Biphasic Calcium Phosphate and Activated Carbon Microparticles in a Plasma Clot for Bone Reconstruction and In Situ Drug Delivery: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biphasic Calcium Phosphate (BCP) Particles
2.2. Activated Carbon (AC) Particles
2.3. Drug Adsorption into AC Particles
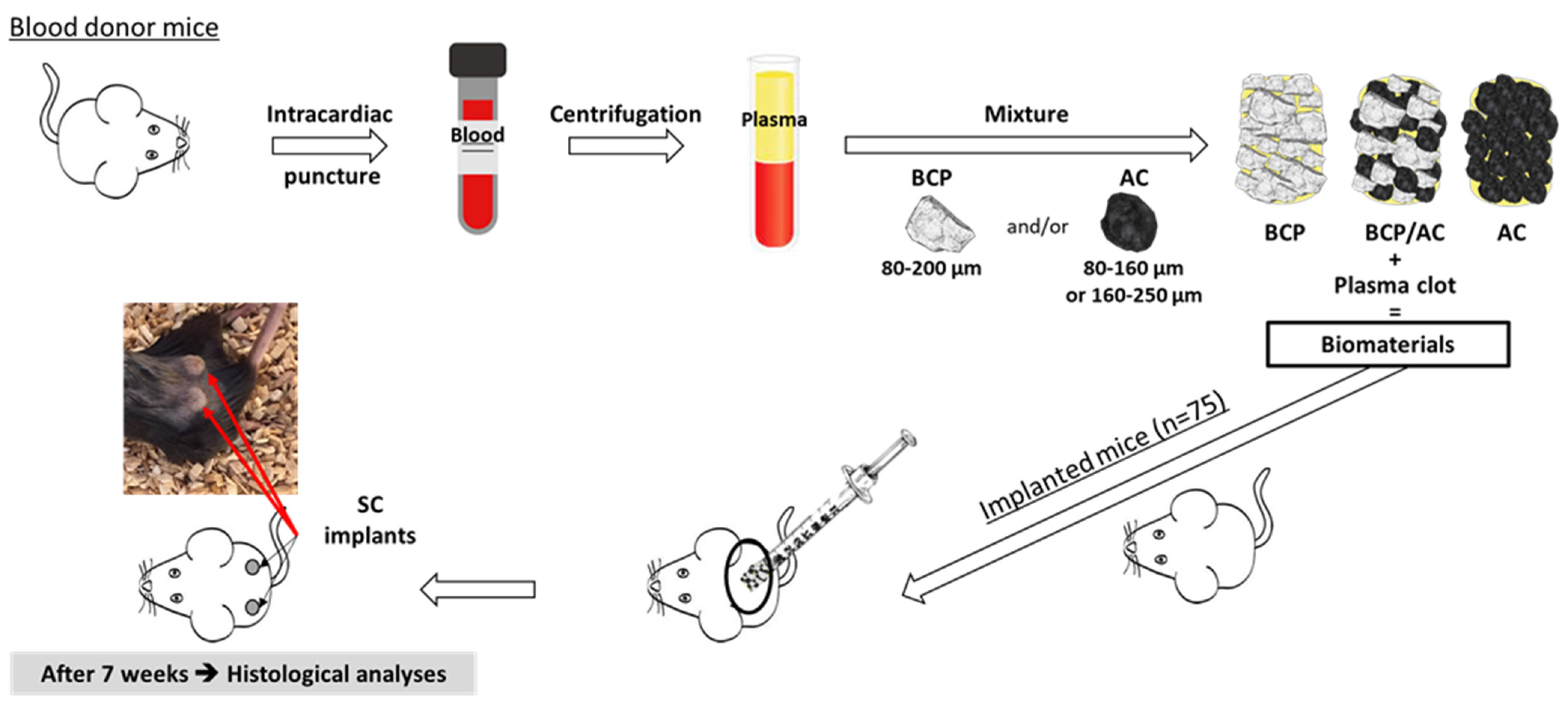
2.4. Preparation of Particles/Plasma Composites and Subcutaneous Implantation
2.5. Morphological Characterization of the Composites
2.6. Histological Analyses
2.7. Porosity Analysis of AC and BCP Particles
2.8. Statistical Analysis
3. Results
3.1. Comparison of Subcutaneous IMMATURE Bone Formation Induced by BCP, BCP/AC and AC Composites
3.2. Influence of Biomaterial Impregnation with Solvents on Subcutaneous Immature Bone Formation
3.3. Particle Porosity and Tacrolimus (TA) Adsorption
3.4. Effect of Tacrolimus (TA) on Subcutaneous Bone-like Tissue Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, D.; Kumar, V.; Sharma, S. Drug-Loaded Biomaterials for Orthopedic Applications: A Review. J. Control. Release 2022, 344, 113–133. [Google Scholar] [CrossRef] [PubMed]
- VK, A.D.; Ray, S.; Arora, U.; Mitra, S.; Sionkowska, A.; Jaiswal, A.K. Dual Drug Delivery Platforms for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 969843. [Google Scholar] [CrossRef]
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic Calcium Phosphate Ceramics for Bone Reconstruction: A Review of Biological Response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Chu, K.-T.; Ou, S.-F.; Chen, S.-Y.; Chiou, S.-Y.; Chou, H.-H.; Ou, K.-L. Research of Phase Transformation Induced Biodegradable Properties on Hydroxyapatite and Tricalcium Phosphate Based Bioceramic. Ceram. Int. 2013, 39, 1455–1462. [Google Scholar] [CrossRef]
- Houmard, M.; Fu, Q.; Genet, M.; Saiz, E.; Tomsia, A.P. On the Structural, Mechanical, and Biodegradation Properties of HA/β-TCP Robocast Scaffolds: Bone-Substitute Material in Various Tissue-Engineering Applications. J. Biomed. Mater. Res. 2013, 101, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.; Pajares, A.; Saiz, E.; Tomsia, A.P.; Guiberteau, F. Mechanical Properties of Calcium Phosphate Scaffolds Fabricated by Robocasting. J. Biomed. Mater. Res. 2008, 85A, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Passuti, N.; Martin, S.; Deudon, C.; Legeros, R.Z.; Raher, S. Macroporous Calcium Phosphate Ceramic for Long Bone Surgery in Humans and Dogs. Clinical and Histological Study. J. Biomed. Mater. Res. 1990, 24, 379–396. [Google Scholar] [CrossRef]
