Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
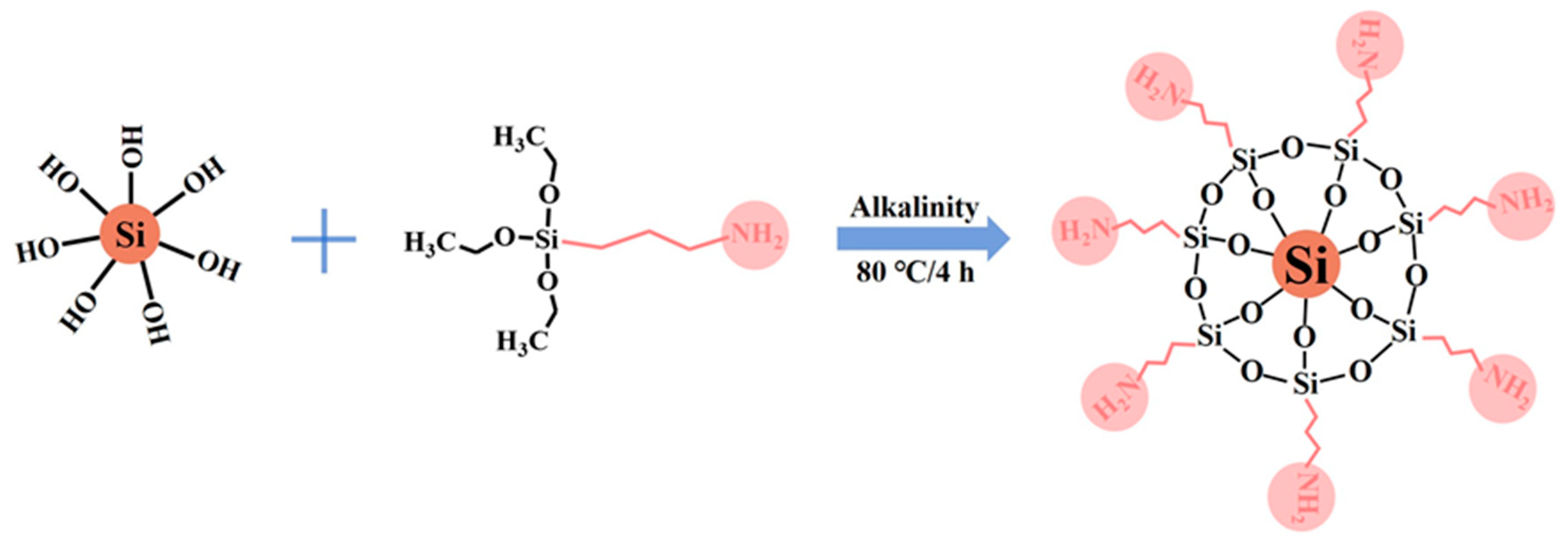
2.2. Preparation of ANS
2.3. Characterization of ANS
2.4. Pressure Penetration Test
2.5. BET Test
2.6. Expansion Inhibition Test
2.7. Dispersion Inhibition Test
2.8. Clay Zeta Potential and Particle Size Test
3. Results
3.1. Characterization of ANS
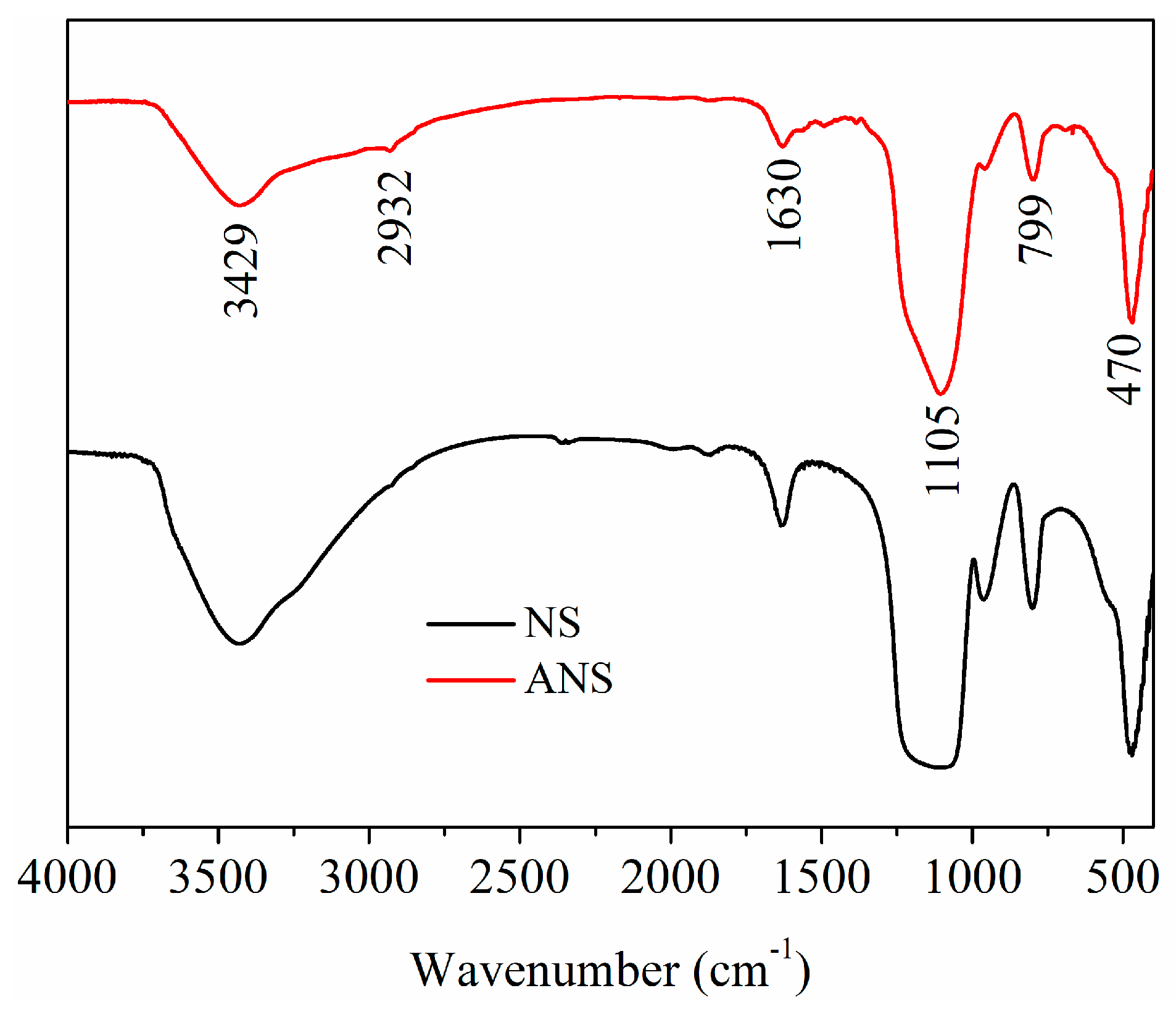
3.1.1. FT-IR
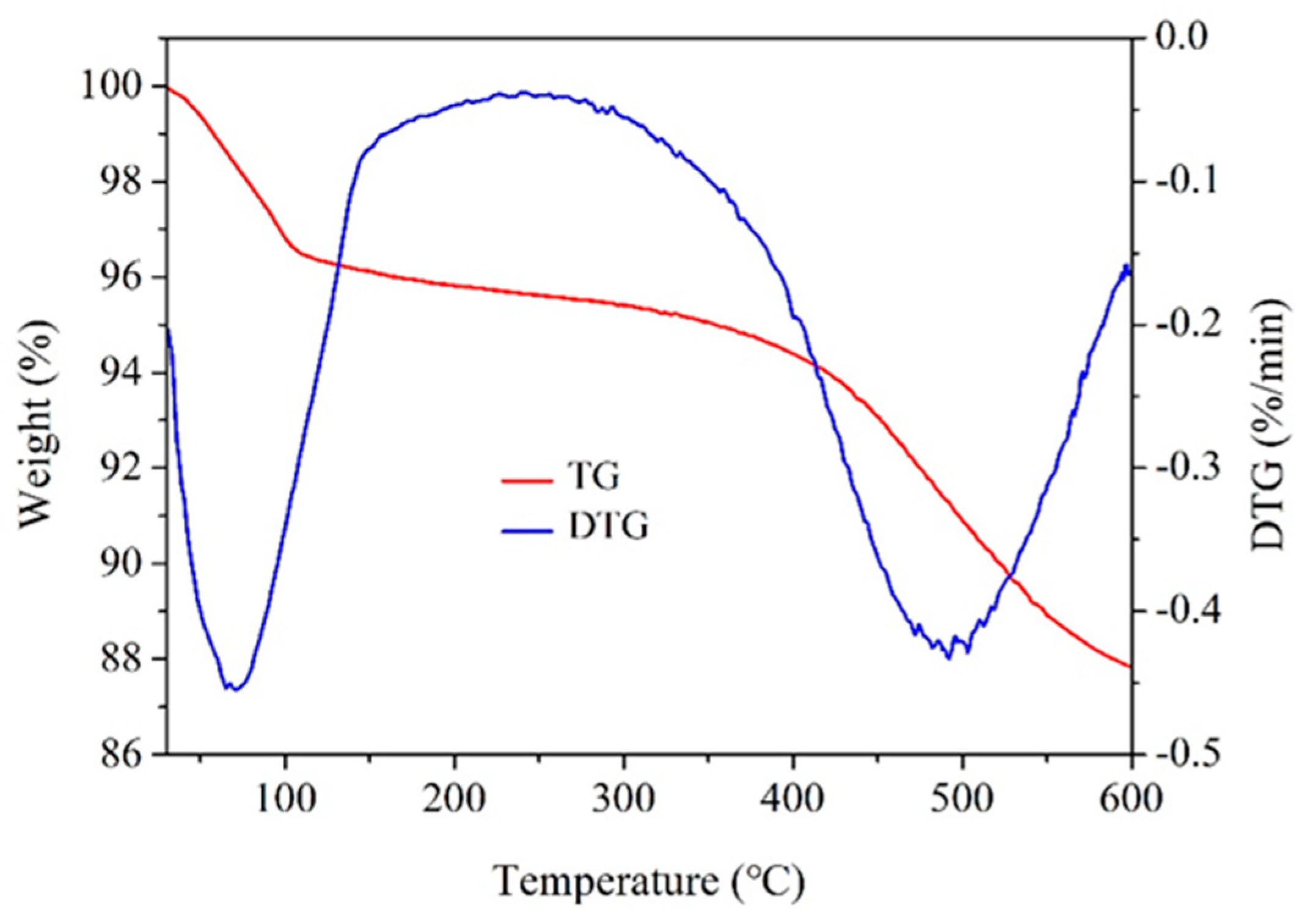
3.1.2. TGA
3.1.3. PST
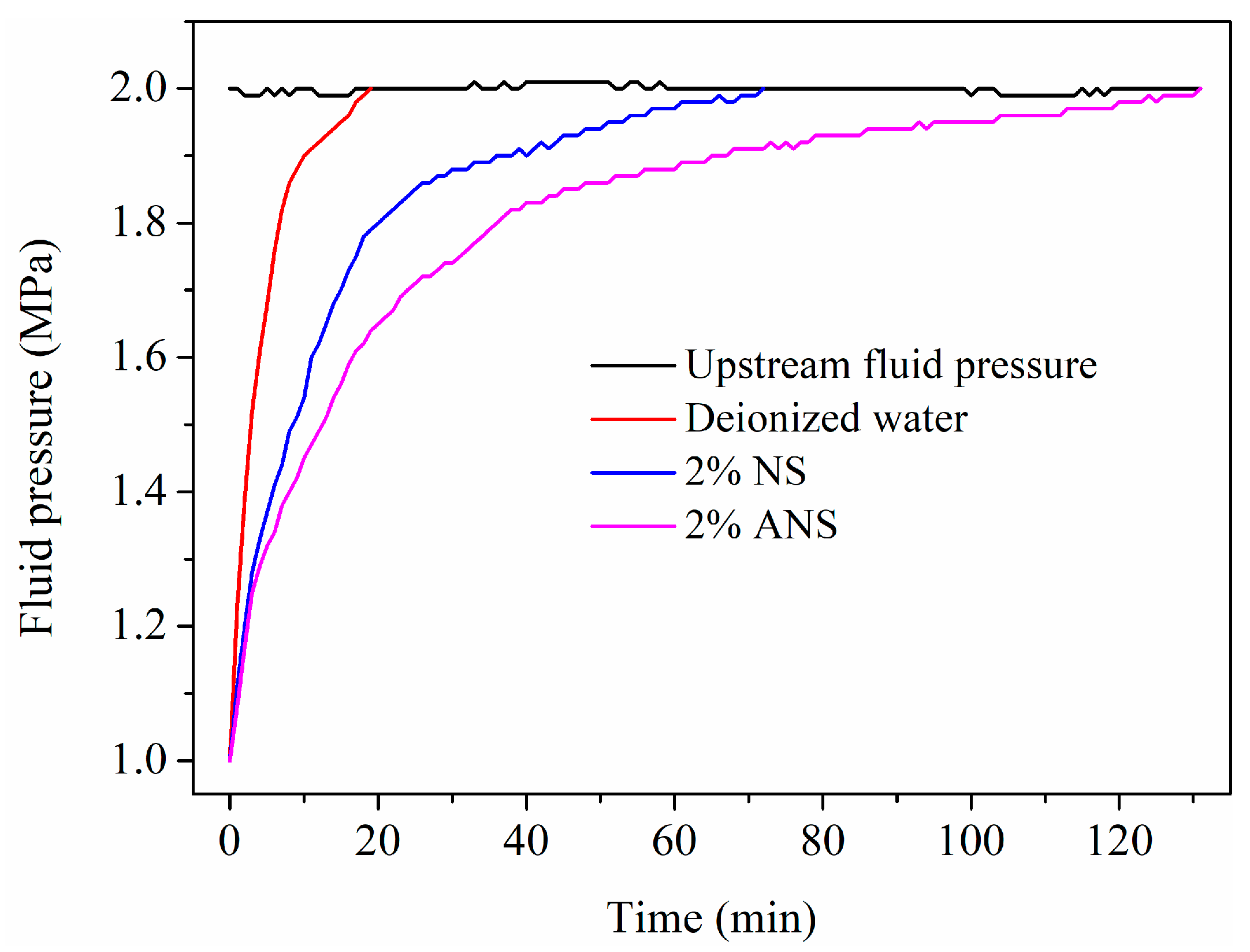
3.2. Pressure Penetration
3.3. BET Investigation
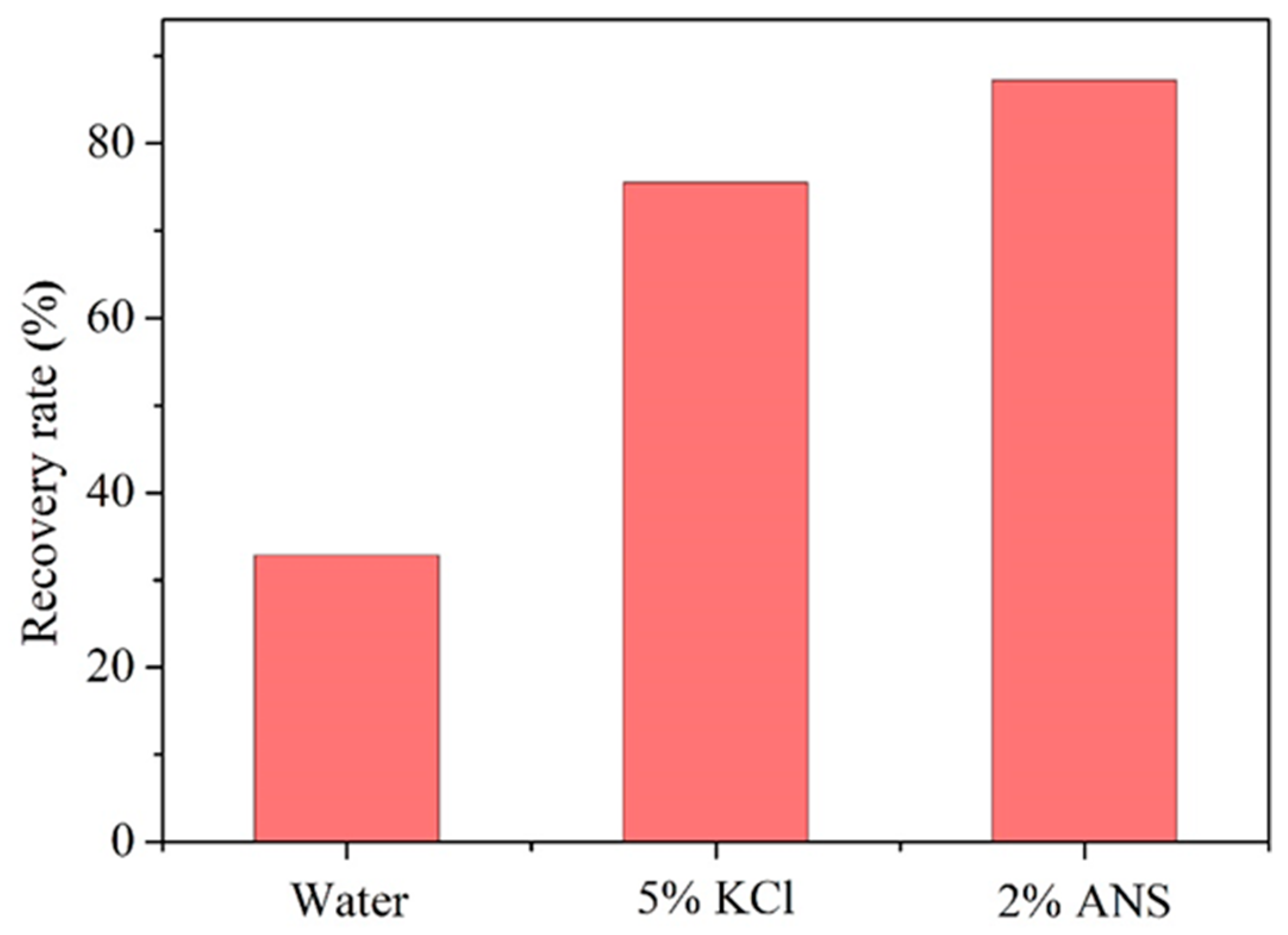
3.4. Expansion Inhibition
3.5. Dispersion Inhibition
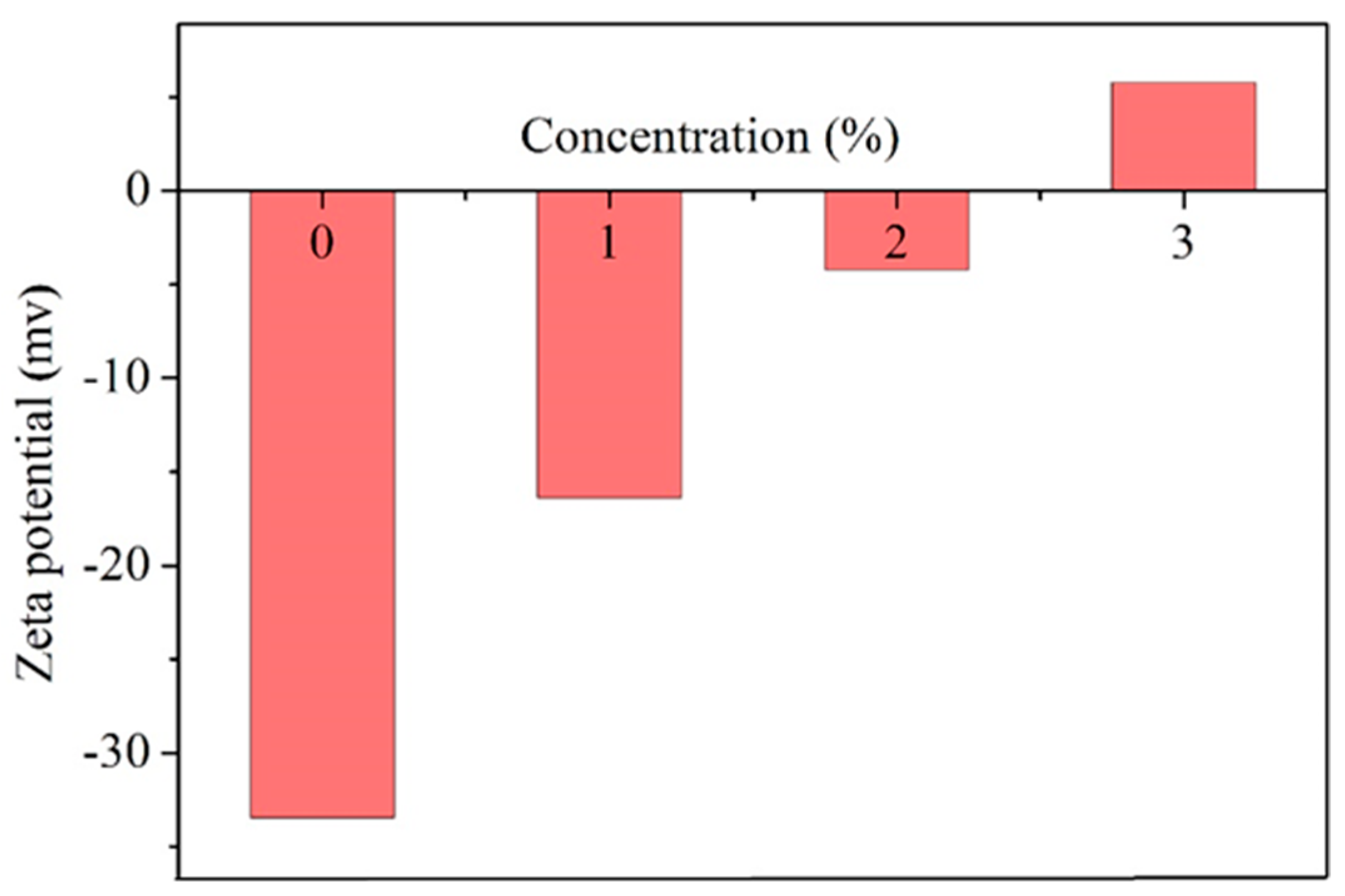
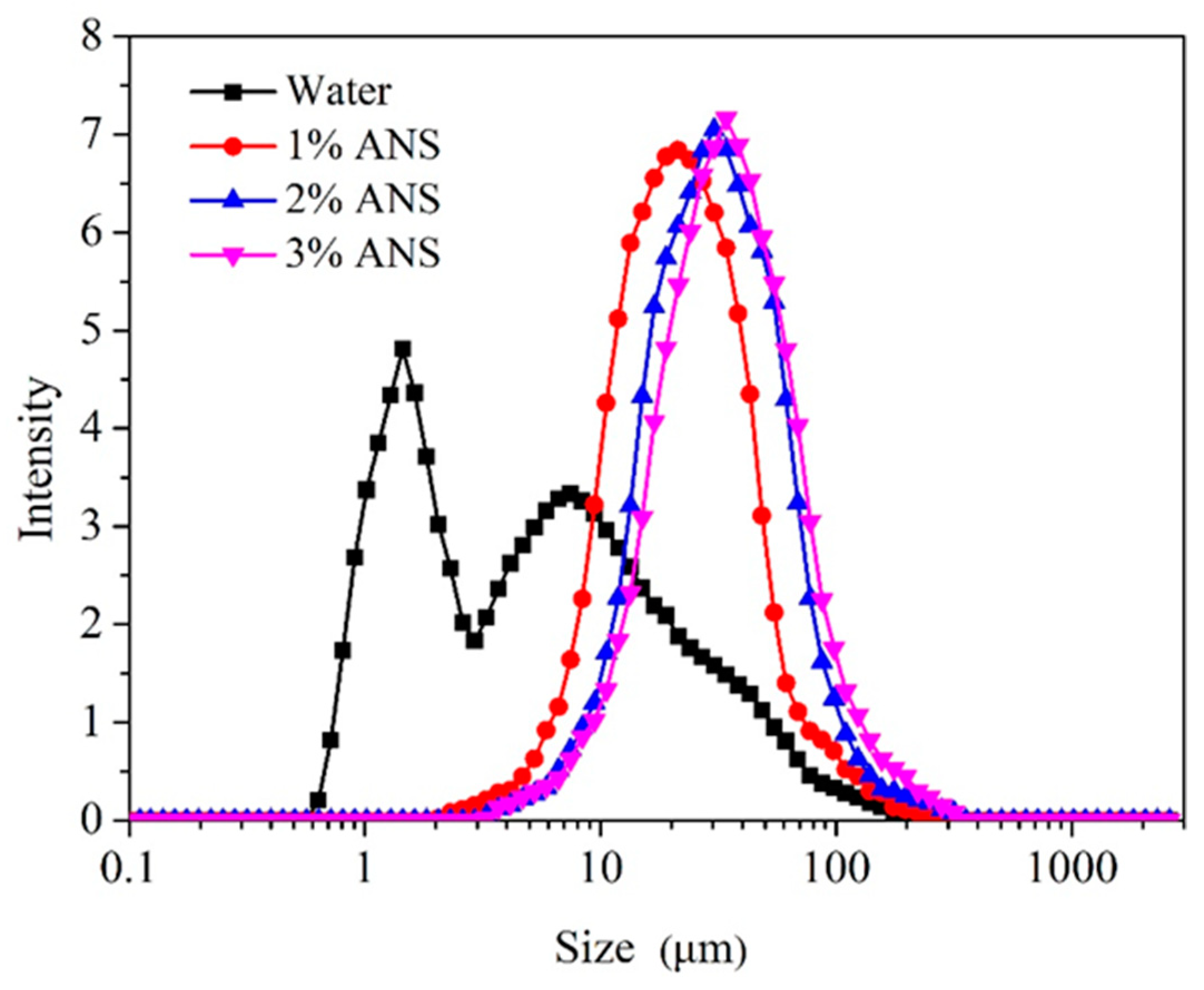
3.6. Clay Zeta Potential and Particle Size
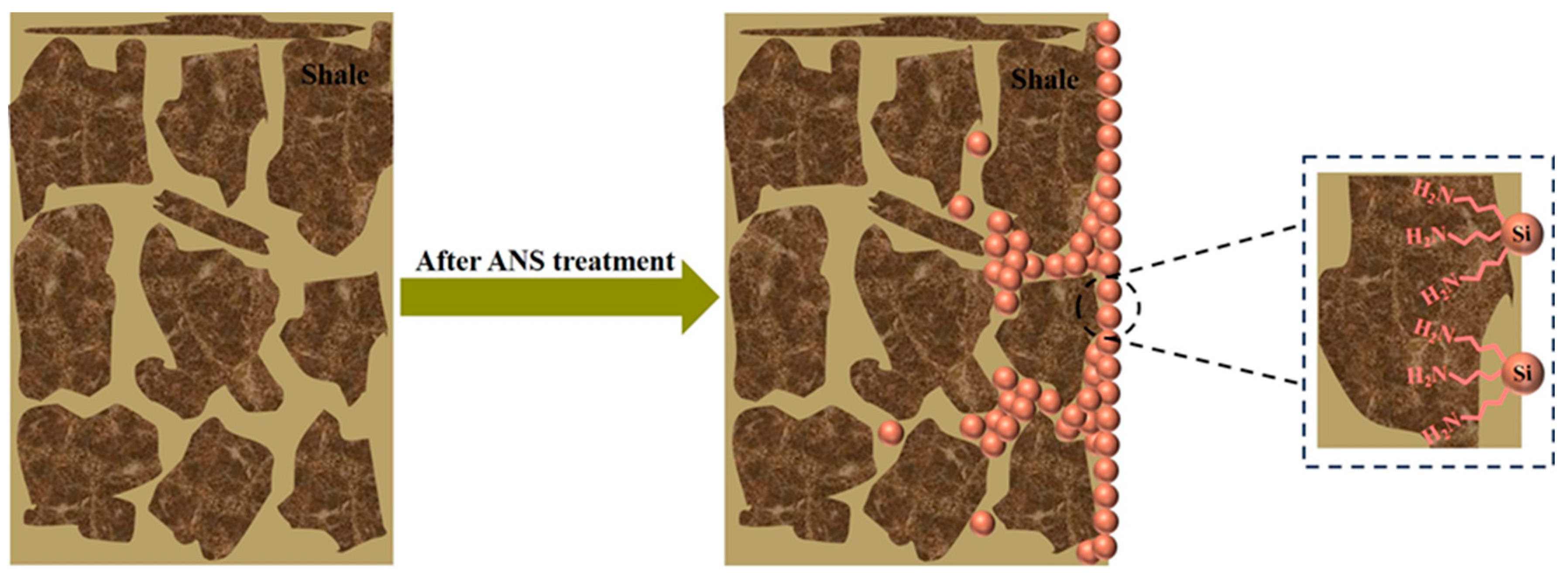
3.7. Wellbore Stability Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Aminated nano-silica |
| APTES | (3-Aminopropyl) triethoxysilane |
| FT-IR | Fourier transforms infrared spectroscopy |
| TGA | Thermo-gravimetric analysis |
| PST | Particle size tests |
| KCl | Potassium chloride |
| HPAM | Partially hydrolyzed polyacrylamide |
| NS | Nano-silica |
| BET | Brunauer-Emmett-Teller |
References
- Allawi, R.H.; Al-Jawad, M.S. Wellbore instability management using geomechanical modeling and wellbore stability analysis for Zubair shale formation in Southern Iraq. J. Pet. Explor. Prod. Technol. 2021, 11, 4047–4062. [Google Scholar] [CrossRef]
- Zhao, X.; Geng, Q.; Zhang, Z.; Qiu, Z.; Fang, Q.; Wang, Z.; Yan, C.; Ma, Y.; Li, Y. Phase change material microcapsules for smart temperature regulation of drilling fluids for gas hydrate reservoirs. Energy 2023, 263, 125715. [Google Scholar] [CrossRef]
- Chang, Y.; Xiao, S.; Ma, R.; Zhang, Z.; He, J. Atomistic insight into oil displacement on rough surface by Janus nanoparticles. Energy 2022, 245, 123264. [Google Scholar] [CrossRef]
