Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Preparation and Characterization of Calendulae flos Lyophilized Extracts, and Investigation of Biological Activity
2.4. Electrospun Nanofiber Preparation
2.5. Identification of the Electrospun Nanofibers
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. X-ray Diffraction (XRPD)
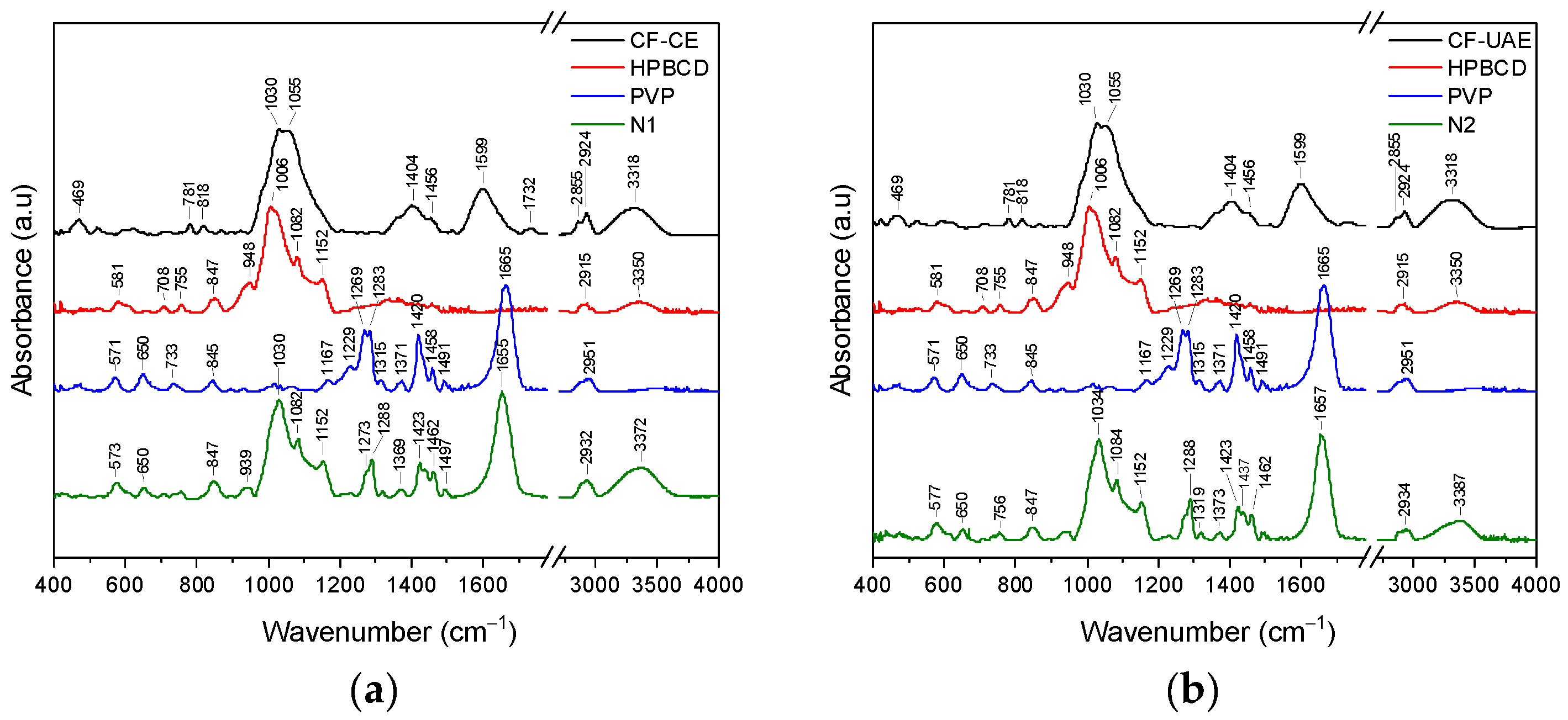
2.5.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (ATR-FTIR) and DFT Study
2.6. Studies of Electrospun Nanofiber’s Functionality
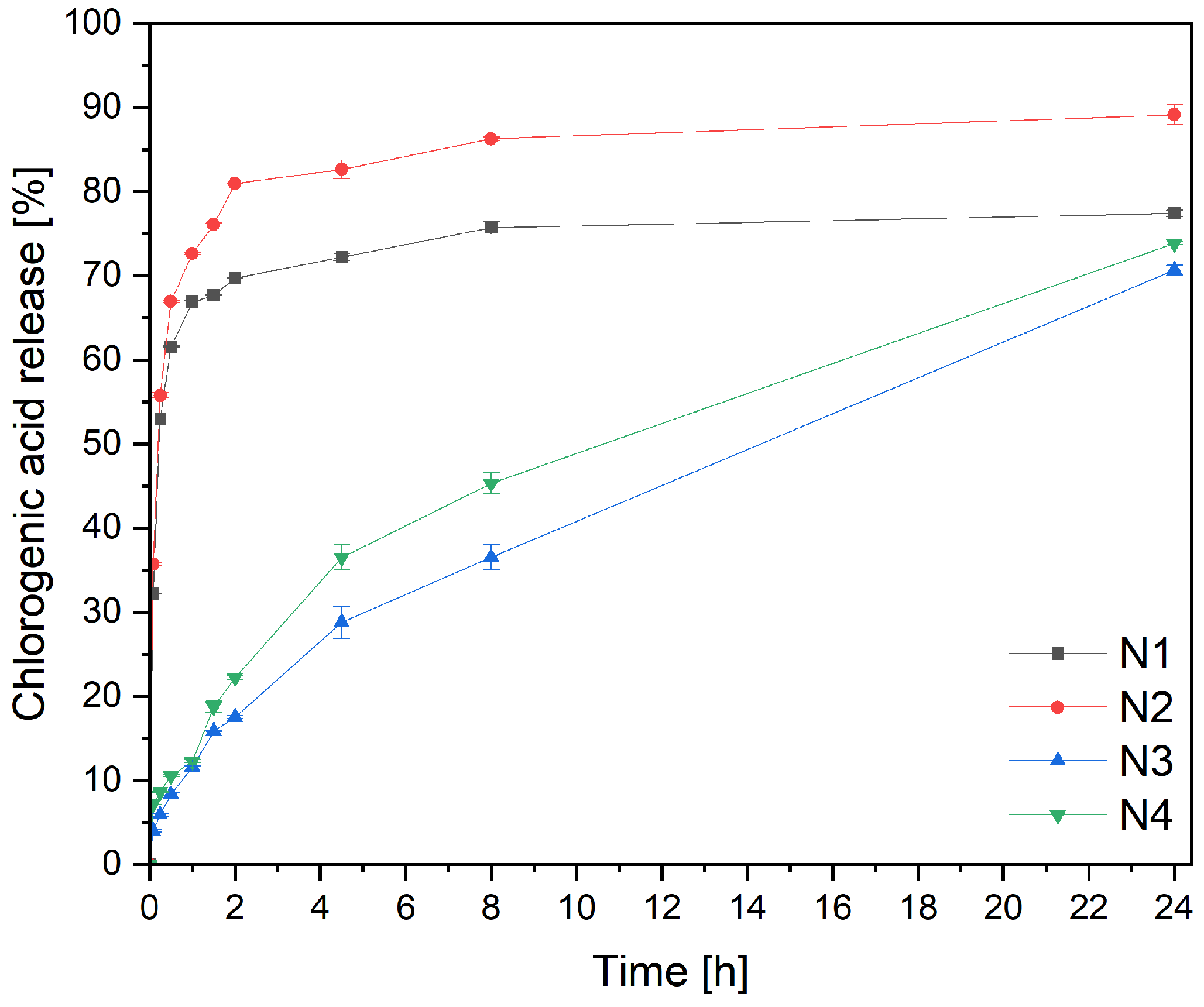
2.6.1. Release of Active Components
2.6.2. Microbiological Activity
2.6.3. Wound Healing Assay
2.7. Statistical Analysis
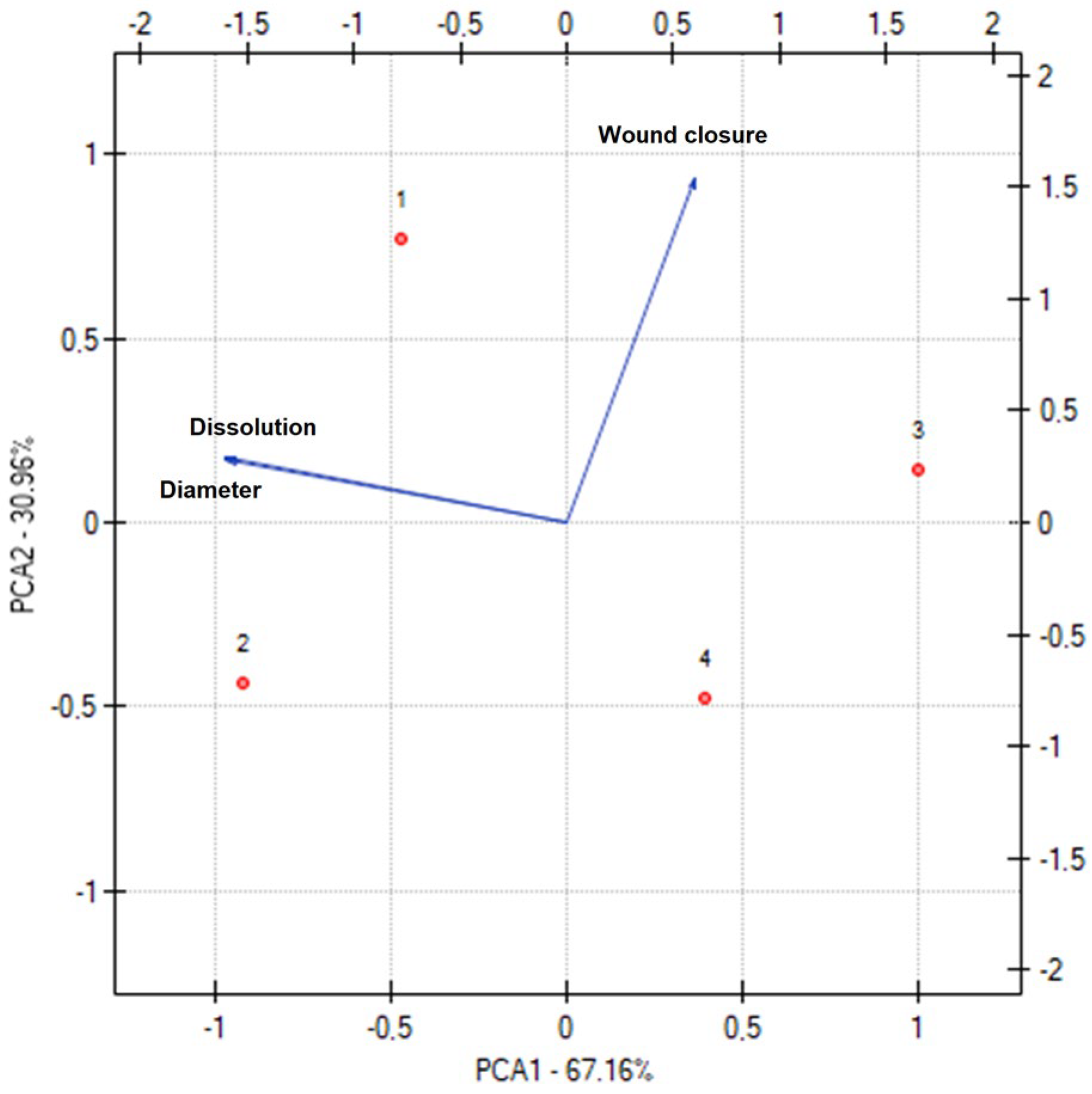
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic Wounds: Current Status, Available Strategies and Emerging Therapeutic Solutions. J. Control Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent Advances on Biomedical Applications of Scaffolds in Wound Healing and Dermal Tissue Engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Ersanli, C.; Voidarou, C.; Tzora, A.; Fotou, K.; Zeugolis, D.I.; Skoufos, I. Electrospun Scaffolds as Antimicrobial Herbal Extract Delivery Vehicles for Wound Healing. J. Funct. Biomater. 2023, 14, 481. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Therapeutic Applications of Electrospun Nanofibers Impregnated with Various Biological Macromolecules for Effective Wound Healing Strategy—A Review. Biomed. Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Plech, T.; Szymanowska, D.; Michniak-Kohn, B.; Cielecka-Piontek, J. Chitosan-Based Hydrogels for Controlled Delivery of Asiaticoside-Rich Centella Asiatica Extracts with Wound Healing Potential. Int. J. Mol. Sci. 2023, 24, 17229. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal Plants and Their Components for Wound Healing Applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaproń, B.; Plech, T.; Szymanowska, D.; Karaźniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae Flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Calendulae Flos—Herbal Medicinal Product | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/herbal/calendulae-flos (accessed on 9 January 2024).
