The Influence of Precipitated Particles on the Grain Size in Cold-Rolled Al–Mn Alloy Foils upon Annealing at 100–550 °C
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Experimental Methods and Data Analyses
3. Results and Discussion
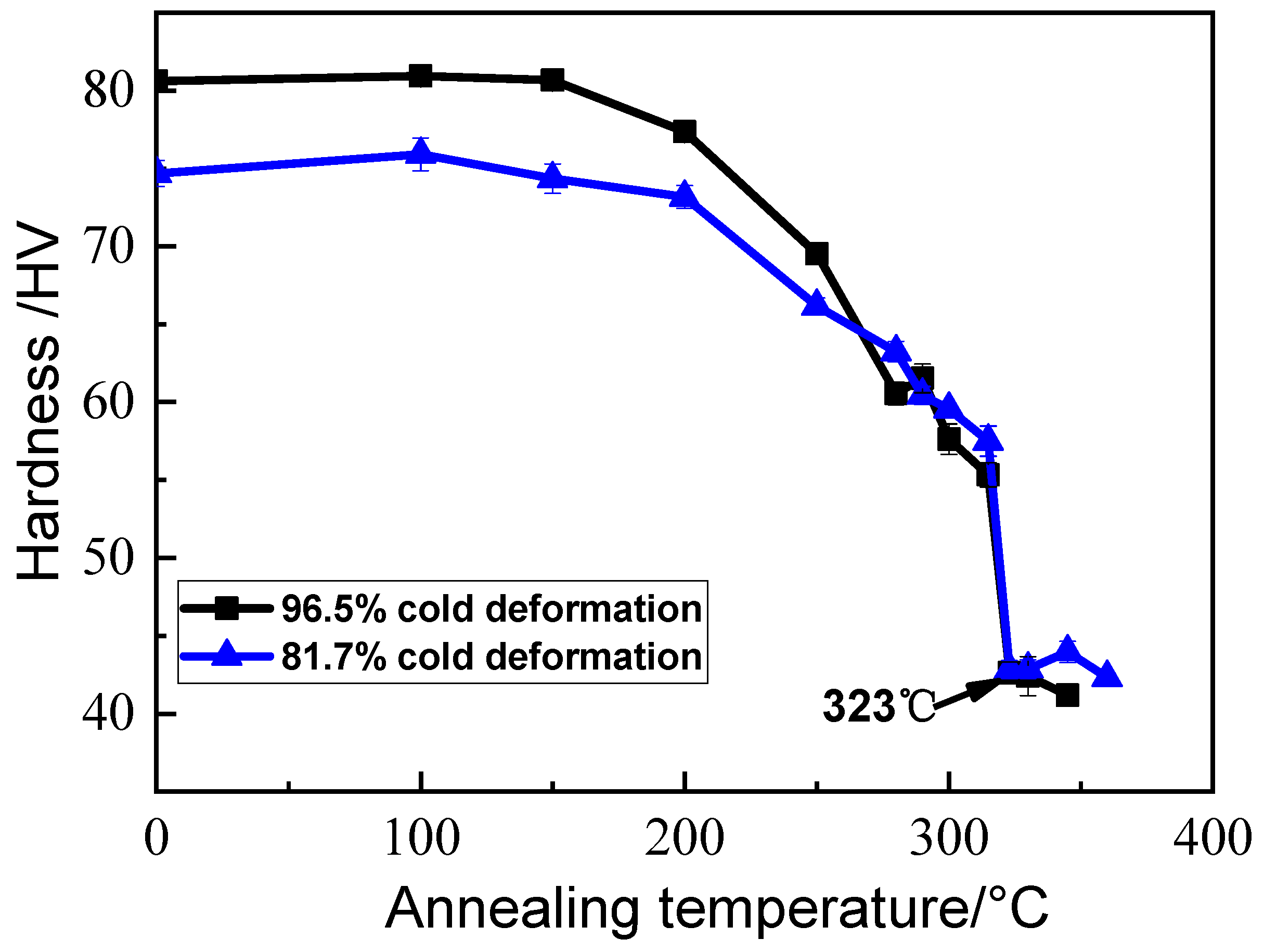
3.1. Micro-Hardness Measurement of Alloys Annealed at 100–360 °C
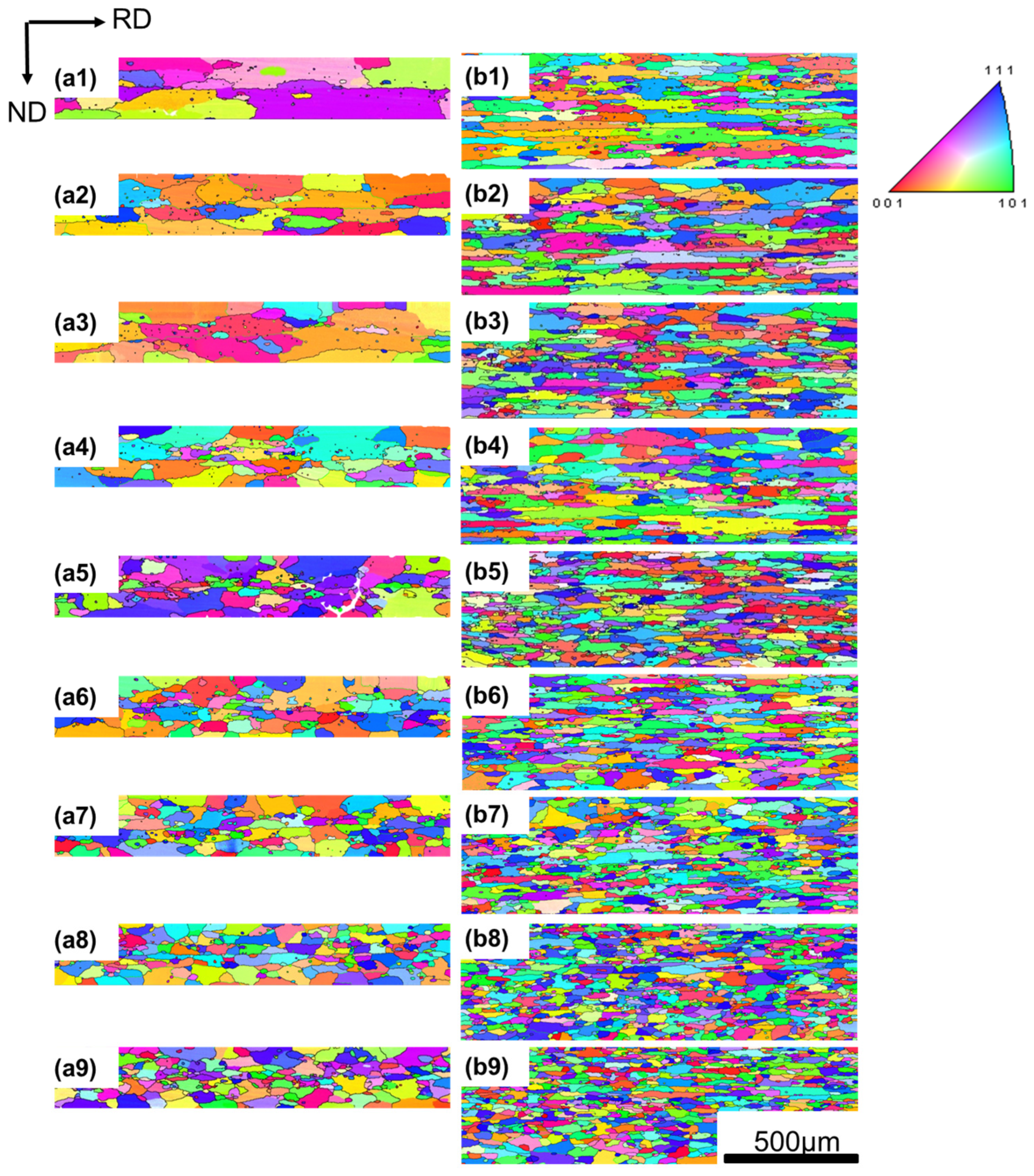
3.2. Grain Size Change with Annealing Temperature
3.3. Abnormal Relationship between Recrystallized Grain Sizes and Temperature
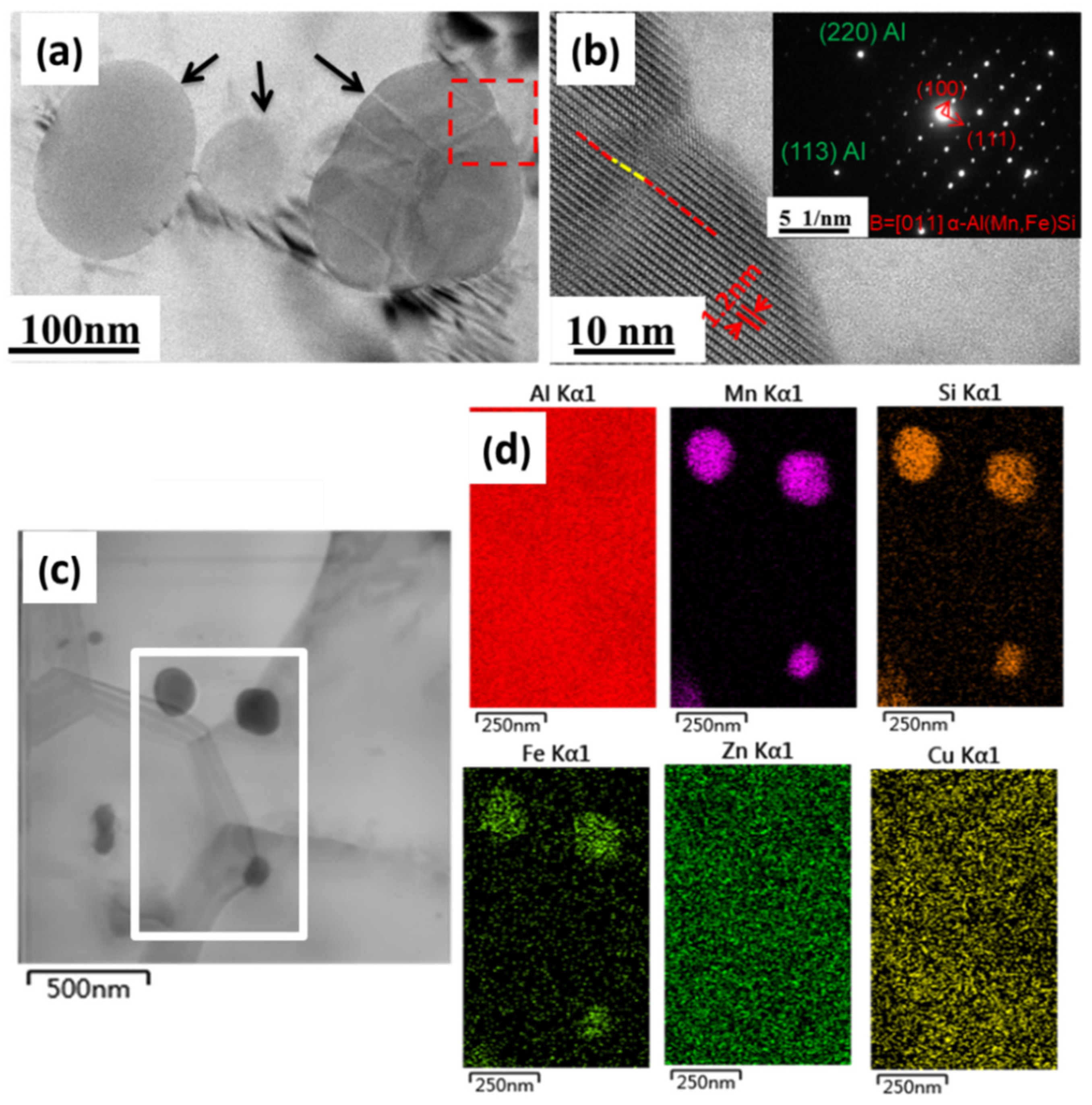
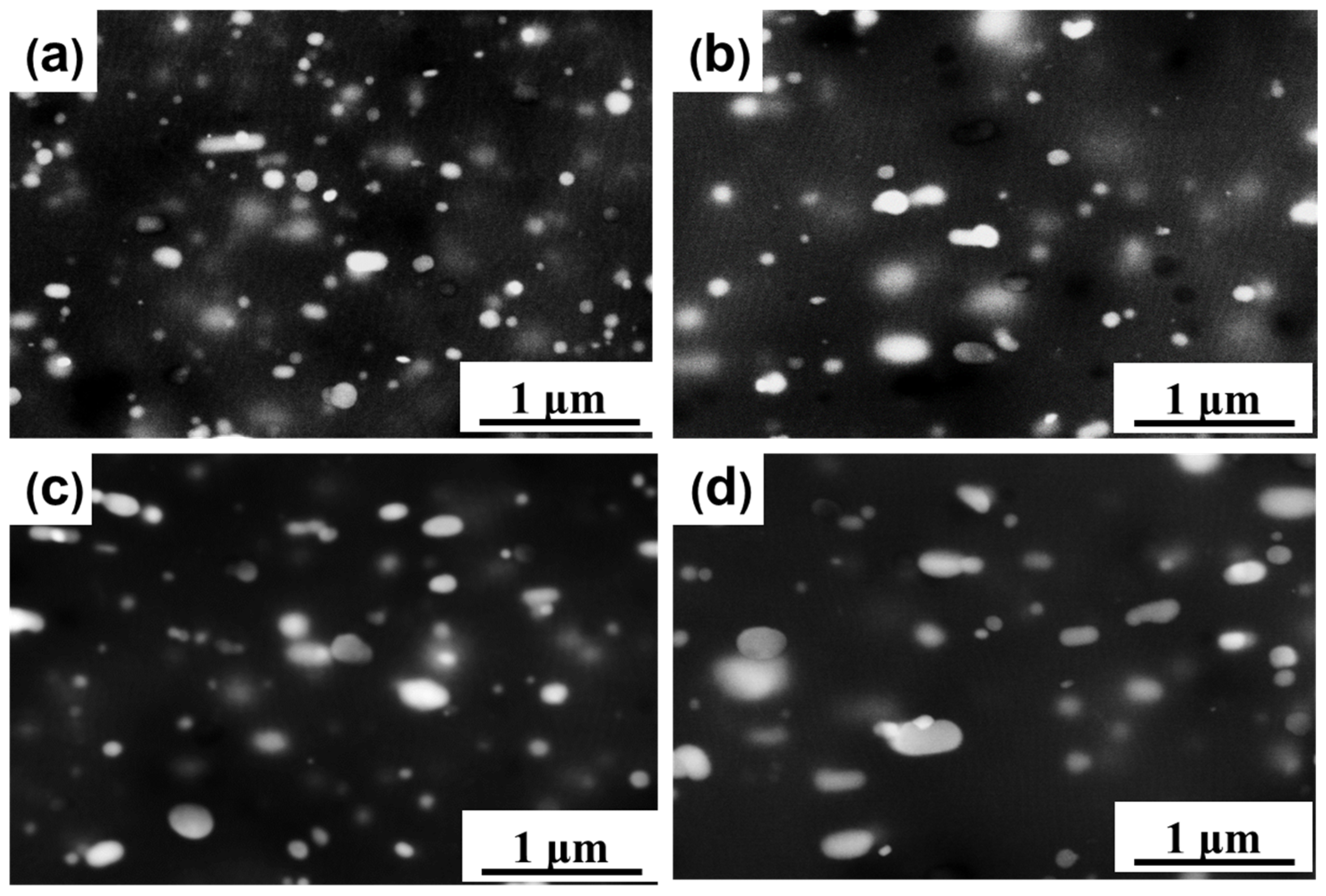
3.4. Microstructural Characterization of the Precipitates before and after Annealing Treatment
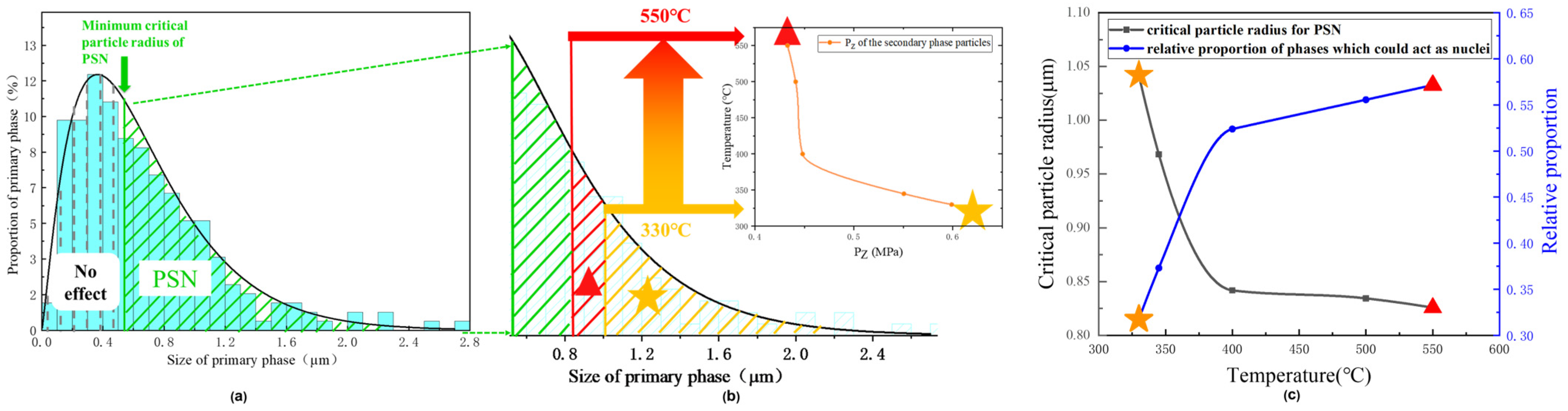
3.5. The Effect of Precipitation on the Recrystallized Grain Sizes after Annealing
4. Conclusions
- (1)
- The recrystallization finishing temperature of the Al–Mn alloy cold-rolled by 81.7% and 96.5% reductions were determined to be the same, around 323 °C. When annealed at temperatures from 330 to 550 °C, the recrystallized grain size showed an abnormal variation with the annealing temperature, i.e., the lower the annealing temperature, the larger the grain size. This was more obvious in the temperature range of 330–400 °C.
