New Adsorption Materials for Deep Desulfurization of Fuel Oil
Abstract
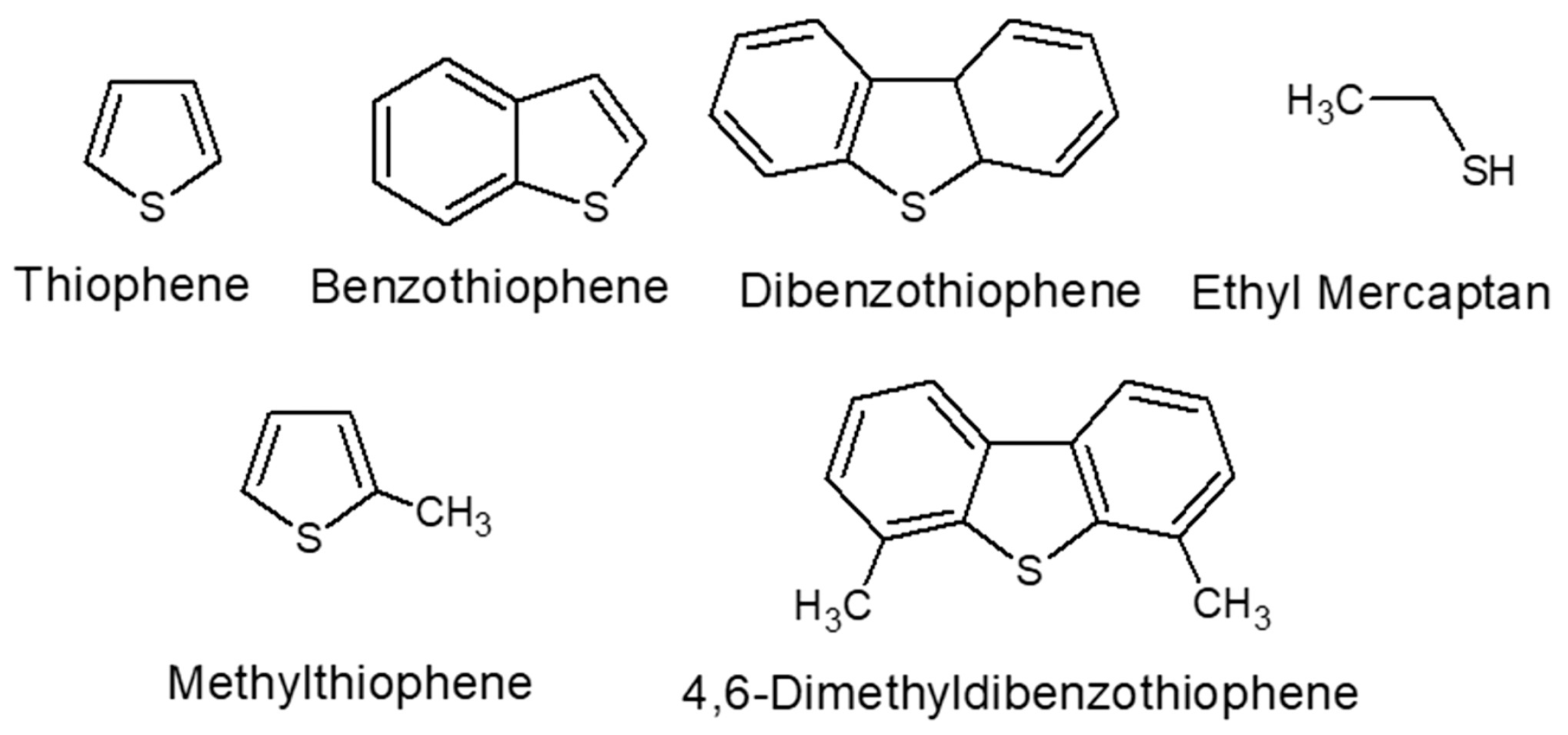
:1. Introduction
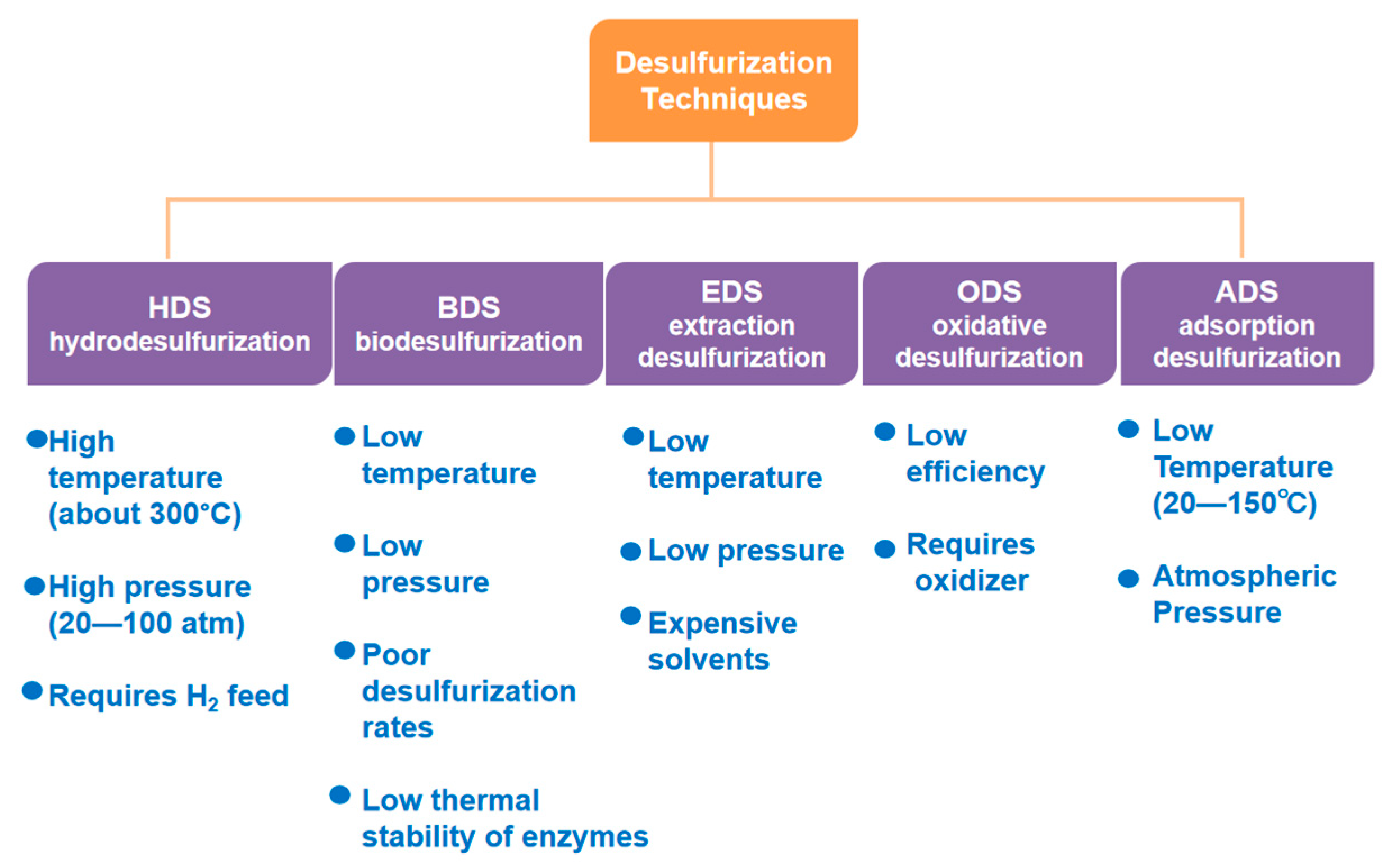
