Synthesis and Transport Properties of ZnSnP2-yAsy Chalcopyrite Solid Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical and Structural Characterization
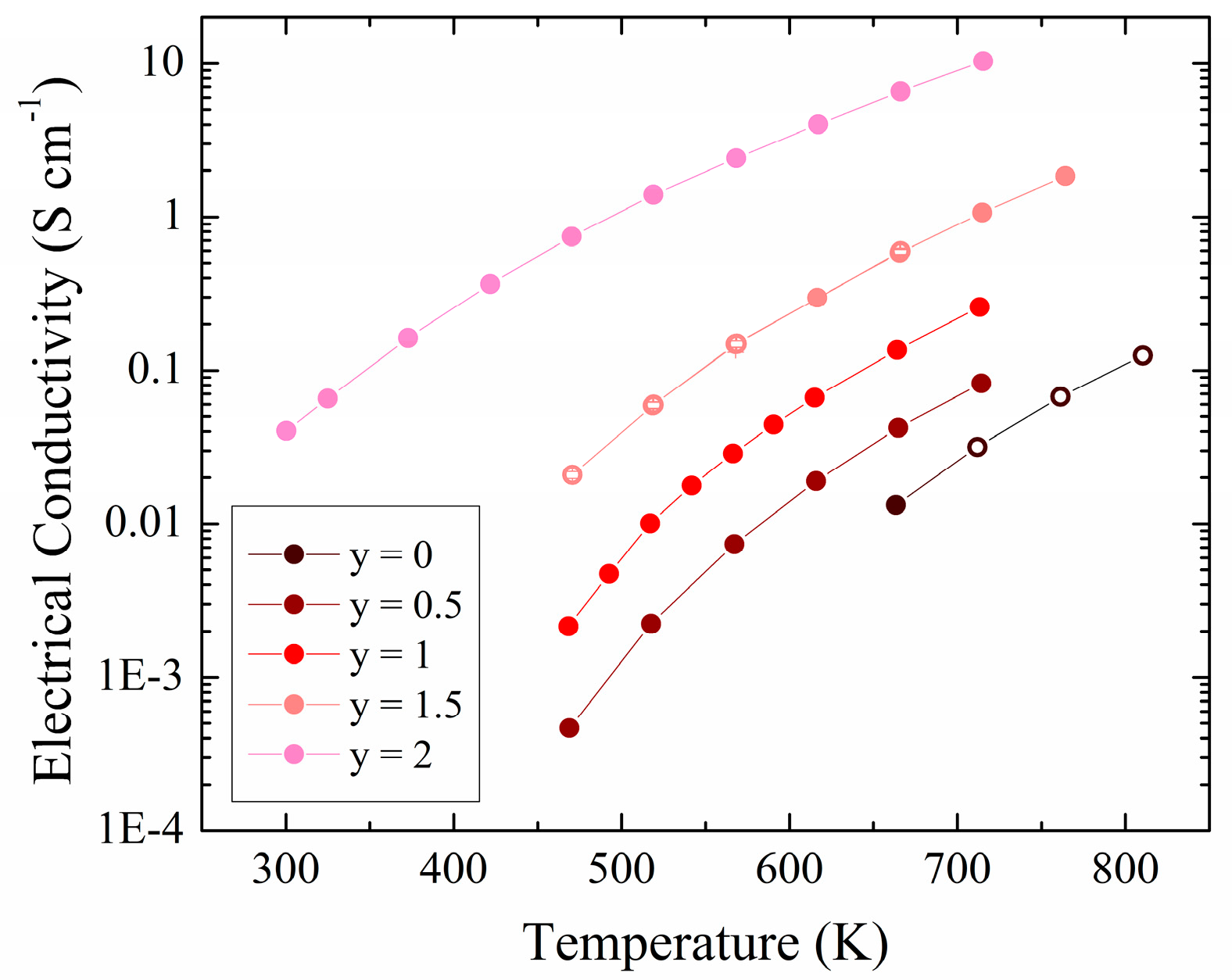
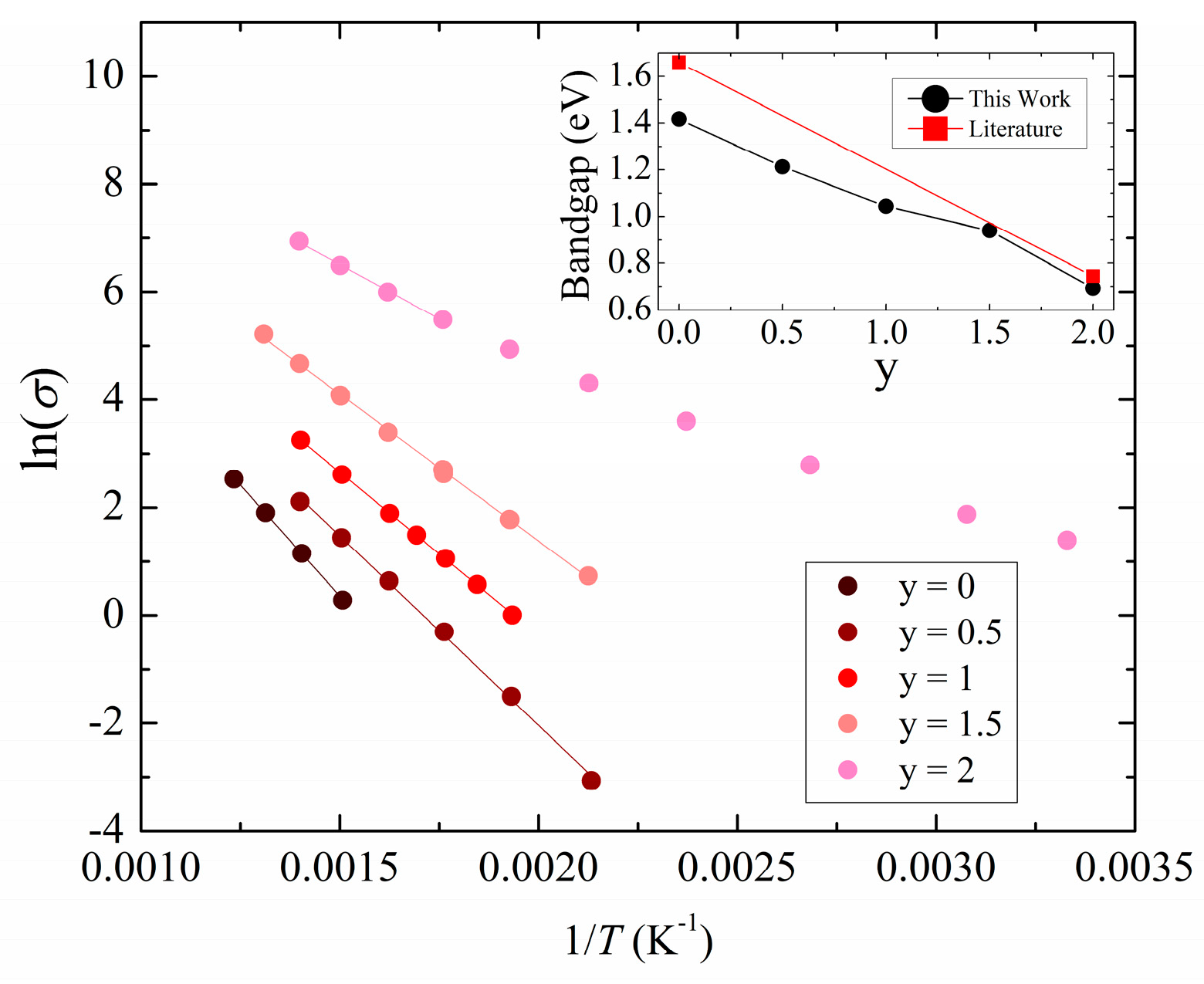
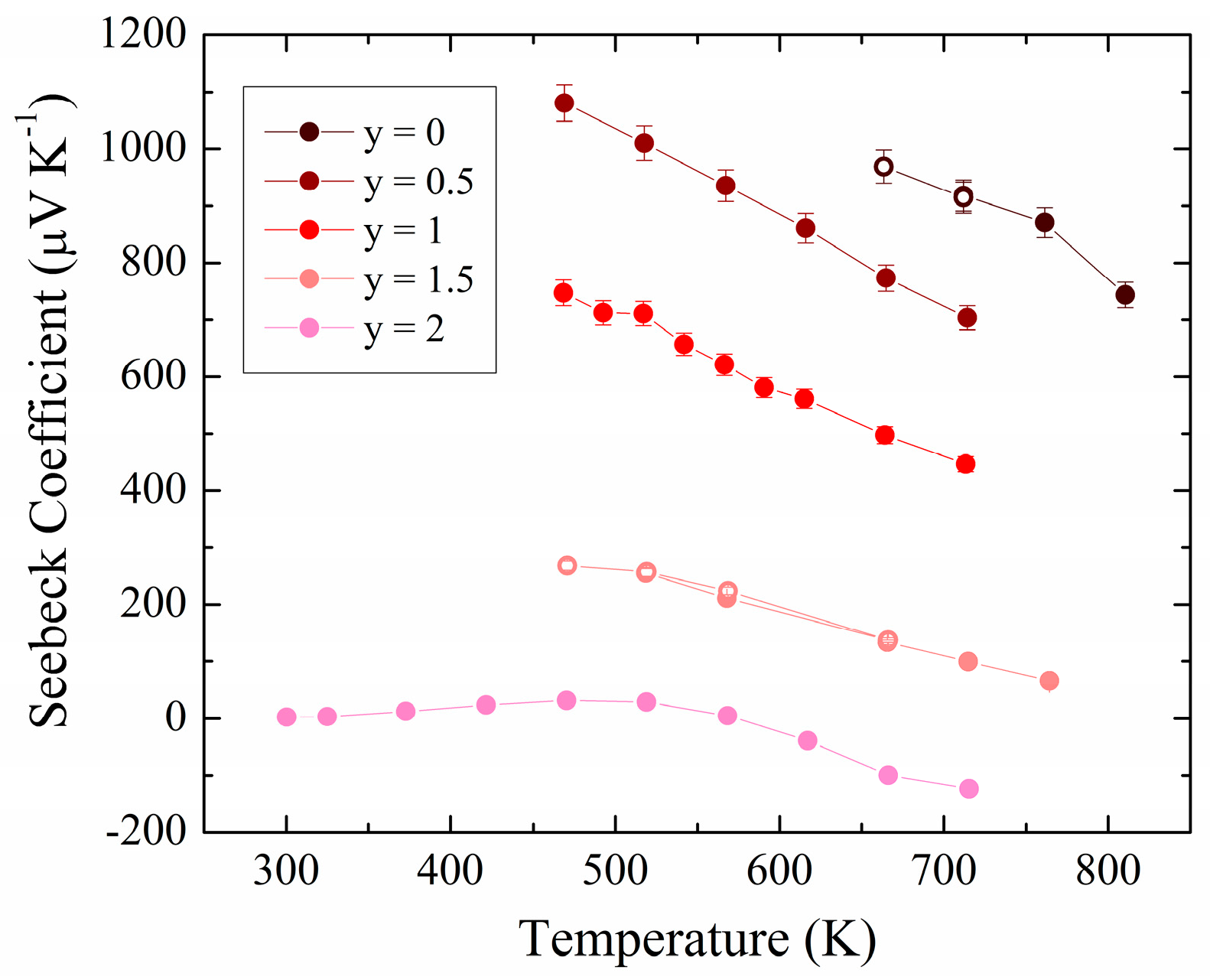
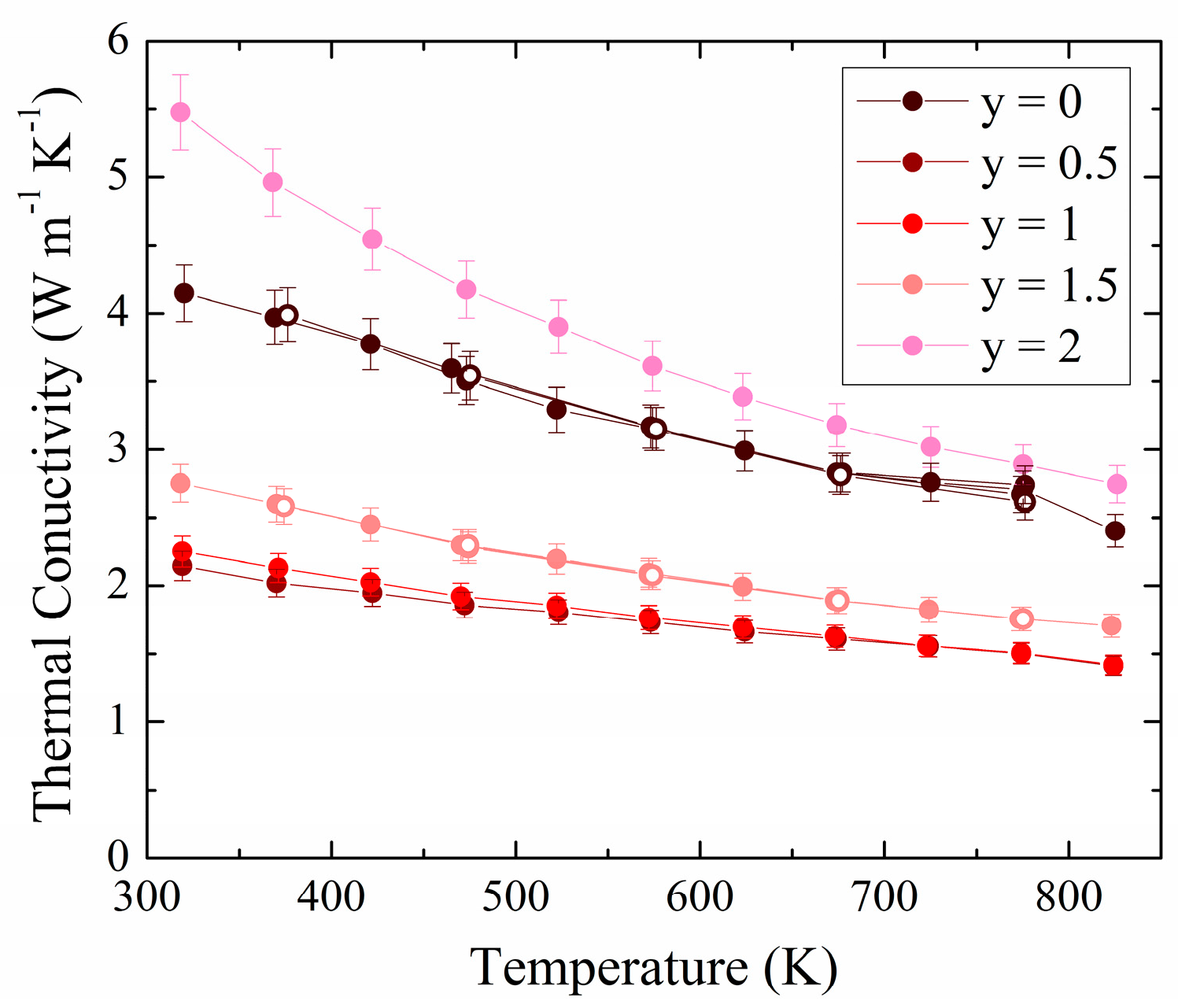
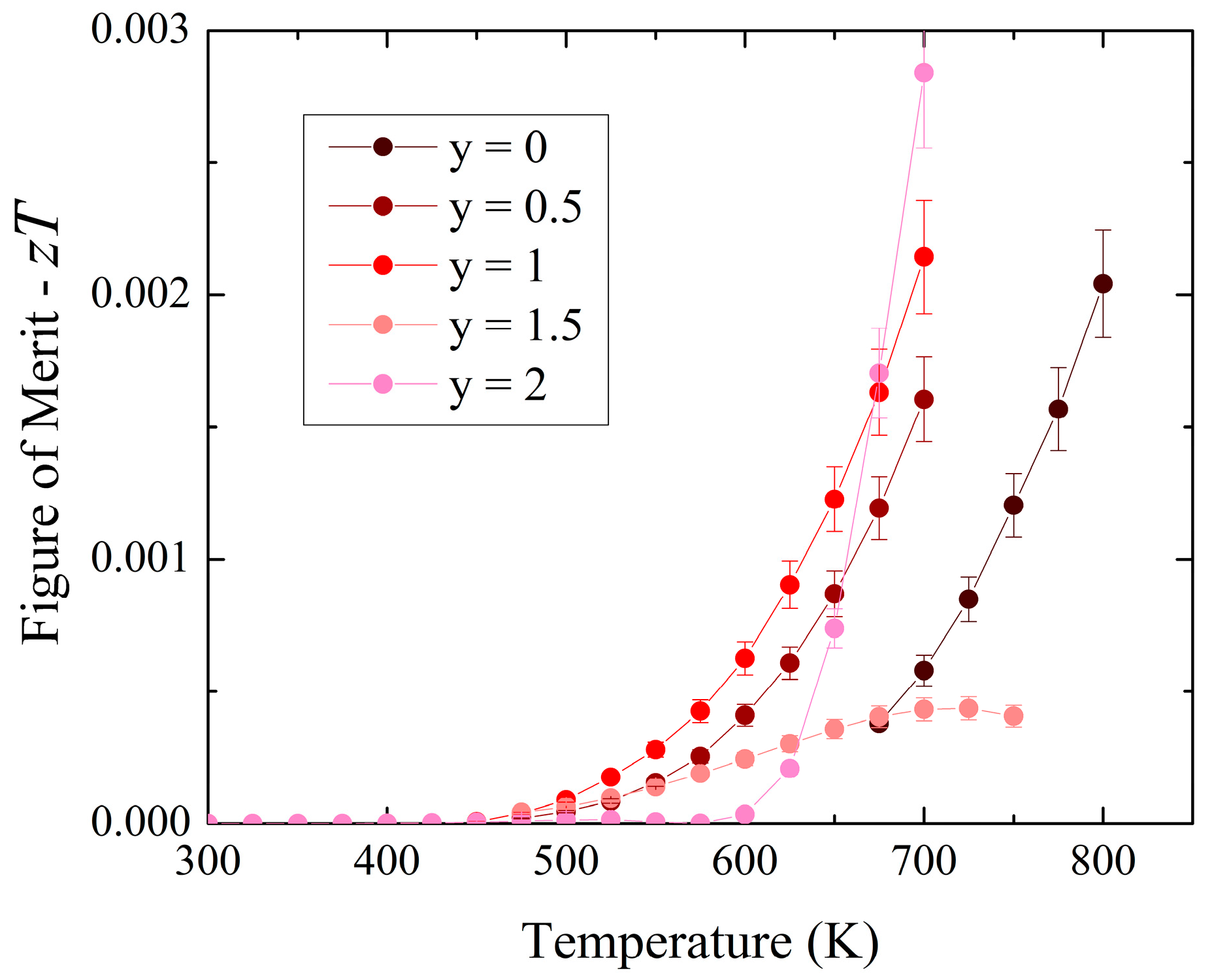
3.2. Experimental Physical Properties
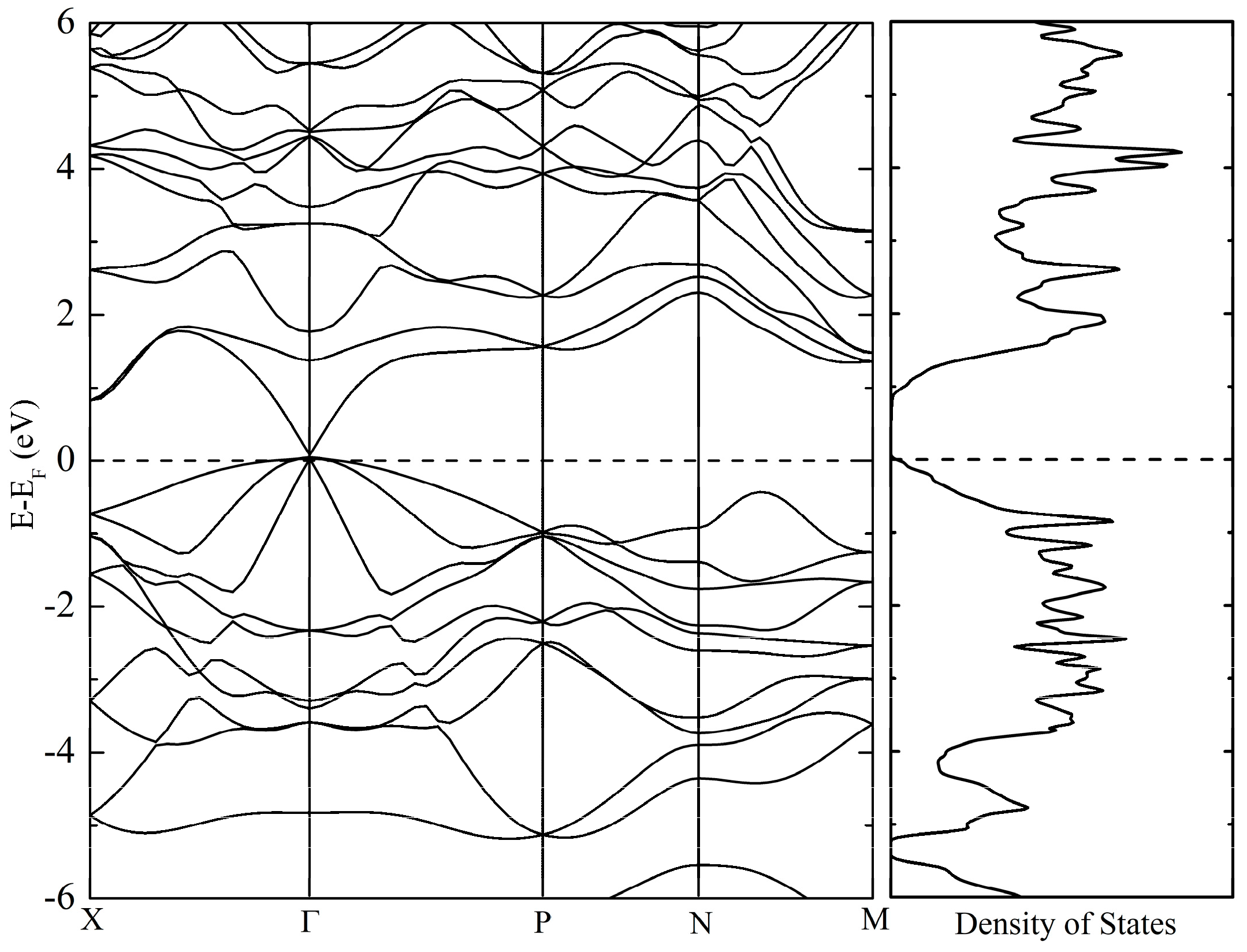
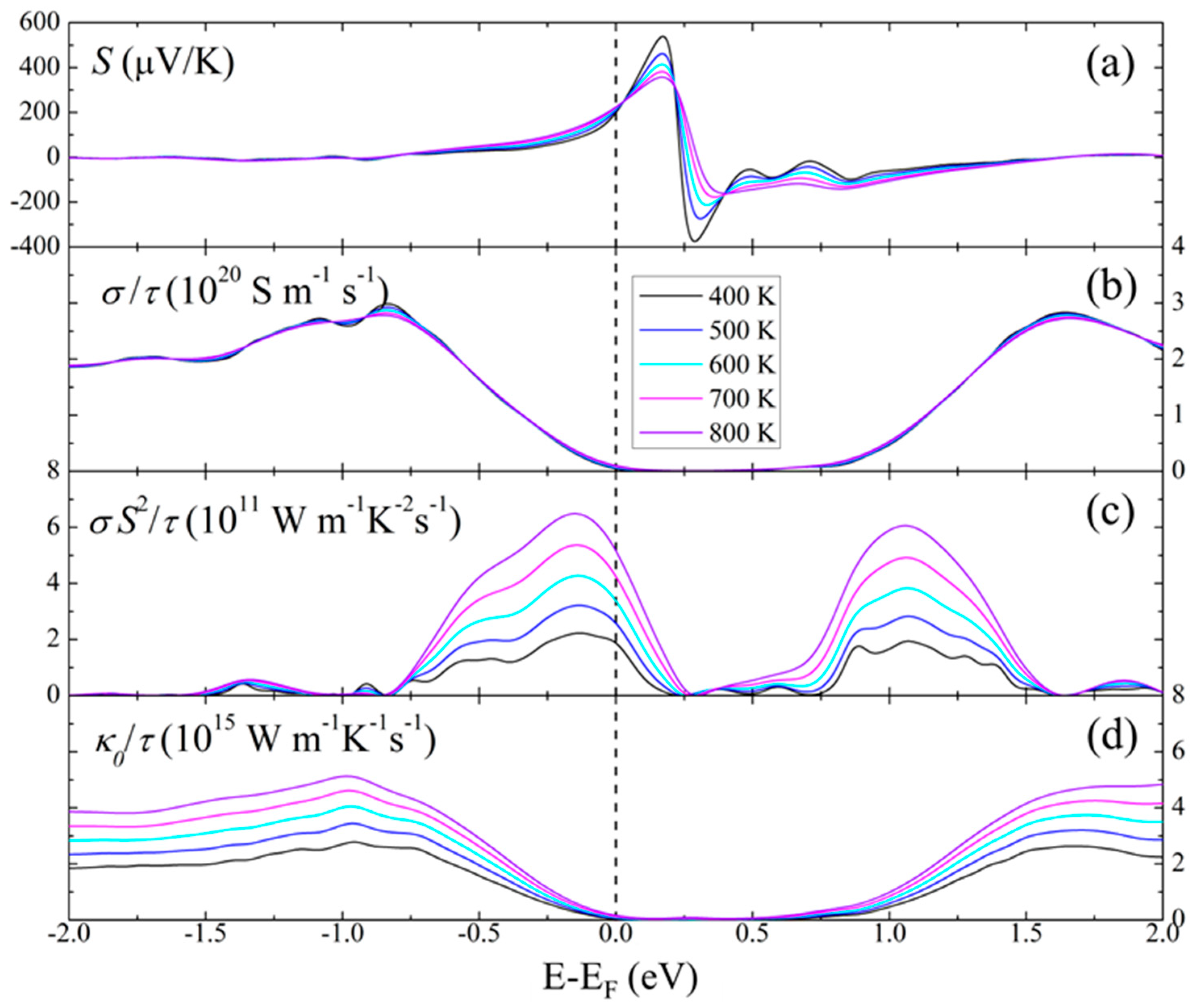
3.3. Calculated Physical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, S.R.; Chen, C.W.; Date, M.; Wang, Y.C.; Tsai, H.W.; Wang, Z.M.; Chueh, Y.L. Recent Developments in the Synthesis of Nanostructured Chalcopyrite Materials and Their Applications: A Review. RSC Adv. 2016, 6, 60643–60656. [Google Scholar] [CrossRef]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and Nonlinear Optical Properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Schunemann, P.G.; Schepler, K.L.; Budni, P.A. Nonlinear Frequency Conversion Performance of AgGaSe2, ZnGeP2, and CdGeAs2. MRS Bull. 1998, 23, 45–49. [Google Scholar] [CrossRef]
- Yang, S.; Lin, C.; Chen, K.; Fan, H.; Chen, J.; Fang, S.; Ye, N.; Luo, M. Trade-Off for Better Balanced Nonlinear Optical Performance with Disordered Si in ZnGeP2. Chem. Mater. 2022, 34, 11007–11013. [Google Scholar] [CrossRef]
- Kildal, H.; Mikkelsen, J.C. The Nonlinear Optical Coefficient, Phasematching, and Optical Damage in the Chalcopyrite AgGaSe2. Opt. Commun. 1973, 9, 315–318. [Google Scholar] [CrossRef]
