Biomechanical Fatigue Behavior of a Dental Implant Due to Chewing Forces: A Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
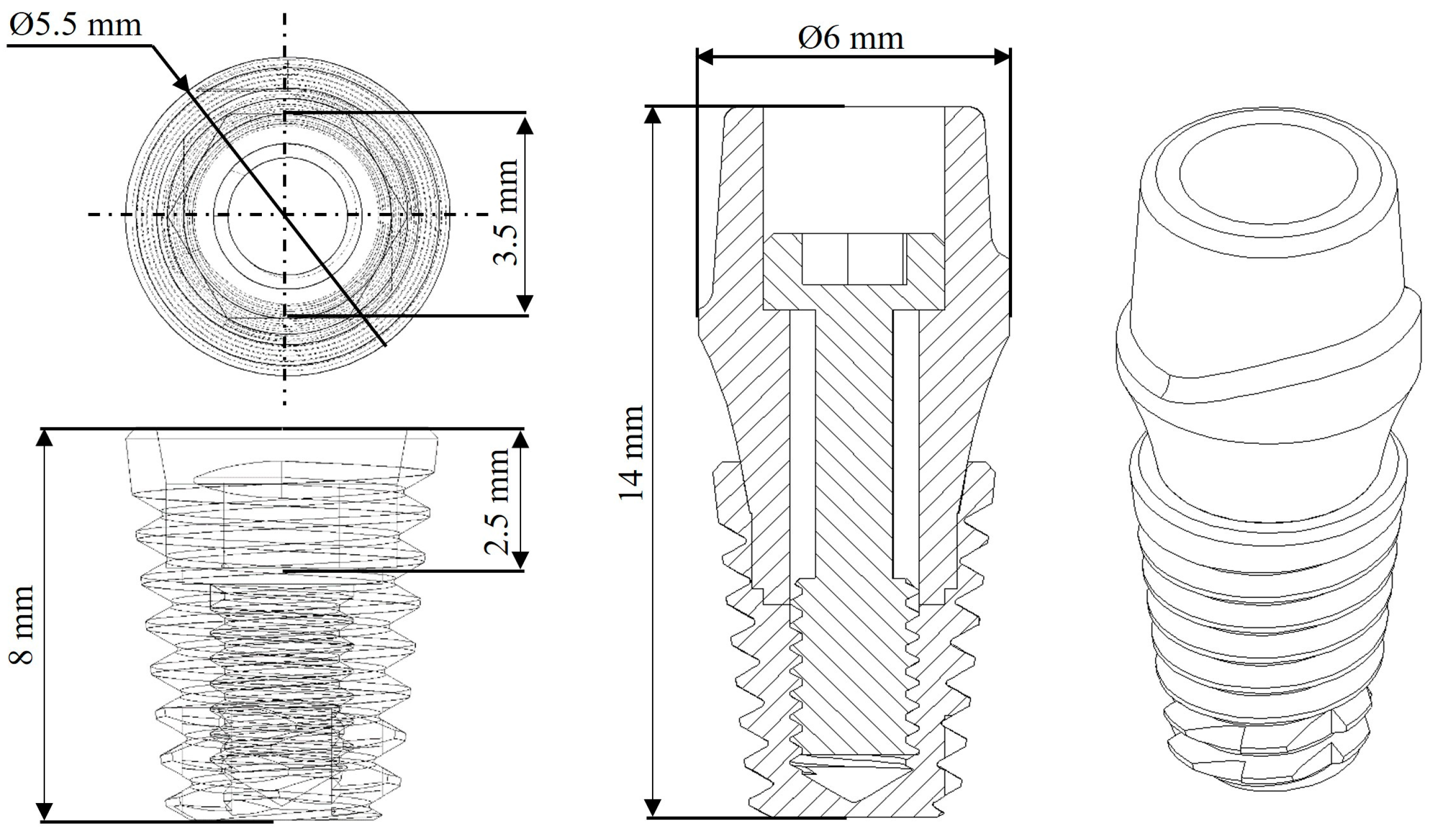
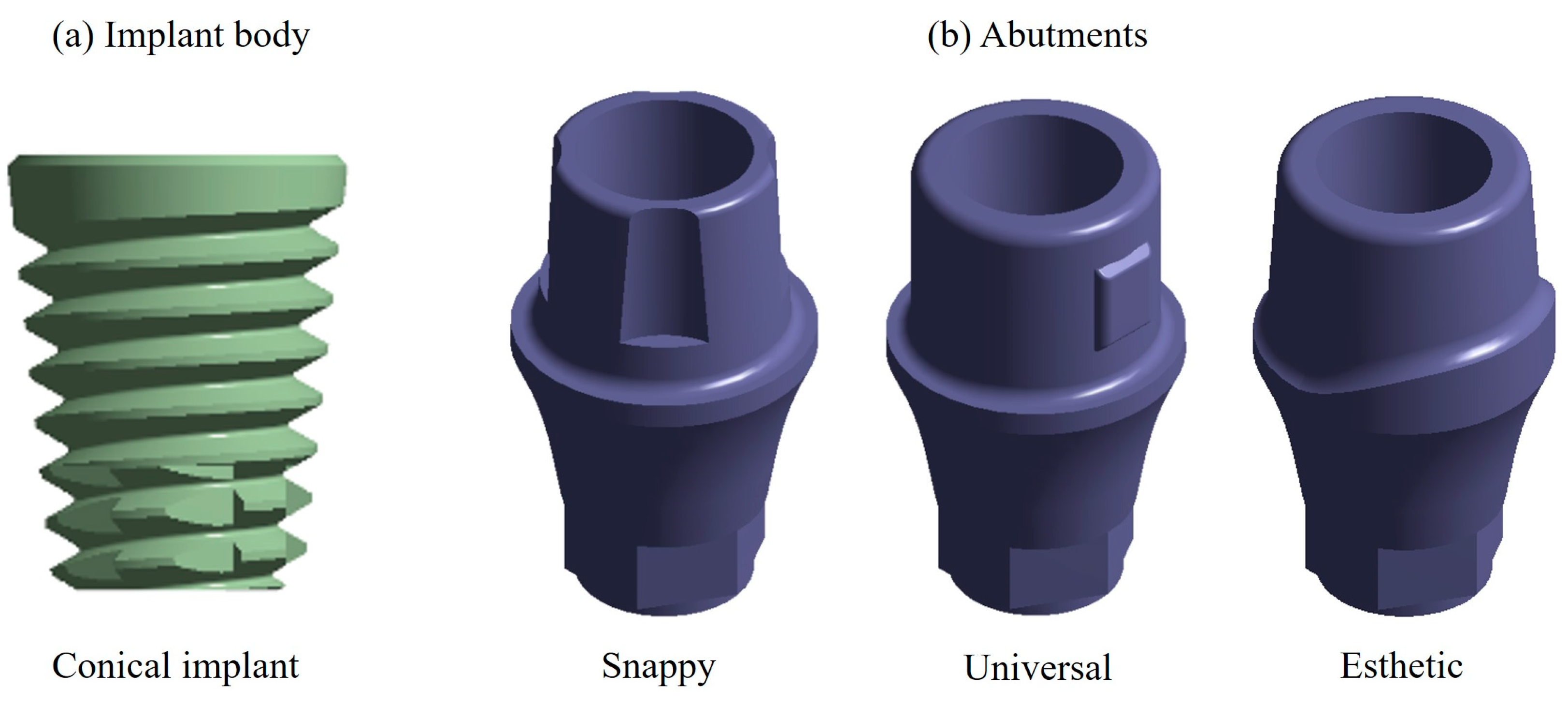
2.1. Characteristics of the Dental Implant
2.2. Dental Implant Modeling
2.3. Material Properties
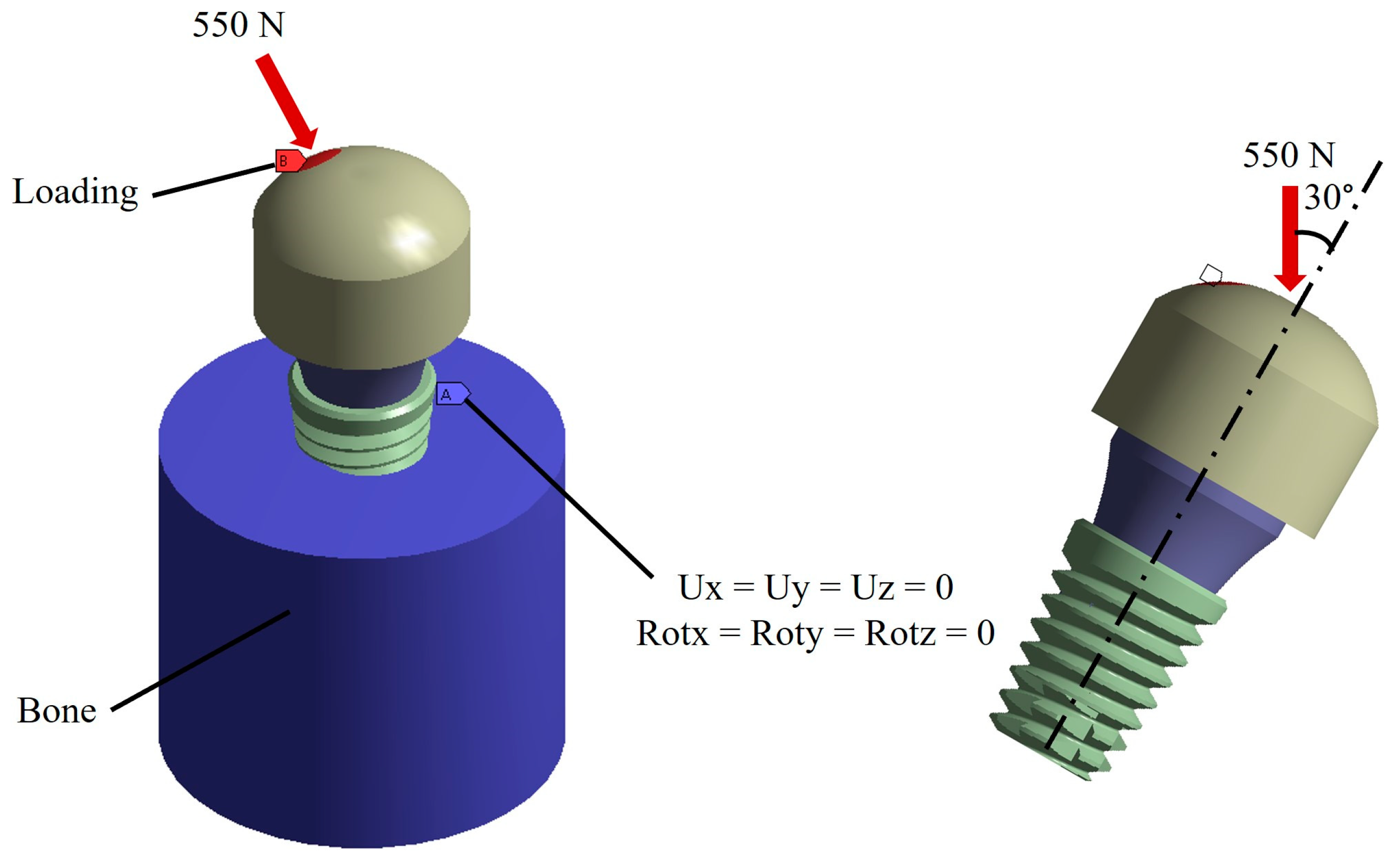
2.4. Boundary and Loading Conditions
2.5. Analysis
3. Results
3.1. Displacements and Deformations
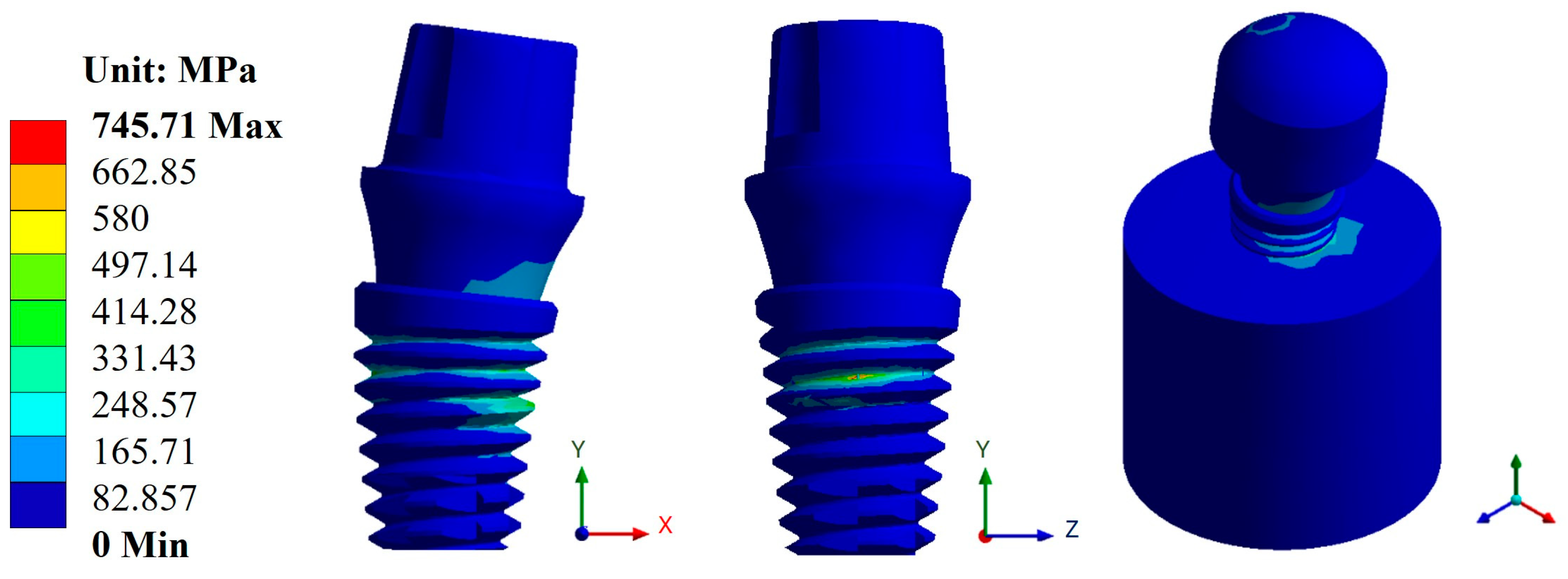
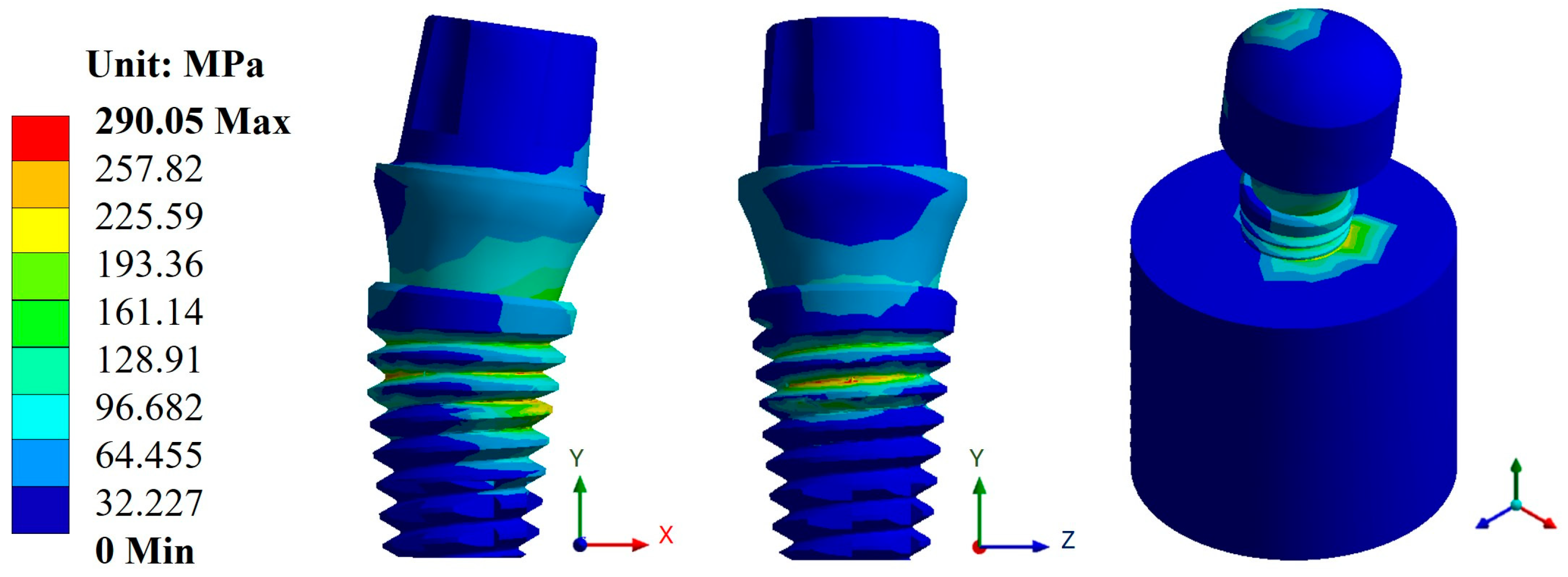
3.2. von Mises Stress
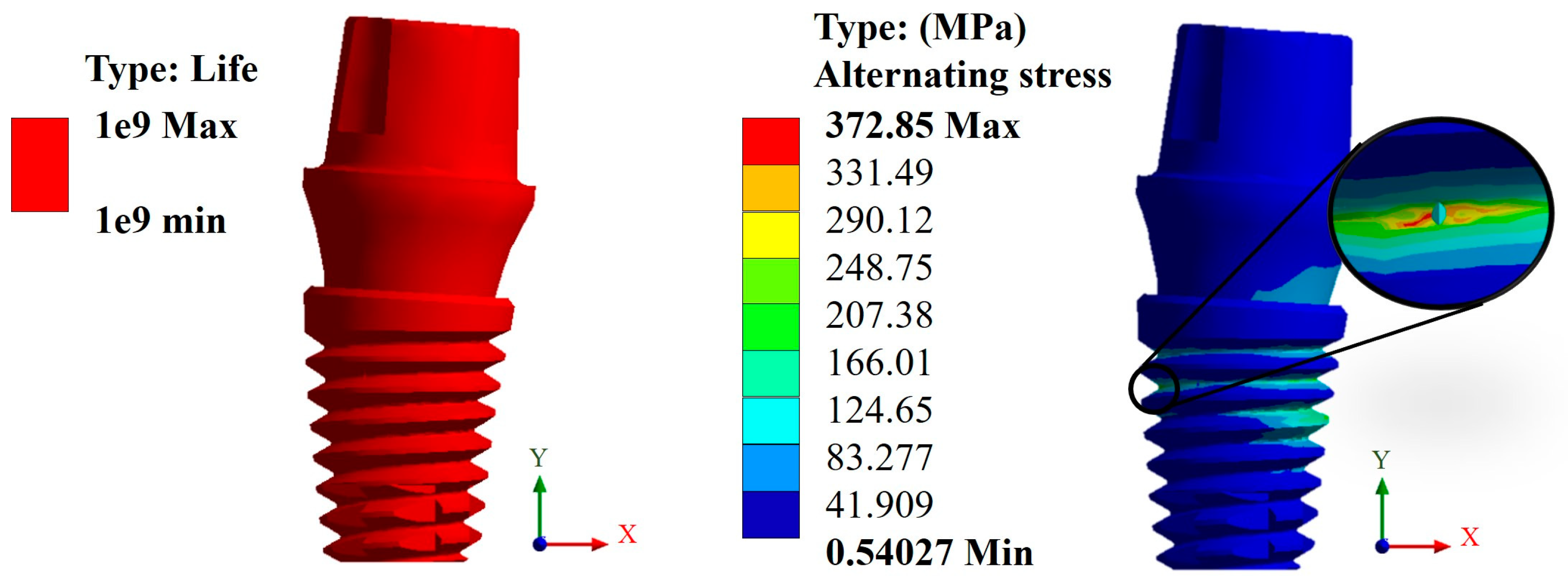
3.3. Life Cycles to Fatigue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Results of Displacements and Unit Deformations
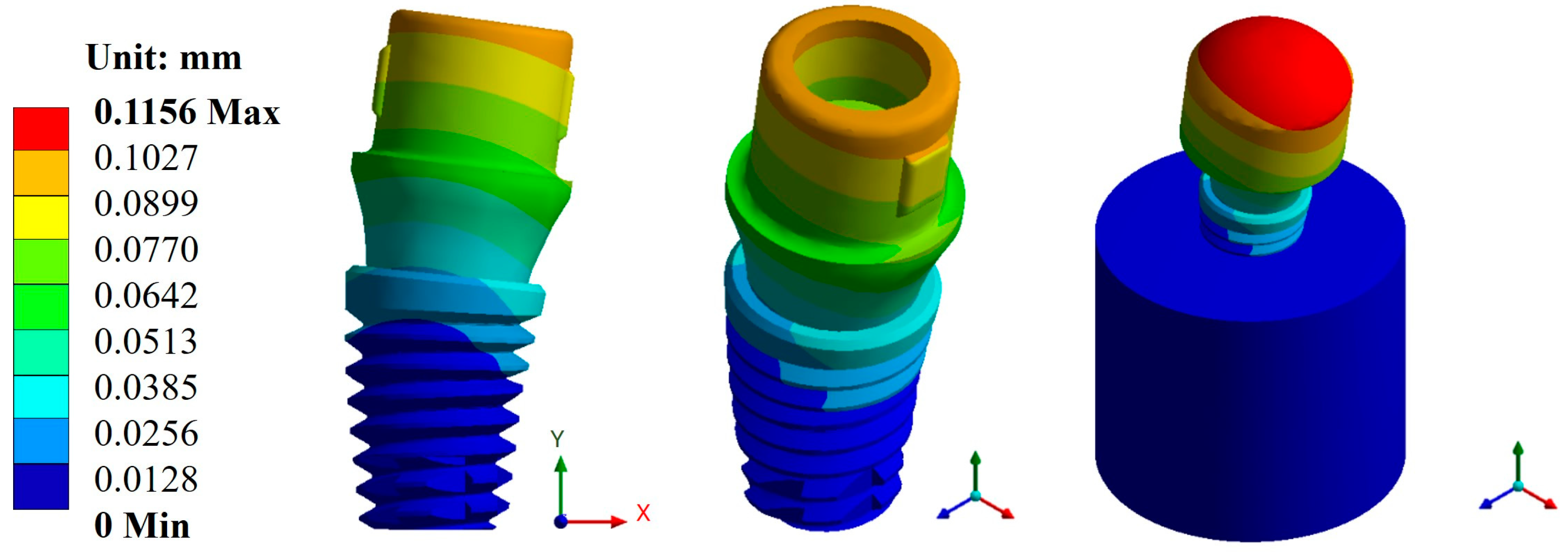

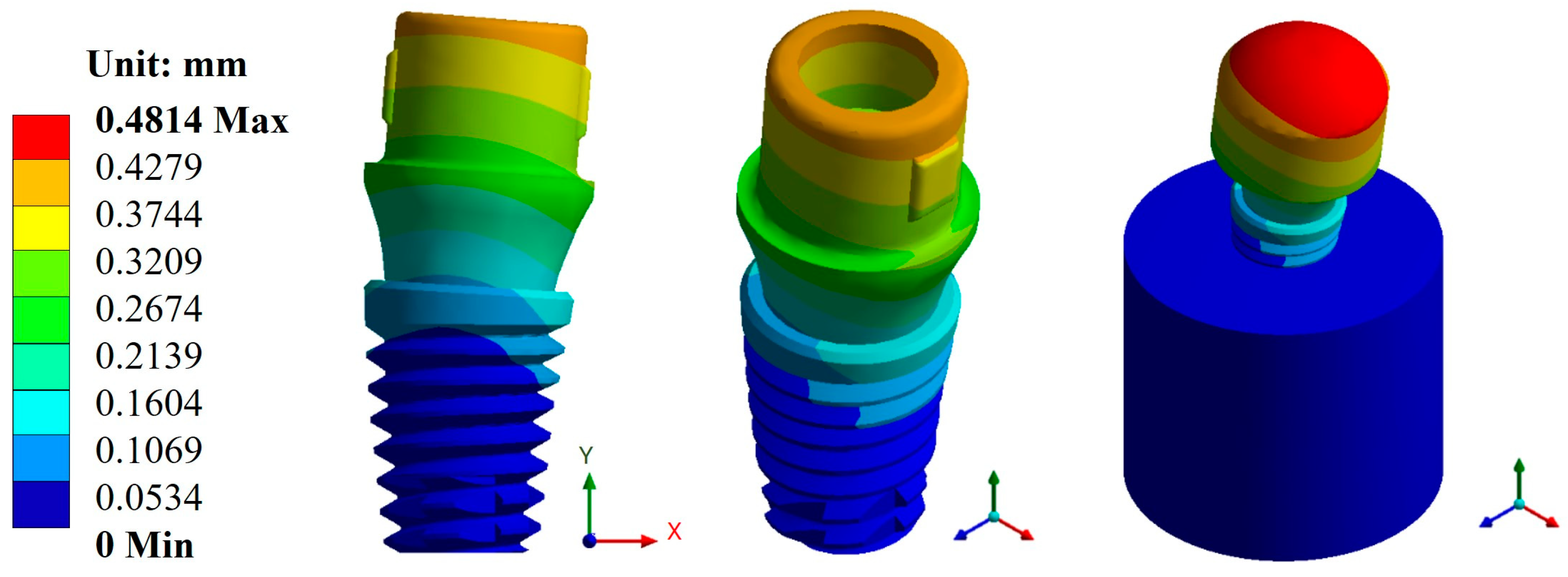
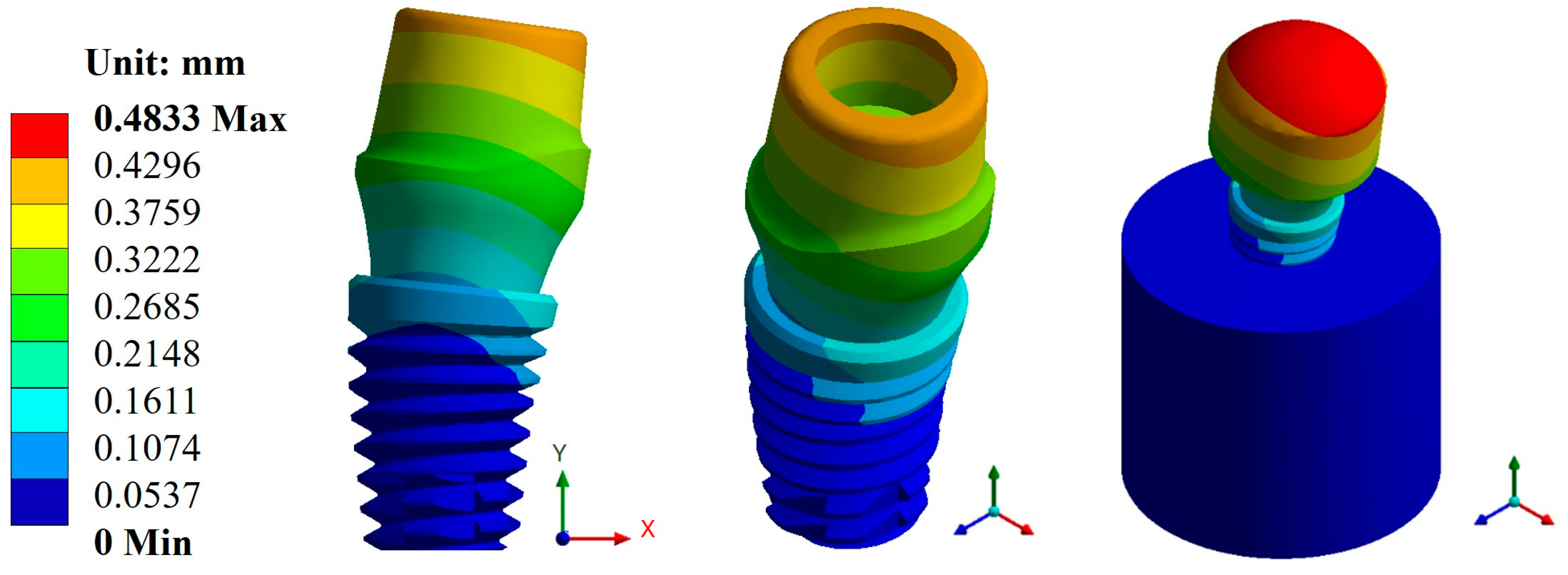
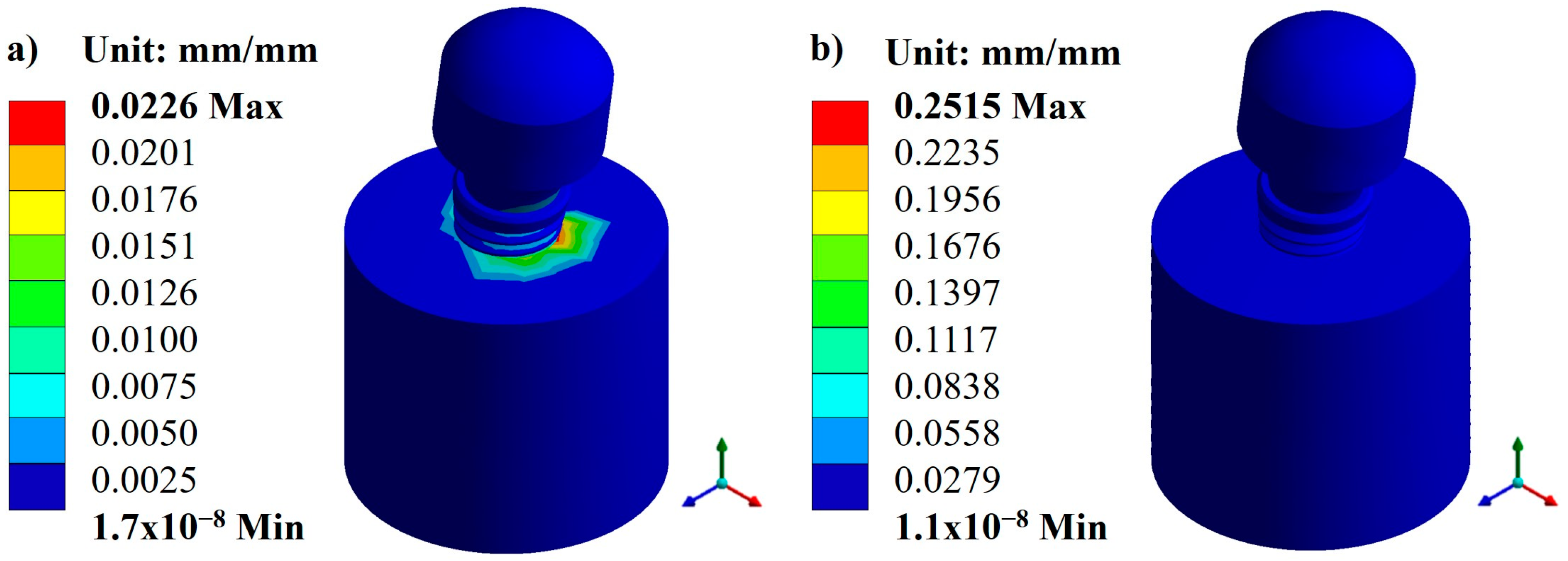
Appendix A.2. Results of von Mises Stress

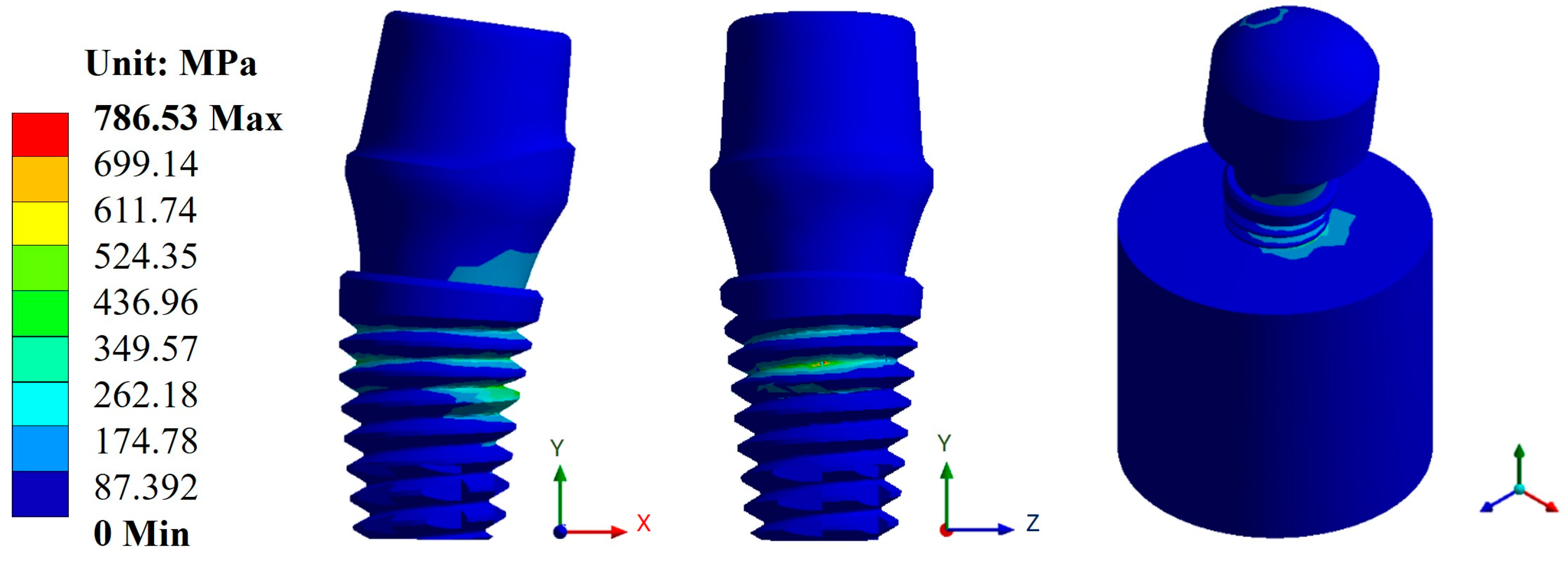

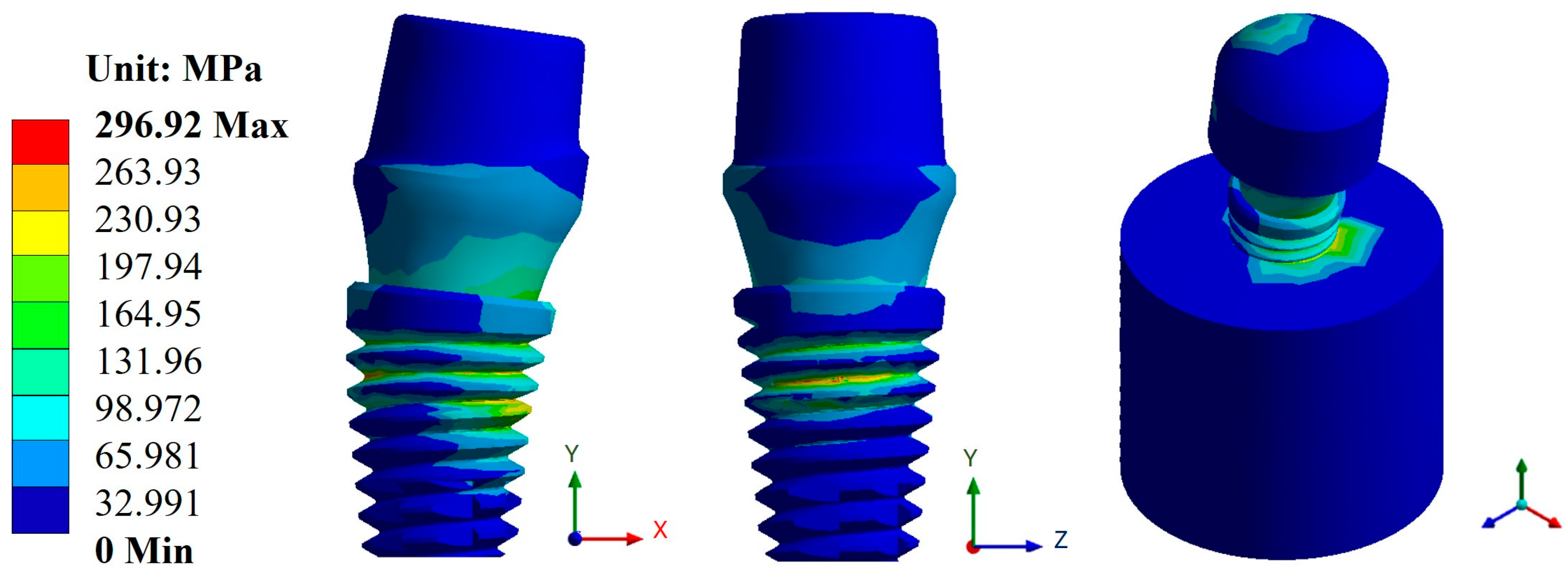
Appendix A.3. Results of Life-to-Fatigue and Alternating Stress
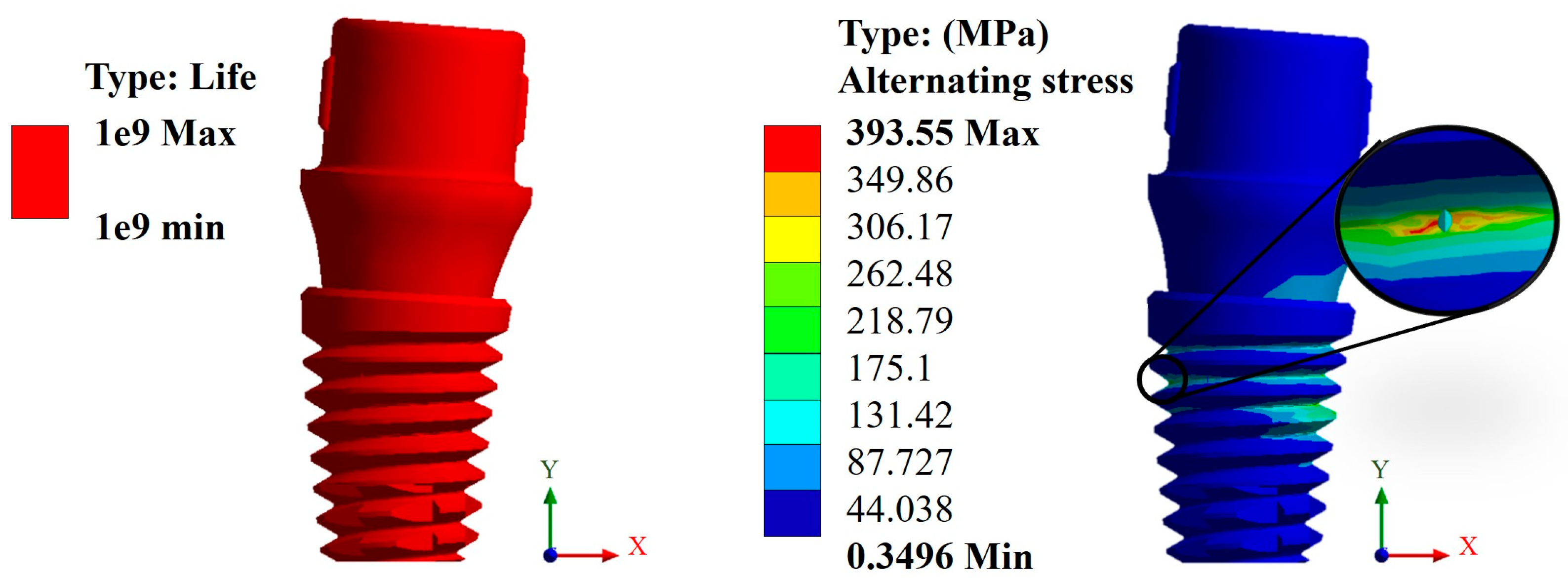



References
- Chen, X.; Ma, R.; Min, J.; Li, Z.; Yu, P.; Yu, H. Effect of PEEK and PTFE coatings in fatigue performance of dental implant retaining screw joint: An in vitro study. J. Mech. Behav. Biomed. Mater. 2020, 103, 103530. [Google Scholar] [CrossRef]
- Sun, F.; Wang, L.; Li, X.C.; Cheng, W.; Lin, Z.; Ba, D.C.; Song, G.Q.; Sun, C.S. Effect of surface modification on the long-term stability of dental implant abutment screws by plasma nitriding treatment. Surf. Coat. Technol. 2020, 399, 126089. [Google Scholar] [CrossRef]
- Boehm, M.W.; Yakubov, G.E.; Stokes, J.R.; Baier, S.K. The role of saliva in oral processing: Reconsidering the breakdown path paradigm. J. Texture Stud. 2020, 51, 67–77. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamanishi, Y.; Machado, L.S.; Matsumoto, S.; Tovar, N.; Coelho, P.G.; Thompson, V.P.; Imazato, S. In vitro fatigue tests and in silico finite element analysis of dental implants with different fixture/abutment joint types using computer-aided design models. J. Prosthodont. Res. 2018, 62, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Yamaguchi, S.; Imazato, S.; Nakano, T.; Yatani, H. Influences of implant neck design and implant-abutment joint type on peri-implant bone stress and abutment micromovement: Three-dimensional finite element analysis. Dent. Mater. 2012, 28, 1126–1133. [Google Scholar] [CrossRef]