- (2)
- During the annealing process, the micro-scale primary-phase particles remained almost unchanged, providing nucleation sites for recrystallization. The coarsening and dissolution of the nano-sized secondary phase particles were observed to hinder the recrystallization process by reducing the nucleation rate and pinning the grain boundaries.
- (3)
- The critical particle radii of PSN at different temperatures were calculated. With increasing the annealing temperature from 330 to 550 °C, the critical particle radius of PSN decreased, which increased the percentage of the effective large particles that could act as nucleation sites, leading to more grains and smaller grain sizes. In addition, the foil surface could facilitate the recrystallization process, which is more effective for the thinner foil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Connor, Z.; Murty, G.S. Recent improvements in aluminum brazing sheet for use in heat exchanger applications, especially radiator tube stock. J. Mater. Manuf. 2005, 114, 852–858. [Google Scholar]
- Goodrich, H.S.; Connor, Z.M.; Murty, G.S. Aging Response and Elevated Temperature Strengthening in Brazing Sheet Core Alloys of 3xxx Series Aluminum; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Ikeda, H.; Shoji, Y. Progress in aluminum materials for automobile heat exchangers. Sumitomo Light Met. Tech. Rep. 1994, 35, 43–56. [Google Scholar]
- Tu, Y.; Tong, Z.; Jiang, J. Effect of Microstructure on Diffusional Solidification of 4343/3005/4343 Multi-Layer Aluminum Brazing Sheet. Metall. Mater. Trans. A 2013, 44, 1760–1766. [Google Scholar] [CrossRef]
- Marshall, G.J.; Bolingbroke, R.K.; Gray, A. Microstructural control in an aluminum core alloy for brazing sheet applications. Metall. Mater. Trans. A 1993, 24, 1935–1942. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Kim, M.-S.; Jung, D. Brazeability of cold rolled three layer Al–7.5Si/Al–1.2Mn–2Zn–(0.04–1.0)Si/Al–7.5Si (wt.%) clad sheets. J. Mater. Process. Technol. 2002, 130, 240–244. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, M.S. Effect of cold rolling condition on the brazing characteristic for Al 4343/3003/4343 brazing sheet. J. Korean Inst. Met. Mater. 1999, 37, 992–999. [Google Scholar]