- Balaguer, T.; Boukhechba, F.; Clavé, A.; Bouvet-Gerbettaz, S.; Trojani, C.; Michiels, J.-F.; Laugier, J.-P.; Bouler, J.-M.; Carle, G.F.; Scimeca, J.-C.; et al. Biphasic Calcium Phosphate Microparticles for Bone Formation: Benefits of Combination with Blood Clot. Tissue Eng. Part A 2010, 16, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, T.; Fellah, B.H.; Boukhechba, F.; Traverson, M.; Mouska, X.; Ambrosetti, D.; Dadone, B.; Michiels, J.; Amri, E.; Trojani, C.; et al. Combination of Blood and Biphasic Calcium Phosphate Microparticles for the Reconstruction of Large Bone Defects in Dog: A Pilot Study. J. Biomed. Mater. Res. 2018, 106, 1842–1850. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of Non-Steroidal Anti-Inflammatory Drugs from Aqueous Solution Using Activated Carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The Use of Activated Carbon for the Removal of Pharmaceuticals from Aqueous Solutions: A Review. Rev. Environ. Sci. Biotechnol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Do, D.D.; Junpirom, S.; Do, H.D. A New Adsorption–Desorption Model for Water Adsorption in Activated Carbon. Carbon 2009, 47, 1466–1473. [Google Scholar] [CrossRef]
- Aschermann, G.; Schröder, C.; Zietzschmann, F.; Jekel, M. Organic Micropollutant Desorption in Various Water Matrices-Activated Carbon Pore Characteristics Determine the Reversibility of Adsorption. Chemosphere 2019, 237, 124415. [Google Scholar] [CrossRef] [PubMed]
- Lopez De Armentia, S.; Del Real, J.C.; Paz, E.; Dunne, N. Advances in Biodegradable 3D Printed Scaffolds with Carbon-Based Nanomaterials for Bone Regeneration. Materials 2020, 13, 5083. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jin, X.; Wu, C.; Zhang, W. Injectable Composite Hydrogel Based on Carbon Particles for Photothermal Therapy of Bone Tumor and Bone Regeneration. J. Mater. Sci. Technol. 2022, 118, 64–72. [Google Scholar] [CrossRef]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Wu, X.; Cong, X.; Wang, L.; Wang, Y.; Yang, Y.; Li, W.; Sun, T. The Influence of Tumor-Induced Immune Dysfunction on the Immune Cell Distribution of Gold Nanoparticles In Vivo. Biomater. Sci. 2017, 5, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Usui, Y.; Narita, N.; Ogiwara, N.; Iashigaki, N.; Nakamura, K.; Kato, H.; Sano, K.; Ogiwara, N.; Kametani, K.; et al. A Thin Carbon-Fiber Web as a Scaffold for Bone-Tissue Regeneration. Small 2009, 5, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, D.; Łudzik, K.; Jażdżewska, M. Carbon Fibres as Potential Bone Implants with Controlled Doxorubicin Release. Sci. Rep. 2022, 12, 2607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Z.; Liu, Q.; Yang, P.; Wang, P.; Wei, S.; Liu, A.; Zhao, Z. Potential Load-Bearing Bone Substitution Material: Carbon-Fiber-Reinforced Magnesium-Doped Hydroxyapatite Composites with Excellent Mechanical Performance and Tailored Biological Properties. ACS Biomater. Sci. Eng. 2022, 8, 921–938. [Google Scholar] [CrossRef]