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In Vitro Studies to Evaluate the Wound Healing Properties of Calendula officinalis Extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A Systematic Review of Calendula officinalis Extract for Wound Healing. Wound Repair. Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula Flower Extract Loaded PVA Hydrogel Sheet for Wound Management: Optimization, Characterization and in-Vivo Study. J. Drug Deliv. Sci. Technol. 2022, 68, 103035. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef] [PubMed]
- Kharat, Z.; Amiri Goushki, M.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO Nanofibers Containing Calendula officinalis Extract: Preparation, Characterization, in Vitro and in Vivo Evaluation for Wound Healing Applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Osanloo, M.; Noori, F.; Tavassoli, A.; Ataollahi, M.R.; Davoodi, A.; Seifalah-Zade, M.; Taghinezhad, A.; Fereydouni, N.; Goodarzi, A. Effect of PCL Nanofiber Mats Coated with Chitosan Microcapsules Containing Cinnamon Essential Oil for Wound Healing. BMC Complement. Med. Ther. 2023, 23, 84. [Google Scholar] [CrossRef]
- Azizi, M.; Azimzadeh, M.; Afzali, M.; Alafzadeh, M.; Hossein, S. Characterization and Optimization of Using Calendula officinalis Extract in The Fabrication of Polycaprolactone/Gelatin Electrospun Nanofibers for Wound Dressing Applications. J. Adv. Mater. Process. 2018, 6, 34–46. [Google Scholar]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and Characterization of Calendula Officinalis-Loaded PCL/Gum Arabic Nanocomposite Scaffolds for Wound Healing Applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- European Medicines Agency. European Pharmacopoeia. Available online: https://scholar.google.com/scholar_lookup?title=European+Pharmacopoeia+(01/2011:1297)+Monograph+Calendulae+flos&author=European+Medicines+Agency&publication_year=2011 (accessed on 3 January 2024).
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Cielecka-Piontek, J. Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni Cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin? Nutrients 2022, 14, 3897. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Michniak-Kohn, B.; Cielecka-Piontek, J. The Antioxidant Potential of Resveratrol from Red Vine Leaves Delivered in an Electrospun Nanofiber System. Antioxidants 2023, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Golchin, A.; Mahmoodinia Maymand, M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun Polycaprolactone Nanofibers: Current Research and Applications in Biomedical Application. Adv. Pharm. Bull. 2022, 12, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Qian, Y.; Lv, L.; Li, X.; Liu, Y. Characteristics of MgO/PCL/PVP Antibacterial Nanofiber Membranes Produced by Electrospinning Technology. Surf. Interfaces 2022, 28, 101661. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, C.; Yang, Z.; Cai, Z.; Deng, Z.; Wu, D. Polycaprolactone/Polyvinyl Pyrrolidone Nanofibers Developed by Solution Blow Spinning for Encapsulation of Chlorogenic Acid. Food Qual. Saf. 2022, 6, fyac014. [Google Scholar] [CrossRef]
