Antibacterial Zirconia Surfaces from Organocatalyzed Atom-Transfer Radical Polymerization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Yttria Tetragonal Zirconia Polycrystal (Y-TZP) Substrates
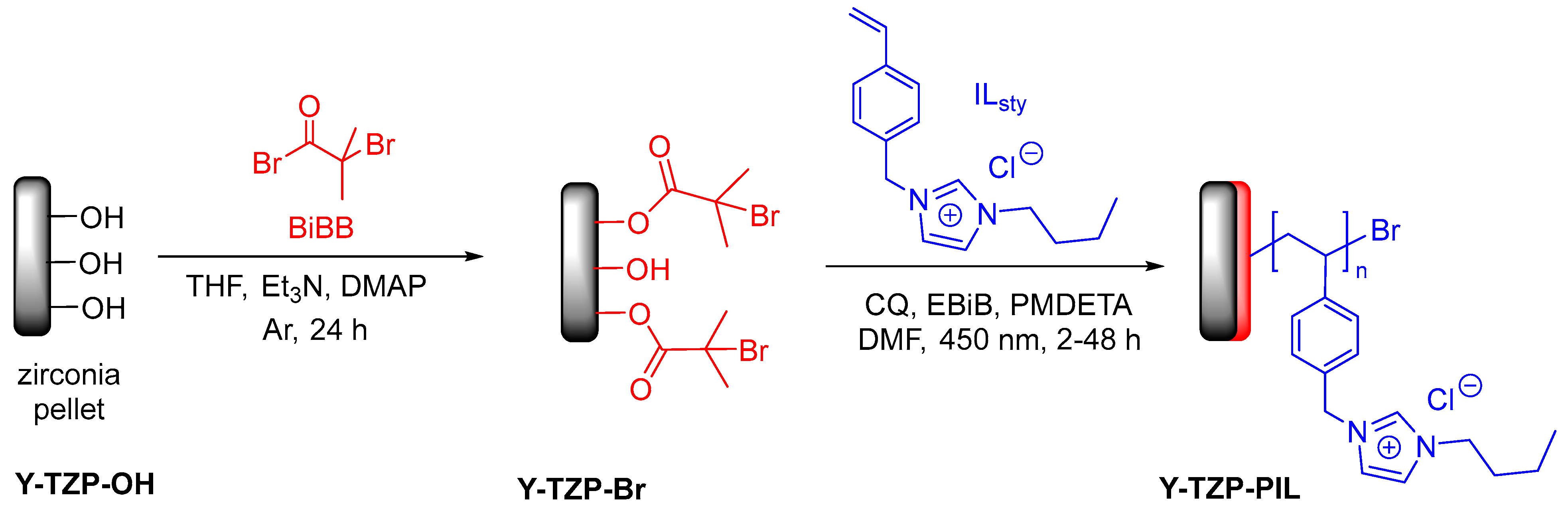
2.3. Immobilization of ATRP Initiators on the Zirconia Substrates
2.4. Surface-Initiated Photo-ATRP from Y-TZP-Br Substrates
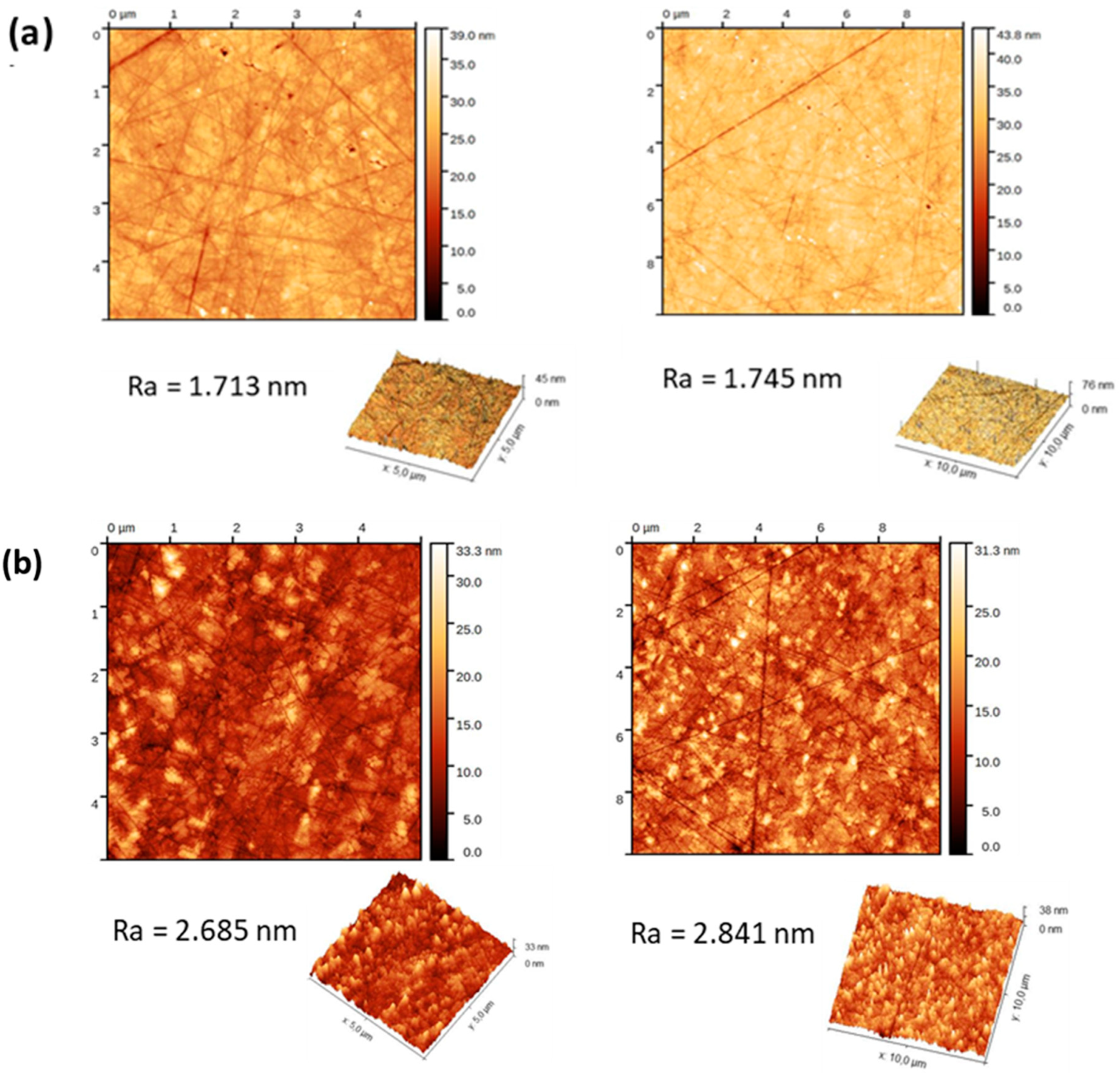
2.5. Characterizations
2.6. Measurements of the Antibacterial Activities
- (a)
- Bacterial culture
- (b)
- Bacterial adhesion test
- (c)
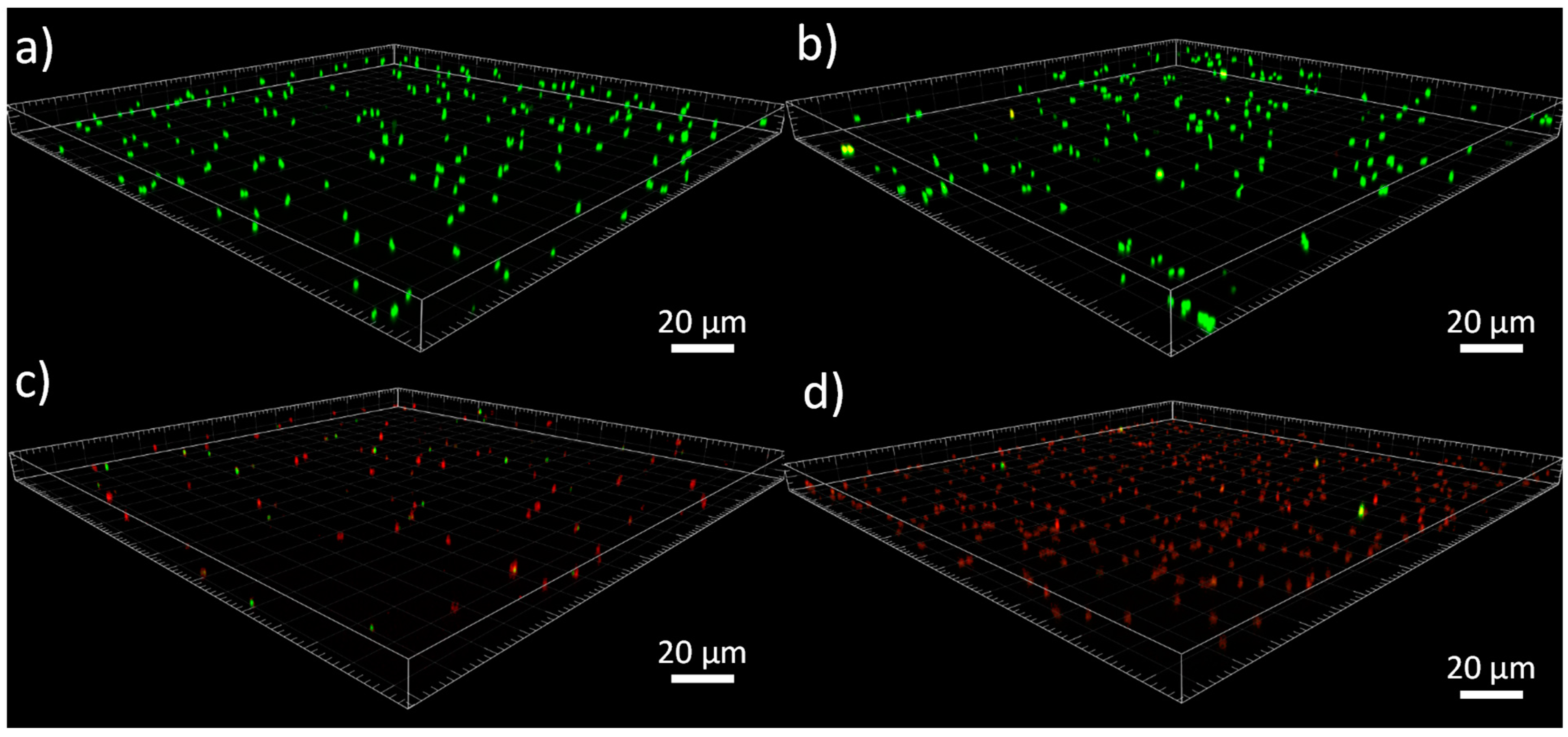
- Confocal microscopy analysis
3. Results and Discussion
3.1. Preparation of Zirconia Pellets, Ionic Liquid Monomer Synthesis and Initiator Grafting
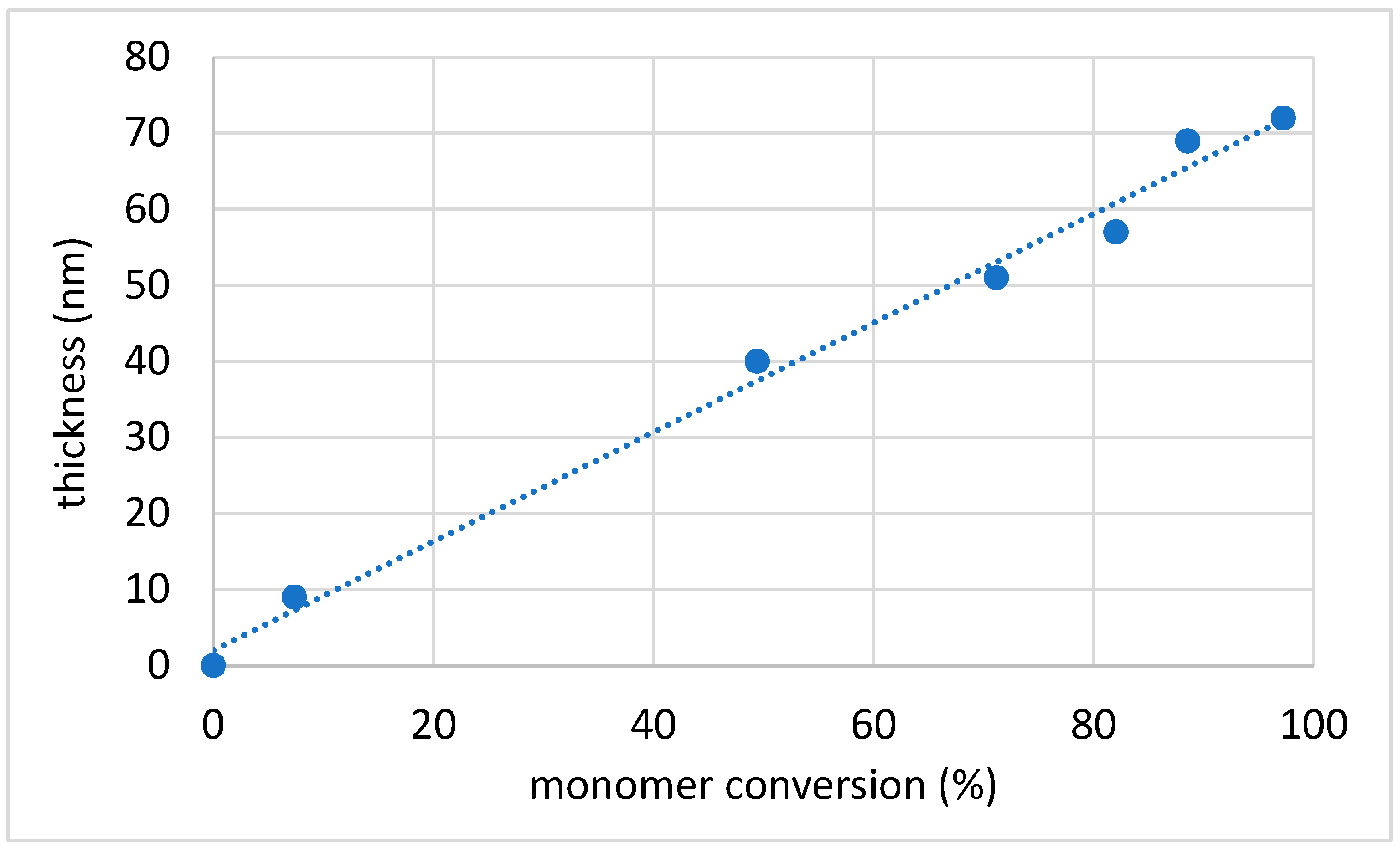
3.2. Ionic Liquid Monomer Polymerization Initiated from the Zirconia Surface
3.3. Antibacterial Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishihara, H.; Haro Adanez, M.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hong, G.; Matsui, H.; Song, Y.-J.; Sasaki, K. The Effects of Syndecan on Osteoblastic Cell Adhesion Onto Nano-Zirconia Surface. Int. J. Nanomed. 2020, 15, 5061–5072. [Google Scholar] [CrossRef]
- Stadlinger, B.; Hennig, M.; Eckelt, U.; Kuhlisch, E.; Mai, R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int. J. Oral Maxillofac. Surg. 2010, 39, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Lawrence, J.; Chian, K.S. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J. Mater. Sci. Mater. Med. 2005, 16, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Treccani, L.; Klein, T.Y.