- Holt, R.M.; Larsen, I.; Fjær, E.; Stenebråten, J.F. Comparing mechanical and ultrasonic behaviour of a brittle and a ductile shale: Relevance to prediction of borehole stability and verification of shale barriers. J. Petrol. Sci. Eng. 2020, 187, 106746. [Google Scholar] [CrossRef]
- Chang, Y.; Xiao, S.; Fu, Y.; Wang, X.; Zhang, Z.; He, J. Nanomechanical characteristics of trapped oil droplets with nanoparticles: A molecular dynamics simulation. J. Petrol. Sci. Eng. 2021, 203, 108649. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Zhu, H.; Yang, L.; Li, Y.; Wang, T.; Wu, X. Improved shale hydration inhibition with combination of gelatin and KCl or EPTAC, an environmentally friendly inhibitor for water-based drilling fluids. J. Appl. Polym. Sci. 2019, 136, 47585. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.; Zhao, X.; Zhang, Y.; Li, G.; Huang, W. Application of nano-polymer emulsion for inhibiting shale self-imbibition in water-based drilling fluids. J. Surfactants Deterg. 2018, 21, 155–164. [Google Scholar] [CrossRef]
- Xie, G.; Xiao, Y.; Deng, M.; Luo, Y.; Luo, P. Low Molecular Weight Branched Polyamine as a Clay Swelling Inhibitor and Its Inhibition Mechanism: Experiment and Density Functional Theory Simulation. Energy Fuels 2020, 34, 2169–2177. [Google Scholar] [CrossRef]
- Sun, J.S.; Wang, Z.L.; Liu, J.P.; Lv, K.H.; Zhang, F.; Shao, Z.H.; Dong, X.D.; Dai, Z.W.; Zhang, X.F. Notoginsenoside as an environmentally friendly shale inhibitor in water-based drilling fluid. Petrol. Sci. 2022, 19, 608–618. [Google Scholar] [CrossRef]
- Huang, X.; Dai, Z.; Zhang, C.; Lv, K.; Meng, X.; Wang, R.; Lalji, S.M.; Khan, M.A. Improving the performance of a polyamine shale inhibitor by introducing a hydrophobic group in oil and gas development. Fuel 2024, 355, 129435. [Google Scholar] [CrossRef]
- Saleh, T.A.; Rana, A. Surface-modified biopolymer as an environment-friendly shale inhibitor and swelling control agent. J. Mol. Liq. 2021, 342, 117275. [Google Scholar] [CrossRef]