- Kumar, V.; Tripathy, S.K.; Jha, V. Second Order Nonlinear Optical Properties of A IB IIIC 2VI Chalcopyrite Semiconductors. Appl. Phys. Lett. 2012, 101, 192105. [Google Scholar] [CrossRef]
- Yoneda, R.; Beppu, K.; Wada, T. CuInSe2 and Related I–III–VI2 Chalcopyrite Compounds for Photovoltaic Application. Jpn. J. Appl. Phys. 2021, 60, 080101. [Google Scholar] [CrossRef]
- Wagner, S. Preparation and Properties of Green-light-emitting CdS–CuGaS2 Heterodiodes. J. Appl. Phys. 1974, 45, 246–251. [Google Scholar] [CrossRef]
- Carr, W.D.; Morelli, D.T. Influence of Doping and Solid Solution Formation on the Thermoelectric Properties of Chalcopyrite Semiconductors. J. Alloys Compd. 2015, 630, 277–281. [Google Scholar] [CrossRef]
- Mukherjee, M.; Yumnam, G.; Singh, A.K. High Thermoelectric Figure of Merit via Tunable Valley Convergence Coupled Low Thermal Conductivity in AIIBIVC2V Chalcopyrites. J. Phys. Chem. C 2018, 122, 29150–29157. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kleinke, H. New Bulk Materials for Thermoelectric Power Generation: Clathrates and Complex Antimonides. Chem. Mater. 2010, 22, 604–611. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as Thermoelectric Materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Zaia, E.W.; Gordon, M.P.; Yuan, P.; Urban, J.J. Progress and Perspective: Soft Thermoelectric Materials for Wearable and Internet-of-Things Applications. Adv. Electron. Mater. 2019, 5, 1800823. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Park, G.; Park, S.; Khan, S.; Kim, J.; Kim, W. Energy Harvesting Using Thermoelectricity for IoT (Internet of Things) and E-Skin Sensors. J. Phys. Energy 2019, 1, 42001. [Google Scholar] [CrossRef]