- Varsei, M.; Tanha, N.R.; Gorji, M.; Mazinani, S. Fabrication and Optimization of PCL/PVP Nanofibers with Lawsonia Inermis for Antibacterial Wound Dressings. Polym. Polym. Compos. 2021, 29 (Suppl. 9), S1403–S1413. [Google Scholar] [CrossRef]
- Valkama, E.; Haluska, O.; Lehto, V.-P.; Korhonen, O.; Pajula, K. Production and Stability of Amorphous Solid Dispersions Produced by a Freeze-Drying Method from DMSO. Int. J. Pharm. 2021, 606, 120902. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, E.M.; Abdelghany, A.M.; Badr, S.I.; Morsi, M.A. Structural, Optical, Morphological and Thermal Properties of PEO/PVP Blend Containing Different Concentrations of Biosynthesized Au Nanoparticles. J. Mater. Res. Technol. 2018, 7, 419–431. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers 2019, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Chanaj-Kaczmarek, J.; Rosiak, N.; Szymanowska, D.; Rajewski, M.; Wender-Ozegowska, E.; Cielecka-Piontek, J. The Chitosan-Based System with Scutellariae Baicalensis Radix Extract for the Local Treatment of Vaginal Infections. Pharmaceutics 2022, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia Glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Pagès, M.; Violleau, F.; Marsan, O.; Manero, M.-H.; Richard, R.; Torré, J.-P. Ozonized 2-Hydroxypropyl-β-Cyclodextrins as Novel Materials with Oxidative and Bactericidal Properties. Carbohydr. Polym. 2022, 291, 119516. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Rosiak, N.; Tykarska, E.; Kozak, M.; Jenczyk, J.; Szulc, P.; Kobus-Cisowska, J.; Lewandowska, K.; Płazińska, A.; Płaziński, W.; et al. Combinations of Piperine with Hydroxypropyl-β-Cyclodextrin as a Multifunctional System. Int. J. Mol. Sci. 2021, 22, 4195. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Inclusion Complexes of Bendamustine with β-CD, HP-β-CD and Epi-β-CD: In-Vitro and in-Vivo Evaluation. Drug Dev. Ind. Pharm. 2015, 41, 1978–1988. [Google Scholar] [CrossRef]
- Freitas, D.F.S.; Mattos, G.C.; Mendes, L.C. The Role of Octadecylamine as Zirconium Phosphate Intercalating Agent on Poly(Vinyl Alcohol)/Poly(N-Vinyl-2-Pyrrolidone) Biodegradable Systems. J. Therm. Anal. Calorim. 2022, 147, 315–325. [Google Scholar] [CrossRef]
- Teoh, X.-Y.; Yeoh, Y.; Yoong, L.-K.; Chan, S.-Y. Sustainable Dissolution Performance of a Carrier Tailored Electrospun. Pharm. Res. 2020, 37, 28. [Google Scholar] [CrossRef]
- Gayo, Z.; Lucida, H.; Zaini, E. Solid dispersion of quercetin-PVP K-30 and its effects on the antioxidant activity. JIF 2020, 16, 144–154. [Google Scholar] [CrossRef]
- Erizal, E.; Tjahyono, T.; Dian, P.P.; Darmawan, D. Synthesis of Polyvinyl Pirrolidone (PVC)/Κ-Carrageenan Hydrogel Prepared by Gamma Radiation Processing As a Function of Dose and PVP Concentration. Indones. J. Chem. 2013, 13, 41–46. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. Available online: https://www.semanticscholar.org/paper/Kinetic-Thermal-Degradation-of-Cellulose%2C-Succinate-Abderrahim-Abderrahman/5c99b0eb0b3947274161326b963f12931bf42940 (accessed on 9 January 2024).