- Murtaza, M.; Kamal, M.S.; Mahmoud, M. Application of a novel and sustainable silicate solution as an alternative to sodium silicate for clay swelling inhibition. ACS Omega 2020, 5, 17405–17415. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.; Zhao, X.; Mou, T.; Zhong, H.; Huang, W. A polymer microsphere emulsion as a high-performance shale stabilizer for water-based drilling fluids. RSC Adv. 2018, 8, 20852–20861. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Sun, B.; Liu, S.; Xing, X.; Wang, M. Formation damage mechanisms associated with drilling and completion fluids for deepwater reservoirs. J. Petrol. Sci. Eng. 2019, 173, 112–121. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.; Haq, B.; Patil, S. Insights into the application of surfactants and nanomaterials as shale inhibitors for water-based drilling fluid: A review. J. Nat. Gas. Sci. Eng. 2021, 92, 103987. [Google Scholar] [CrossRef]
- Khodja, M.; Canselier, J.P.; Bergaya, F.; Fourar, K.; Khodja, M.; Cohaut, N.; Benmounah, A. Shale problems and water-based drilling fluid optimization inthe Hassi Messaoud Algerian oil field. Appl. Clay Sci. 2010, 49, 383–393. [Google Scholar] [CrossRef]
- Sehly, K.; Chiew, H.L.; Li, H.; Song, A.; Leong, Y.K.; Huang, W. Stability and ageing behaviour and the formulation of potassium-based drilling muds. Appl. Clay Sci. 2015, 104, 309–317. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water-based drilling fluid system. J. Petrol. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Gholami, R.; Elochukwu, H.; Fakhari, N.; Sarmadivaleh, M. A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors. Earth Sci. Rev. 2018, 177, 2–13. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M.; Ayoub, M.; Zamir, A.; Abbas, M.A. A review of the usage of deep eutectic solvents as shale inhibitors in drilling mud. J. Mol. Liq. 2022, 361, 119673. [Google Scholar] [CrossRef]