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef] [PubMed]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Gerlach, J.W.; Maendl, S.; Rezwan, K. Magnesium-containing mixed coatings on zirconia for dental implants: Mechanical characterization and in vitro behavior. J. Biomater. Appl. 2015, 30, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Mahdavi, H. Grafting of zwitterion polymer on polyamide nanofiltration membranes via surface-initiated RAFT polymerization with improved antifouling properties as a new strategy. Sep. Purif. Technol. 2021, 254, 117605–117618. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Q.; Yuan, S.; Zhuang, X.; Zhang, F. Enhanced Antifouling and Anticorrosion Properties of Stainless Steel by Biomimetic Anchoring PEGDMA-Cross-Linking Polycationic Brushes. Ind. Eng. Chem. Res. 2019, 58, 7107–7119. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Z.; Guo, Y.; Ju, Y.; Liu, Y.; Feng, R.; Xiong, C.; Ober, C.K.; Dong, L. Flexible Hydrophobic Antifouling Coating with Oriented Nanotopography and Nonleaking Capsaicin. ACS Appl. Mater. Interfaces 2018, 10, 9718–9726. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Sun, X.; Hu, J.; Shi, P.; Huang, X. Semifluorinated Synergistic Nonfouling/Fouling-Release Surface. ACS Appl. Mater. Interfaces 2017, 9, 16517–16523. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Qin, J.; Ren, Y.; Wang, B.; Cui, H.; Ding, Y.; Mao, H.; Yan, F. Antibacterial activity of cationic polymers: Side-chain or main-chain type? Polym. Chem. 2018, 9, 4611–4616. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H. Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer. Polymers 2015, 7, 2290–2303. [Google Scholar] [CrossRef]
