Optical Temperature-Sensing Performance of La2Ce2O7:Ho3+ Yb3+ Powders
Abstract
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auzel, F. Stimulated emission of Er1s in a fluorophosphate glass. Comptes Rendus I Acad. Sci. 1966, 263, 765. [Google Scholar]
- Zhang, J.; Jin, C. Electronic structure, upconversion luminescence and optical temperature sensing behavior of Yb3+-Er3+/Ho3+ doped NaLaMgWO6. J. Alloys Compd. 2019, 783, 84–94. [Google Scholar] [CrossRef]
- Plöschner, M.; Denkova, D.; De Camillis, S.; Das, M.; Parker, L.M.; Zheng, X.; Lu, Y.; Ojosnegros, S.; Piper, J.A. Simultaneous super-linear excitation-emission and emission depletion allows imaging of upconversion nanoparticles with higher sub-diffraction resolution. Opt. Express 2020, 28, 24308–24326. [Google Scholar] [CrossRef]
- Tadge, P.; Yadav, R.S.; Vishwakarma, P.K.; Rai, S.B.; Chen, T.M.; Sapra, S.; Ray, S. Enhanced photovoltaic performance of Y2O3: Ho3+/Yb3+ upconversion nanophosphor based DSSC and investigation of color tunability in Ho3+/Tm3+/Yb3+ tridoped Y2O3. J. Alloys Compd. 2020, 821, 153230. [Google Scholar] [CrossRef]
- Fischer, L.H.; Harms, G.S.; Wolfbeis, O.S. Upconverting nanoparticles for nanoscale thermometry. Angew. Chem. Int. Ed. 2011, 50, 4546–4551. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, S.P.; Sardar, A.; Kumar, K.; da Silva, J.C.E. Role of Ca2+ co-dopants on structural and optical properties of YF3: Tm3+/Yb3+ upconversion phosphor for improved optical thermometry. Sens. Actuators A Phys. 2018, 280, 179–187. [Google Scholar] [CrossRef]