- Meenarathi, B.; Chen, H.-H.; Chen, P.-H.; Anbarasan, R. Synthesis and Characterization of Fluorescent Bio-Degradable Poly (ε-Caprolactone). Int. J. Plast. Technol. 2014, 18, 135–145. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef]
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun Nanofibers of Polycaprolactone/Collagen as a Sustained-Release Drug Delivery System for Artemisinin. Pharmaceutics 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S.; et al. Staphylococcus Aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q. Current Research on Fungi in Chronic Wounds. Front. Mol. Biosci. 2023, 9, 1057766. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Z.; Mao, X.; Cheng, J.; Huang, L.; Tang, J. Advances in Wound Dressing Based on Electrospinning Nanofibers. J. Appl. Polym. Sci. 2024, 141, e54746. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Z.; Wan, X.; Fang, H.; Yu, D.-G.; Chen, X.; Liu, P. The Relationships between Process Parameters and Polymeric Nanofibers Fabricated Using a Modified Coaxial Electrospinning. Nanomaterials 2019, 9, 843. [Google Scholar] [CrossRef] [PubMed]
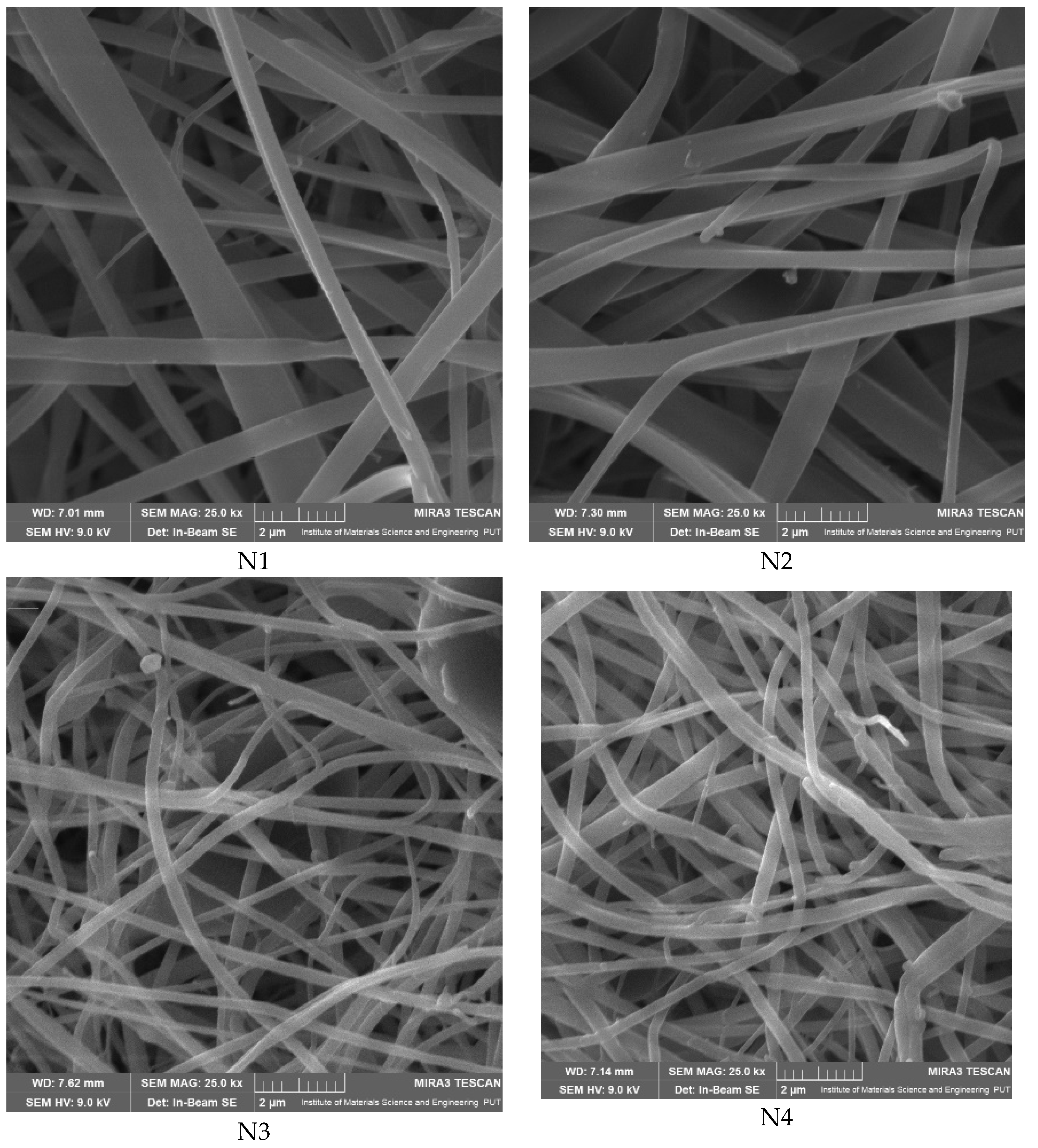





| Nanofiber (N1) | Nanofiber (N2) | Nanofiber (N3) | Nanofiber (N4) | |
|---|---|---|---|---|
| CF-CE | 0.5 g | - | 0.5 g | - |
| CF-UAE | - | 0.5 g | - | 0.5 g |
| PVP | 2.0 g | 2.0 g | 1.9 g | 1.9 g |
| HPβCD | 2.0 g | 2.0 g | - | - |
| PCL | - | - | 0.5 g | 0.5 g |
| Methanol | 10.0 mL | 10.0 mL | - | - |
| Methanol/dichloromethane | - | - | 10.0 mL | 10.0 mL |
| TPC [mg GAE/1 g Plant Material] | Chlorogenic Acid Content [µg/1 mg of Lyophilized Extract] | Narcissin Content [µg/1 mg of Lyophilized Extract] | Antioxidant Activity IC50 [mg/mL] | Anti-Inflammatory Activity IC50 [mg/mL] | |
|---|---|---|---|---|---|
| CF-CE | 8.53 ± 1.15 a | 9.96 ± 0.24 b | 0.26 ± 0.02 a | 1.37 ± 0.08 a | 10.44 ± 0.45 a |
