Use of Limestone Sludge in the Preparation of ɩ-Carrageenan/Alginate-Based Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterization of Crosslinking Solution from Limestone Sludge
2.3. Preparation of Alginate Films
2.4. Preparation of ɩ-Carrageenan/Alginate Films
2.5. Chemical Characterization by Spectroscopy Techniques
2.5.1. Inductively Coupled Plasma Optical Emission Spectroscopy
2.5.2. Nuclear Magnetic Resonance
2.5.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.6. Antifungal Assays
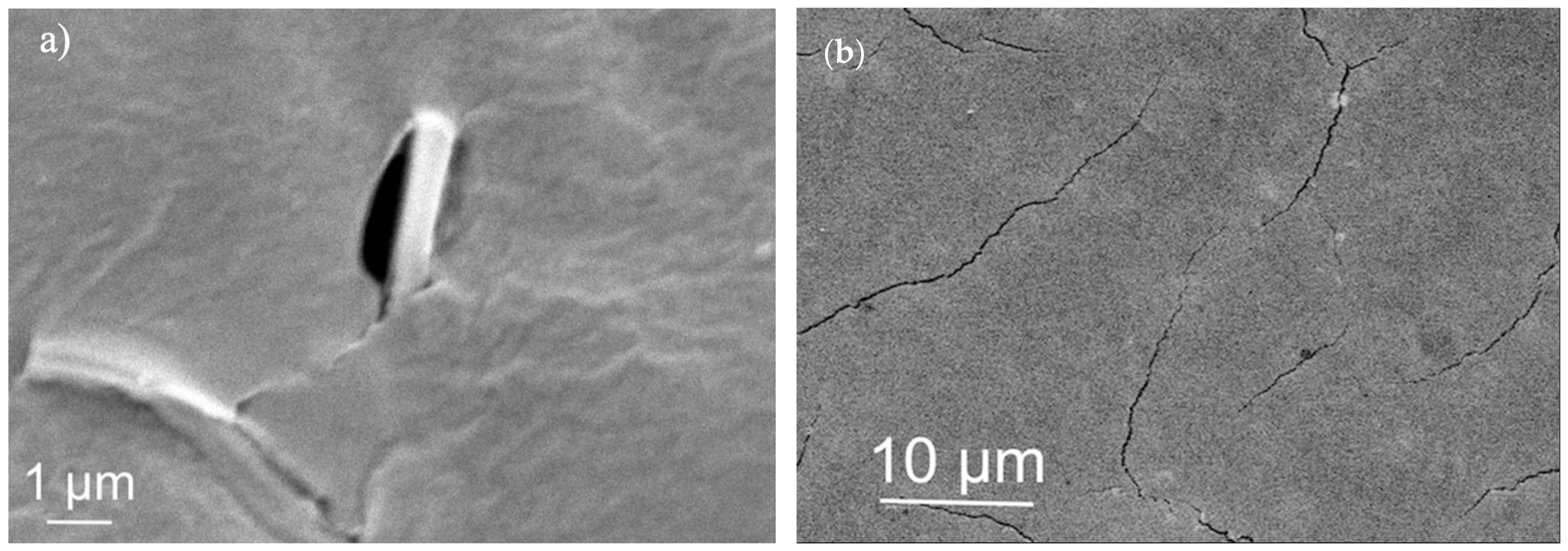
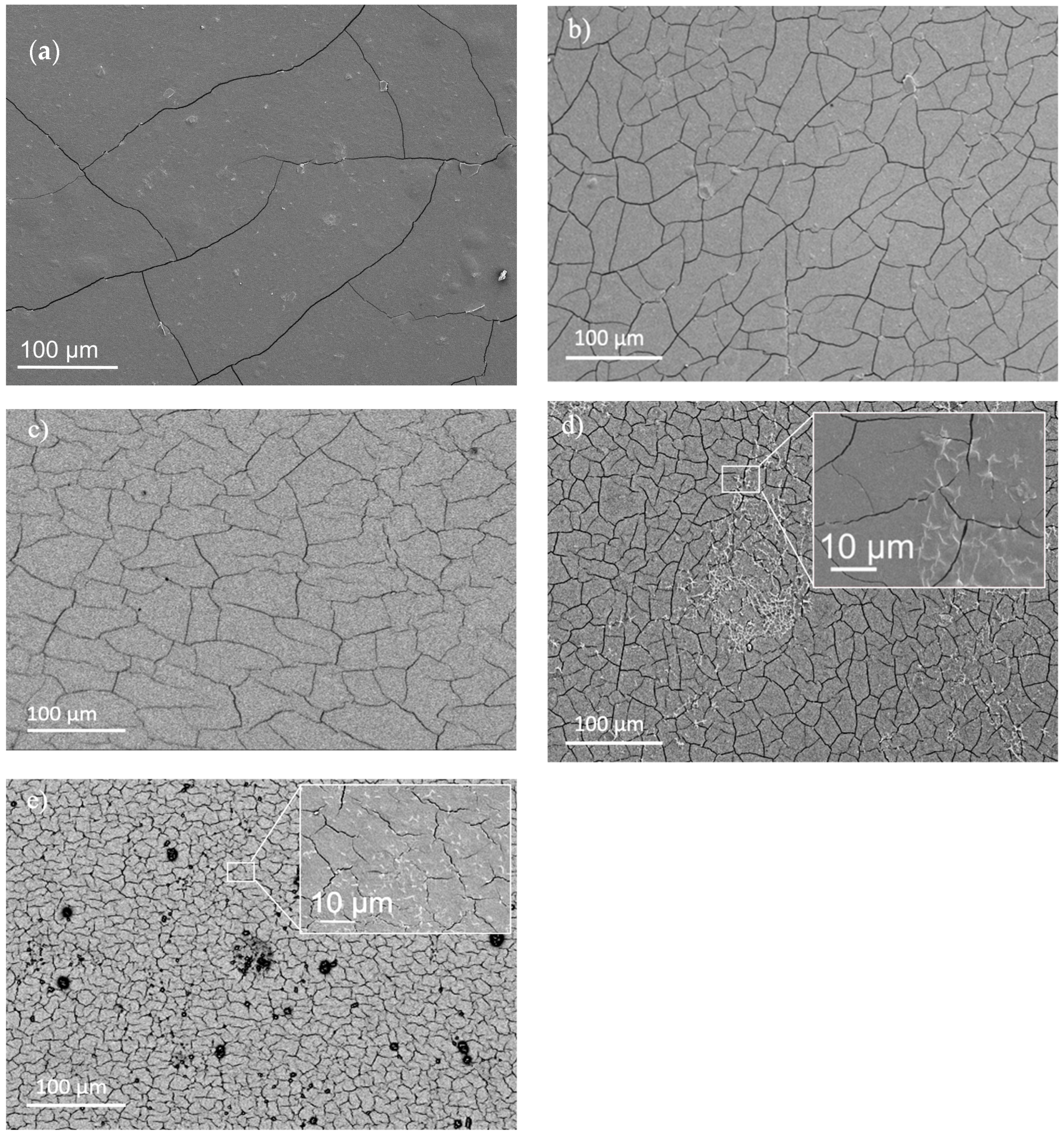
2.7. Morphologic Characterization
2.8. Characterization of Mechanical Performance
3. Results and Discussion
3.1. Composition of the Stone Sludge and of Crosslinking Solution
3.2. Characterization of the Produced Crosslinked Films
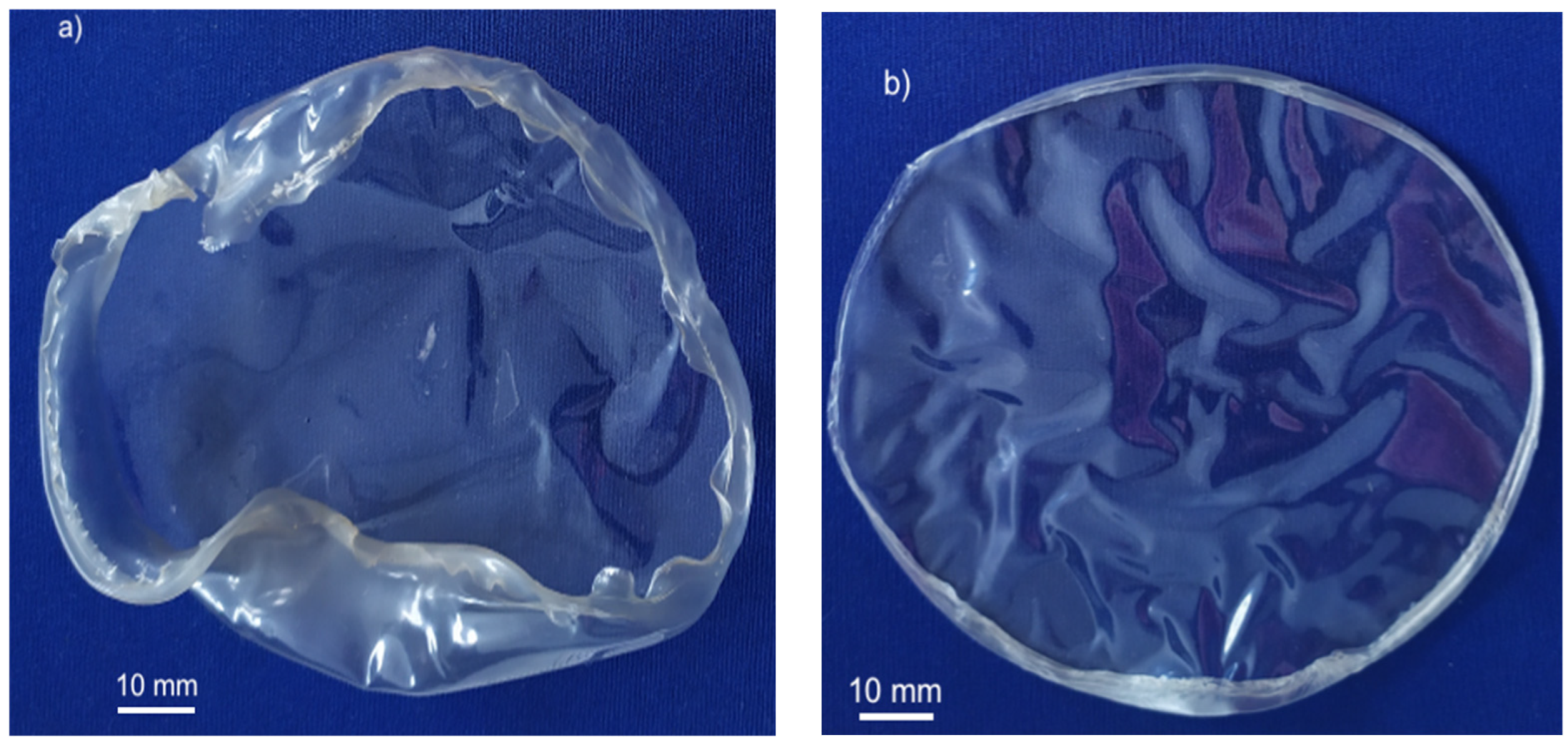
3.2.1. Alginate Films
3.2.2. Films of ɩ-Carrageenan/Alginate
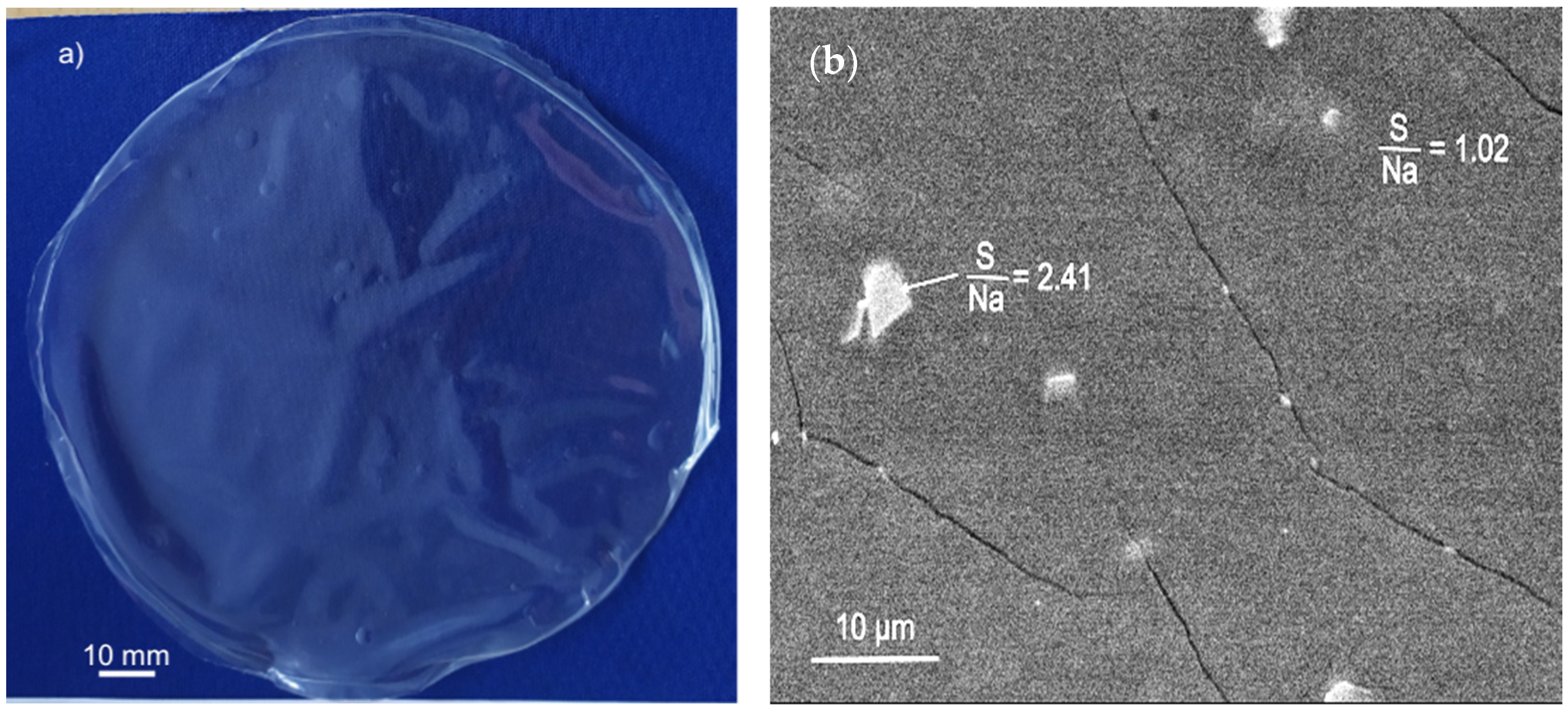
3.2.3. Compositional Analysis
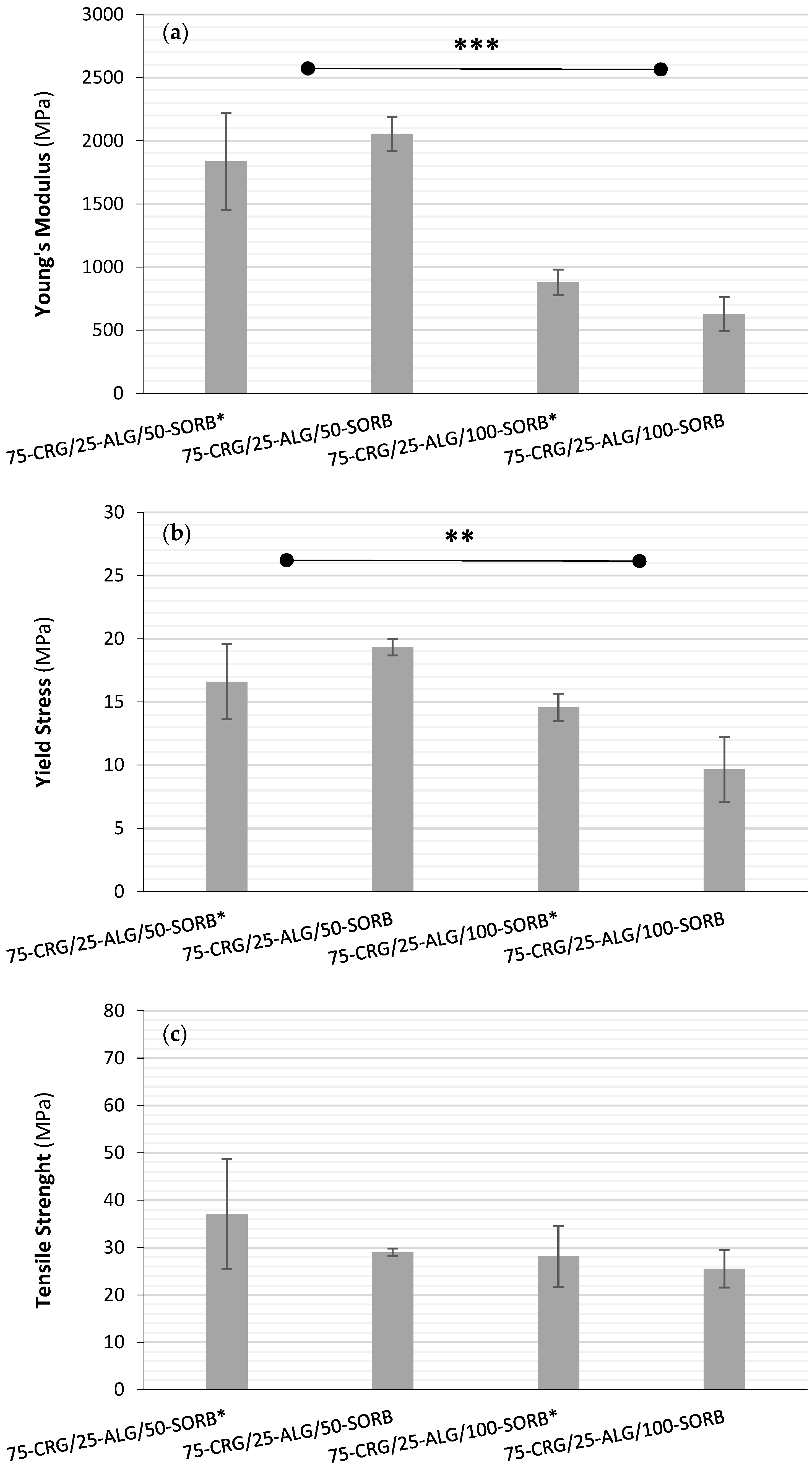
3.2.4. Films’ Mechanical Performance
3.2.5. Anti-Fungal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubatova, D.; Khongova, I.; Krejci Kotlanova, M.; Zezulova, A.; Bohac, M. The use of limestone sludge for the geopolymer preparation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1205, 012002. [Google Scholar] [CrossRef]
- Lasemi, Z.; Butler, S.; Ansari, S. Limestone Fines and Lime Sludge: From By-Product Waste to Potential Beneficial Use—A Key to Sustainability. In Illinois State Geological Survey; University of Minnesota: Minneapolis, MN, USA, 2015; pp. 51–60. [Google Scholar]
- Hart, M.L.; Shakoor, A.; Wilson, T.P. Characterization of lime sludge for engineering applications. Waste Manag. 1993, 13, 55–63. [Google Scholar] [CrossRef]
- Zichella, L.; Bellopede, R.; Spriano, S.; Marini, P. Preliminary investigations on stone cutting sludge processing for a future recovery. J. Clean. Prod. 2018, 178, 866–876. [Google Scholar] [CrossRef]
- Choudhary, J.; Kumar, B.; Gupta, A. Feasible utilization of waste limestone sludge as filter in bituminous concrete. Constr. Buiding Mater. 2020, 239, 117781. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, A.; Bhunia, D. An Investigation on Effect of Partial Replacement of Cement by Waste Marble Slurry. Constr. Build. Mater. 2017, 134, 471–488. [Google Scholar] [CrossRef]
