Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.1.1. Raw Material
2.1.2. Pretreatment
2.1.3. Enzymatic Hydrolysis
2.2. PHA Production
2.2.1. Microorganisms and Media
2.2.2. Inoculum Preparation
2.2.3. Shake Flask Assays
2.2.4. Bioreactor Cultivation
2.2.5. Analytical Techniques
2.2.6. Calculations
2.3. PHA Extraction
3. Results and Discussion
3.1. Feedstock Characterisation
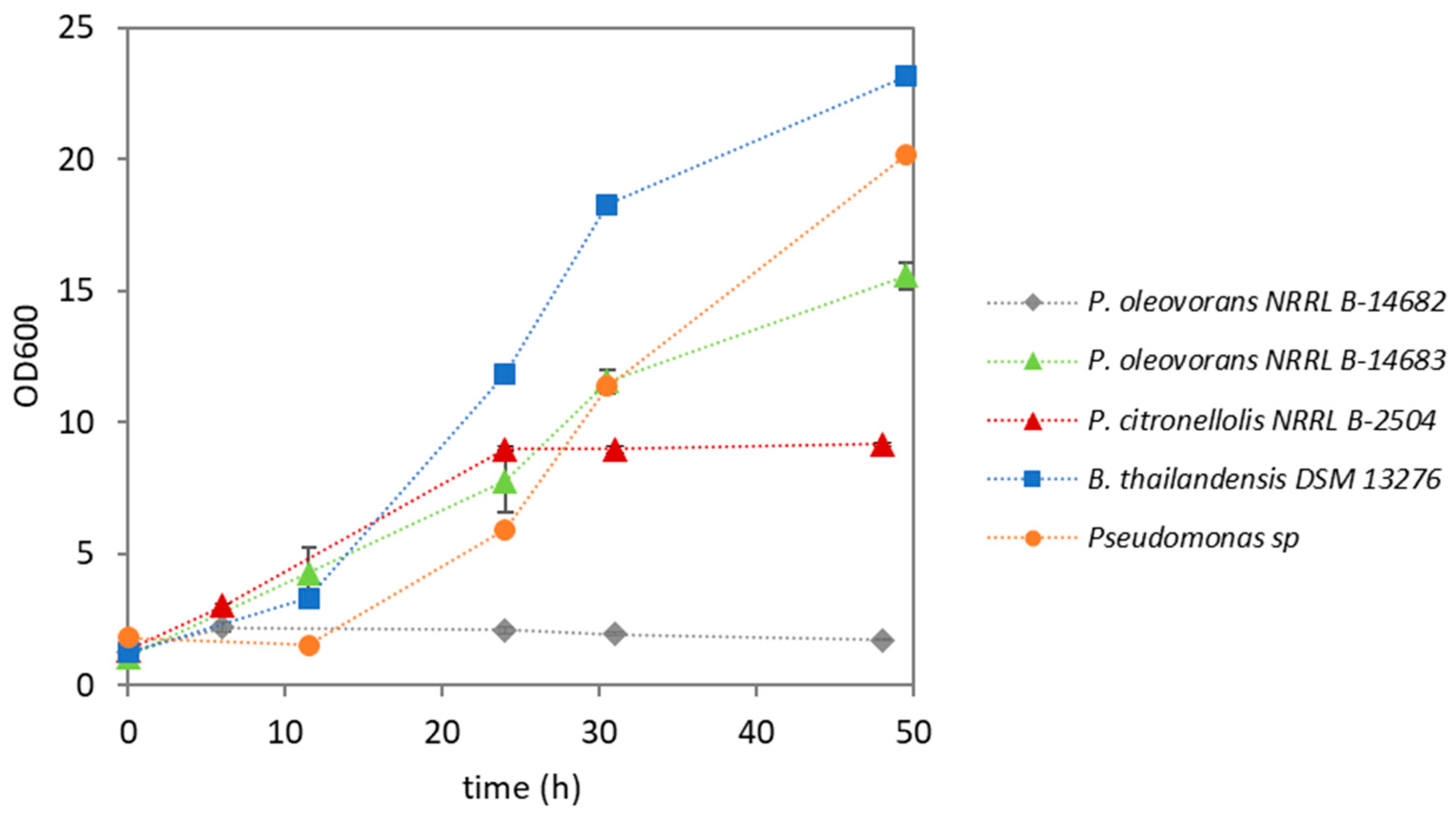
3.2. Shake Flask Screening
3.3. Bioreactor Assays
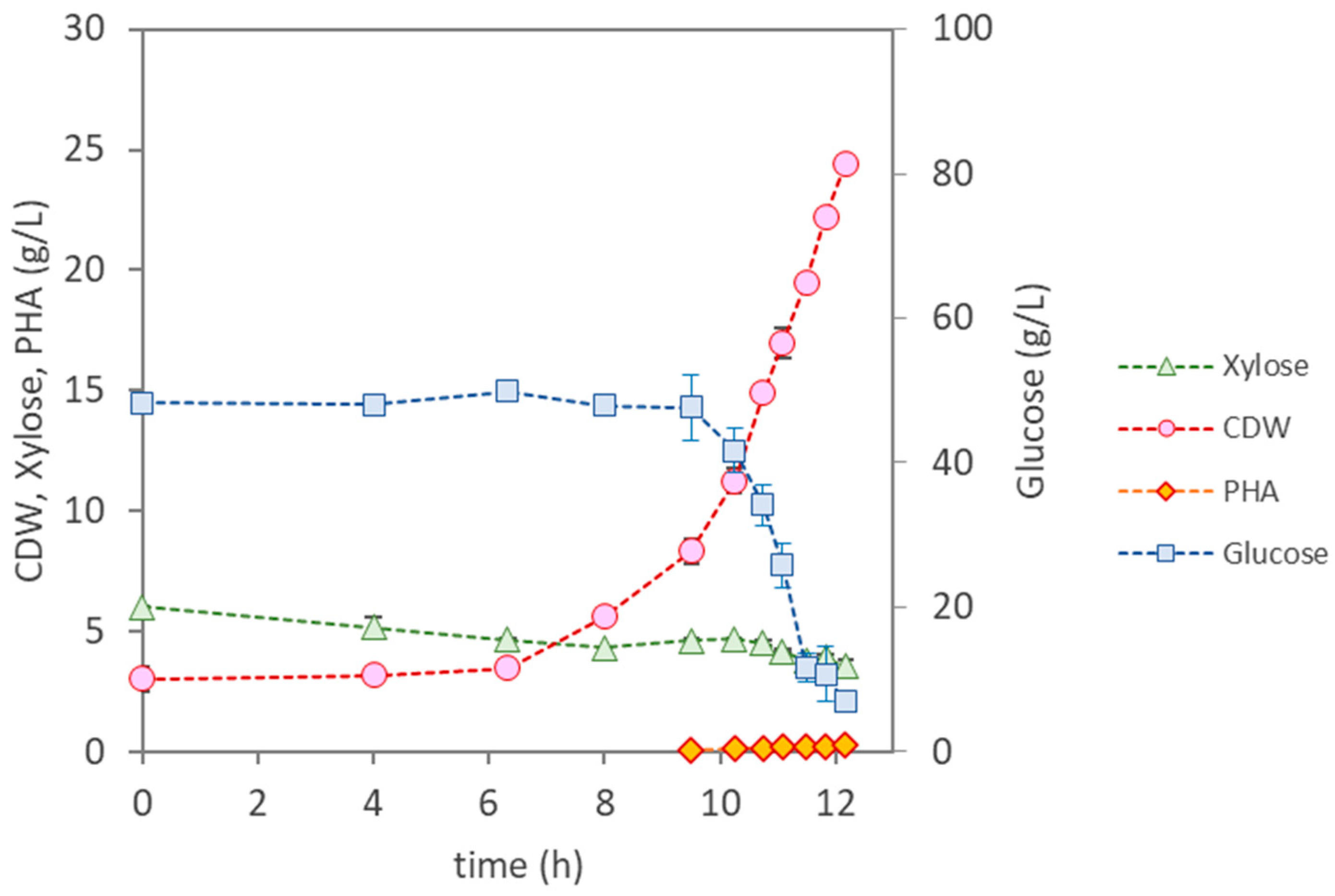
3.3.1. Bioreactor Cultivation of Pseudomonas citronellolis NRRL B-2504
3.3.2. Bioreactor Cultivation of Burkholderia thailandensis DSM 13276
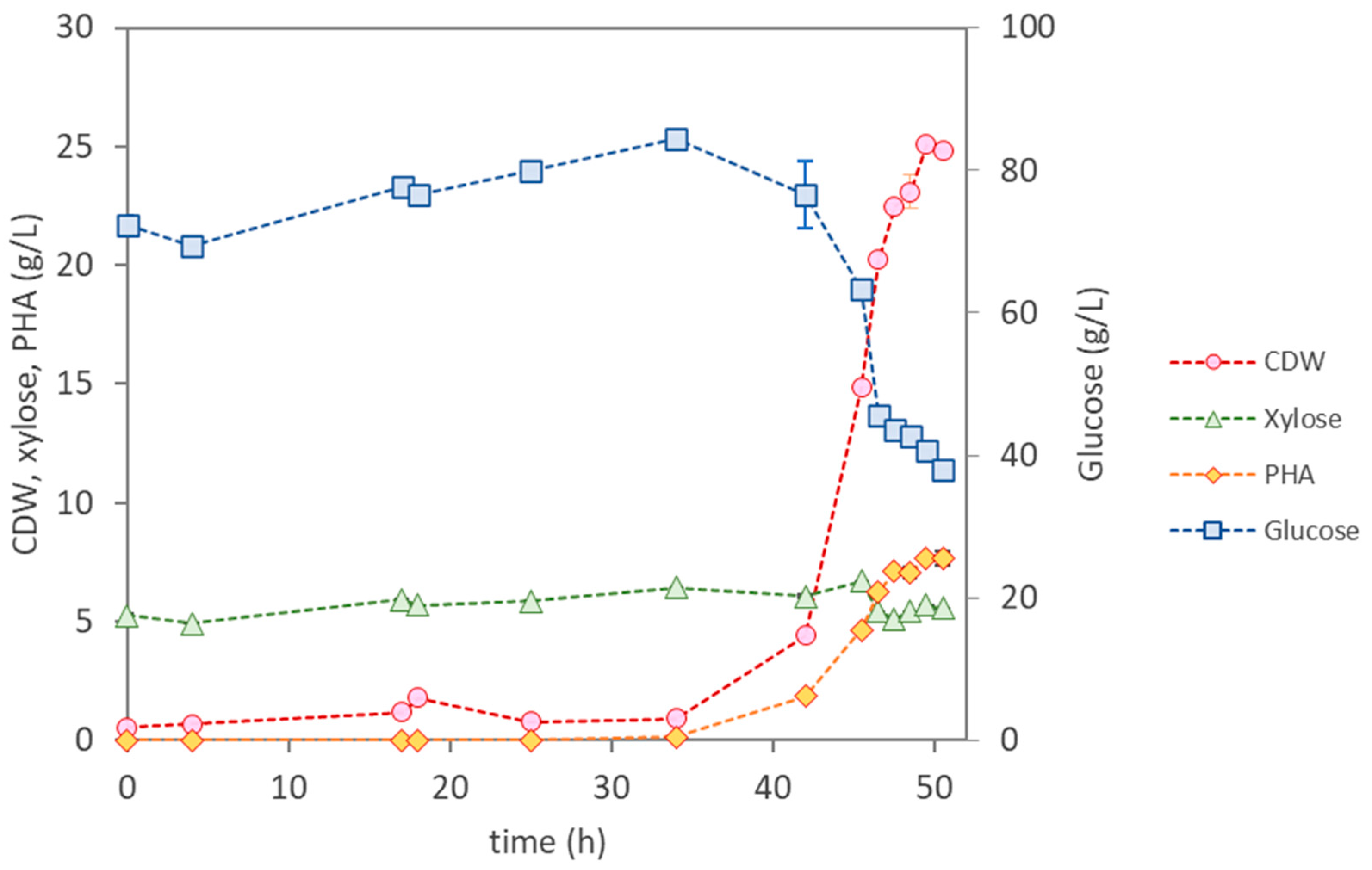
3.3.3. Bioreactor Cultivation of Pseudomonas sp.
3.3.4. Overall Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopf, S.; Åkesson, D.; Skrifvars, M. Textile Fiber Production of Biopolymers—A Review of Spinning Techniques for Polyhydroxyalkanoates in Biomedical Applications. Politics Rev. 2023, 63, 200–245. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Brahmananda, R.C.V.S.; Sivaramakrishna, A. Recent Advances in Biodegradable Polymers—Properties, Applications and Future Prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramarao, B.V.; Misra, M. A Review on Polyhydroxyalkanoate (PHA) Production through the Use of Lignocellulosic Biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- González-Rojo, S.; Díez-Antolínez, R. Production of Polyhydroxyalkanoates as a Feasible Alternative for an Integrated Multiproduct Lignocellulosic Biorefinery. Bioresour. Technol. 