- Freer, R.; Powell, A.V. Realising the Potential of Thermoelectric Technology: A Roadmap. J. Mater. Chem. C 2020, 8, 441–463. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.-J.; Liu, J. Suitability of Thermoelectric Power Generator for Implantable Medical Devices. J. Phys. D Appl. Phys. 2007, 40, 5790–5800. [Google Scholar] [CrossRef]
- Leonov, V.; Vullers, R.J.M. Wearable Electronics Self-Powered by Using Human Body Heat: The State of the Art and the Perspective. J. Renew. Sustain. Energy 2009, 1, 062701. [Google Scholar] [CrossRef]
- Francioso, L.; De Pascali, C.; Farella, I.; Martucci, C.; Cretì, P.; Siciliano, P.; Perrone, A. Flexible Thermoelectric Generator for Ambient Assisted Living Wearable Biometric Sensors. J. Power Sources 2011, 196, 3239–3243. [Google Scholar] [CrossRef]
- Tian, R.; Liu, Y.; Koumoto, K.; Chen, J. Body Heat Powers Future Electronic Skins. Joule 2019, 3, 1399–1403. [Google Scholar] [CrossRef]
- Slack, G.A. New Materials and Performance Limits for Thermoelectric Cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance Bulk Thermoelectrics with All-Scale Hierarchical Architecture. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Gahtori, B.; Bathula, S.; Tyagi, K.; Jayasimhadri, M.; Srivastava, A.K.; Singh, S.; Budhani, R.C.; Dhar, A. Giant Enhancement in Thermoelectric Performance of Copper Selenide by Incorporation of Different Nanoscale Dimensional Defect Features. Nano Energy 2015, 13, 36–46. [Google Scholar] [CrossRef]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial Indium Solubility Induces Chemical Stability and Colossal Thermoelectric Figure of Merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Wei, W.; Chang, C.; Yang, T.; Liu, J.; Tang, H.; Zhang, J.; Li, Y.; Xu, F.; Zhang, Z.; Li, J.F.; et al. Achieving High Thermoelectric Figure of Merit in Polycrystalline SnSe via Introducing Sn Vacancies. J. Am. Chem. Soc. 2018, 140, 499–505. [Google Scholar] [CrossRef]
- Chandra, S.; Biswas, K. Realization of High Thermoelectric Figure of Merit in Solution Synthesized 2D SnSe Nanoplates via Ge Alloying. J. Am. Chem. Soc. 2019, 141, 6141–6145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lee, Y.K.; Yu, Y.; Byun, S.; Luo, Z.Z.; Lee, H.; Ge, B.; Lee, Y.L.; Chen, X.; Lee, J.Y.; et al. Polycrystalline SnSe with a Thermoelectric Figure of Merit Greater than the Single Crystal. Nat. Mater. 2021, 20, 1378–1384. [Google Scholar] [CrossRef]
- Nuss, J.; Wedig, U.; Xie, W.; Yordanov, P.; Bruin, J.; Hübner, R.; Weidenkaff, A.; Takagi, H. Phosphide–Tetrahedrite Ag6Ge10P12: Thermoelectric Performance of a Long-Forgotten Silver-Cluster Compound. Chem. Mater. 2017, 29, 6956–6965. [Google Scholar] [CrossRef]