- Olivier, F.; Sarou-Kanian, V.; Fayon, F.; Bonnamy, S.; Rochet, N. In Vivo Effectiveness of Carbonated Calcium-deficient Hydroxyapatite-coated Activated Carbon Fiber Cloth on Bone Regeneration. J. Biomed. Mater. Res. 2022, 110, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Li, F.; Xu, D.; Liu, J.; Li, J.; Zhong, L.; Liu, Y.; Bai, N. Performance of 3D Printed Porous Polyetheretherketone Composite Scaffolds Combined with Nano-Hydroxyapatite/Carbon Fiber in Bone Tissue Engineering: A Biological Evaluation. Front. Bioeng. Biotechnol. 2024, 12, 1343294. [Google Scholar] [CrossRef] [PubMed]
- Olivier, F.; Drouet, C.; Marsan, O.; Sarou-Kanian, V.; Rekima, S.; Gautier, N.; Fayon, F.; Bonnamy, S.; Rochet, N. Long-Term Fate and Efficacy of a Biomimetic (Sr)-Apatite-Coated Carbon Patch Used for Bone Reconstruction. J. Funct. Biomater. 2023, 14, 246. [Google Scholar] [CrossRef]
- Olivier, F.; Bonnamy, S.; Rochet, N.; Drouet, C. Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. Int. J. Mol. Sci. 2021, 22, 12247. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Krebsbach, P.H.; Satomura, K.; Kerr, J.; Riminucci, M.; Benayahu, D.; Robey, P.G. Single-Colony Derived Strains of Human Marrow Stromal Fibroblasts Form Bone after Transplantation In Vivo. J. Bone Miner. Res. 1997, 12, 1335–1347. [Google Scholar] [CrossRef]
- Boukhechba, F.; Balaguer, T.; Bouvet-Gerbettaz, S.; Michiels, J.-F.; Bouler, J.-M.; Carle, G.F.; Scimeca, J.-C.; Rochet, N. Fate of Bone Marrow Stromal Cells in a Syngenic Model of Bone Formation. Tissue Eng. Part A 2011, 17, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Bouvet-Gerbettaz, S.; Boukhechba, F.; Balaguer, T.; Schmid-Antomarchi, H.; Michiels, J.-F.; Scimeca, J.-C.; Rochet, N. Adaptive Immune Response Inhibits Ectopic Mature Bone Formation Induced by BMSCs/BCP/Plasma Composite in Immune-Competent Mice. Tissue Eng. Part A 2014, 20, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption Models for Carbon Slit-Shaped Pores with Surface Energetical Heterogeneity and Geometrical Corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Parra, J.B.; Carvalho, A.P.; Ania, C.O. Waste-Derived Activated Carbons for Removal of Ibuprofen from Solution: Role of Surface Chemistry and Pore Structure. Bioresour. Technol. 2009, 100, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of Emerging Pollutants on Activated Carbon. Rev. Chem. Eng. 2017, 33, 491–522. [Google Scholar] [CrossRef]