- Scaizer Lopes, G.R.; Melo de Matos, J.D.; Queiroz, D.A.; Mendes Tribst, J.P.; Ramos, N.C.; Garcia Rocha, M.; Baldotto Barbosa, A.; Bottino, M.A.; Souto Borges, A.L.; Sussumu Nishioka, R. Influence of abutment design on biomechanical behavior to support a screw-retained 3-unit fixed partial denture. Materials 2022, 15, 6235. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Vivek, R.; Chaturvedi, T.P.; Chauhan, P.; Gupta, S. SEM analysis and management of fracture dental implant. Case Rep. Dent. 2013, 2013, 270385. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Ríos-Santos, J.V.; Herrero-Climent, M. Review of the mechanical behavior of different implant-abutment connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Prasad Gibson, M.; Kim, H.; Monaco, E.A. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Gosavi, S.; Gosavi, S.; Alla, R. Titanium: A miracle metal in dentistry. Trends Biomater. Artif. Organs 2013, 27, 42–46. [Google Scholar]
- Messer, R.; Wataha, J. Dental Materials: Biocompatibility. Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–10. [Google Scholar]
- Alrabeah, G.O.; Brett, P.; Knowles, J.C.; Petridis, H. The effect of metal ions released from different dental implant-abutment couples on osteoblast function and secretion of bone resorbing mediators. J. Dent. 2017, 66, 91–101. [Google Scholar] [CrossRef]
- Romero-Resendiz, L.; Gómez-Sáez, P.; Vicente-Escuder, A.; Amigó-Borrás, V. Development of Ti-In alloys by powder metallurgy for application as dental biomaterial. J. Mater. Res. Technol. 2021, 11, 1719–1729. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, W.; Gao, R.; Zhang, H.; Dong, L.; Qin, J.; Wang, B.; Jia, W.; Li, X. Fatigue behavior and osseointegration of porous Ti-6Al-4V scaffolds with dense core for dental application. Mater. Des. 2020, 195, 108994. [Google Scholar] [CrossRef]
- Poggio, C.E.; Ercoli, C.; Rispoli, L.; Maiorana, C.; Esposito, M. Metal-free materials for fixed prosthodontic restorations. Cochrane Database Syst. Rev. 2017, 12, CD009606. [Google Scholar] [CrossRef]