- Alyamac, K.E.; Ghafari, E.; Ince, R. Development of Eco-Efficient Self-Compacting Concrete with Waste Marble Powder Using the Response Surface Method. J. Clean. Prod. 2017, 144, 192–202. [Google Scholar] [CrossRef]
- Munir, M.J.; Kazmi, S.M.S.; Wu, Y.-F.; Hanif, A.; Khan, M.U.A. Thermally Efficient Fired Clay Bricks Incorporating Waste Marble Sludge: An Industrial-Scale Study. J. Clean. Prod. 2018, 174, 1122–1135. [Google Scholar] [CrossRef]
- Buyuksagis, I.S.; Uygunoglu, T.; Tatar, E. Investigation on the Usage of Waste Marble Powder in Cement-Based Adhesive Mortar. Constr. Build. Mater. 2017, 154, 734–742. [Google Scholar] [CrossRef]
- Jayakody, M.; Gunathilake, M.; Wijesekara, I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: A review. J. Food Meas. Charact. 2022, 16, 1195–1227. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Champion, D.; Voilley, A. Wetting properties at the surface of iota-carrageenan-based edible films. J. Colloid Interface Sci. 2006, 294, 400–410. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Budiman, M.A.; Uju; Tarman, K. A Review on the difference of physical and mechanical properties of bioplastic from seaweed hydrocolloids with various plasticizers. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012012. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Natural polymers in bio-degradable/edible film: A review on environmental concerns, cold plasma technology and nanotechnology application on food packaging—A recent trends. Food Chem. Adv. 2022, 1, 100135. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Draget, K.I. Alginates: Properties and Applications. Polym. Sci. A Compr. Ref. 2012, 1–10, 213–220. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Maiti, S.; Kumari, L. 3—Smart Nanopolysaccharides for the Delivery of Bioactives. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 67–94. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Yu, F.; Cui, T.; Yang, C.; Dai, X.; Ma, J. κ-Carrageenan/Sodium alginate double-network hydrogel with enhanced mechanical properties, anti-swelling, and adsorption capacity. Chemosphere 2019, 237, 124417. [Google Scholar] [CrossRef] [PubMed]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Gaudin, S.; Lourdin, D.; Le Botlan, D.; Ilari, J.L.; Forssell, P.; Colonna, P. Effect of polymer-plasticizer interactions on the oxygen permeability of starch-sorbitol-water films. Macromol. Symp. 1999, 138, 245–248. [Google Scholar] [CrossRef]
- Patton, J.; Reeder, W. New Indicator for Titration of Calcium with (Ethylenedinitrilo) Tetraacetate. Anal. Chem. 1956, 28, 1026–1028. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Cross-linked alginate edible coatings incorporated with hexyl acetate: Film characteristics and its application on fresh-cut rose apple. Food Biosci. 2023, 52, 102410. [Google Scholar] [CrossRef]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; He, J.; Huang, Y. A new insight to the effect of calcium concentration on gelation process and physical properties of alginate films. J. Mater. Sci. 2016, 51, 5791–5801. [Google Scholar] [CrossRef]
- van Meerten, S.G.J.; Franssen, W.M.J.; Kentgens, A.P.M. ssNake: A cross-platform open-source NMR data processing and fitting application. J. Magn. Reson. 2019, 301, 56–66. [Google Scholar] [CrossRef]
- Sousa AM, M.; Borges, J.; Silva, F.; Ramos, A.M.; Cabrita, E.J.; Gonçalves, M.P. Shaping the molecular assemblies of native and alkali-modified agars in dilute and concentrated aqueous media via microwave-assisted extraction. Soft Matter 2013, 9, 3131. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. Enemy of the (immunosuppressed) state: An update on the pathogenesis of Aspergillus fumigatus infection. Br. J. Haematol. 2010, 150, 406–417. [Google Scholar] [CrossRef]
- Brandl, J.; Andersen, M.R. Aspergilli: Models for systems biology in filamentous fungi. Curr. Opin. Syst. Biol. 2017, 6, 67–73. [Google Scholar] [CrossRef]
- Küçük, G.S.; Çelik, Ö.F.; Mazi, B.G.; Türe, H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packag. Technol Sci. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Silva, M.A.; Iamanaka, B.T.; Taniwaki, M.H.; Kieckbusch, T.G. Evaluation of the Antimicrobial Potential of Alginate and Alginate/Chitosan Films Containing Potassium Sorbate and Natamycin. Packag. Technol. Sci. 2013, 26, 479–492. [Google Scholar] [CrossRef]