- Wu, Y.; Suo, H.; He, D.; Guo, C. Highly sensitive up-conversion optical thermometry based on Yb3+-Er3+ co-doped NaLa (MoO4)2 green phosphors. Mater. Res. Bull. 2018, 106, 14–18. [Google Scholar] [CrossRef]
- Quintanilla, M.; Cantelar, E.; Cussó, F.; Villegas, M.; Caballero, A.C. Temperature sensing with up-converting submicron-sized LiNbO3: Er3+/Yb3+ particles. Appl. Phys. Express 2011, 4, 22601. [Google Scholar] [CrossRef]
- Haro-González, P.; Martín, I.R.; Martín, L.L.; León-Luis, S.F.; Pérez-Rodríguez, C.; Lavín, V. Characterization of Er3+ and Nd3+ doped Strontium Barium Niobate glass ceramic as temperature sensors. Opt. Mater. 2011, 33, 742–745. [Google Scholar] [CrossRef]
- Soni, A.K.; Rai, V.K.; Kumar, S. Cooling in Er3+: BaMoO4 phosphor on codoping with Yb3+ for elevated temperature sensing. Sens. Actuators B Chem. 2016, 229, 476–482. [Google Scholar] [CrossRef]
- Fan, H.; Lu, Z.; Meng, Y.; Chen, P.; Zhou, L.; Zhao, J.; He, X. Optical temperature sensor with superior sensitivity based on Ca2LaSbO6: Mn4+, Eu3+ phosphor. Opt. Laser Technol. 2022, 148, 107804. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Rao, G.V.S. Oxide pyrochlores-a review. Prog. Solid State Chem. 1983, 1, 55–143. [Google Scholar] [CrossRef]
- Cao, X.; Vassen, R.; Fischer, W.; Tietz, F.; Jungen, W.; Stöver, D. Lanthanum-cerium oxide as a thermal barrier-coating material for high-temperature applications. Adv. Mater. 2003, 15, 1438–1442. [Google Scholar] [CrossRef]
- Patel, M.; Aguiar, J.A.; Sickafus, K.E.; Baldinozzi, G. Radiation effects in Gd2Ce2O7: Role of anion sublattice in disordering and limitations of Gibbons model for damage evolution in disordered systems. In Proceedings of the 29th Conference of the Condensed Matter Division of the European Physical Society, Manchester, UK, 24 August 2022. [Google Scholar]