2. Desulfurization Process
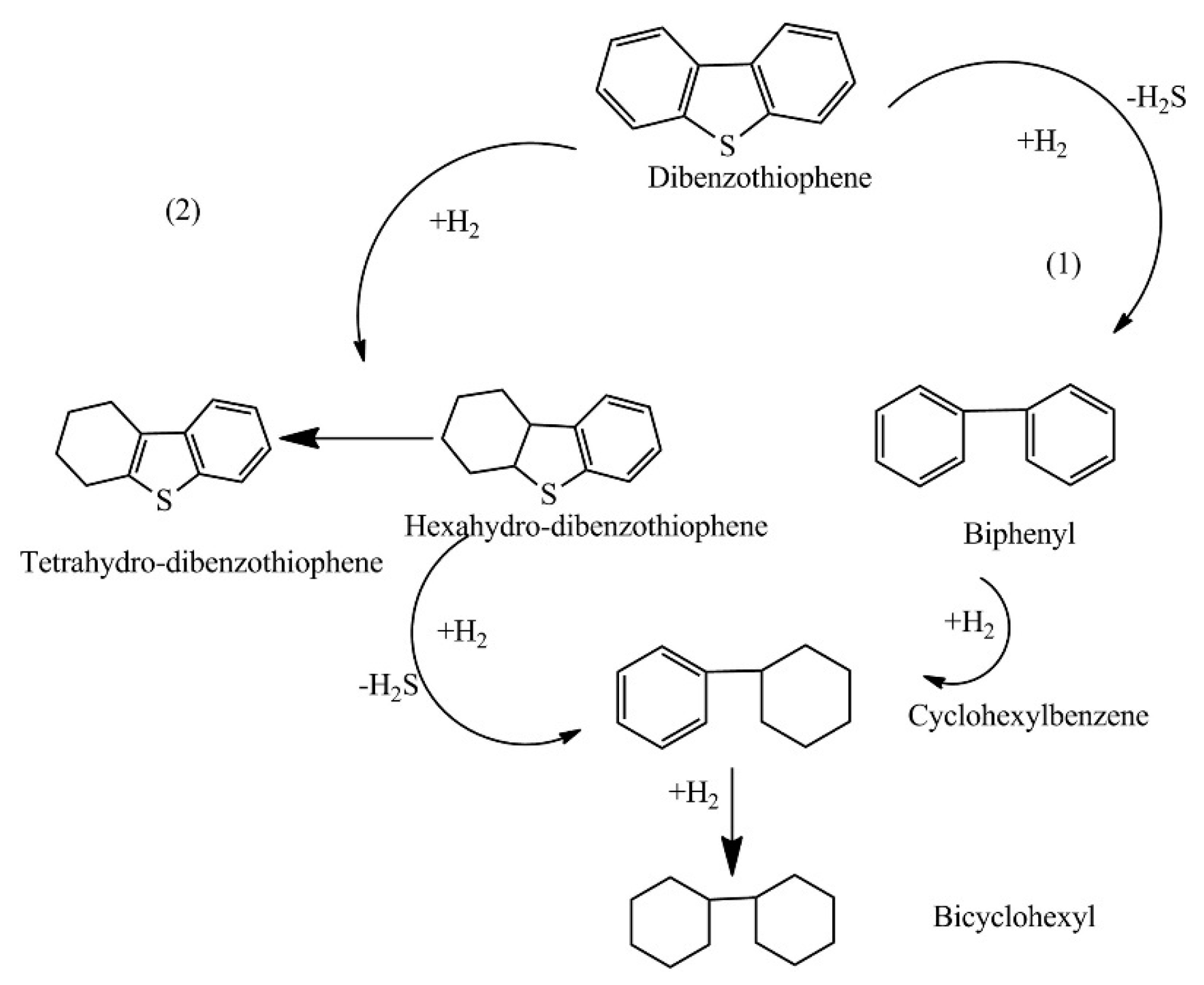
2.1. Hydrodesulfurization