- Scherer, G.W. Drying gels: II. Film and flat plate. J. Non-Cryst. Solids 1987, 89, 217–238. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Time dependent gelling properties of cuboid alginate gels made by external gelation method: Effects of alginate-CaCl2 solution ratios and pH. Food Hydrocoll. 2019, 90, 232–240. [Google Scholar] [CrossRef]
- Reed, J.S. Principles of Ceramics Processing; Wiley Interscience: Hoboken, NJ, USA, 1995. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Reinecke, H.; Navarro, R.; Pérez, M. Plasticizers. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2011; pp. 498–525. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef]
- Janaswamy, S.; Chandrasekaran, R. Effect of calcium ions on the organization of iota-carrageenan helices: An X-ray investigation. Carbohydr. Res. 2002, 337, 523–535. [Google Scholar] [CrossRef]
- Espíndola, S.P.; Norder, B.; Koper, G.J.M.; Picken, S.J. The Glass Transition Temperature of Heterogeneous Biopolymer Systems. Biomacromolecules 2023, 24, 1627–1637. [Google Scholar] [CrossRef]
- Vinceković, M.; Pustak, A.; Tušek-Božić, L.; Liu, F.; Ungar, G.; Bujan, M.; Šmit, I.; Filipović-Vinceković, N. Structural and thermal study of mesomorphic dodecylammonium carrageenates. J. Colloid Interface Sci. 2010, 341, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Correia, N.T.; Alvarez, C.; Moura Ramos, J.J.; Descamps, M. The β−α Branching in d-Sorbitol as Studied by Thermally Stimulated Depolarization Currents (TSDC). J. Phys. Chem. B 2001, 105, 5663–5669. [Google Scholar] [CrossRef]
- Michon, C. Gelatin/iota-carrageenan interactions in non-gelling conditions. Food Hydrocoll. 2000, 14, 203–208. [Google Scholar] [CrossRef]
- Kindle, E.M. Some Factors Affecting the Development of Mud-Cracks. J. Geol. 1917, 25, 135–144. [Google Scholar] [CrossRef]
- Beattie, J.; Kelso, M. Equilibrium and dynamics of the binding of calcium ion to sorbitol (D-glucitol). Aust. J. Chem. 1981, 34, 2563. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte Polysaccharide–Gelatin Complexes: Rheology and Structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Nunes, M.C.; Raymundo, A.; Gouveia, L.; Sousa, I.; Cordobés, F.; Guerrero, A.; Franco, J.M. Microalgae biomass interaction in biopolymer gelled systems. Food Hydrocoll. 2011, 25, 817–825. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, PL, USA, 2018. [Google Scholar]