- Iwai, N.; Nakayama, K.; Kitazume, T. Antibacterial activities of imidazolium, pyrrolidinium and piperidinium salts. Bioorg. Med. Chem. Lett. 2011, 21, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, Q.; Guo, J.; Qin, J.; Mao, H.; Wang, B.; Yan, F. Structure–Antibacterial Activity Relationships of Imidazolium-Type Ionic Liquid Monomers, Poly(ionic liquids) and Poly(ionic liquid) Membranes: Effect of Alkyl Chain Length and Cations. ACS Appl. Mater. Interfaces 2016, 8, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, J.; Li, Y.; Zhao, X.; Chen, L.; Li, G.; Wang, L.; Jia, Z.; Ge, X. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Pei, X. Preparation, Characterization, and Tunable Wettability of Poly(ionic liquid) Brushes via Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2008, 41, 4615–4621. [Google Scholar] [CrossRef]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Olivier, A.; Meyer, F.; Raquez, J.-M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 1, 157–181. [Google Scholar] [CrossRef]
- Bech, L.; Elzein, T.; Meylheuc, T.; Ponche, A.; Brogly, M.; Lepoittevin, B.; Roger, P. Atom transfer radical polymerization of styrene from different poly(ethylene terephthalate) surfaces: Films, fibers and fabrics. Eur. Polym. J. 2009, 45, 246–255. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Elzein, T.; Dragoe, D.; Bejjani, B.; Lemée, F.; Levillain, J.; Bazin, P.; Roger, P.; Dez, I. Hydrophobization of chitosan films by surface grafting with fluorinated polymer brushes. Carbohydr. Polym. 