- Qiu, Z.; Xu, J.; Yang, P.; Zhao, X.; Mou, T.; Zhong, H.; Huang, W. Effect of amphiphilic polymer/nano-silica composite on shale stability for water-based muds. Appl. Sci. 2018, 8, 1839. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Zhang, X.; Qiu, Z.; Zhao, C.; Zhang, X.; Jin, J. Improving the shale stability with nano-silica grafted with hyperbranched polyethyleneimine in water-based drilling fluid. J. Nat. Gas. Sci. Eng. 2020, 83, 103624. [Google Scholar] [CrossRef]
- Xu, J.G.; Su, K.; Li, M.; Lyu, X.; Zhu, S.; Huang, Y. Hydration inhibition and physical plugging to enhance shale stability using zwitterionic polymer/nano-silica composite. J. Mol. Liq. 2023, 387, 122642. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Y.; Zhang, Y.; Lan, K. A novel self-healing and degradable plugging material for high-temperature gas well. J. Mol. Liq. 2023, 376, 121473. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Moslemizadeh, A.; Dezaki, A.S. A novel nonionic surfactant for inhibiting shale hydration. Appl. Clay Sci. 2015, 118, 74–86. [Google Scholar] [CrossRef]
- Yang, L.; Kong, D.; Chang, X.; Jiang, G.; Ao, T.; Xie, C.; Moukoko, A.D.K.; Ma, J. Counterion-specific shale hydration inhibiting performance of vinylimdazolium ionic liquids. J. Mol. Liq. 2021, 335, 116544. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Luo, P.; Huang, J.; Xiong, J.; Liang, L.; Li, W. Study of a polyamine inhibitor used for shale water-based drilling fluid. ACS Omega 2021, 6, 15448–15459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, Y.; She, J.; Kuang, J. Experimental study of nano-drilling fluid based on nano temporary plugging technology and its application mechanism in shale drilling. Appl. Nanosci. 2019, 9, 1637–1648. [Google Scholar] [CrossRef]
- Yang, X.; Cai, J.; Jiang, G.; Zhang, Y.; Shi, Y.; Chen, S.; Yue, Y.; Wei, Z.; Yin, D.; Li, H. Modeling of nanoparticle fluid microscopic plugging effect on horizontal and vertical wellbore of shale gas. Energy 2022, 239, 122130. [Google Scholar] [CrossRef]