- Mohammed, A.; Gaduan, A.; Chaitram, P.; Pooran, A.; Lee, K.-Y.; Ward, K. Sargassum inspired, optimized calcium alginate bioplastic composites for food packaging. Food Hydrocoll. 2023, 135, 108192. [Google Scholar] [CrossRef]
- Salisu, A.; Sanagi, M.M.; Naim, A.; Wan Ibrahim, W.A.; Abd Karim, K.J. Removal of lead ions from aqueous solutions using sodium alginate-graft-poly(methyl methacrylate) beads. Desalination Water Treat. 2015, 57, 15353–15361. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–Carboxylate Interactions in Metal–Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Kacurakova, M.; Wilson, R. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Rosa, A.D. Synthesis and Characterization of Carboxymethyl Derivatives of Kappa-Carrageenan. Carbohydr. Polym. 2012, 87, 1810. [Google Scholar] [CrossRef]
- Jumaah, F.N.; Mobarak, N.N.; Ahmad, A.; Ghani, M.A.; Rahman, M.Y.A. Derivative of Iota-Carrageenan as Solid Polymer Electrolyte. Ionics 2015, 21, 1311. [Google Scholar] [CrossRef]
- Paula, G.A.; Benevides, N.M.B.; Cunha, A.P.; de Oliveira, A.V.; Pinto, A.M.B.; Morais, J.P.S.; Azeredo, H.M.C. Development and characterization of edible films from mixtures of κ-carrageenan, ɩ-carrageenan, and alginate. Food Hydrocoll. 2015, 47, 140–145. [Google Scholar] [CrossRef]
- de Castro, E.d.S.G.; Cassella, R.J. Direct determination of sorbitol and sodium glutamate by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) in the thermostabilizer employed in the production of yellow-fever vaccine. Talanta 2016, 152, 33–38. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Kokova, V.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Murdjeva, M.; Apostolova, E. Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Mar. Drugs 2023, 21, 245. [Google Scholar] [CrossRef]
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR Characterization of Alginates from the Main Alginophyte Species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C High Resolution NMR Spectroscopy of Carrageenans: Application in Research and Industry. Trends Food Sci. Technol. 2002, 13, 73. [Google Scholar] [CrossRef]