- Yoon, J.S.; Lee, S.H.; Kim, M.S. Sagging resistance of cold rolled aluminum 4343/3N03/4343 clad sheet. J. Mater. Sci. Lett. 2001, 20, 229–232. [Google Scholar] [CrossRef]
- Wang, N.; Flatoy, J.E.; Li, Y.J.; Marthinsen, K. Evolution in microstructure and mechanical properties during back-annealing of AlMnFeSi alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 1878–1883. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Jung, D.S.; Han, J.W.; You, B.D. Fabrication and Sagging Behavior of Three-Layer Al-Si/Al-Mn-Zn/Al-Si Clad Sheets for Automotive Heat Exchanger. Mater. Sci. Forum 2003, 439, 221–226. [Google Scholar] [CrossRef]
- Liao, L.H.; Jin, H.O.; Gallerneault, M.; Esmaeili, S. Recrystallization behavior of a co-cast AA3xxx–AA6xxx aluminum alloy laminate—Effect of annealing conditions and cold reduction levels. Mater. Charact. 2014, 94, 215–226. [Google Scholar] [CrossRef]
- Huang, K.; Wang, N.; Li, Y.J.; Marthinsen, K. The influence of microchemistry on the softening behavior of two cold-rolled Al–Mn–Fe–Si alloys. Mater. Sci. Eng. A 2014, 94, 215–226. [Google Scholar]