2.2. Biodesulfurization
2.3. Extraction Desulfurization
2.4. Oxidative Desulfurization
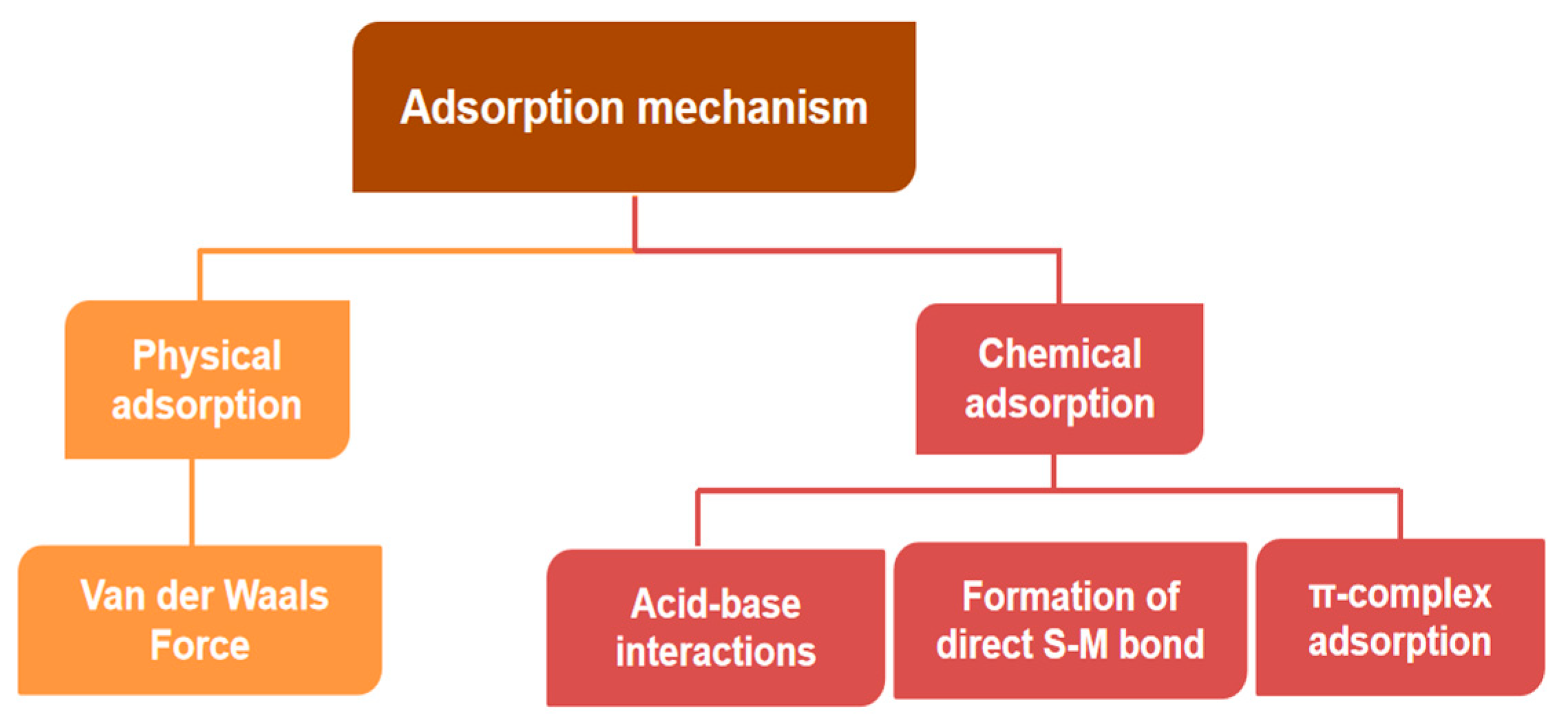
2.5. Adsorption Desulfurization
3. Different Types