2023, 386, 129496. [Google Scholar] [CrossRef] [PubMed]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic Feedstock: A Review of a Sustainable Platform for Cleaner Production of Nature’s Plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Mielenz, J.R. Biofuels, Methods and Protocols; Humana: Totowa, NJ, USA, 2009; 293p. [Google Scholar]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Pereira, J.R.; Araújo, D.; Freitas, P.; Marques, A.C.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Production of Medium-Chain-Length Polyhydroxyalkanoates by Pseudomonas chlororaphis Subsp. aurantiaca: Cultivation on Fruit Pulp Waste and Polymer Characterization. Int. J. Biol. Macromol. 2021, 167, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.C.; Pereira, H. Potential of Eucalyptus globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Gominho, J.; Costa, R.A.; Lourenço, A.; Quilhó, T.; Pereira, H. Eucalyptus globulus Stumps Bark: Chemical and Anatomical Characterization Under a Valorisation Perspective. Waste Biomass Valoriz. 2021, 12, 1253–1265. [Google Scholar] [CrossRef]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-Production of Biofuels and Value-Added Compounds from Industrial Eucalyptus globulus Bark Residues Using Hydrothermal Treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Ramsay, J.A.; Hassan, M.-C.A.; Ramsay, B.A. Hemicellulose as a potential substrate for production of poly(β-hydroxyalkanoates). Can. J. Microbiol. 1995, 41, 262–266. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Production of Medium-Chain Length Polyhydroxyalkanoates by Pseudomonas citronellolis Grown in Apple Pulp Waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar]
- de Meneses, L.; Pereira, J.R.; Sevrin, C.; Grandfils, C.; Paiva, A.; Reis, M.A.M.; Freitas, F. Pseudomonas chlororaphis as a multiproduct platform: Conversion of glycerol into high-value biopolymers and phenazines. New Biotechnol. 