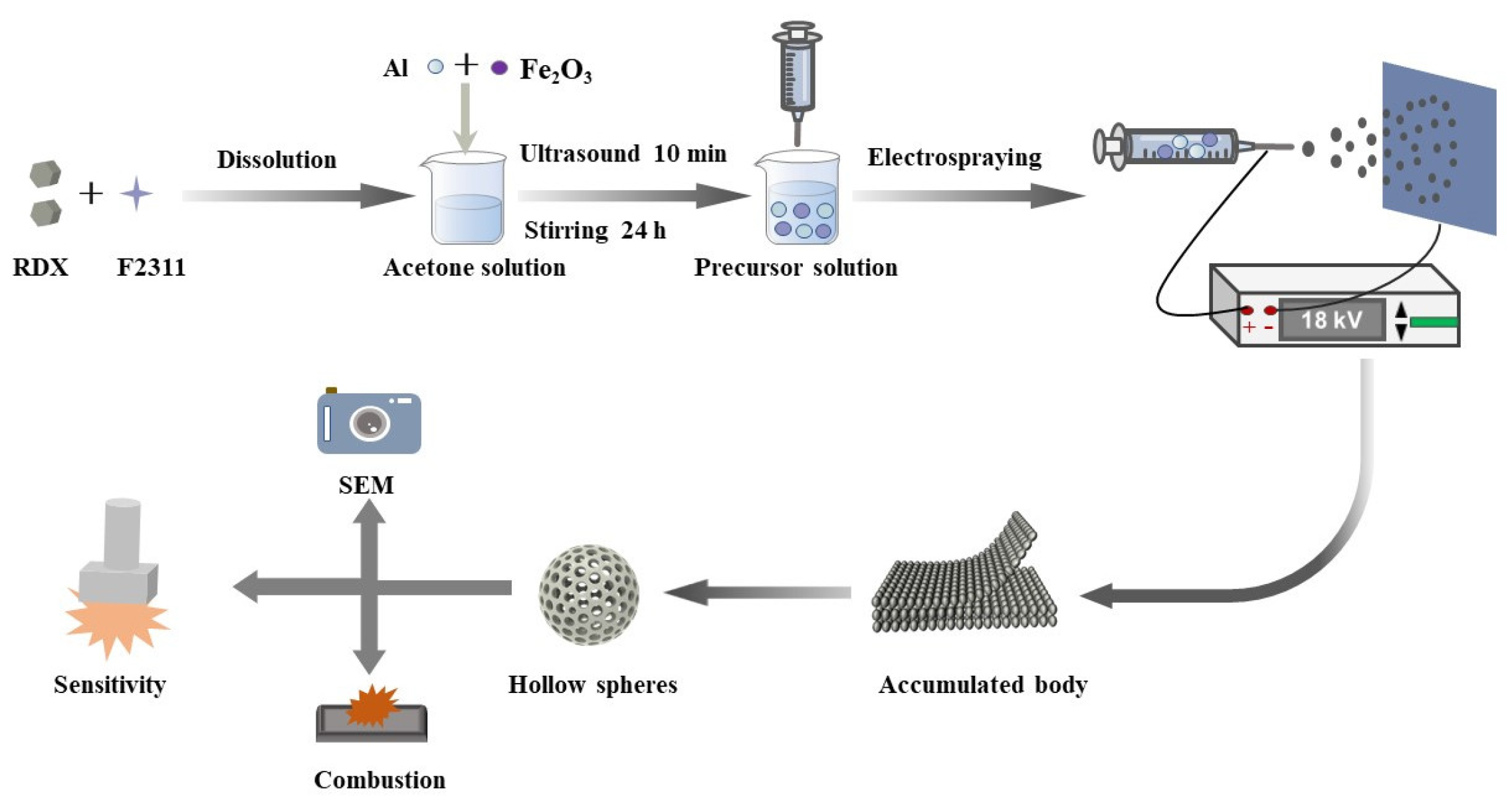
2.3. RDX/F2311/Fe2O3/Al Composite Microsphere Preparation
2.4. Characterization
3. Results and Discussion
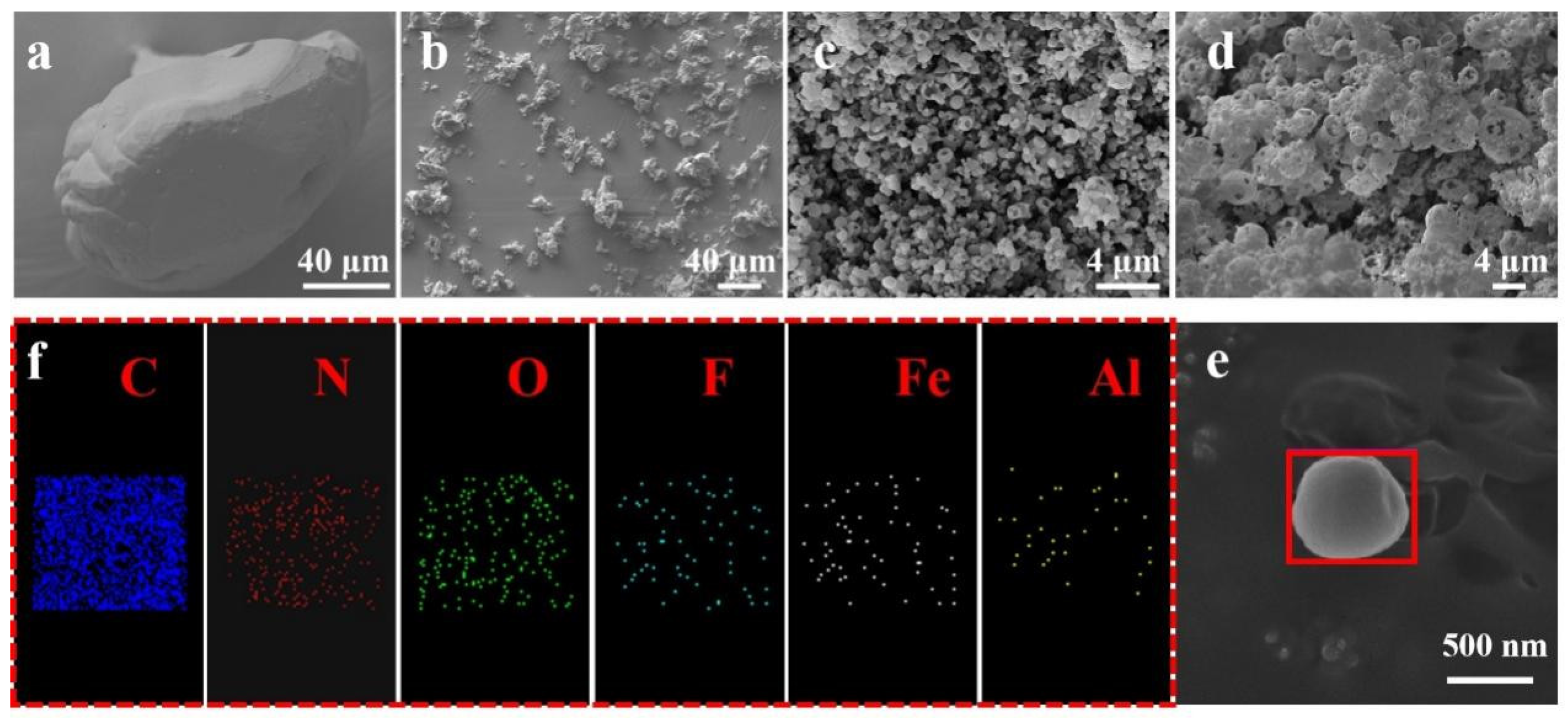
3.1. Morphological Analysis
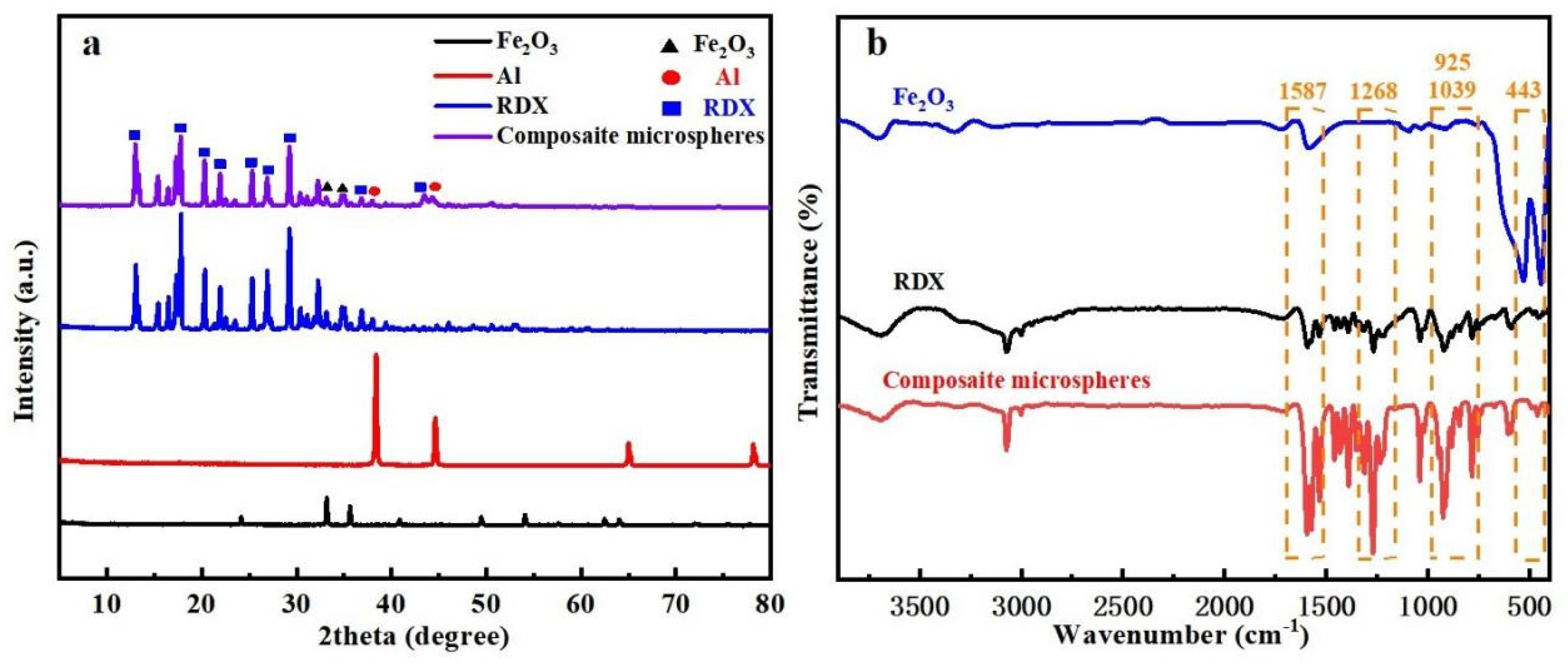
3.2. Compositional Analysis
3.3. Combustion Performance
- (1)
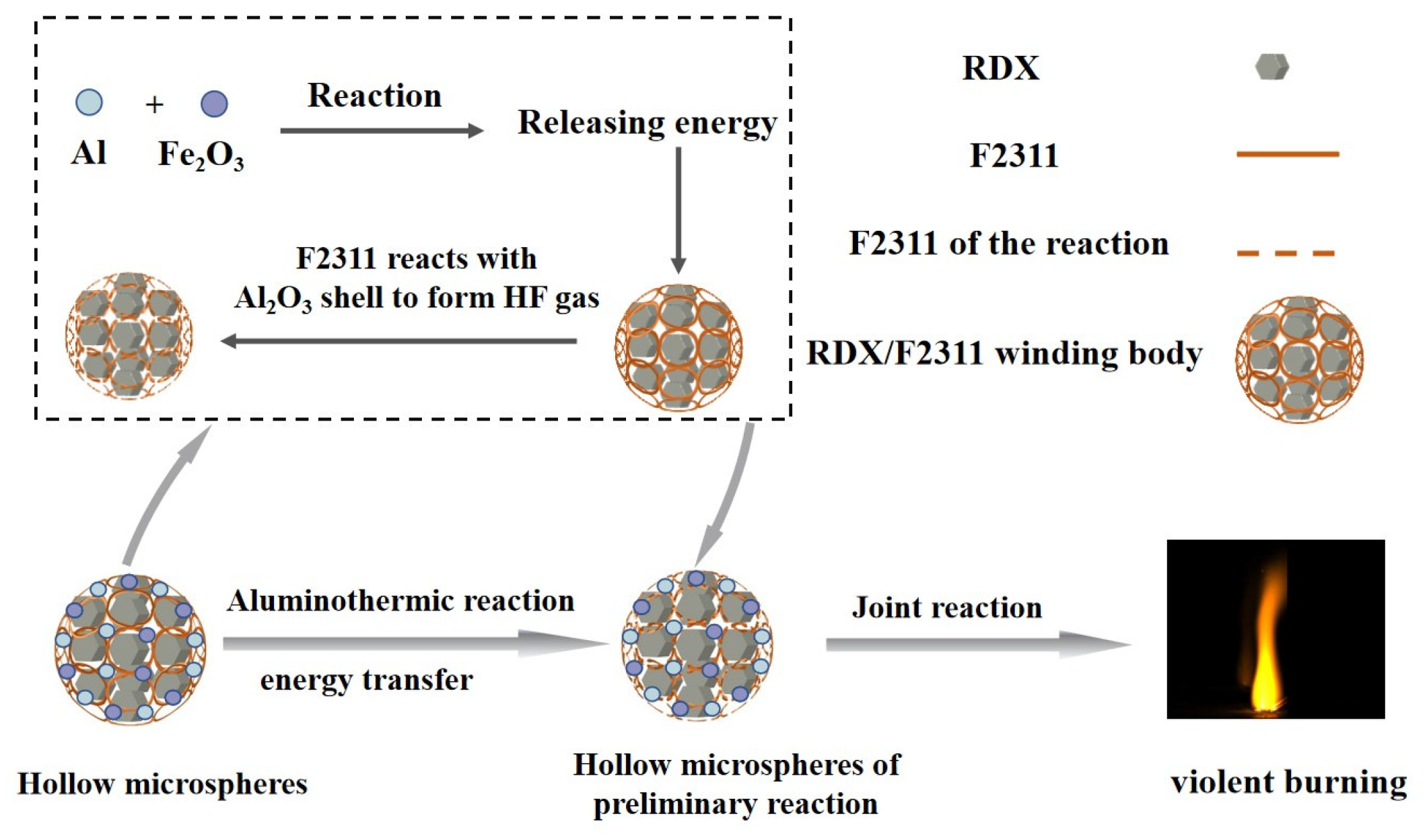
- The combustion of conventional nano-alumina powder results in the formation of a dense inert layer on the powder’s surface. This layer hinders the diffusion of oxidizing gases within the powder. Increasing fluorine content leads to the destruction of more of the alumina shell layer, and the sublimation temperature of the aluminum fluoride produced (1276 °C) is lower than the combustion temperature of the nano-alumina. The exposed surface of the aluminum fluoride post-sublimation serves as reactant for further oxidation consequently accelerating the combustion time of the composite microspheres and reaching peak combustion intensity faster.
- (2)
- The scanning electron microscope (SEM) image in Figure 2 reveals a significant number of composite microspheres arranged in stacks. Within these composite microspheres, F2311 serves as a binder that consolidates the other components. Additionally, Figure 6 illustrates the burning times of various samples. A correlation between the F2311 content and burning time is evident, highlighting the advantages of the preparation method. Specifically, an increase in the F2311 content results in closer component contact, shorter heat and mass transfer distances, and ultimately, shorter burning times. Notably, no noticeable difference in combustion duration was observed when the mass fraction of F2311 was raised to 5 wt%.