- Pourkhalil, H.; Nakhaee, A. Effect of nano ZnO on wellbore stability in shale: An experimental investigation. J. Pet. Sci. Eng. 2019, 173, 880–888. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Lu, Y.; Shen, X.; Zhang, H.; Peng, J.; Jiang, S.; Duan, M. Compatibility and efficiency of hydrophilic/hydrophobic nano silica as rheological modifiers and fluid loss reducers in water-based drilling fluids. Geoenergy Sci. Eng. 2024, 234, 212628. [Google Scholar] [CrossRef]
- Ma, L.; Luo, P.; He, Y.; Zhang, L.; Fan, Y.; Jiang, Z. Ultra-stable silica nanoparticles as nano-plugging additive for shale exploitation in harsh environments. Nanomaterials 2019, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Tchameni, A.P.; Djouonkep LD, W.; Nagre, R.D.; Wang, X. Thermo-responsive polymer-based Janus biogenic-nanosilica composite, part B: Experimental study as a multi-functional synergistic shale stabilizer for water-based drilling fluids. J. Mol. Liq. 2024, 395, 123921. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A.; Rabiei, M.; Fakhari, N.; Balasubramaniam, P.; Rasouli, V.; Nagarajan, R. An approach to improve wellbore stability in active shale formations using nanomaterials. Petroleum 2020, 7, 24–32. [Google Scholar] [CrossRef]
- Xu, J.G.; Luo, T.; Wang, J.; Zhu, S.; Azadbakht, S.; Lyu, X.; Li, M.; Wang, L. Investigation of alcohol-based deep eutectic solvents for inhibiting hydration in shale formations. J. Mol. Liq. 2023, 392, 123551. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, Z.; Wang, X.; Li, X.; Zhang, Z.; Wang, X.; Dai, X.; Xin, Y.; Liu, Q.; Yao, H.; et al. Novel modified nano-silica/polymer composite in water-based drilling fluids to plug shale pores. Energ. Source. Part A 2022, 44, 8662–8678. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, H.; Wang, P. Polyampholyte-grafted silica nanoparticles for shale plugging under high-temperature and extreme-salinity conditions. Geoenergy Sci. Eng. 2024, 233, 212576. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Iqbal, T.; Al Harthi, M.A.; Kamal, M.S. Synergistic effect of polymer and nanoparticles on shale hydration and swelling performance of drilling fluids. J. Petrol. Sci. Eng. 2021, 205, 108763. [Google Scholar] [CrossRef]