- Dyrby, M.; Petersen, R.V.; Larsen, J.; Rudolf, B.; Nørgaard, L.; Engelsen, S.B. Towards On-Line Monitoring of the Composition of Commercial Carrageenan Powders. Carbohydrate. Polymers 2004, 57, 337. [Google Scholar] [CrossRef]
- van de Velde, F.; Rollema, H.S. High Resolution NMR of Carrageenans. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1605–1610. [Google Scholar] [CrossRef]
- Sánchez, R.A.R.; Matulewicz, M.C.; Ciancia, M. NMR Spectroscopy for Structural Elucidation of Sulfated Polysaccharides from Red Seaweeds. Int. J. Biol. Macromol. 2022, 199, 386. [Google Scholar] [CrossRef]
- Barbut, S.; Harper, B.A. Dried Ca-alginate films: Effects of glycerol, relative humidity, soy fibers, and carrageenan. LWT 2019, 103, 260–265. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Effect of alginate and λ-carrageenan on tensile properties and water vapour permeability of sodium caseinate-lipid based films. Carbohydr. Polym. 2008, 74, 419–426. [Google Scholar] [CrossRef]
- Santana, A.A.; Kieckbusch, T.G. Physical evaluation of biodegradable films of calcium alginate plasticized with polyols. Braz. J. Chem. Eng. 2013, 30, 835–845. [Google Scholar] [CrossRef]
- Cha, D.S.; Choi, J.H.; Chinnan, M.S.; Park, H.J. Antimicrobial films based on Na-alginate and κ-carrageenan. LWT 2002, 35, 715–719. [Google Scholar] [CrossRef]
- Teles, M.; Adão, P.; Afonso, C.; Bernardino, R.; Guedes, M.; Baptista, R.; Bernardino, S. Development and Characterization of Films for Food Application Incorporating Porphyran Extracted from Porphyra dioica. Coatings 2022, 12, 148. [Google Scholar] [CrossRef]
- Baptista, R.S.; Teles, M.; Adão, P.; Afonso, C.; Bernardino, R.; Bernardino, S.; Ferro, A.C.; Elias, S.; Guedes, M. Morphological and Mechanical Characterization of Films Incorporating Porphyran Extracted from Porphyra Dioica. Coatings 2022, 12, 1720. [Google Scholar] [CrossRef]
- Soares, F.; Trovão, J.; Gil, F.; Catarino, L.; Tiago, I.; Portugal, A.; Cardoso, S.M. Potential Use of Carrageenans against the Limestone Proliferation of the Cyanobacterium Parakomarekiella sesnandensis. Appl. Sci. 2021, 11, 10589. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]


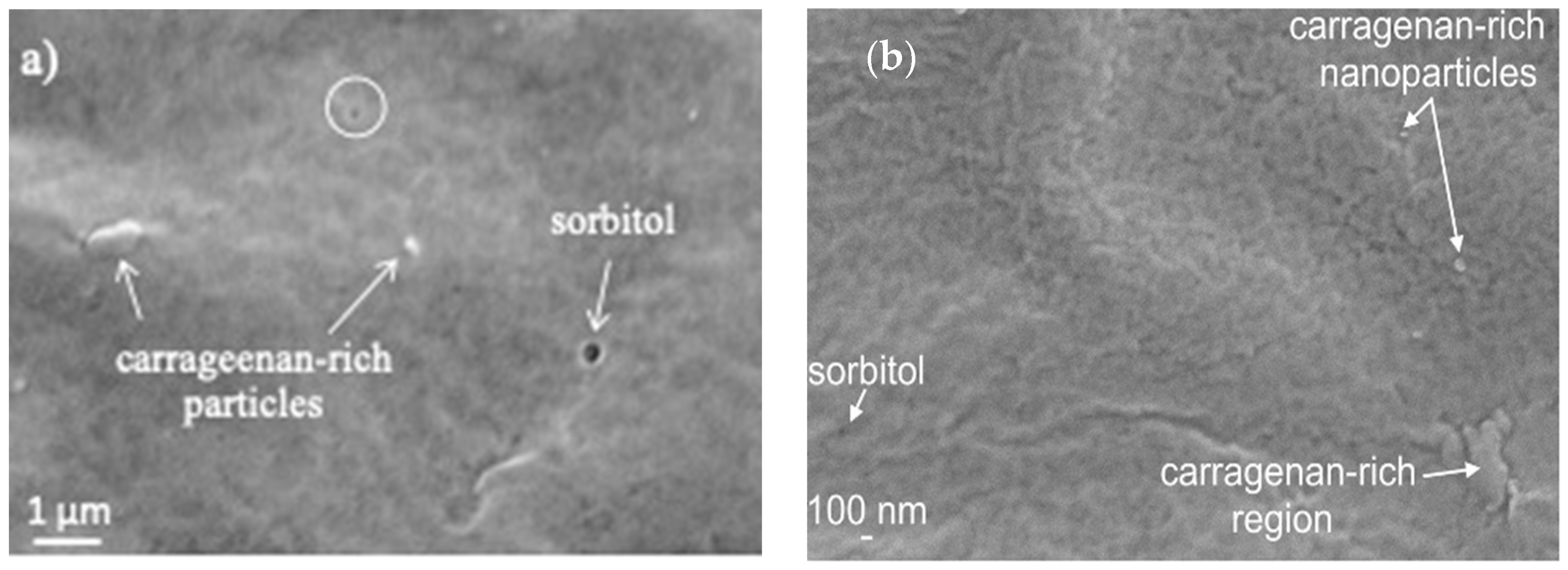