2020, 55, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; Araújo, D.; Marques, A.C.; Neves, L.A.; Grandfils, C.; Sevrin, C.; Alves, V.D.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Demonstration of the Adhesive Properties of the Medium-Chain-Length Polyhydroxyalkanoate Produced by Pseudomonas chlororaphis Subsp. aurantiaca from Glycerol. Int. J. Biol. Macromol. 2019, 122, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; De Jong, H. Acetate Metabolism and the Inhibition of Bacterial Growth by Acetate. J. Bacteriol. 2019, 201, 13. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-López, C.; Colorado-Arias, S.; Ramírez-Carmona, M. Modeling of Microbial Growth and Ammonia Consumption at Different Temperatures in the Production of a Polyhydroxyalkanoate (PHA) Biopolymer. J. Appl. Res. Technol. 2015, 13, 498–503. [Google Scholar] [CrossRef]
- Blunt, W.; Blanchard, C.; Doyle, C.; Vasquez, V.; Ye, M.; Adewale, P.; Liu, Y.; Morley, K.; Monteil-Rivera, F. The Potential of Burkholderia thailandensis E264 for Co-Valorization of C5 and C6 Sugars into Multiple Value-Added Bio-Products. Bioresour. Technol. 2023, 387, 129595. [Google Scholar] [CrossRef] [PubMed]
- Silva, J. Production of Medium-Chain Length Polyhydroxyalkanoates (PHA) from Sugar-Rich Extracts and Hydrolysates from White Wine Grape Pomace. Master’s Thesis, Universidade NOVA de Lisboa, Lisbon, Portugal, 2019. [Google Scholar]
- Lopes, M.S.G.; Gomez, J.G.C.; Taciro, M.K.; Mendonça, T.T.; Silva, L.F. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. J. Ind. Microbiol. Biotechnol. 2014, 41, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sukruansuwan, V.; Napathorn, S.C. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain A-04. Biotechnol. Biofuels 2018, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Moorkoth, D.; Nampoothiri, K.M. Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by a novel halotolerant mangrove isolate. Bioresour. Technol. 2016, 201, 253–260. [Google Scholar] [CrossRef] [PubMed]