- Patwe, S.J.; Ambekar, B.R.; Tyagi, A.K. Synthesis, characterization and lattice thermal expansion of some compounds in the system Gd2CexZr2−xO7. J. Alloys Compd. 2005, 389, 243–246. [Google Scholar] [CrossRef]
- Ma, W.; Gong, S.; Xu, H.; Cao, X. On improving the phase stability and thermal expansion coefficients of lanthanum cerium oxide solid solutions. Scr. Mater. 2006, 54, 1505–1508. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Kornienk, O.A.; Sameljuk, A.V.; Sayir, A. Phase relation studies in the CeO2-La2O3 system at 1100–1500 °C. J. Eur. Ceram. Soc. 2011, 31, 1277–1283. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Fang, M.; Fu, L.; Carlos, L.D.; Ferreira, R.A.; Wang, S. Blue-light excitable La2Ce2O7:Eu3+ red powders for white light-emitting diodes. J. Alloys Compd. 2020, 814, 152226. [Google Scholar] [CrossRef]
- Banwal, A.; Bokolia, R. Enhanced upconversion luminescence and optical temperature sensing performance in Er3+ doped BaBi2Nb2O9 ferroelectric ceramic. Ceram. Int. 2022, 48, 2230–2240. [Google Scholar] [CrossRef]
- Galvao, R.; dos Santos, L.F.; de Oliveira Lima, K.; Goncalves, R.R.; de Souza Menezes, L. Single Er3+/Yb3+-codoped yttria nanocrystals for temperature sensing: Experimental characterization and theoretical modeling. J. Phys. Chem. C 2021, 125, 14807–14817. [Google Scholar] [CrossRef]