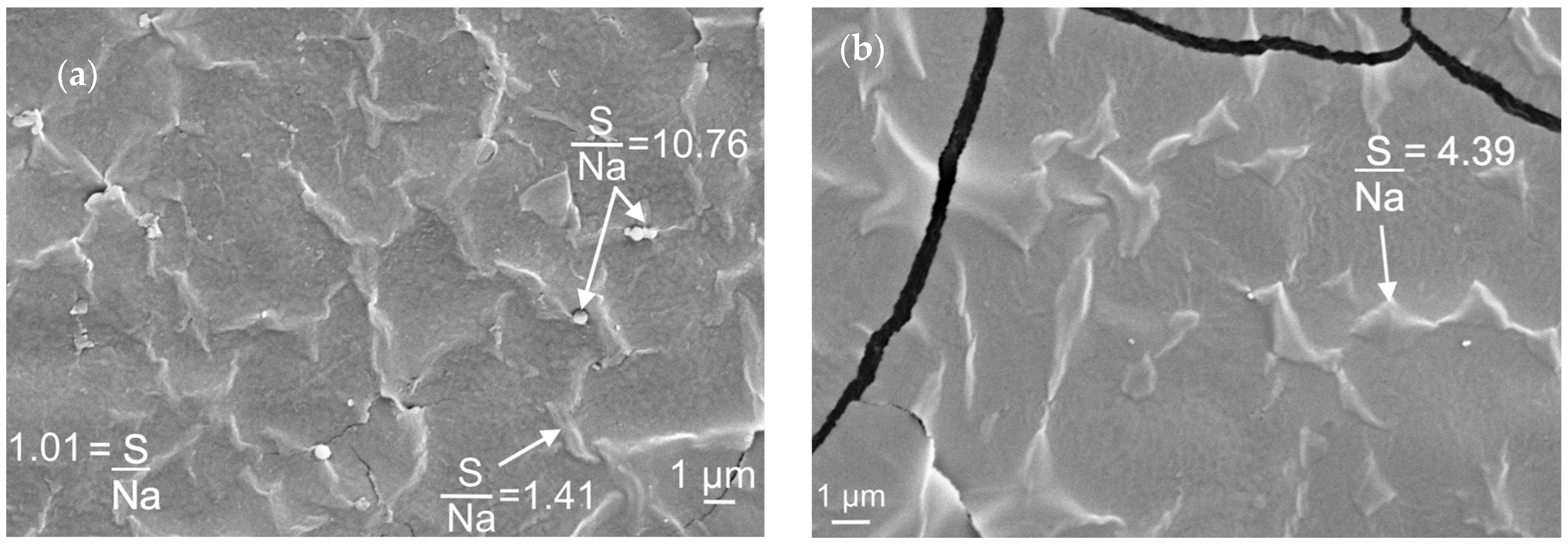
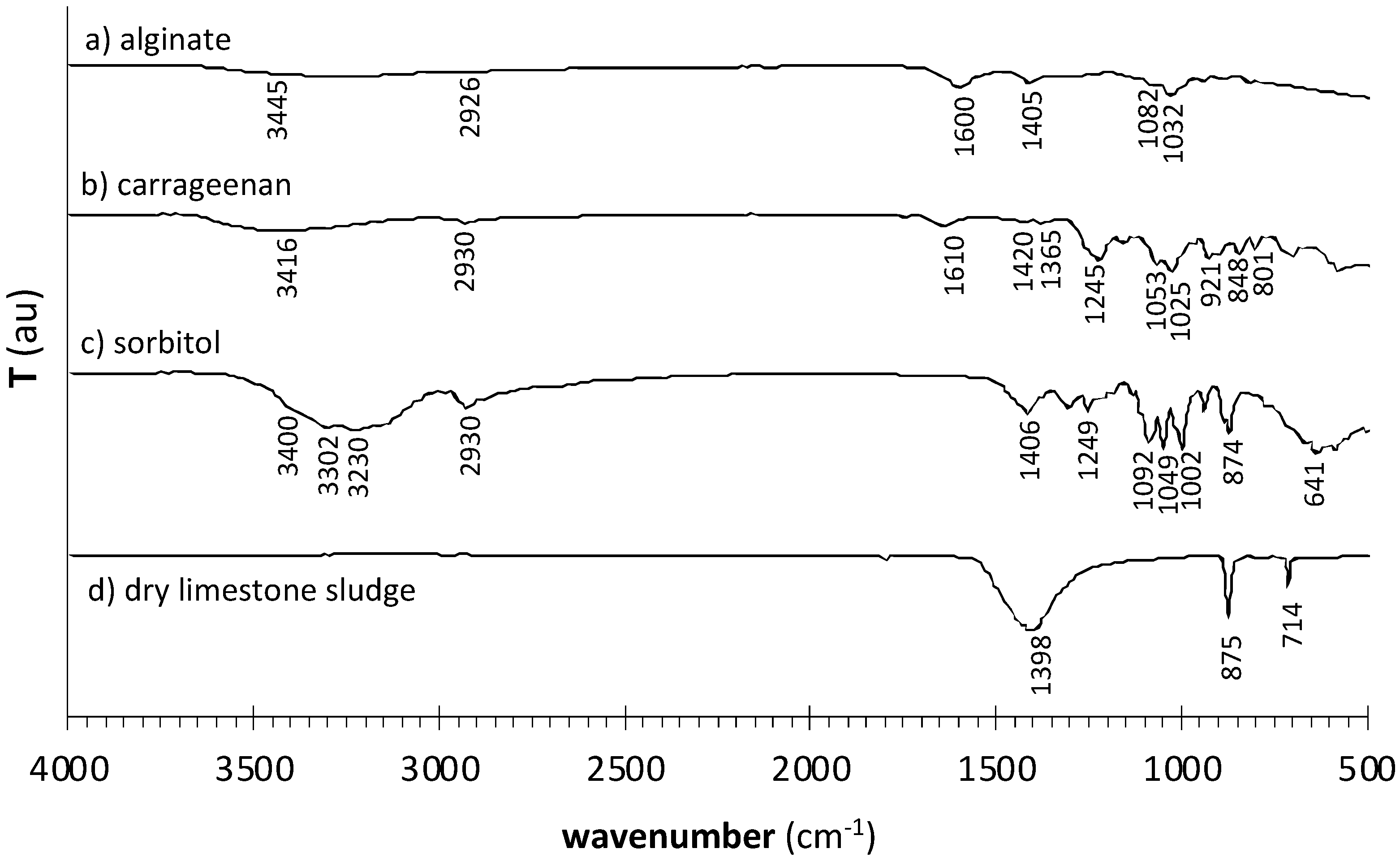
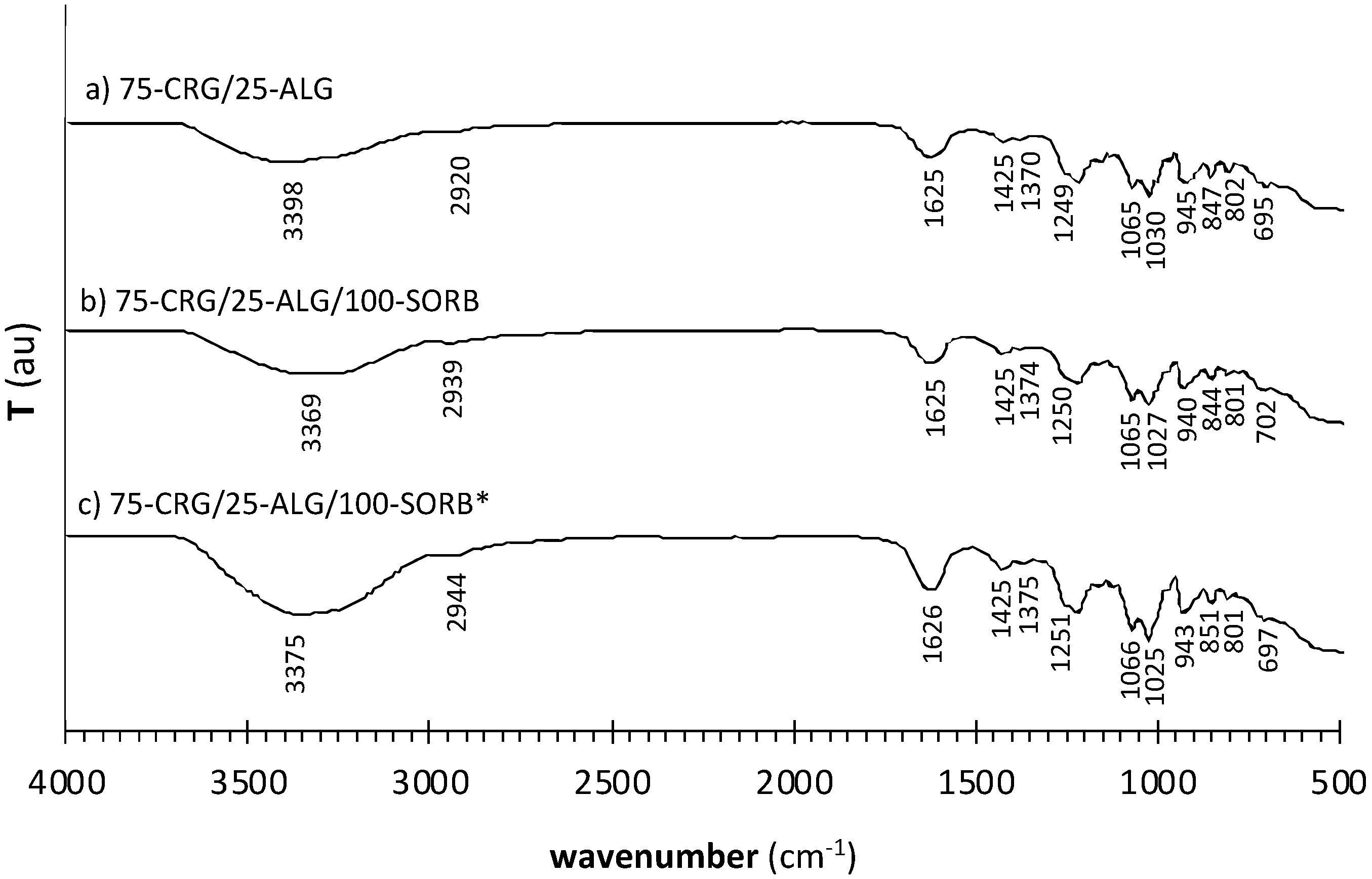
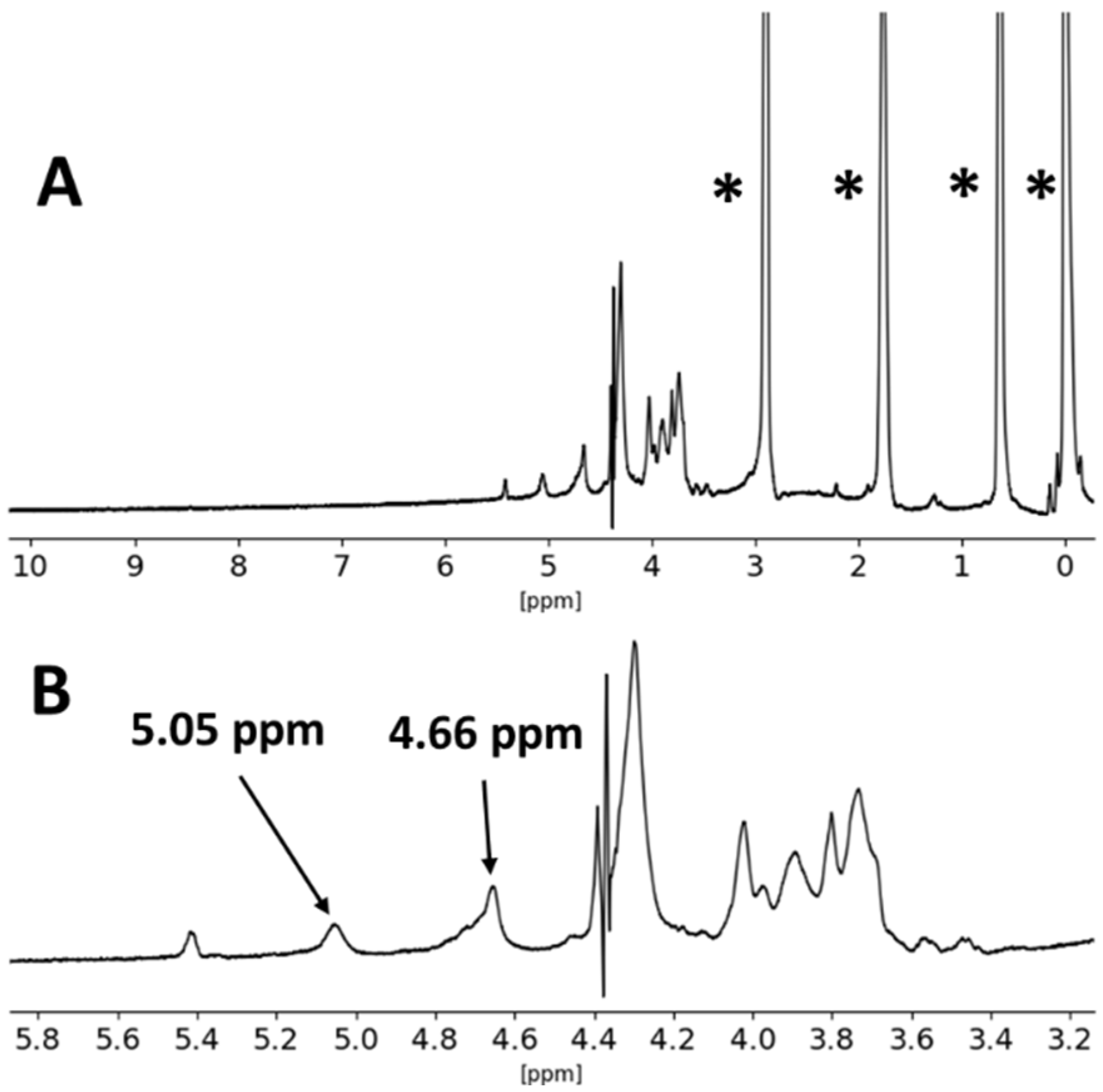
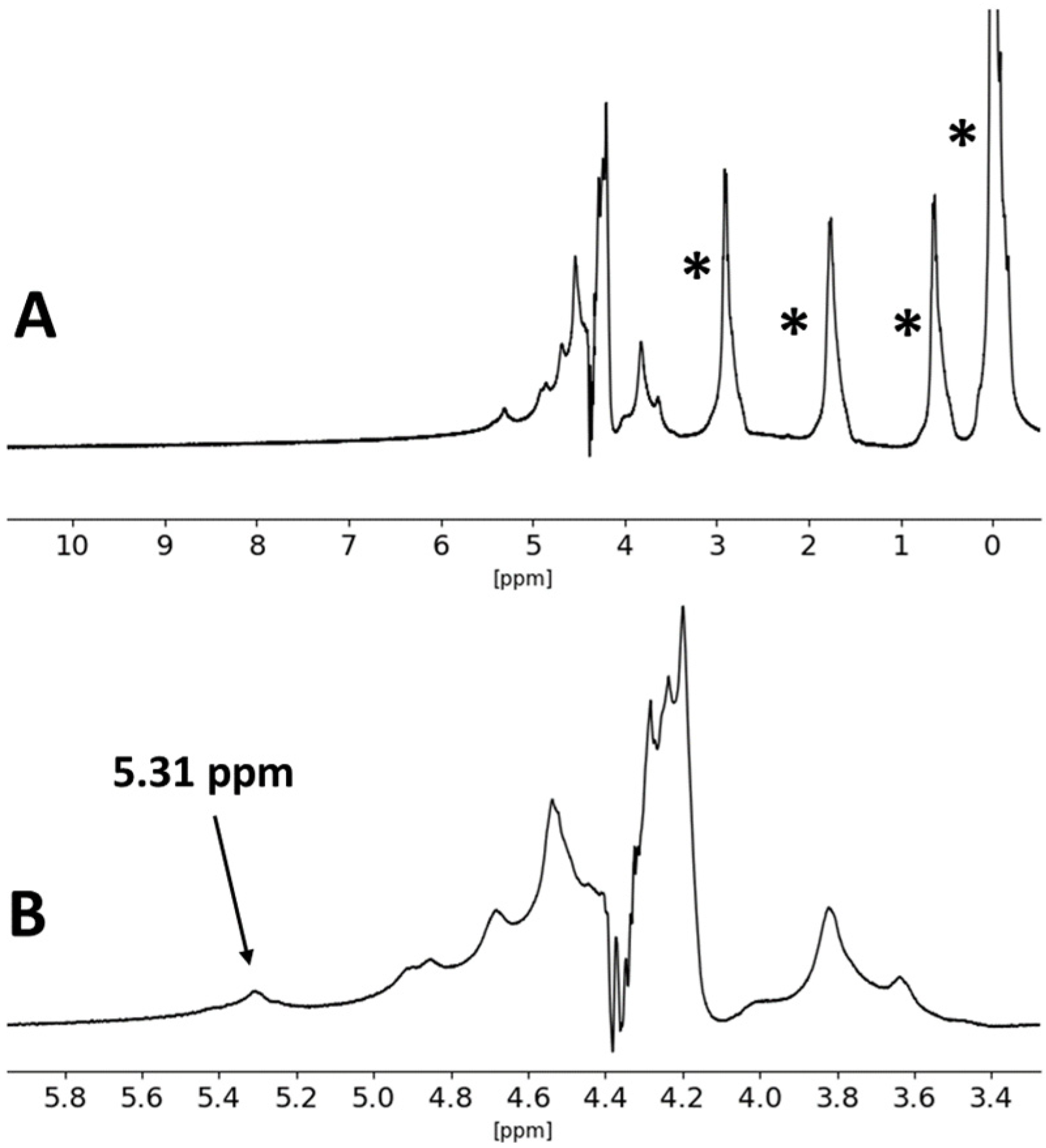


| Formulation | Crosslinking Solution | ALG/Spolysach (Weight Ratio) | CRG/Spolysach (Weight Ratio) | SORB/Spolysach (Weight Ratio) |
|---|---|---|---|---|
| 100-ALG/100-SORB * | Previous hydration Ca2+ + sorbitol | 100 | 0 | 100 |
| Ca2+ + sorbitol | 100 | 0 | 100 | |
| 75-CRG/25-ALG | Ca2+ | 75 | 25 | 0 |
| 75-CRG/25-ALG/50-SORB | Ca2+ | 75 | 25 | 50 |
| 75-CRG/25-ALG/100-SORB | Ca2+ | 75 | 25 | 100 |
| 75-CRG/25-ALG/50-SORB * | Ca2+ + sorbitol | 75 | 25 | 50 |
| 75-CRG/25-ALG/100-SORB * | Ca2+ + sorbitol | 75 | 25 | 100 |
| Formulation | Film Thickness (µm) | Linear Retraction (Radial Direction) (%) | Polygon Average Size (µm) |
|---|---|---|---|
| 75-CRG/25-ALG | 44.7 ± 5.0 | 14.2 ± 4.9 | 205.7 ± 124.0 |
| 75-CRG/25-ALG/50-SORB | 50.8 ± 7.6 | 8.3 ± 2.4 | 54.4 ± 22.0 |
| 75-CRG/25-ALG/100-SORB | 52.1 ± 5.8 | 5.1 ± 2.5 | 38.8 ± 11.1 |
| 75-CRG/25-ALG/50-SORB * | 48.1 ± 5.2 | 4.4 ± 1.9 | 25.6 ± 10.1 |
| 75-CRG/25-ALG/100-SORB * | 55.0 ± 4.2 | 3.5 ± 2.1 | 14.4 ± 4.9 |
| Band Maximum Position (cm−1) | FWHM (cm−1) | T (%) | |
|---|---|---|---|
| Na-alginate | 3445 | 352 ± 6 | 7.6 ± 0.3 |
| ɩ-carrageenan | 3416 | 340 ± 1 | 9.4 ± 0.8 |
| D-(-)sorbitol | 3400 | 364 ± 3 | 34.0 ± 1.8 |
| 75-CRG/25-ALG | 3398 | 415 ± 9 | 24.2 ± 0.9 |
| 75-CRG/25-ALG/100-SORB | 3369 | 424 ± 5 | 22.6 ± 1.0 |
| 75-CRG/25-ALG/100-SORB * | 3375 | 425 ± 3 | 46.8 ± 1.4 |
| ALG | CRG | SORB | CRG/ALG | CRG/ALG/SORB | CRG/ALG/SORB* | Vibration Assignment |
|---|---|---|---|---|---|---|
| 3445 | 3416 | 3400 | 3378 | 3369 | 3371 | O–H stretch |
| 2926 | 2930 | 2930 | 2920 | 2916 | 2914 | C–H asymmetric stretch of alkanes |
| 1600 | 1625 | 1626 | 1626 | O–C–O asymmetric stretch of –COO− (alginate) | ||
| 1610 | Water deformation | |||||
| 1405 | 1450 | 1450 | 1450 | O–C–O symmetric stretch of –COO− (alginate) | ||
| 1406 | Out-of-plane bend of O–H of C–OH linkage | |||||
| 1245 | 1249 | 1250 | 1251 | S=O stretch of ester sulfate | ||
| 1249 | In-plane bend of O–H of C–OH linkage | |||||
| 1082 | Asymmetric stretch of C–O of alcohol | |||||