| Component | As Received | Pretreated | |
|---|---|---|---|
| (g/100 go.d.) | (g/100 go.d.) | (Percentage Recovered) | |
| Glucan | 33.3 ± 0.4 | 38.1 ± 0.6 | 97.3 |
| Xylan | 12.2 ± 0.2 | 6.0 ± 0.3 | 41.8 |
| Arabinan | 1.2 ± 0.1 | 0.0 | 0 |
| Acetyl Groups | 3.2 ± 0.2 | 0.4 ± 0.1 | - |
| Klason Lignin | 23.2 ± 0.6 | 27.5 ± 0.9 | - |
| Acid-soluble Lignin | 0.5 ± 0.1 | 0.1 ± 0.0 | - |
| Ash | 8.5 ± 0.4 | 10.5 ± 0.5 | - |
| Others | 17.9 | 17.4 | - |
| Strain | CDW (g/L) | PHA (wt.%) | Type of PHA |
|---|---|---|---|
| Pseudomonas oleovorans NRRL B-14682 | 1.31 ± 0.19 | n.d. | --- |
| Pseudomonas oleovorans NRRL B-14683 | 4.05 ± 0.42 | 1.1 ± 0.1 | PHB |
| Pseudomonas citronellolis NRRL B-2504 | 2.60 ± 0.20 | 3.6 ± 0.1 | mcl-PHA |
| Pseudomonas sp. | 7.37 ± 0.58 | 38.9 ± 4.1 | PHB |
| Burkholderia thailandensis DSM 13276 | 6.30 ± 0.50 | 25.4 ± 2.0 | PHB |
| Parameter | P. citronellolis NRRL B-2504 | B. thailandensis E264 | Pseudomonas sp. | |
|---|---|---|---|---|
| Assay P1 | Assay P2 | |||
| Cultivation Time (h) | 12 | 47 | 49 | 50 |
| µmax (h−1) | 0.37 | 0.13 | 0.22 | 0.22 |
| CDW (g/L) | 24.4 ± 0.15 | 8.87 ± 0.34 | 11.30 ± 0.19 | 24.83 ± 0.04 |
| PHA (wt.%) | <1 | 12.3 | 18 | 31 |
| PHA (g/L) | 0.25 | 1.10 | 2.10 | 7.70 |
| rPHA (gPHA/(L·h)) | - | 0.023 | 0.110 | 0.150 |
| [Glucose] Uptake (g/L) | 41.0 | 59.7 | 22.1 | 46.6 |
| [Xylose] Uptake (g/L) | 1.6 | 1.9 | 0 | 0.85 |
| YPHA/S (gPHA/gsugar) | 0.0058 | 0.018 | 0.27 | 0.16 |
| YX/S (gX/gsugar) | 0.49 | 0.13 | 0.24 | 0.35 |
| PHA Composition | HD (65%); HDd (25%); HTd (14%) | HB (98.6%); HV (1.4%) | HB (100%) | HB (100%) |