2019, 205, 437–446. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhong, M.; Luebke, D.; Nulwala, H.; Matyjaszewski, K. Atom Transfer Radical Polymerization of Ionic Liquid Monomer: The Influence of Salt/Counterion on Polymerization. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2175–2184. [Google Scholar] [CrossRef]
- Boulif, N.; Sebakhy, K.O.; Joosten, H.; Raffa, P. Design and synthesis of novel di- and triblock amphiphilic polyelectrolytes: Improving salt-induced viscosity reduction of water solutions for potential application in enhanced oil recovery. J. Appl. Polym. Sci. 2021, 138, 50366. [Google Scholar] [CrossRef]
- Shanmugam, S.; Boyer, C. Organic photocatalysts for cleaner polymer synthesis. Science 2016, 352, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Dadashi-Silab, S.; Matyjaszewski, K.; Spencer, N.D.; Benetti, E.M. Surface-Initiated Photoinduced ATRP: Mechanism, Oxygen Tolerance, and Temporal Control during the Synthesis of Polymer Brushes. Macromolecules 2020, 53, 2801–2810. [Google Scholar] [CrossRef]
- Discekici, E.H.; Anastasaki, A.; Read de Alaniz, J.; Hawker, C.J. Evolution and Future Directions of Metal-Free Atom Transfer Radical Polymerization. Macromolecules 2018, 51, 7421–7434. [Google Scholar] [CrossRef]