- Dinić, I.; Vuković, M.; Rabanal, M.E.; Milošević, M.; Bukumira, M.; Tomić, N.; Tomić, M.; Mančić, L.; Ignjatović, N. Temperature Sensing Properties of Biocompatible Yb/Er-Doped GdF3 and YF3 Mesocrystals. J. Funct. Biomater. 2023, 15, 6. [Google Scholar] [CrossRef]
- Van Swieten, T.P.; Yu, D.; Yu, T.; Vonk, S.J.; Suta, M.; Zhang, Q.; Rabouw, F.T. A Ho3+-based luminescent thermometer for sensitive sensing over a wide temperature range. Adv. Opt. Mater. 2021, 9, 2001518. [Google Scholar] [CrossRef]
- Saidi, K.; Chaabani, W.; Dammak, M. Highly sensitive optical temperature sensing based on pump-power-dependent upconversion luminescence in LiZnPO4: Yb3+-Er3+/Ho3+ phosphors. RSC Adv. 2021, 11, 30926–30936. [Google Scholar] [CrossRef]
- Doğan, A.; Erdem, M.; Esmer, K.; Eryürek, G. Upconversion luminescence and temperature sensing characteristics of Ho3+/Yb3+ co-doped tellurite glasses. J. Non-Cryst. Solids 2021, 571, 121055. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, R.S.; Singh, P.; Rai, S.B. Upconversion and downshifting emissions of Ho3+-Yb3+ co-doped ATiO3 perovskite phosphors with temperature sensing properties in Ho3+-Yb3+ co-doped BaTiO3 phosphor. J. Alloys Compd. 2021, 855, 157452. [Google Scholar] [CrossRef]
- Dey, R.; Kumari, A.; Soni, A.K.; Rai, V.K. CaMoO4:Ho3+-Yb3+-Mg2+ upconverting powder for application in lighting devices and optical temperature sensing. Sens. Actuators B Chem. 2015, 210, 581–588. [Google Scholar] [CrossRef]
- Nair, G.B.; Sharma, A.K.; Dhoble, S.J.; Swart, H.C. Upconversion process in BaY2F8:Yb3+, Ho3+ powder for optical thermometry. Luminescence 2021, 36, 1847–1850. [Google Scholar] [CrossRef]