| 1082 | C–O–C stretch | |||||
| 1053 | 1065 | 1072 | 1066 | C–O–C stretch of 3,6-anhydrogalactose | ||
| 1032 | 1030 | 1027 | 1025 | C–O stretch | ||
| 921 | 922 | 920 | 931 | C–O stretch of 3,6-anhydrogalactose | ||
| 848 | 847 | 844 | 851 | C–O-SO3 stretch of sulfate on C-4 of the galactose | ||
| 801 | C–O-SO3 stetch of sulfate on C-2 of the 3,6-anhydrogalactose |
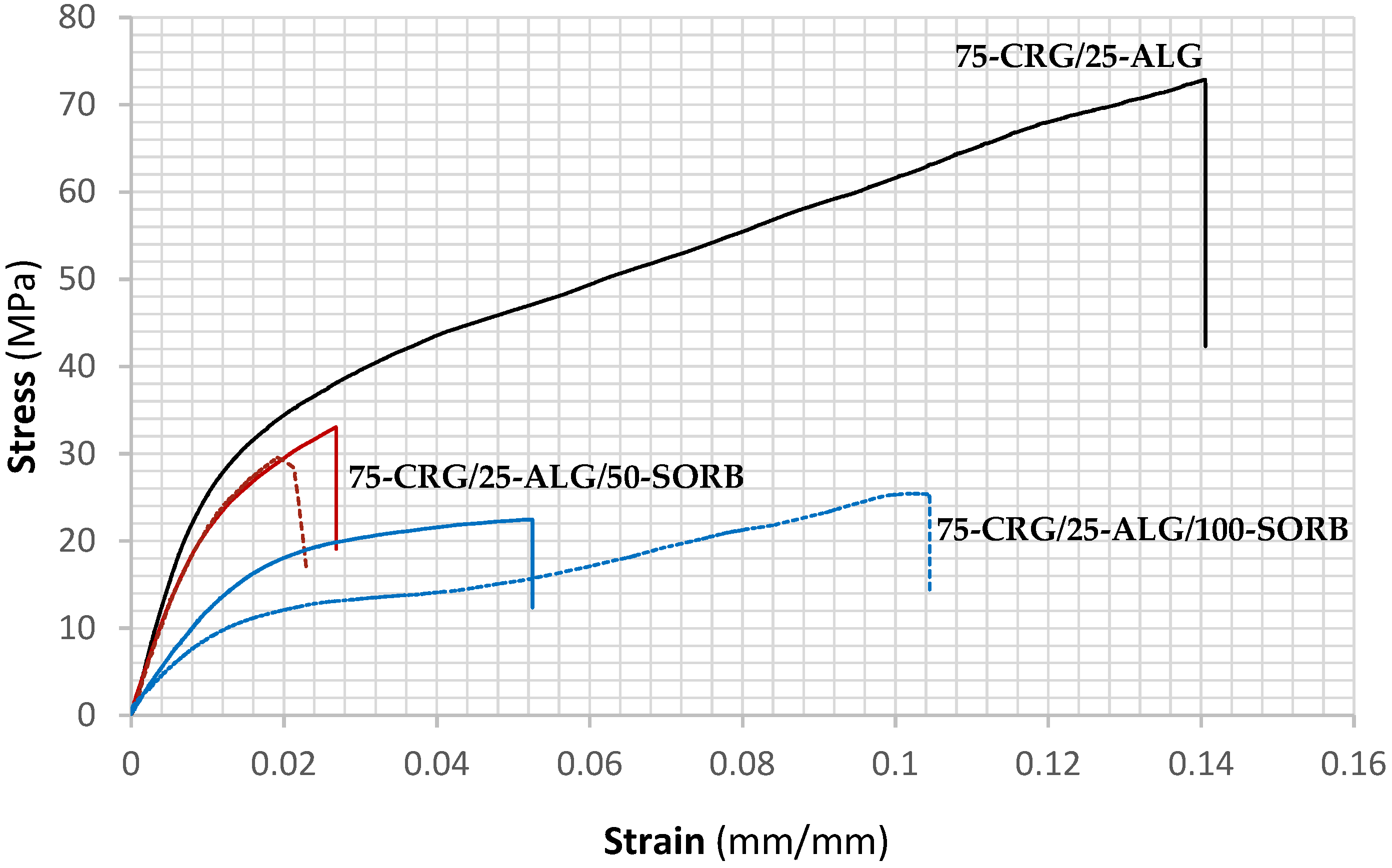
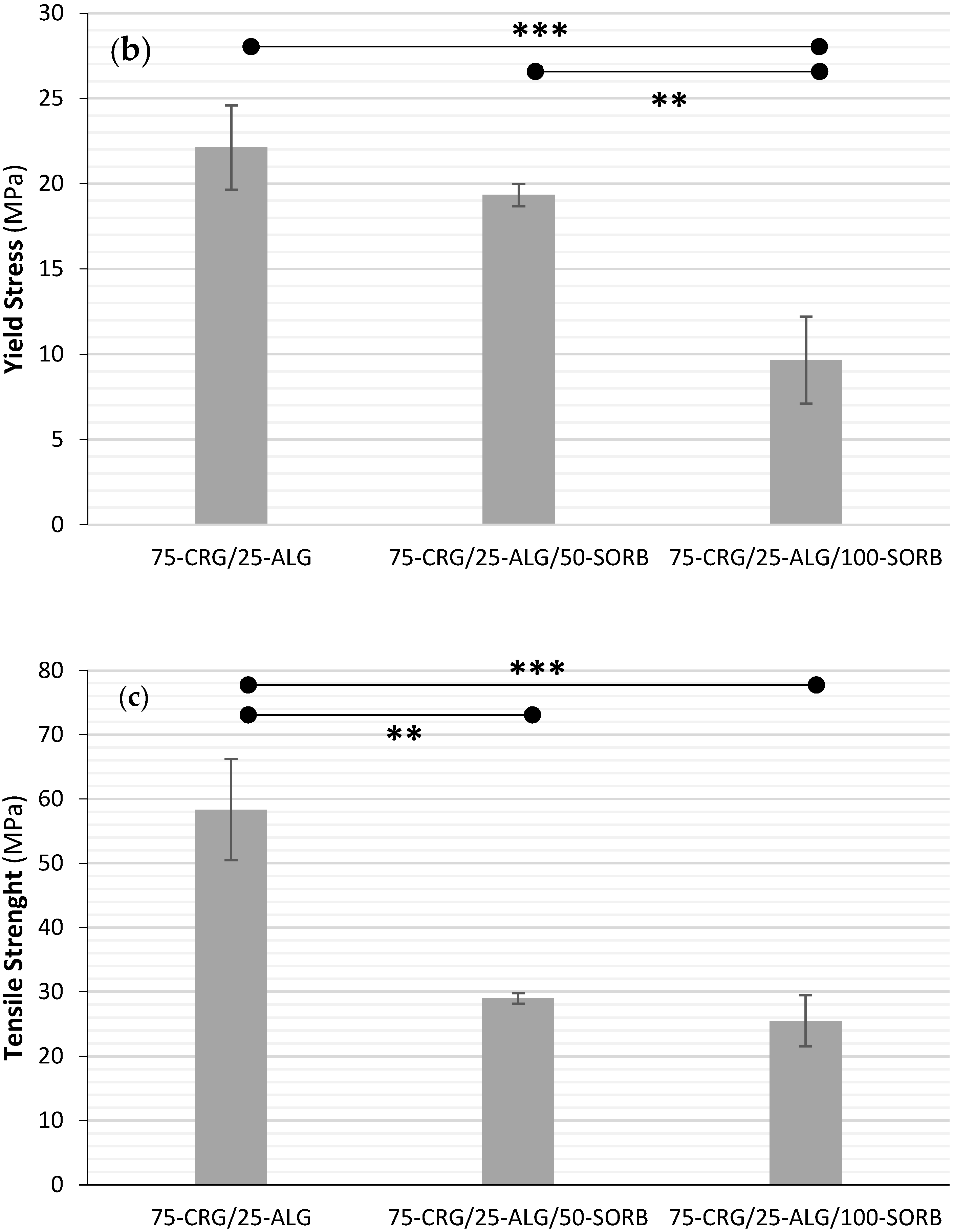
| Film Designation | Young’s Modulus (MPa) | Yield Stress (MPa) | Tensile Strength (MPa) | Fracture Strain (%) |
|---|---|---|---|---|
| 75-CRG/25-ALG | 2430 ± 343 | 22.1 ± 2.5 | 58.3 ± 7.9 | 12.6 ± 3.0 |
| 75-CRG/25-ALG/50-SORB | 2056 ± 135 | 19.3 ± 0.7 | 29.0 ± 0.8 | 1.8 ± 0.5 |
| 75-CRG/25-ALG/100-SORB | 627 ± 134 | 9.7 ± 2.5 | 25.5 ± 3.9 | 12.7 ± 4.2 |
| 75-CRG/25-ALG/50-SORB * | 1837 ± 386 | 16.6 ± 3.0 | 37.1 ± 11.6 | 9.2 ± 4.1 |
| 75-CRG/25-ALG/100-SORB * | 879 ± 101 | 14.6 ± 1.1 | 28.2 ± 6.4 | 14.7 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adão, P.; Calado, M.d.L.; Fernandes, W.; Alves, L.G.; Côrte-Real, L.; Guedes, M.; Baptista, R.; Bernardino, R.; Gil, M.M.; Campos, M.J.; et al. Use of Limestone Sludge in the Preparation of ɩ-Carrageenan/Alginate-Based Films. Materials 2024, 17, 1668. https://doi.org/10.3390/ma17071668
Adão P, Calado MdL, Fernandes W, Alves LG, Côrte-Real L, Guedes M, Baptista R, Bernardino R, Gil MM, Campos MJ, et al. Use of Limestone Sludge in the Preparation of ɩ-Carrageenan/Alginate-Based Films. Materials. 2024; 17(7):1668. https://doi.org/10.3390/ma17071668
Chicago/Turabian StyleAdão, Pedro, Maria da Luz Calado, Wilson Fernandes, Luís G. Alves, Leonor Côrte-Real, Mafalda Guedes, Ricardo Baptista, Raul Bernardino, Maria M. Gil, Maria Jorge Campos, and et al. 2024. "Use of Limestone Sludge in the Preparation of ɩ-Carrageenan/Alginate-Based Films" Materials 17, no. 7: 1668. https://doi.org/10.3390/ma17071668
APA StyleAdão, P., Calado, M. d. L., Fernandes, W., Alves, L. G., Côrte-Real, L., Guedes, M., Baptista, R., Bernardino, R., Gil, M. M., Campos, M. J., & Bernardino, S. (2024). Use of Limestone Sludge in the Preparation of ɩ-Carrageenan/Alginate-Based Films. Materials, 17(7), 1668. https://doi.org/10.3390/ma17071668