3.4. Sensitivity Characterization
3.5. Mechanism Analysis
4. Conclusions
Matters Needing Attention
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zimmer-Galler, R. Correlations between deflagration characteristics and surface properties of nitramine-based propellants. AIAA J. 1968, 6, 2107–2110. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, G.; Zhu, M.; Jiang, X. Constant volume combustion properties of Al/Fe2O3/RDX nanocomposite: The effects of its particle size and chemical constituents. Combust. Flame 2022, 238, 111938. [Google Scholar] [CrossRef]
- Son, S.F.; Yetter, R.A.; Yang, V. Introduction: Nanoscale Composite Energetic Materials. J. Propuls. Power 2007, 23, 643–644. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Ku, J.H.; Cho, M.H.; Cha, J.K.; Kim, J.M.; Lee, H.W.; Kim, S.H. Effects of metal oxide nanoparticles on combustion and gas-generating performance of NaN3/Al composite powders ignited using a microhotplate platform. Adv. Powder Technol. 2020, 31, 1023–1031. [Google Scholar] [CrossRef]
- Mukhtar, A.; Nasir, H.; Rashid, B. Study of zirconium and ammonium perchlorate base igniter for composite solid base bleed propellant. Int. J. Chem. Eng. Appl. 2020, 11, 29–33. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X.; Xue, Y.; Xie, R.; Liu, L. Design, fabrication and performance of enhanced reactive igniter through depositing Al/CuO nanolaminates onto NiCr micro-heater. Mod. Phys. Lett. B 2021, 35, 2140022. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M.; Hamed, A.; Mokhtar, M.; Gobara, M.; Saleh, A.; Elsaka, E.; El-Sayyad, G.S. Synergistic catalytic effect of thermite nanoparticles on HMX thermal decomposition. J. Inorg. Organomet. Polym. Mater 2021, 31, 2293–2305. [Google Scholar] [CrossRef]
- Pang, W.; Li, Y.; DeLuca, L.T.; Liang, D.; Qin, Z.; Liu, X.; Xu, H.; Fan, X. Effect of metal nanopowders on the performance of solid rocket propellants: A review. Nanomaterials 2021, 11, 2749. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Cheng, L.; Zhu, X.; Li, Y.; Song, D. Preparation and characterization of n-Al/FeF3 nanothermite. Chem. Eng. J. 2018, 331, 850–855. [Google Scholar] [CrossRef]
- Wang, J.; Cao, W.; Liu, R.; Xu, R.; Chen, X. Graphite fluoride as a new oxidizer to construct nano-Al based reactive material and its combustion performance. Combust. Flame 2021, 229, 111393. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Li, G.; Gong, F.; Liu, Y.; Yan, Q.; Yang, Z.; Nie, F. Surface fluorination of n-Al particles with improved combustion performance and adjustable reaction kinetics. Chem. Eng. J. 2021, 425, 131619. [Google Scholar] [CrossRef]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Young, G.; Wilson, D.P.; Kessler, M.; DeLisio, J.B.; Zachariah, M.R. Ignition and Combustion Characteristics of Al/RDX/NC Nanostructured Microparticles. Combust. Sci. Technol. 2021, 193, 2259–2275. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Zhao, L.; Nan, F.; Liu, J.; Wang, X.; Chen, F.; Shao, Z.; He, W. Enhancement strategy of mechanical property by constructing of energetic RDX@CNFs composites in propellants, and investigation on its combustion and sensitivity behavior. Combust. Flame 2022, 244, 112249. [Google Scholar] [CrossRef]
- Yao, J.; Li, B.; Xie, L.-F.; Peng, J. Electrospray Preparation and Properties of RDX/F2604 Composites. J. Energetic Mater. 2018, 36, 223–235. [Google Scholar] [CrossRef]
- Li, F.; Zhu, B.; Sun, Y.; Shi, W. Reaction characteristics of micron-sized Al powder with adding Al/CuO nano-thermite in heated steam. Energy Sources Recovery Util. Env. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Caicedo FM, C.; López, E.V.; Agarwal, A.; Drozd, V.; Durygin, A.; Hernandez, A.F.; Wang, C. Synthesis of graphene oxide from graphite by ball milling. Diam. Relat. Mater. 2020, 109, 108064. [Google Scholar] [CrossRef]