- De Siqueira, R.P.; Sandim, H.R.Z.; Raabe, D. Particle stimulated nucleation in coarse-grained ferritic stainless steel. Metall. Mater. Trans. A 2013, 44, 469–478. [Google Scholar] [CrossRef]
- Robson, J.D.; Henry, D.T.; Davis, B. Particle effects on recrystallization in magnesium–manganese alloys: Particle-stimulated nucleation. Acta Mater. 2009, 57, 2739–2747. [Google Scholar] [CrossRef]
- Tangen, S.; Sjølstad, K.; Furu, T.; Nes, E. Effect of concurrent precipitation on recrystallization and evolution of the P-texture component in a commercial Al-Mn alloy. Metall. Mater. Trans. A 2010, 41A, 2970–2983. [Google Scholar] [CrossRef]
- Ikuo, O.; Tadashi, T.; Kei, U. Brazability and Erosion by Molten Filler Alloy of Aluminum Base Plates with Intermetallic Compounds (Materials, Metallurgy & Weldability). Trans. JWRI 1983, 12, 57–64. [Google Scholar]
- Smith, C.S. Introduction to grains, phases, and interfaces: An interpretation of microstructure. Trans. Am. Inst. Min. Eng. 1948, 175, 15–51. [Google Scholar]
- Nes, E.; Ryum, N.; Hunderi, O. On the Zener drag. Acta Metall. 1985, 33, 11–22. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five decades of the Zener equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Howe, J.M. Metallographic and differential scanning calorimetry analyses of precipitation and recrystallization in an Al-Mn alloy. Metall. Trans. A 1986, 17, 593–605. [Google Scholar] [CrossRef]