- Pan, X.; Lamson, M.; Yan, J.; Matyjaszewski, K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- de Ávila Gonçalves, S.; Rodrigues, P.R.; Pioli Vieira, R. Metal-Free Organocatalyzed Atom Transfer Radical Polymerization: Synthesis, Applications, and Future Perspectives. Macromol. Rapid Commun. 2021, 42, 2100221–2100242. [Google Scholar] [CrossRef] [PubMed]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. J. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Dezanet, C.; Dragoe, D.; Marie, P.; Harfouche, N.; Froissart, S.; Fouchet, A.; Rouden, J.; Lecourt, J.; Harnois, C.; Thébault, P.; et al. Zirconia surface polymer grafting via dopamine-assisted co-deposition and radical photopolymerization. Prog. Org. Coat. 2022, 173, 107202–107210. [Google Scholar] [CrossRef]
- Ben-Hadj-Salem, J.; Dragoe, D.; Marie, P.; Froissart, S.; Fouchet, A.; Rouden, J.; Lecourt, J.; Harnois, C.; Touil, S.; Baudoux, J.; et al. Amido bisphosphonic acid as anchoring agent and photopolymerization initiator onto zirconium oxide surface. Eur. Polym. J. 2023, 195, 112207–112215. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifschitz-van der Waals and Polar Interactions in Macroscopic Systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical dispersion relations for amorphous semiconductors and amorphous dielectrics. Phys. Rev. B 1986, 34, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105, 135–149. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Steinkoenig, J.; Bloesser, F.R.; Huber, B.; Welle, A.; Trouillet, V.; Weidner, S.M.; Barner, L.; Roesky, P.W.; Yuan, J.; Goldmann, A.S.; et al. Controlled radical polymerization and in-depth mass-spectrometric characterization of poly(ionic liquid)s and their photopatterning on surfaces. Polym Chem. 2016, 7, 451–461. [Google Scholar] [CrossRef]