of Adsorption Materials
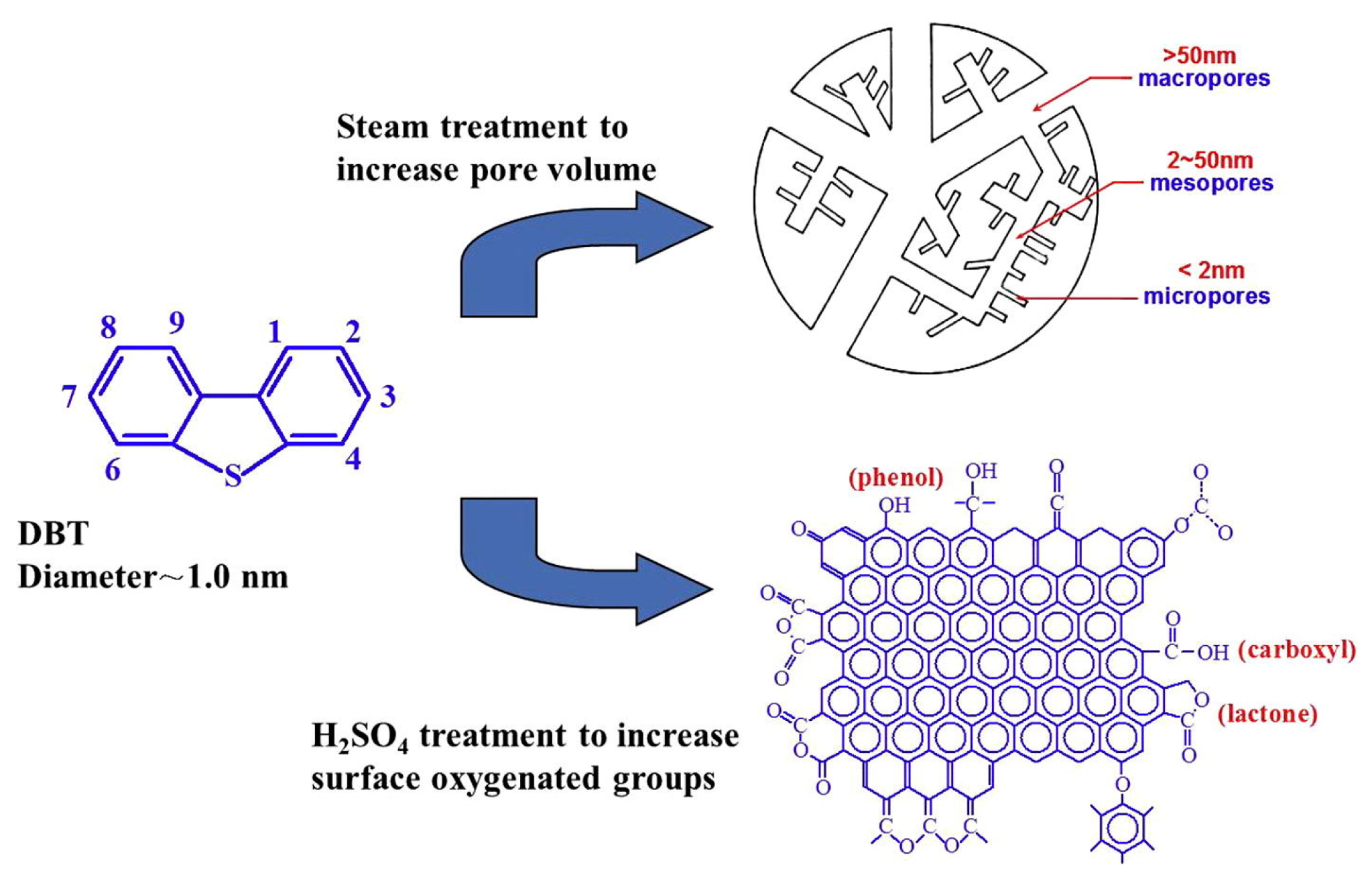
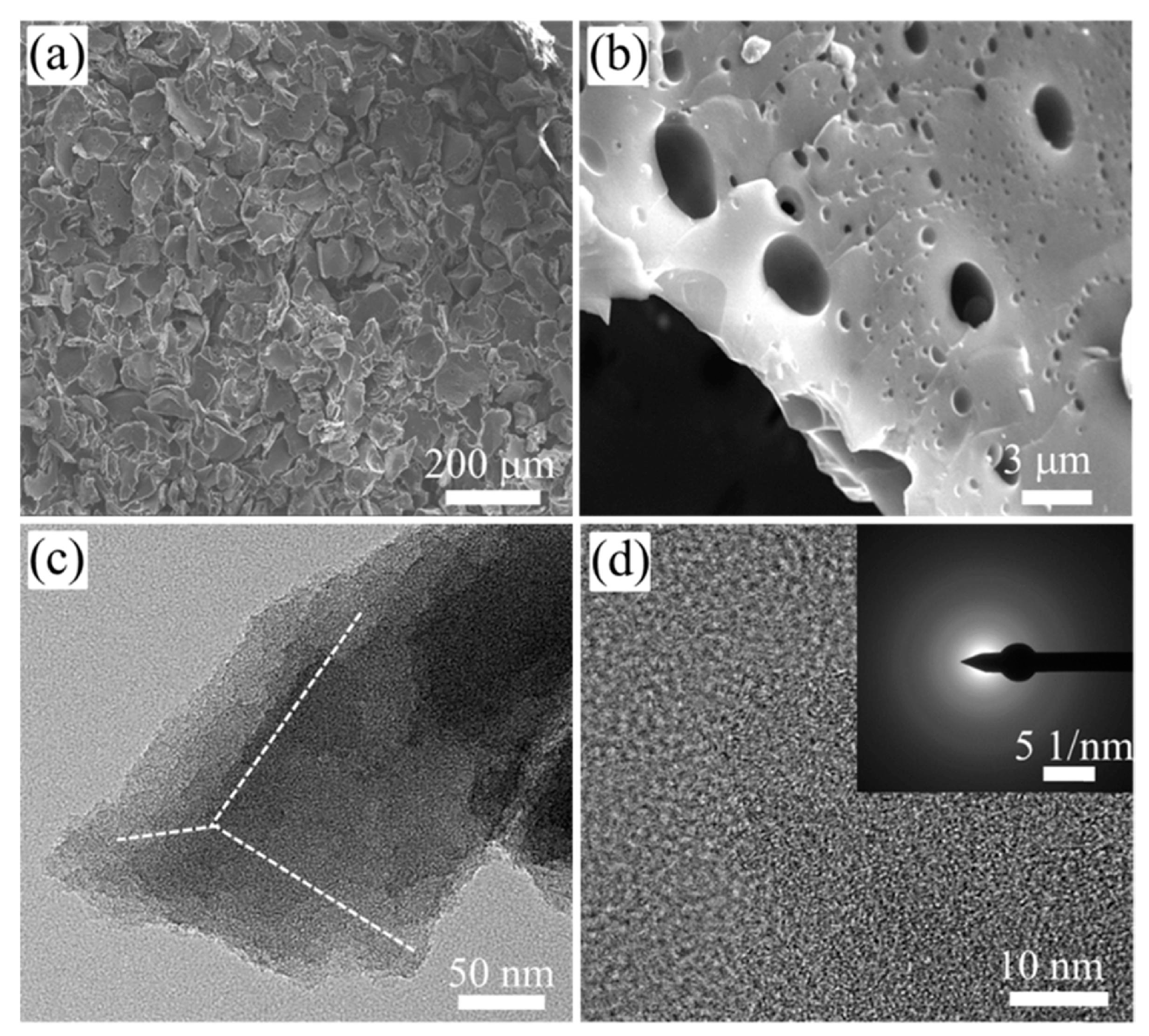