- Yadav, R.S.; Rai, E.; Purohit, L.P.; Rai, S.B. Realizing enhanced downconversion photoluminescence and high color purity in Dy3+ doped MgTiO3 powder in presence of Li+ ion. J. Lumin. 2020, 217, 116810. [Google Scholar] [CrossRef]
- Perrella, R.V.; Debasu, M.L.; Rezende, T.K.; Carneiro, J.A.; Barbosa, H.P.; Ferreirab, R.A.; Carlos, L.D.; Ferrari, J.L. Temperature sensing based on upconversion properties of Yb3+/Ho3+/Tm3+ tri-doped Y2O3 micro particles phosphors obtained by conventional precipitation method. Mater. Sci. Eng. B 2023, 297, 116780. [Google Scholar] [CrossRef]
- Trupke, T.; Green, M.A.; Würfel, P. Improving solar cell efficiencies by down-conversion of high-energy photons. J. Appl. Phys. 2002, 92, 1668–1674. [Google Scholar] [CrossRef]
- Ankur, S.; Yadav, R.S.; Gupta, A.K.; Sreenivas, K. Temperature-dependent light upconversion and thermometric properties of Er 3+/Yb 3+-codoped SrMoO4 sintered ceramics. J. Mater. Sci. 2021, 56, 12716–12731. [Google Scholar]
- Zhang, X.; Wang, M.; Ding, J.; Song, X.; Liu, J.; Shao, J.; Li, Y. LiYF4:Yb3+, Er3+ upconverting submicro-particles: Synthesis and formation mechanism exploration. RSC Adv. 2014, 4, 40223–40231. [Google Scholar] [CrossRef]
- Xue, X.; Uechi, S.; Tiwari, R.N.; Duan, Z.; Liao, M.; Yoshimura, M.; Ohishi, Y. Size-dependent Upconversion Luminescence in Er3+/Yb3+ Codoped LiYF4 Nano/Microcrystals. In Proceedings of the Conference on Lasers and Electro-Optics/Pacific Rim, Kyoto, Japan, 30 June–4 July 2013; TuPI_28. Optica Publishing Group: Washington, DC, USA, 2013. [Google Scholar]
- Blasse, G.; Grabmaier, B.C.; Blasse, G.; Grabmaier, B.C. A General Introduction to Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–9. [Google Scholar]