- Krieger, A.; Zika, A.; Gröhn, F. Functional nano-objects by electrostatic self-assembly: Structure, switching, and photocatalysis. Front. Chem. 2021, 9, 779360. [Google Scholar] [CrossRef] [PubMed]
- Kolay, S.; Bain, D.; Maity, S.; Devi, A.; Patra, A.; Antoine, R. Self-Assembled Metal Nanoclusters: Driving Forces and Structural Correlation with Optical Properties. Nanomaterials 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Review—Influence of Processing Parameters to Control Morphology and Optical Properties of Sol-Gel Synthesized ZnO Nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Marsh, B.M.; Iyer, K.; Cooks, R.G. Reaction Acceleration in Electrospray Droplets: Size, Distance, and Surfactant Effects. J. Am. Soc. Mass Spectrom. 2019, 30, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ren, H.; Liu, J.; Jiao, Q. Facile preparation and synergetic energy releasing of nano-Al@RDX@Viton hollow microspheres. Chem. Eng. J. 2020, 379, 122333. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, L.; Ke, X.; Zhang, T.; Hao, G.; Hu, Y.; Zhang, G.; Guo, H.; Jiang, W. Energetic metastable Al/CuO/PVDF/RDX microspheres with enhanced combustion performance. Chem. Eng. Sci. 2021, 231, 116302. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Huang, H.; Zhao, Y.; Song, K.; Wang, H.; Xie, W.; Cheng, Y.; Fan, X. Preparation and characterization of the Al/Fe2O3/RDX/NC nanocomposites by electrospray. J. Therm. Anal. Calorim. 2019, 137, 1615–1620. [Google Scholar] [CrossRef]
- Collard, D.N.; Fleck, T.J.; Rhoads, J.F.; Son, S.F. Tailoring the reactivity of printable Al/PVDF filament. Combust. Flame 2021, 223, 110–117. [Google Scholar] [CrossRef]
- Wang, H.; Rehwoldt, M.; Kline, D.J.; Wu, T.; Wang, P.; Zachariah, M.R. Comparison study of the ignition and combustion characteristics of directly-written Al/PVDF, Al/Viton and Al/THV composites. Combust. Flame 2019, 201, 181–186. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion in the future: The importance of chemistry. Proc. Combust. Inst. 2020, 38, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Kalal, R.K.; Jangid, S.K.; Shekhar, H.; Alegaonkar, P.S.; Kumar, A. Thermo-physical Properties and Combustion Wave Aspects of RDX Contain Low Aluminium Composite Propellant. Combust. Flame 2020, 218, 12–17. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, S.; Li, G.; Luo, Y. Effect of fluoropolymer content on thermal and combustion performance of direct writing high-solid nanothermite composite. RSC Adv. 2022, 12, 5612–5618. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.T.; Min, T.S.; Kim, S.H. Improved Energetic-Behaviors of Spontaneously Surface-Mediated Al Particles. Sci. Rep. 2017, 7, 4659. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jiang, D.; Yang, L.; Song, W.; Wang, R.; Huang, Q. Preparation of RDX/F2311/Fe2O3/Al Composite Hollow Microspheres by Electrospray and Synergistic Energy Release during Combustion between Components. Materials 2024, 17, 1623. https://doi.org/10.3390/ma17071623
Zhang Z, Jiang D, Yang L, Song W, Wang R, Huang Q. Preparation of RDX/F2311/Fe2O3/Al Composite Hollow Microspheres by Electrospray and Synergistic Energy Release during Combustion between Components. Materials. 2024; 17(7):1623. https://doi.org/10.3390/ma17071623
Chicago/Turabian StyleZhang, Zhenwei, Dong Jiang, Lanting Yang, Wenkui Song, Ruihao Wang, and Qiuan Huang. 2024. "Preparation of RDX/F2311/Fe2O3/Al Composite Hollow Microspheres by Electrospray and Synergistic Energy Release during Combustion between Components" Materials 17, no. 7: 1623. https://doi.org/10.3390/ma17071623
APA StyleZhang, Z., Jiang, D., Yang, L., Song, W., Wang, R., & Huang, Q. (2024). Preparation of RDX/F2311/Fe2O3/Al Composite Hollow Microspheres by Electrospray and Synergistic Energy Release during Combustion between Components. Materials, 17(7), 1623. https://doi.org/10.3390/ma17071623