- Hariram, V. A Brief Review on PEEK as biomaterial, Importance of Implant Design, 3D Printing and FEA in Dental Implant. E3S Web Conf. 2024, 491, 01017. [Google Scholar] [CrossRef]
- AlOtaibi, N.; Naudi, K.; Conway, D.; Ayoub, A. The current state of PEEK implant osseointegration and future perspectives: A systematic review. Eur. Cells Mater. 2020, 40, 1–20. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Xu, X.; He, L.; Zhu, B.; Li, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Huang, H.Y.; Feng, S.W.; Chiang, K.Y.; Li, Y.C.; Peng, T.Y.; Nikawa, H. Effects of various functional monomers’ reaction on the surface characteristics and bonding performance of polyetheretherketone. J. Prosthodont. Res. 2023, JPR_D_23_00063. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Yaqin Syed, A.U.; Rashid Habib, S.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEEK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Zimmermann, T.; Spinting, T.; Kallage, I.; Müller, W.D. Fatigue limits of different PEEK materials for dental implants. J. Mech. Behav. Biomed. Mater. 2017, 69, 163–168. [Google Scholar] [CrossRef]
- Sun, C.; Kang, J.; Yang, C.; Zheng, J.; Su, Y.; Dong, E.; Liu, Y.; Yao, S.; Shi, C.; Pang, H.; et al. Additive Manufactured Polyether–Ether–Ketone Implants for Orthopaedic Applications: A Narrative Review. Biomater. Transl. 2022, 3, 116–133. [Google Scholar] [CrossRef]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y. gang PEEK (Polyether–Ether–Ketone) and Its Composite Materials in Orthopedic Implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Wang, B.; Huang, M.; Dang, P.; Xie, J.; Zhang, X.; Yan, X. PEEK in Fixed Dental Prostheses: Application and Adhesion Improvement. Polymers 2022, 14, 2323. [Google Scholar] [CrossRef]
- Shala, K.; Tmava-Dragusha, A.; Dula, L.; Pustina-Krasniqi, T.; Bicaj, T.; Ahmedi, E.; Lila, Z. Evaluation of maximum bite force in patients with complete dentures. Open Access Maced. J. Med. Sci. 2018, 6, 559–563. [Google Scholar] [CrossRef]
- Fontolliet, A.; Al-Haj Husain, N.; Özcan, M. Wear analysis and topographical properties of monolithic zirconia and CoCr against human enamel after polishing and glazing procedures. J. Mech. Behav. Biomed. Mater. 2020, 105, 103712. [Google Scholar] [CrossRef]