- Saleh, T.A.; Nur, M.M.; Satria, M.; Al-Arfaj, A.A. Synthesis of novel hydrophobic nanocomposite-modified silica as efficient shale inhibitor in fuel industry. Surf. Interfaces 2023, 38, 102837. [Google Scholar] [CrossRef]
- Ni, X.; Jiang, G.; Li, Y.; Yang, L.; Li, W.; Wang, K.; Deng, Z. Synthesis of superhydrophobic nanofluids as shale inhibitor and study of the inhibition mechanism. Appl. Surf. Sci. 2019, 484, 957–965. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.; Zhao, X.; Huang, W. Hydrophobic modified polymer based silica nanocomposite for improving shale stability in water-based drilling fluids. J. Petrol. Sci. Eng. 2017, 153, 325–330. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, Z.; Huang, W.; Zhao, X. Preparation and performance properties of polymer latex SDNL in water-based drilling fluids for drilling troublesome shale formations. J. Nat. Gas. Sci. Eng. 2017, 37, 462–470. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Wang, J.; Zhang, J. Oil occurrence mechanism in nanoporous shales: A theoretical and experimental study. Mar. Petrol. Geol. 2023, 156, 106422. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.S.; Zhao, X.; Zhong, H.Y.; Li, G.R.; Huang, W.A. Synthesis and characterization of shale stabilizer based on polyethylene glycol grafted nano-silica composite in water-based drilling fluids. J. Petrol. Sci. Eng. 2018, 163, 371–377. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Chang, X.; Lin, Z.; Chen, G.; Li, J.; Liu, J.; Tian, S. Evaluating microdistribution of adsorbed and free oil in a lacustrine shale using nuclear magnetic resonance: A theoretical and experimental study. J. Petrol. Sci. Eng. 2022, 212, 110208. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Wang, J.; Zhang, J.; Yin, Y. Pore Structure Characterizations of Shale Oil Reservoirs with Heat Treatment: A Case Study from Dongying Sag, Bohai Bay Basin, China. ACS Omega 2023, 8, 26508–26525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, G.; Shen, X.; Li, G. Poly-L-arginine as a high-performance and biodegradable shale inhibitor in water-based drilling fluids for stabilizing wellbore. ACS Sustain. Chem. Eng. 2020, 8, 1899–1907. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Osman, M.M.; Yakout, A.A.; Abdelfattah, A.M. Water and soil decontamination of toxic heavy metals using aminosilica-functionalized-ionic liquid nanocomposite. J. Mol. Liq. 2018, 266, 834–845. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, L.; Wu, S.; Dong, L.J. Synthesis and characterization of compartmented Ca-alginate/silica self-healing fibers containing bituminous rejuvenator. Constr. Build. Mater. 2018, 190, 623–631. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, J.; Xie, S.; Huang, X.; Wang, R.; Wang, J.; Wang, Q. Novel use of a superhydrophobic nanosilica performing wettability alteration and plugging in water-based drilling fluids for wellbore strengthening. Energy Fuels 2022, 36, 6144–6158. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Huang, W.; Sun, D.; Zhang, D.; Cao, J. Synergistic stabilization of shale by a mixture of polyamidoamine dendrimers modified bentonite with various generations in water-based drilling fluid. Appl. Clay. Sci. 2015, 114, 359–369. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, J.; Liu, X. Experimental study on crack propagation in shale formations considering hydration and wettability. J. Nat. Gas. Sci. Eng. 2015, 23, 492–499. [Google Scholar] [CrossRef]
- Liang, L.; Luo, D.; Liu, X.; Xiong, J. Experimental study on the wettability and adsorption characteristics of Longmaxi Formation shale in the Sichuan Basin, China. J. Nat. Gas. Sci. Eng. 2016, 33, 1107–1118. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Tang, Z.; Zhang, X.; Zhang, D.; Huang, W. Minimization shale hydration with the combination of hydroxyl-terminated PAMAM dendrimers and KCl. J. Mater. Sci. 2016, 51, 8484–8501. [Google Scholar] [CrossRef]