- Suwardi, A.; Hu, L.; Wang, X.; Tan, X.Y.; Repaka, D.V.M.; Wong, L.-M.; Ni, X.; Liew, W.H.; Lim, S.H.; Yan, Q.; et al. Origin of High Thermoelectric Performance in Earth-Abundant Phosphide–Tetrahedrite. ACS Appl. Mater. Interfaces 2020, 12, 9150–9157. [Google Scholar] [CrossRef]
- Mark, J.; Mori, T. Thermoelectric Performance Enhancement of the Cost-Effective Phosphide ZnCu2P8. ACS Appl. Energy Mater. 2021, 4, 4861–4866. [Google Scholar] [CrossRef]
- Quinn, R.J.; Bos, J.W.G. Recent Progress in Phosphide Materials for Thermoelectric Conversion. J. Mater. Chem. A 2023, 11, 8453–8469. [Google Scholar] [CrossRef]
- Sreeparvathy, P.C.; Kanchana, V.; Vaitheeswaran, G. Thermoelectric Properties of Zinc Based Pnictide Semiconductors. J. Appl. Phys. 2016, 119, 85701. [Google Scholar] [CrossRef]
- Ramirez, D.; Menezes, L.T.; Kleinke, H. Thermoelectric Properties of the Chalcopyrite Solid Solutions ZnGe1−XSnxP2. ACS Appl. Electron. Mater. 2024, in press. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. GSAS—General Structure Analysis System; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Wang, H.; Porter, W.D.; Böttner, H.; König, J.; Chen, L.; Bai, S.; Tritt, T.M.; Mayolet, A.; Senawiratne, J.; Smith, C.; et al. Transport Properties of Bulk Thermoelectrics—An International Round-Robin Study, Part I: Seebeck Coefficient and Electrical Resistivity. J. Electron. Mater. 2013, 42, 654–664. [Google Scholar] [CrossRef]