- Shinya, A. Dental material research in prosthodontics—Towards developing better and efficient biomimetic materials. J. Prosthodont. Res. 2023, 67, vi–vii. [Google Scholar] [CrossRef]
- Suphangul, S.; Rokaya, D.; Kanchanasobhana, C.; Rungsiyakull, P.; Chaijareenont, P. PEEK Biomaterial in Long-Term Provisional Implant Restorations: A Review. J. Funct. Biomater. 2022, 13, 33. [Google Scholar] [CrossRef]
- Souza, J.C.; Pinho, S.S.; Braz, M.P.; Silva, F.S.; Henriques, B. Carbon fiber-reinforced PEEK in implant dentistry: A scoping review on the finite element method. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1355–1367. [Google Scholar] [CrossRef]
- Altiparmak, N.; Polat, S.; Onat, S. Finite Element Analysis of the biomechanical effects of titanium and CFR-PEEK additively manufactured subperiosteal jaw implant (AMSJI) on maxilla. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101290. [Google Scholar] [CrossRef]
- Naghavi, S.A.; Lin, C.; Sun, C.; Tamaddon, M.; Basiouny, M.; Garcia-Souto, P.; Taylor, S.; Hua, J.; Li, D.; Wang, L.; et al. Stress Shielding and Bone Resorption of Press-Fit Polyether–Ether–Ketone (PEEK) Hip Prosthesis: A Sawbone Model Study. Polymers 2022, 14, 4600. [Google Scholar] [CrossRef]
- Verma, V.; Hazari, P.; Verma, P. Do implants made of polyetheretherketone and its composites have reduced stress shielding effects compared to other dental implant materials? A systematic review. Evid.-Based Dent. 2023, 24, 193–194. [Google Scholar] [CrossRef]
- Parra Reyes, D. Systematic review of the literature on the evaluation of the chewing process. Areté 2021, 20, 39–47. [Google Scholar]
- Ayllón, J.M.; Navarro, C.; Vázquez, J.; Domínguez, J. Fatigue life estimation in dental implants. Eng. Fract. Mech. 2014, 123, 34–43. [Google Scholar] [CrossRef]
- Slavicek, G. Human mastication. Int. J. Stomatol. Occlusion Med. 2010, 3, 29–41. [Google Scholar] [CrossRef]
- Kayabasi, O.; Yuzbasioglu, E.; Erzincanli, F. Static, dynamic and fatigue behaviors of dental implant using finite element method. Adv. Eng. Softw. 2006, 37, 649–658. [Google Scholar] [CrossRef]
- Voumard, B.; Maquer, G.; Heuberger, P.; Zysset, P.K.; Wolfram, U. Peroperative estimation of bone quality and primary dental implant stability. J. Mech. Behav. Biomed. Mater. 2019, 22, 24–32. [Google Scholar] [CrossRef]