| CF-UAE | 8.95 ± 1.51 a | 10.44 ± 0.08 a | 0.27 ± 0.03 a | 1.28 ± 0.04 a | 9.75 ± 0.50 a |
| N1 | N2 | N3 | N4 | |
|---|---|---|---|---|
| Fiber distribution | 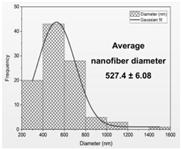 | 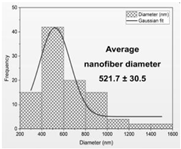 |  | 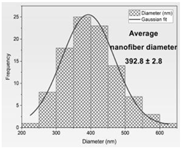 |
| Diameter of nanofibers [nm] | 527.40 ± 6.08 c | 521.70 ± 30.50 c | 298.40 ± 7.00 a | 392.80 ± 2.80 b |
| Pathogen | MICs (mg/mL) | |||||
|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N4 | Chlorogenic Acid | Lyophilized Extract [11] | |
| Staphylococcus aureus | 200 | 100 | 100 | 100 | 2.5 | 8 |
| Pseudomonas aeruginosa | >200 | >200 | 200 | 100 | 2.5 | 4 |
| Candida albicans | 200 | 100 | 100 | 100 | 1.25–2.5 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Rosiak, N.; Plech, T.; Karpiński, T.M.; Miklaszewski, A.; Witkowska, K.; Jaskólski, M.; Erdem, C.; Cielecka-Piontek, J. Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications. Materials 2024, 17, 1736. https://doi.org/10.3390/ma17081736
Paczkowska-Walendowska M, Rosiak N, Plech T, Karpiński TM, Miklaszewski A, Witkowska K, Jaskólski M, Erdem C, Cielecka-Piontek J. Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications. Materials. 2024; 17(8):1736. https://doi.org/10.3390/ma17081736
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Natalia Rosiak, Tomasz Plech, Tomasz M. Karpiński, Andrzej Miklaszewski, Katarzyna Witkowska, Maciej Jaskólski, Cansu Erdem, and Judyta Cielecka-Piontek. 2024. "Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications" Materials 17, no. 8: 1736. https://doi.org/10.3390/ma17081736
APA StylePaczkowska-Walendowska, M., Rosiak, N., Plech, T., Karpiński, T. M., Miklaszewski, A., Witkowska, K., Jaskólski, M., Erdem, C., & Cielecka-Piontek, J. (2024). Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications. Materials, 17(8), 1736. https://doi.org/10.3390/ma17081736