- Wang, H.; Porter, W.D.; Böttner, H.; König, J.; Chen, L.; Bai, S.; Tritt, T.M.; Mayolett, A.; Senawiratne, J.; Smith, C.; et al. Transport Properties of Bulk Thermoelectrics: An International Round-Robin Study, Part II: Thermal Diffusivity, Specific Heat, and Thermal Conductivity. J. Electron. Mater. 2013, 42, 1073–1084. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D.; Luitz, J. WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties; Schwarz, K., Ed.; Technische Universität Wien: Vienna, Austria, 2001; ISBN 3-9501031-1-2. [Google Scholar]
- Schwarz, K. DFT Calculations of Solids with LAPW and WIEN2k. J. Solid State Chem. 2003, 176, 319–328. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.K.H.; Marks, L.D. WIEN2k: An APW+lo Program for Calculating the Properties of Solids. J. Chem. Phys. 2020, 152, 074101. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Carrete, J.; Verstraete, M.J. BoltzTraP2, a Program for Interpolating Band Structures and Calculating Semi-Classical Transport Coefficients. Comput. Phys. Commun. 2018, 231, 140–145. [Google Scholar] [CrossRef]
- Bilc, D.I.; Hautier, G.; Waroquiers, D.; Rignanese, G.M.; Ghosez, P. Low-Dimensional Transport and Large Thermoelectric Power Factors in Bulk Semiconductors by Band Engineering of Highly Directional Electronic States. Phys. Rev. Lett. 2015, 114, 136601. [Google Scholar] [CrossRef]
- Amsler, M.; Ward, L.; Hegde, V.I.; Goesten, M.G.; Yi, X.; Wolverton, C. Ternary Mixed-Anion Semiconductors with Tunable Band Gaps from Machine-Learning and Crystal Structure Prediction. Phys. Rev. Mater. 2019, 3, 035404. [Google Scholar] [CrossRef]