- Ma, J.; Pang, S.; Zhou, W.; Xia, B.; An, Y. Novel deep eutectic solvents for stabilizing clay and inhibiting shale hydration. Energy Fuels 2021, 35, 7833–7843. [Google Scholar] [CrossRef]
- Huang, X.-B.; Sun, J.-S.; Huang, Y.; Yan, B.-C.; Dong, X.-D.; Liu, F.; Wang, R. Laponite: A promising nanomaterial to formulate high-performance water-based drilling fluids. Pet. Sci. 2021, 18, 579–590. [Google Scholar] [CrossRef]
- Blkoor, S.O.; Norddin, M.N.A.B.M.; Ismail, I.; Oseh, J.O.; Basaleh, S.S.; Risal, A.R.B.; Sariman, M.F.B.; Ngouangna, E.N. Enhancing the stability of shale in water-based fluid with polyethylene glycol/nanosilica composite grafted with sodium dodecyl sulfate. Arab. J. Geosci. 2023, 16, 541. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Huang, W.; Cao, J. Poly(oxypropylene)-amidoamine modified bentonite as potential shale inhibitor in water-based drilling fluids. Appl. Clay. Sci. 2012, 67, 36–43. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.; Zhao, X.; Zhong, H.; Huang, W. Study of 1-Octyl-3-methylimidazolium bromide for inhibiting shale hydration and dispersion. J. Petrol. Sci. Eng. 2019, 177, 208–214. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S. A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. J. Petrol. Sci. Eng. 2021, 206, 109043. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xu, J.; Pei, D.; Su, K.; Wang, L. Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability. Materials 2024, 17, 1776. https://doi.org/10.3390/ma17081776
Li M, Xu J, Pei D, Su K, Wang L. Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability. Materials. 2024; 17(8):1776. https://doi.org/10.3390/ma17081776
Chicago/Turabian StyleLi, Meng, Jiangen Xu, Dongdong Pei, Kanhua Su, and Liang Wang. 2024. "Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability" Materials 17, no. 8: 1776. https://doi.org/10.3390/ma17081776