- Steiner, J.A.; Ferguson, S.J.; Harry, G. Screw insertion in trabecular bone causes peri-implant bone damage. Med. Eng. Phys. 2016, 38, 417–422. [Google Scholar] [CrossRef]
- Gomes, C.; Mesnard, M.; Ramos, A. Bone density and proximal support effects on dental implant stability—Finite Element Analysis and in vitro experiments. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101512. [Google Scholar] [CrossRef]
- Falcinelli, C.; Valente, F.; Vasta, M.; Traini, T. Finite element analysis in implant dentistry: State of the art and future directions. Dent. Mater. 2023, 39, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Kudagi, V.S.; Talwade, P. Applications of finite element analysis in dentistry: A review. J. Int. Oral Health 2021, 13, 415–422. [Google Scholar] [CrossRef]
- Lisiak-Myszke, M.; Marciniak, D.; Bieliński, M.; Sobczak, H.; Garbacewicz, Ł.; Drogoszewska, B. Application of Finite Element Analysis in Oral and Maxillofacial Surgery—A Literature Review. Materials 2020, 13, 3063. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Bitencourt, S.B.; Rosa, C.D.D.R.D.; Vieira, A.B.; Dos Santos, D.M.; Goiato, M.C. Influence of different restoring materials on stress distribution in prosthesis on implants: A review of finite element studies. Eur. J. Dent. 2023, 17, 001–006. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Gallardo, E.A.; Morán, A.; Bravo, J.A.; Moreno, M.; Vite, M. Microabrasion on dental restorative porcelains and amalgam. Tribol.—Mater. Surf. Interfaces 2013, 7, 74–82. [Google Scholar] [CrossRef]
- Romanyk, D.L.; Vafaeian, B.; Addison, O.; Adeeb, S. The use of finite element analysis in dentistry and orthodontics: Critical points for model development and interpreting results. Semin. Orthod. 2020, 26, 162–173. [Google Scholar] [CrossRef]
- Zeno, K.G.; Ammoury, M.J. The surge of finite element analysis in the study of orthodontic mechanics: Are the findings applicable in practice? Semin. Orthod. 2023, 29, 308–316. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Xu, G. Progress of finite element analysis method for oral implantology. Acad. J. Med. Health Sci. 2023, 4, 65–70. [Google Scholar] [CrossRef]