- Ansari, A.A.; Parchur, A.K.; Nazeeruddin, M.K.; Tavakoli, M.M. Luminescent lanthanide nanocomposites in thermometry: Chemistry of dopant ions and host matrices. Coord. Chem. Rev. 2021, 444, 214040. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, G.; Li, X.; Jiang, S.; Wei, X.; Chen, Y.; Duan, C. Strategy for thermometry via Tm3+-doped NaYF4 core-shell nanoparticles. Opt. Lett. 2014, 39, 6687–6690. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, A.; Gavrilović, T.; Dramićanin, M.D. Luminescence intensity ratio thermometry with Er3+: Performance overview. Crystals 2021, 11, 189. [Google Scholar] [CrossRef]
- Jahanbazi, F.; Mao, Y. Recent advances on metal oxide-based luminescence thermometry. J. Mater. Chem. C 2021, 9, 16410–16439. [Google Scholar] [CrossRef]
- Li, W.; Hu, L.; Chen, W.; Sun, S.; Guzik, M.; Boulon, G. The effect of temperature on green and red upconversion emissions of LiYF4: 20Yb3+, 1Ho3+ and its application for temperature sensing. J. Alloys Compd. 2021, 866, 158813. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, S.; Wang, M.; Ren, H.; Tang, X.; Zou, Y.; Dou, R.; Liu, W. Upconversion luminescence and optical thermometry behaviors of Yb3+ and Ho3+ co-doped GYTO crystal. Front. Optoelectron. 2023, 16, 31. [Google Scholar] [CrossRef]
- Nonaka, T.; Sugiura, T.; Tsukamoto, T.; Yamamoto, S.I. Green upconversion luminescence and temperature sensitivity of LaOF: Yb, Ho phosphors. J. Korean Ceram. Soc. 2022, 59, 889–894. [Google Scholar] [CrossRef]