- Yasunaga, I.; Tomoki, Y. Aluminum Alloy Brazing Sheet. United States Patent 0141166, 24 May 2018. [Google Scholar]
- Poková, M.Š.; Zimina, M.; Cieslar, M. Effect of pre-annealing on microstructure evolution of TRC AA3003 aluminum alloy subjected to ECAP. Trans. Nonferrous Met. Soc. China 2016, 26, 627–633. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, K.; Marthinsen, K.; Logé, R.E. Controlling grain structure and texture in Al-Mn from the competition between precipitation and recrystallization. Acta Mater. 2017, 141, 360–373. [Google Scholar] [CrossRef]
- Liu, W.C.; Radhakrishnan, B. Recrystallization behavior of a supersaturated Al–Mn alloy. Mater. Lett. 2010, 64, 1829–1832. [Google Scholar] [CrossRef]
- Somerday, M.; Humphreys, F.J. Recrystallisation behaviour of supersaturated Al–Mn alloys Part 1–Al–1.3 wt-% Mn. Mater. Sci. Technol. 2003, 19, 20–29. [Google Scholar] [CrossRef]
- Birol, Y. Impact of homogenization on recrystallization of a supersaturated Al–Mn alloy. Scr. Mater. 2009, 60, 5–8. [Google Scholar] [CrossRef]