- Poli, O.; Manzon, L.; Niglio, T.; Ettorre, E.; Vozza, I. Masticatory force in relation with age in subjects with full permanent dentition: A cross-sectional study. Healthcare 2021, 9, 700. [Google Scholar] [CrossRef]
- Hernández-Vázquez, R.A.; Romero-Ángeles, B.; Urriolagoitia-Sosa, G.; Vázquez-Feijoo, J.A.; Vázquez-López, A.J.; Urriolagoitia-Calderón, G. Numerical análisis of masticatory forces on a lower first molar considering the contact between dental tissues. Appl. Bionics Biomech. 2018, 2018, 4196343. [Google Scholar] [CrossRef]
- da Hora Sales, P.E.; Pessoa Barros, A.W.; Barbosa de Oliveira-Neto, O.; Camello, F.J.; Tavares Carvalho, A.A.; Carneiro Leão, J. Do zirconia dental implants present better clinical results than titanium dental implants? A systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101324. [Google Scholar] [CrossRef]
- Knapic, D.; Muck, M.; Heitz, J.; Baumgartner, W.; Mandare, A.I.; Kleber, C.; Hassel, A.W. Electrochemical and surface characterization of anodized and fs-laser treated Ti6Al4V for osseo-repellent bone screws and dental implants. Electrochim. Acta 2023, 466, 142965. [Google Scholar] [CrossRef]
- Ortega, B.; González, H. Functionalization of polymeric prostheses by thermal spraying: A review. Rev. Colomb. Mater. 2021, 16, 90–103. [Google Scholar] [CrossRef]
- Martinez-Mondragon, M.; Urriolagoitia-Sosa, G.; Romero-Ángeles, B.; Maya-Anaya, D.; Martínez-Reyes, J.; Gallegos-Funes, F.J.; Urriolagoitia-Calderón, G.M. Numerical analysis of Zirconium and Titanium implants under the effect of critical masticatory load. Materials 2022, 15, 7843. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mondragon, M.; Urriolagoitia-Sosa, G.; Romero-Ángeles, B.; Pérez-Partida, J.C.; Cruz-Olivares, I.M.; Urriolagoitia-Calderón, G.M. Bilinear numerical analysis of the structural behavior of a dental implant applied as a biomaterial carbon fiber reinforced polyether-ether-ketone (CFR-PEEK): A finite element analysis. Dent. Hypotheses 2023, 14, 45–48. [Google Scholar] [CrossRef]
- Bachiri, A.; Djebbar, N.; Boutabout, B.; Serier, B. Effect of different impactor designs on biomechanical behavior in the interface bone-implant: A comparative biomechanics study. Comput. Methods Programs Biomed. 2020, 197, 105723. [Google Scholar] [CrossRef]