- Yousuf, S.; Gupta, D.C. Thermoelectric Response of ZrNiSn and ZrNiPb Half-Heuslers: Applicability of Semi-Classical Boltzmann Transport Theory. Results Phys. 2019, 12, 1382–1386. [Google Scholar] [CrossRef]
- Manimaran, M.; Kalkura, S.N.; Ramasamy, P. Crystallization of ZnSnAs2 by Physical Vapour Transport. J. Mater. Sci. Lett. 1995, 14, 1366–1368. [Google Scholar] [CrossRef]
- Masumoto, K.; Isomura, S. The Preparation and Semiconducting Properties of Single Crystals of ZnSnAs2 Compound B. J. Phys. Chem. Solids 1965, 26, 163–171. [Google Scholar] [CrossRef]
- Gasson, D.B.; Holmes, P.J.; Jennings, I.C.; Marathe, B.R.; Parrott, J.E. The Properties of ZnSnAs2 and CdSnAs2. J. Phys. Chem. Solids 1962, 23, 1291–1302. [Google Scholar] [CrossRef]
- Shaban, H.T.; Mobarak, M.; Nassary, M.M. Characterization of CuInSe2 Single Crystal. Phys. B Condens. Matter 2007, 389, 351–354. [Google Scholar] [CrossRef]
- Wang, K.; Qin, P.; Ge, Z.H.; Feng, J. Highly Enhanced Thermoelectric Properties of P-Type CuInSe2 Alloys by the Vacancy Doping. Scr. Mater. 2018, 149, 88–92. [Google Scholar] [CrossRef]
- St-Jean, P.; Seryogin, G.A.; Francoeur, S. Band Gap of Sphalerite and Chalcopyrite Phases of Epitaxial ZnSnP2. Appl. Phys. Lett. 2010, 96, 231913. [Google Scholar] [CrossRef]
- Yao, J.; Takas, N.J.; Schliefert, M.L.; Paprocki, D.S.; Blanchard, P.E.R.; Gou, H.; Mar, A.; Exstrom, C.L.; Darveau, S.A.; Poudeu, P.F.P.; et al. Thermoelectric Properties of P-Type CuInSe2 Chalcopyrites Enhanced by Introduction of Manganese. Phys. Rev. B—Condens. Matter Mater. Phys. 2011, 84, 075203. [Google Scholar] [CrossRef]