- Birol, Y. Recrystallization of a supersaturated Al–Mn alloy. Scr. Mater. 2008, 59, 611–614. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wolf, D. A Read-Shockley model for high-angle grain boundaries. Scr. Metall. 1989, 23, 1713–1718. [Google Scholar] [CrossRef]
- Li, Y.J.; Muggerud, A.M.F.; Olsen, A.; Furu, T. Precipitation of partially coherent α-Al (Mn, Fe) Si dispersoids and their strengthening effect in AA 3003 alloy. Acta Mater. 2012, 60, 1004–1014. [Google Scholar] [CrossRef]
- McLean, M. The kinetics of spheroidization of lead inclusions in aluminium. Philos. Mag. 1973, 27, 1253–1266. [Google Scholar] [CrossRef]


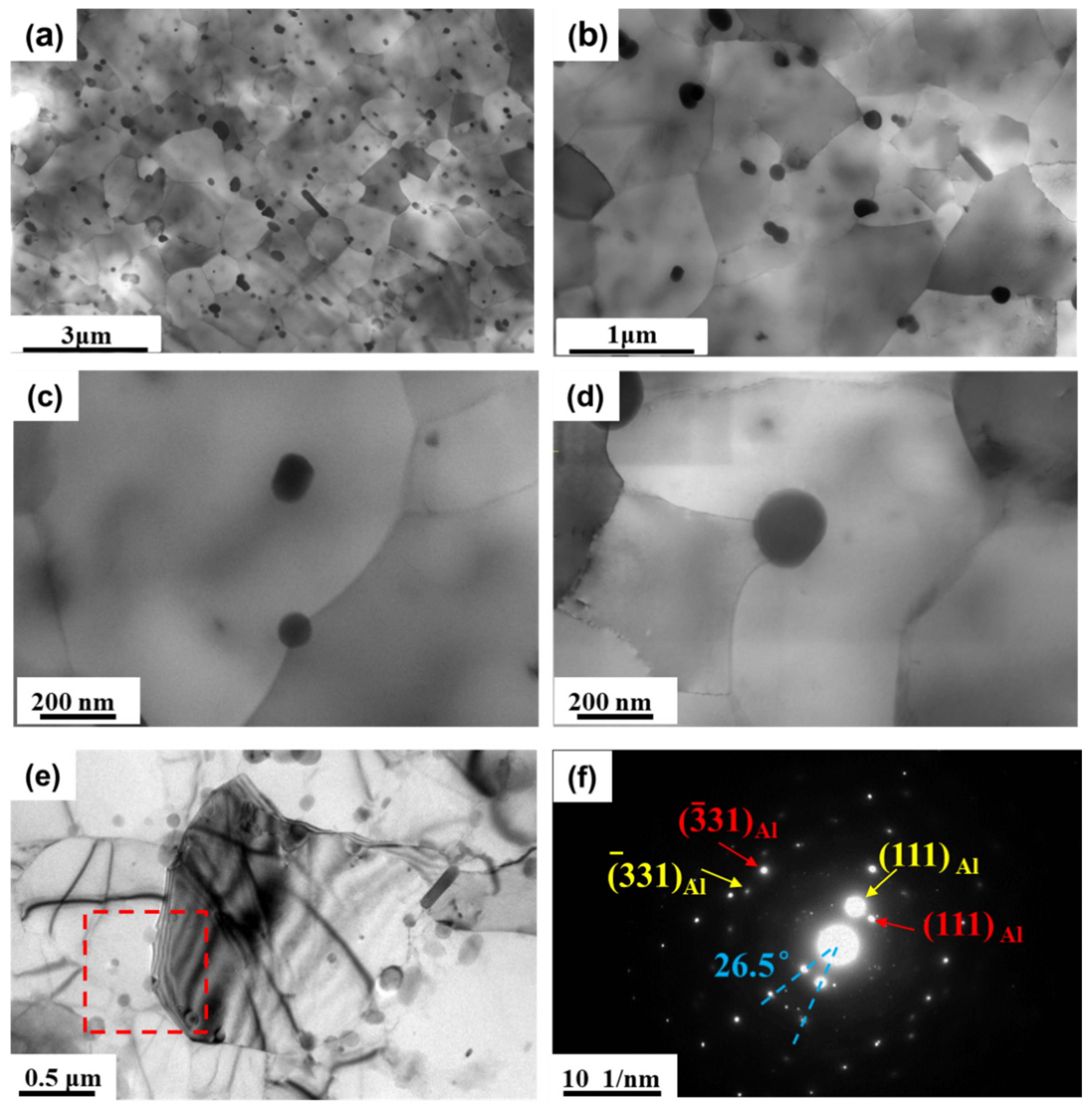
| Element | Si | Fe | Cu | Mn | Zn | Zr | Al |
|---|---|---|---|---|---|---|---|
| Content (wt%) | 0.6 | 0.3 | 0.1 | 1.6 | 1.3 | 0.1 | bal |
| T/°C | PD/MPa | PZ/MPa | rg/μm |
|---|---|---|---|
| 330 | 1.23 | 0.60 | 1.04 |
| 345 | 1.23 | 0.55 | 0.97 |
| 400 | 1.23 | 0.45 | 0.84 |
| 500 | 1.23 | 0.44 | 0.83 |