- Figueroa-Hernández, C.; Pantaleón-Matamoros, E.; Méndez-González, S.; García-Fernández, C.; Gómez-González, R.; Carvajal-de la Osa, J. Análisis del fenómeno de fatiga en implantes dentales monocomponente. Ing. Mecánica 2020, 23, e595. [Google Scholar]
- Shemtov-Yona, K.; Rittel, D. Fatigue of Dental Implants: Facts and Fallacies. Dent. J. 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.; Blasón-González, S.; García-García, I.; Lamela-Rey, M.J.; Fernández-Canteli, A.; Álvarez-Arenal, Á. Optimized Planning and Evaluation of Dental Implant Fatigue Testing: A Specific Software Application. Biology 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Ziaie, B.; Khalili, S.M.R. Evaluation of Fatigue Life for Dental Implants Using FEM Analysis. Prosthesis 2021, 3, 300–313. [Google Scholar] [CrossRef]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical evaluations of the long-term stability of dental implant using finite element modeling method: A systematic review. J. Adv. Prosthodont. 2022, 14, 182–202. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, M.; Lanza, A.; Sbordone, L.; Ruggiero, A. Stress-strain and fatigue life numerical evaluation of two different dental implants considering isotropic and anisotropic human jaw. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2023, 237, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, Z.; Mohammadi, B.; Karimi, H.R. On the fatigue life of dental implants: Numerical and experimental investigation on configuration effect. Med. Eng. Phys. 2024, 123, 104078. [Google Scholar] [CrossRef]








| Material | Young’s Modulus (GPa) | Poisson’s Ratio | Density (Kg/m3) | Yield Strength (MPa) | Ultimate Strength (MPa) |
|---|---|---|---|---|---|
| Ti6Al4V | 114 | 0.36 | 4430 | 1100 | 1170 |
| ZrO2 | 210 | 0.31 | 6100 | 2000 | 900 |
| CFR-PEEK | 24 | 0.38 | 1400 | 300 | 330 |
| Cortical bone * | Ex = 12.6 | νxy = 0.3 | 1700 | - | - |
| Ey = 12.6 | νyx = 0.3 | ||||
| Ez = 19.4 | νyz = 0.253 | ||||
| Gxy = 4850 | νzy = 0.39 | ||||
| Gyz = 5700 | νxz = 0.253 | ||||
| Gxz = 5700 | νzx = 0.39 | ||||
| Trabecular bone * | Ex = 1.148 | νxy = 0.055 | 270 | - | - |
| Ey = 2.70 | νyx = 0.01 | ||||
| Ez = 1.148 | νyz = 0.01 | ||||
| Gxy = 68 | νzy = 0.055 | ||||