- Shivappooja, P.; Ista, L.K.; Canavan, H.E.; Lopez, G.P. ARGET–ATRP Synthesis and Characterization of PNIPAAm Brushes for Quantitative Cell Detachment Studies. Biointerphases 2012, 7, 32. [Google Scholar] [CrossRef]
- Hildebrandt, M.; Shin, E.-y.; Yang, S.; Ali, W.; Altinpinar, S.; Gutmann, J.S. Investigation of Roughness Correlation in Polymer Brushes via X-ray Scattering. Polymers 2020, 12, 2101. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.; Vasudevan, S.; David, H.; Shaktivel, A.; Shanmugam, K.; Neelakantan, P.; Solomon, A.P. Revisiting ESKAPE Pathogens: Virulence, resistance, and combating strategies focusing on quorum sensing. Front. Cell. Infect. Microbiol. 2023, 13, 1159798. [Google Scholar] [CrossRef] [PubMed]



| Surface | Surface Energy Characteristics (mJ·m−2) | Contact Angle (°) | |||||
|---|---|---|---|---|---|---|---|
| δS | δLW | δ+ | δ− | Water | CH2I2 | CH3NO | |
| Y-TZP a | 40.32 | 34.89 | 0.32 | 23.05 | 59.9 | 48.9 | 49.8 |
| Y-TZP-OH a | 57.87 | 36.20 | 2.56 | 45.86 | 21.1 | 48 | 4.4 |
| Y-TZP-Br | 46.81 | 35.31 | 1.72 | 19.23 | 58.8 | 48.1 | 37.5 |
| Y-TZP-PIL b | 50.92 | 37.90 | 1.03 | 41.03 | 35.0 | 43.3 | 28.2 |
| Surface | Polymerization Reaction Time (h) | Film Thickness (nm) | Static Water Contact Angle (deg) |
|---|---|---|---|
| Y-TZP | - | - | 60 (±3) |
| Y-TZP-OH | - | - | 21 (±6) |
| Y-TZP-Br | - | 2 | 59 (±2) |
| Y-TZP-PIL | 4 | 9 | 37 (±1) |
| Y-TZP-PIL | 8 | 40 | 35 (±1) |
| Y-TZP-PIL | 12 | 51 | 35 (±2) |
| Y-TZP-PIL | 18 | 57 | 35 (±2) |
| Y-TZP-PIL | 24 | 69 | 35 (±2) |