- Dewasurendra, V.; Schunemann, P.G.; Bristow, A.D.; Zawilski, K.T.; Piyathilaka, H.P.; Sooriyagoda, R.; Johnson, M.B. Terahertz Generation by Optical Rectification in Chalcopyrite Crystals ZnGeP2, CdGeP2 and CdSiP2. Opt. Express 2019, 27, 16958–16965. [Google Scholar] [CrossRef]

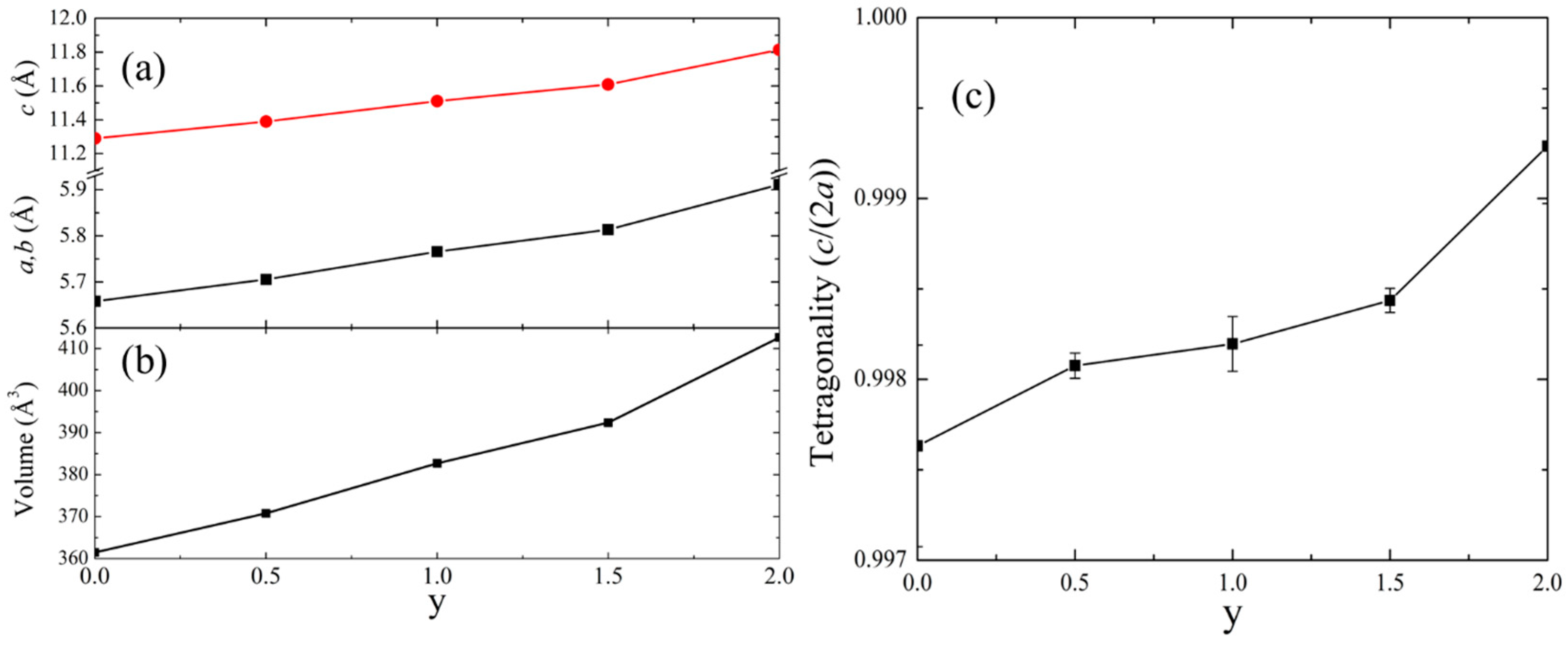

| y | a, b (Å) | c (Å) | V (Å3) | c/(2a) |
|---|---|---|---|---|
| 0.0 | 5.6584(14) | 11.290(4) | 361.48(30) | 0.9976(5) |
| 0.5 | 5.7057(2) | 11.3895(5) | 370.79(1) | 0.9981(1) |
| 1.0 | 5.7659(4) | 11.511(1) | 382.69(3) | 0.9982(2) |
| 1.5 | 5.8133(2) | 11.6085(5) | 392.31(1) | 0.9984(1) |
| 2.0 | 5.9112(8) | 11.814(2) | 412.81(5) | 0.9993(3) |
| Element | y = 0.5 | y = 1.0 | y = 1.5 | y = 2.0 |
|---|---|---|---|---|
| Zn | 24.8 | 23.9 | 24.8 | 21.9 |
| Sn | 25.8 | 24.8 | 25.6 | 32.1 |
| P | 37.5 | 27.1 | 12.7 | - |
| As | 11.9 | 24.2 | 36.9 | 45.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, D.; Menezes, L.T.; Kleinke, H. Synthesis and Transport Properties of ZnSnP2-yAsy Chalcopyrite Solid Solutions. Materials 2024, 17, 1712. https://doi.org/10.3390/ma17081712
Ramirez D, Menezes LT, Kleinke H. Synthesis and Transport Properties of ZnSnP2-yAsy Chalcopyrite Solid Solutions. Materials. 2024; 17(8):1712. https://doi.org/10.3390/ma17081712
Chicago/Turabian StyleRamirez, Daniel, Luke T. Menezes, and Holger Kleinke. 2024. "Synthesis and Transport Properties of ZnSnP2-yAsy Chalcopyrite Solid Solutions" Materials 17, no. 8: 1712. https://doi.org/10.3390/ma17081712