| Gyz = 68 | νxz = 0.322 | ||||
| Gxz = 434 | νzx = 0.055 |
| Element | Total Displacement (mm) | von Mises Stress (MPa) | Fatigue Life (Cycles) | ||||
|---|---|---|---|---|---|---|---|
| Titanium | CFR-PEEK | Titanium | CFR-PEEK | Titanium | CFR-PEEK | ||
| Snappy abutment | Max. | 0.1108 | 0.4653 | 745.71 | 290.05 | 1 × 109 | 5.73 × 105 |
| Min. | 0 | 0 | 0 | 0 | 1 × 109 | 1 × 109 | |
| Universal abutment | Max. | 0.1156 | 0.4814 | 787.1 | 296.94 | 1 × 109 | 5.10 × 105 |
| Min. | 0 | 0 | 0 | 0 | 1 × 109 | 1 × 109 | |
| Esthetic abutment | Max. | 0.1161 | 0.4833 | 786.53 | 296.92 | 1 × 109 | 5.10 × 105 |
| Min. | 0 | 0 | 0 | 0 | 1 × 109 | 1 × 109 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Mondragon, M.; Urriolagoitia-Sosa, G.; Romero-Ángeles, B.; García-Laguna, M.A.; Laguna-Canales, A.S.; Pérez-Partida, J.C.; Mireles-Hernández, J.; Carrasco-Hernández, F.; Urriolagoitia-Calderón, G.M. Biomechanical Fatigue Behavior of a Dental Implant Due to Chewing Forces: A Finite Element Analysis. Materials 2024, 17, 1669. https://doi.org/10.3390/ma17071669
Martinez-Mondragon M, Urriolagoitia-Sosa G, Romero-Ángeles B, García-Laguna MA, Laguna-Canales AS, Pérez-Partida JC, Mireles-Hernández J, Carrasco-Hernández F, Urriolagoitia-Calderón GM. Biomechanical Fatigue Behavior of a Dental Implant Due to Chewing Forces: A Finite Element Analysis. Materials. 2024; 17(7):1669. https://doi.org/10.3390/ma17071669
Chicago/Turabian StyleMartinez-Mondragon, Miguel, Guillermo Urriolagoitia-Sosa, Beatriz Romero-Ángeles, Miguel Angel García-Laguna, Aldo Saul Laguna-Canales, Juan Carlos Pérez-Partida, Jonatan Mireles-Hernández, Francisco Carrasco-Hernández, and Guillermo Manuel Urriolagoitia-Calderón. 2024. "Biomechanical Fatigue Behavior of a Dental Implant Due to Chewing Forces: A Finite Element Analysis" Materials 17, no. 7: 1669. https://doi.org/10.3390/ma17071669
APA StyleMartinez-Mondragon, M., Urriolagoitia-Sosa, G., Romero-Ángeles, B., García-Laguna, M. A., Laguna-Canales, A. S., Pérez-Partida, J. C., Mireles-Hernández, J., Carrasco-Hernández, F., & Urriolagoitia-Calderón, G. M. (2024). Biomechanical Fatigue Behavior of a Dental Implant Due to Chewing Forces: A Finite Element Analysis. Materials, 17(7), 1669. https://doi.org/10.3390/ma17071669