- Inoue, S.; Kiriyama, K.; Hatanaka, Y.; Kanoh, H. Adsorption Properties of an Activated Carbon for 18 Cytokines and HMGB1 from Inflammatory Model Plasma. Colloids Surf. B Biointerfaces 2015, 126, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Ohike, A.; Ibuki, R.; Amidon, G.L.; Yamashita, S. Tacrolimus Is a Class II Low-Solubility High-Permeability Drug: The Effect of P-Glycoprotein Efflux on Regional Permeability of Tacrolimus in Rats. J. Pharm. Sci. 2002, 91, 719–729. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. [Google Scholar] [CrossRef]
- Kang, K.Y.; Ju, J.H.; Song, Y.W.; Yoo, D.-H.; Kim, H.-Y.; Park, S.-H. Tacrolimus Treatment Increases Bone Formation in Patients with Rheumatoid Arthritis. Rheumatol. Int. 2013, 33, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Izumo, N.; Furukawa, M.; Shimakura, T.; Yamamoto, N.; Takahashi, H.E.; Asakura, T.; Wakabayashi, H. Effects of the Calcineurin Inhibitors Cyclosporine and Tacrolimus on Bone Metabolism in Rats. Biomed. Res. 2018, 39, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Nabavi, M.A.; Semyari, H. A Collagen-Based Hydrogel Containing Tacrolimus for Bone Tissue Engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef] [PubMed]









| Particle–[Drug–Solvent] Associated with Plasma | n (Mice) | n (Implants Analyzed) |
|---|---|---|
| BCP | 5 | 9/10 |
| BCP/AC | 6 | 12/12 |
| AC | 5 | 10/10 |
| AC250 | 6 | 8/12 |
| BCP-[NaCl] | 5 | 10/10 |
| BCP-[EtOH] | 5 | 10/10 |
| BCP/AC-[NaCl] | 5 | 9/10 |
| BCP/AC-[EtOH] | 5 | 8/10 |
| AC-[NaCl] | 5 | 10/10 |
| AC-[EtOH] | 5 | 7/10 |
| BCP/AC-[TA1-EtOH] | 6 | 11/12 |
| BCP/AC-[TA2-EtOH] | 6 | 12/12 |
| AC-[TA1-EtOH] | 6 | 11/12 |
| AC-[TA2-EtOH] | 5 | 9/10 |
| SBET (m2/g) | |
|---|---|
| BCP | 2 |
| AC 1 | 1562 |
| BCP/AC—[TA1-EtOH] (800 ppm) | BCP/AC—[TA2-EtOH] (160 ppm) | AC—[TA1-EtOH] (800 ppm) | AC—[TA2-EtOH] (160 ppm) | ||
|---|---|---|---|---|---|
| mTA (µg) | Mean ± SD | 95 ± 10 | 30 ± 4 | 120 ± 10 | 45 ± 8 |
| QTA (mg/g) | Mean ± SD | 19 ± 2 | 6 ± 1 | 24 ± 2 | 9 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekima, S.; Gautier, N.; Bonnamy, S.; Rochet, N.; Olivier, F. Biphasic Calcium Phosphate and Activated Carbon Microparticles in a Plasma Clot for Bone Reconstruction and In Situ Drug Delivery: A Feasibility Study. Materials 2024, 17, 1749. https://doi.org/10.3390/ma17081749
Rekima S, Gautier N, Bonnamy S, Rochet N, Olivier F. Biphasic Calcium Phosphate and Activated Carbon Microparticles in a Plasma Clot for Bone Reconstruction and In Situ Drug Delivery: A Feasibility Study. Materials. 2024; 17(8):1749. https://doi.org/10.3390/ma17081749
Chicago/Turabian StyleRekima, Samah, Nadine Gautier, Sylvie Bonnamy, Nathalie Rochet, and Florian Olivier. 2024. "Biphasic Calcium Phosphate and Activated Carbon Microparticles in a Plasma Clot for Bone Reconstruction and In Situ Drug Delivery: A Feasibility Study" Materials 17, no. 8: 1749. https://doi.org/10.3390/ma17081749