| 550 | 1.23 | 0.43 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, K.; Xiong, X.; Zhang, Y.; Ding, Y.; Wang, J.; Wu, X.; Wen, S.; Huang, H.; Wei, W.; et al. The Influence of Precipitated Particles on the Grain Size in Cold-Rolled Al–Mn Alloy Foils upon Annealing at 100–550 °C. Materials 2024, 17, 1671. https://doi.org/10.3390/ma17071671
Wang J, Gao K, Xiong X, Zhang Y, Ding Y, Wang J, Wu X, Wen S, Huang H, Wei W, et al. The Influence of Precipitated Particles on the Grain Size in Cold-Rolled Al–Mn Alloy Foils upon Annealing at 100–550 °C. Materials. 2024; 17(7):1671. https://doi.org/10.3390/ma17071671
Chicago/Turabian StyleWang, Jianzhu, Kunyuan Gao, Xiangyuan Xiong, Yue Zhang, Yusheng Ding, Jingtao Wang, Xiaolan Wu, Shengping Wen, Hui Huang, Wu Wei, and et al. 2024. "The Influence of Precipitated Particles on the Grain Size in Cold-Rolled Al–Mn Alloy Foils upon Annealing at 100–550 °C" Materials 17, no. 7: 1671. https://doi.org/10.3390/ma17071671
APA StyleWang, J., Gao, K., Xiong, X., Zhang, Y., Ding, Y., Wang, J., Wu, X., Wen, S., Huang, H., Wei, W., Rong, L., Nie, Z., & Zhou, D. (2024). The Influence of Precipitated Particles on the Grain Size in Cold-Rolled Al–Mn Alloy Foils upon Annealing at 100–550 °C. Materials, 17(7), 1671. https://doi.org/10.3390/ma17071671