| Strain | LCB | Hydrolysis | Scale | Cultivation Mode | Type of PHA | CDW (g/L) | PHA (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pseudomonas citronellolis NRR B-2504 | Grape Pomace | Acidic | Shake Flask | Batch | mcl-PHA | 2.83–3.68 | 9.1 | [23] |
| Bioreactor | Fed Batch | 9.3 | 14 | |||||
| Halomonas halophila CCM 3662 | Grape Pomace | Enzymatic | Shake Flask | Batch | PHB | 5.1 | 72 | [8] |
| Bioreactor | 3.1 | 57 | ||||||
| Pseudomonas cepacia | Trembling Aspen | Enzymatic | Shake Flask | Batch | PHB | 2.6 | 60 | [15] |
| Burkholderia sp. F24 | Sugarcane Bagasse | Acidic | Shake Flask | Batch | PHB | 25.0 | 49 | [24] |
| Burkholderia sacchari DSM17165 | Wheat Straw | Enzymatic | Bioreactor | Fed Batch | PHB | 100–140 | 72 | [25] |
| Cupriavidus necator A-04 | Pineapple Core | Acidic | Shake Flask/ Bioreactor | Batch | PHB | 5.3–13.6 | 13.2–56.6 | [26] |
| Bacillus firmus | Rice Straw | Acidic | Shake Flask | Batch | PHB | 1.9 | 89 | [27] |
| Bacillus MG12 | Sugarcane | Acidic | Shake Flask | Batch | PHBHV | 2.89 | 8 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, T.; Torres, C.A.V.; Marques, S.; Gírio, F.; Freitas, F.; Reis, M.A.M. Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate. Materials 2024, 17, 1773. https://doi.org/10.3390/ma17081773
Rodrigues T, Torres CAV, Marques S, Gírio F, Freitas F, Reis MAM. Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate. Materials. 2024; 17(8):1773. https://doi.org/10.3390/ma17081773
Chicago/Turabian StyleRodrigues, Thomas, Cristiana A. V. Torres, Susana Marques, Francisco Gírio, Filomena Freitas, and Maria A. M. Reis. 2024. "Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate" Materials 17, no. 8: 1773. https://doi.org/10.3390/ma17081773