- Makumbane, V.; Yagoub, M.Y.; Xia, Z.; Kroon, R.E.; Swart, H.C. Up-Conversion Luminescence and Optical Temperature Sensing Behaviour of Y2O3: Ho3+, Yb3+ Phosphors. Crystals 2023, 13, 1288. [Google Scholar] [CrossRef]
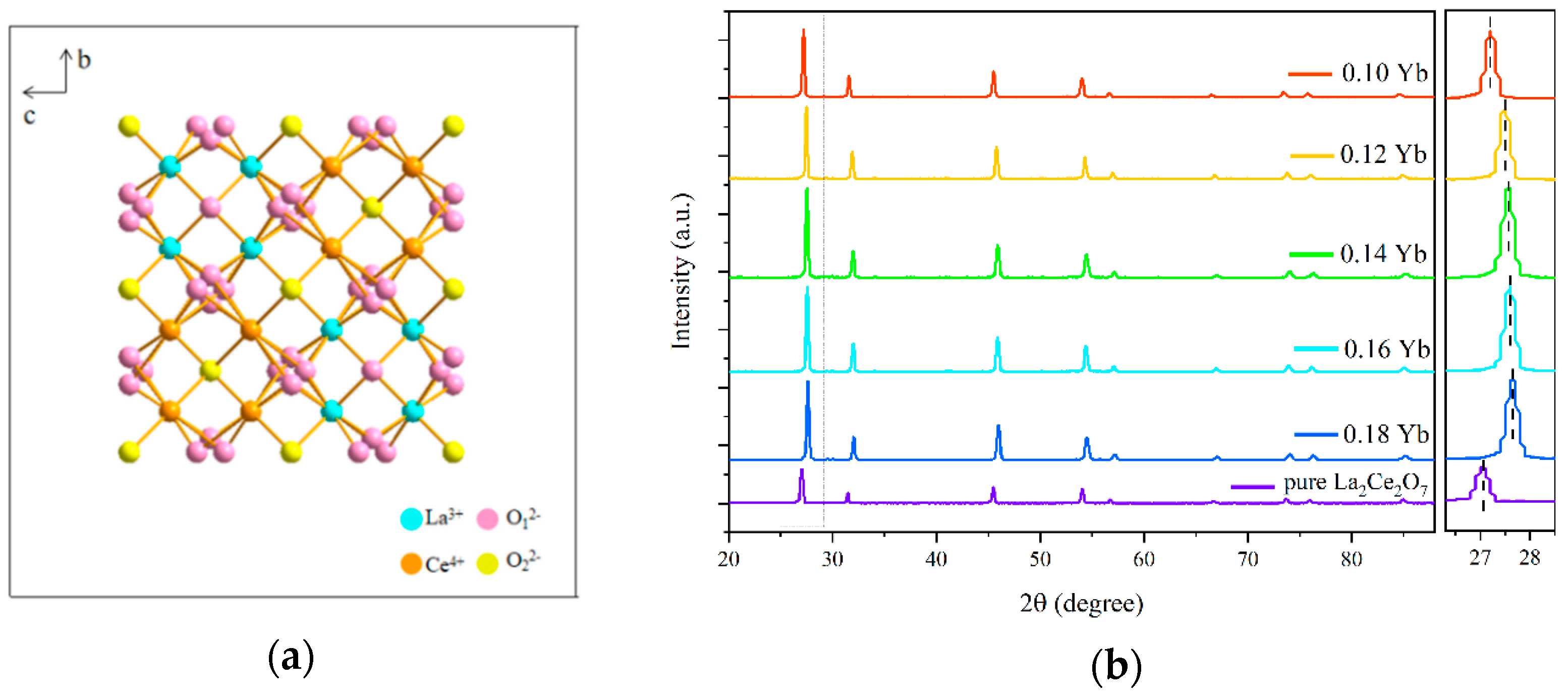
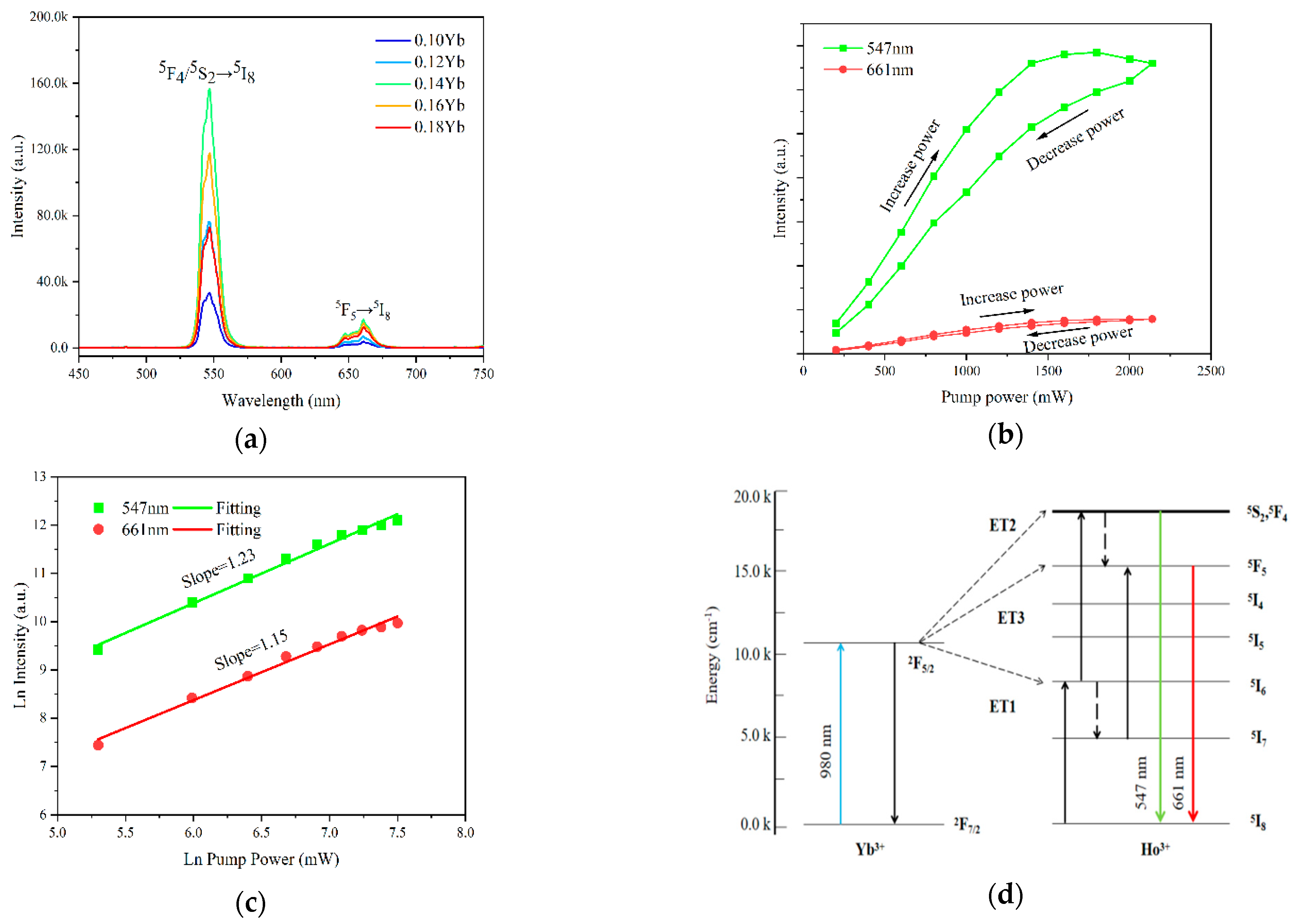
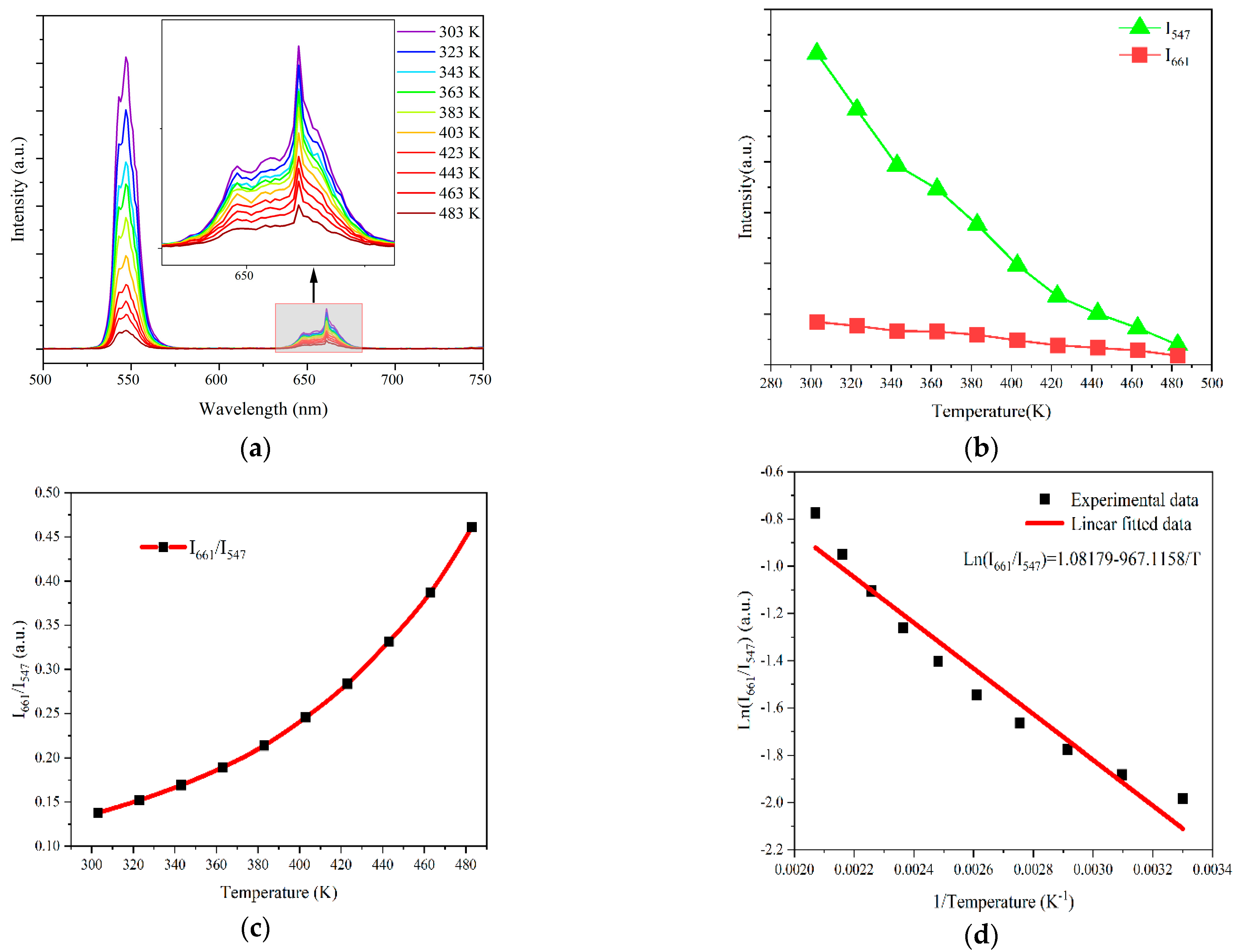
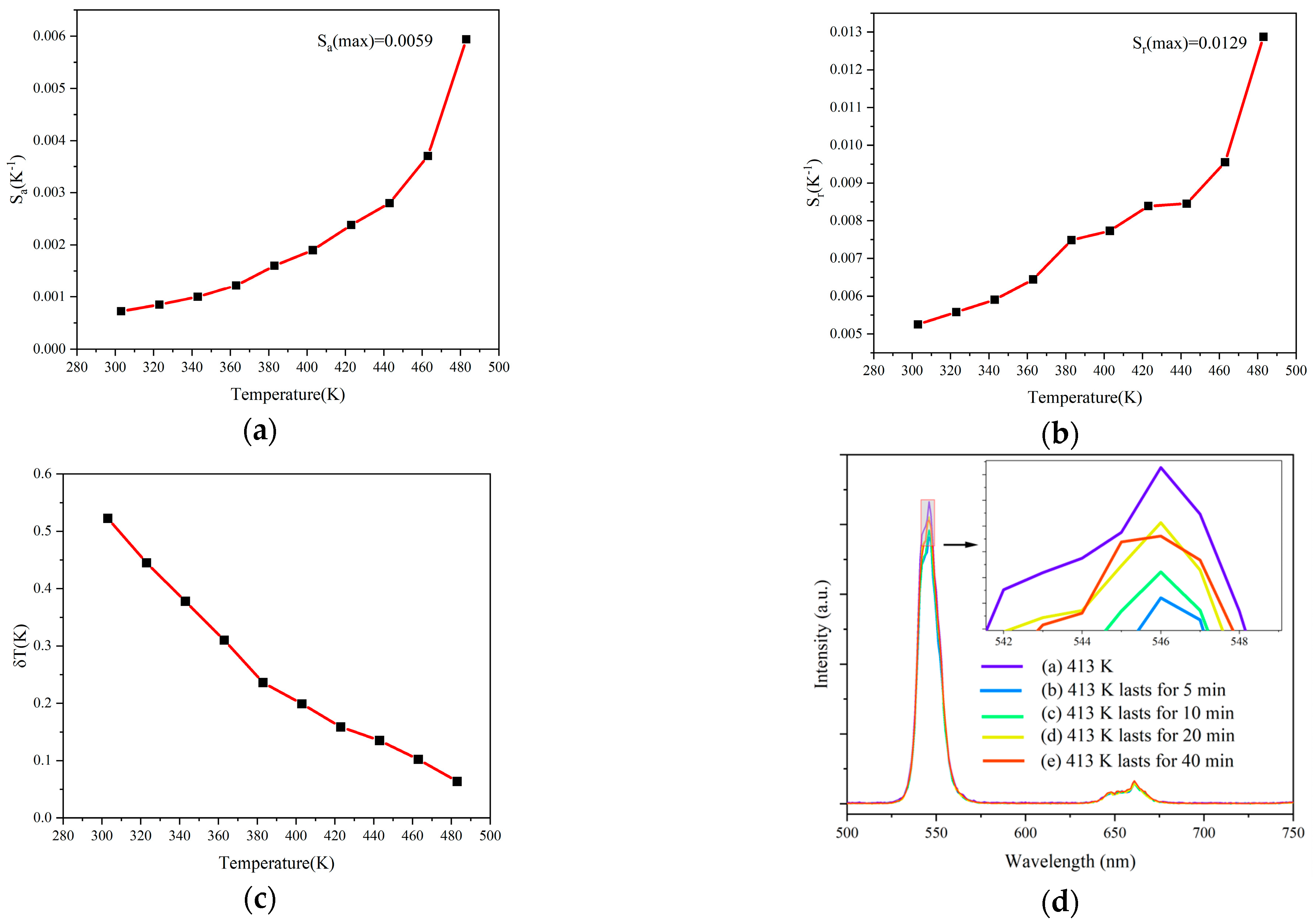
| Materials | Temperature Range (K) | λex (nm) | Sr (K−1) (Max) | References |
|---|---|---|---|---|
| NaLaMgWO6 | 293–553 | 980 | 0.01079 (508 K) | [2] |
| Ba0.77Ca0.23TiO3 | 93–300 | 980 | 0.0053 (93 K) | [10] |
| Tellurite glass | 303–503 | 975 | 0.011 (503 K) | [24] |
| BaTiO3 | 303–513 | 980 | 0.0034 (303 K) | [25] |
| CaMoO4 | 303–543 | 980 | 0.0066 (353 K) | [26] |
| LiYF4 | 100–500 | 976 | 0.0129 (150 K) | [39] |
| Gd0.74Y0.2TaO4 | 330–660 | 980 | 0.0037 (660 K) | [40] |
| LaOF | 298–548 | 980 | 0.00451 (298 K) | [41] |
| Y2O3 | 303–623 | 980 | 0.0064 (423 K) | [42] |
| (Ho0.005Yb0.14La0.855)2Ce2O7 | 293–428 | 980 | 0.0129 (428 K) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, J.; Lin, H.; Yu, D.; Hong, R.; Han, Z.; Tao, C.; Zhang, D. Optical Temperature-Sensing Performance of La2Ce2O7:Ho3+ Yb3+ Powders. Materials 2024, 17, 1692. https://doi.org/10.3390/ma17071692
Chao J, Lin H, Yu D, Hong R, Han Z, Tao C, Zhang D. Optical Temperature-Sensing Performance of La2Ce2O7:Ho3+ Yb3+ Powders. Materials. 2024; 17(7):1692. https://doi.org/10.3390/ma17071692
Chicago/Turabian StyleChao, Jiameng, Hui Lin, Dechao Yu, Ruijin Hong, Zhaoxia Han, Chunxian Tao, and Dawei Zhang. 2024. "Optical Temperature-Sensing Performance of La2Ce2O7:Ho3+ Yb3+ Powders" Materials 17, no. 7: 1692. https://doi.org/10.3390/ma17071692