| Y-TZP-PIL | 48 | 72 | 35(±2) |
| Y-TZP-OH a | 24 | 1 | 24 (±3) |
| Experimental Atomic Ratio % | |||||||
|---|---|---|---|---|---|---|---|
| Surfaces | Zr3d | Y3d | O1s | C1s | Br3d | N1s | Cl2p |
| Y-TZP-OH | 29.9 | 1.8 | 59.7 | 8.6 | - | - | - |
| Y-TZP-PIL a | 6.1 | <1 | 12.1 | 73.2 | <1 | 6.1 | 2.0 |
| Surface | S. aureus | P. aeruginosa | ||
|---|---|---|---|---|
| Number of Adhered Bacteria | Bacterial Adhesion Inhibition | Number of Adhered Bacteria | Bacterial Adhesion Inhibition | |
| Y-TZP-OH | 4.20 × 105 CFU.cm−2 | - | 1.4 × 105 CFU.cm−2 | - |
| Y-TZP-PIL (40 nm) | 2.85 × 104 CFU.cm−2 | 93.2% | 4.9 × 103 CFU.cm−2 | 96.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harfouche, N.; Marie, P.; Dragoe, D.; Le, H.; Thébault, P.; Bilot, C.; Fouchet, A.; Rouden, J.; Baudoux, J.; Lepoittevin, B. Antibacterial Zirconia Surfaces from Organocatalyzed Atom-Transfer Radical Polymerization. Materials 2024, 17, 1775. https://doi.org/10.3390/ma17081775
Harfouche N, Marie P, Dragoe D, Le H, Thébault P, Bilot C, Fouchet A, Rouden J, Baudoux J, Lepoittevin B. Antibacterial Zirconia Surfaces from Organocatalyzed Atom-Transfer Radical Polymerization. Materials. 2024; 17(8):1775. https://doi.org/10.3390/ma17081775
Chicago/Turabian StyleHarfouche, Nesrine, Philippe Marie, Diana Dragoe, Hung Le, Pascal Thébault, Christelle Bilot, Arnaud Fouchet, Jacques Rouden, Jérôme Baudoux, and Bénédicte Lepoittevin. 2024. "Antibacterial Zirconia Surfaces from Organocatalyzed Atom-Transfer Radical Polymerization" Materials 17, no. 8: 1775. https://doi.org/10.3390/ma17081775