3.1. Activated Carbon Materials
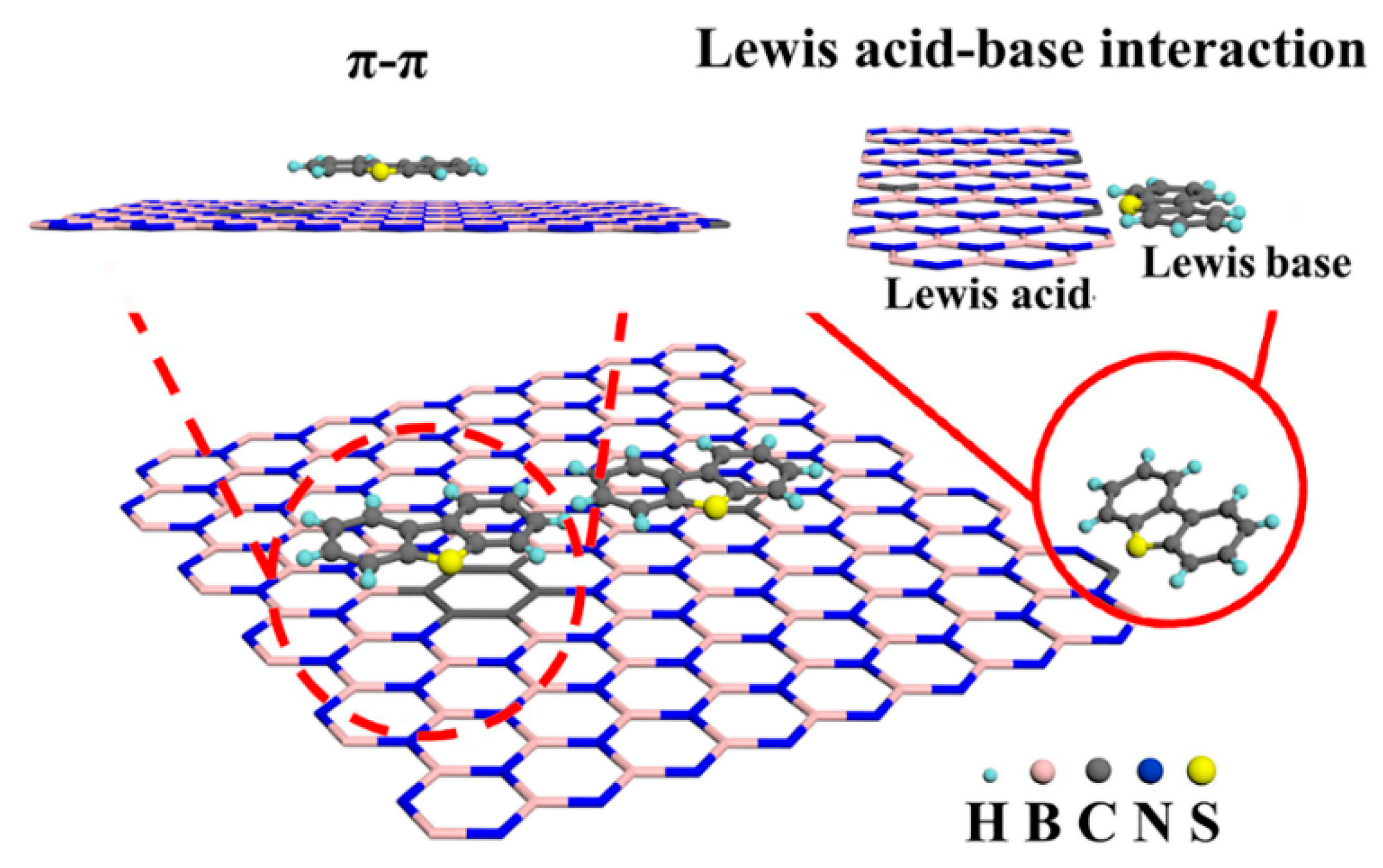
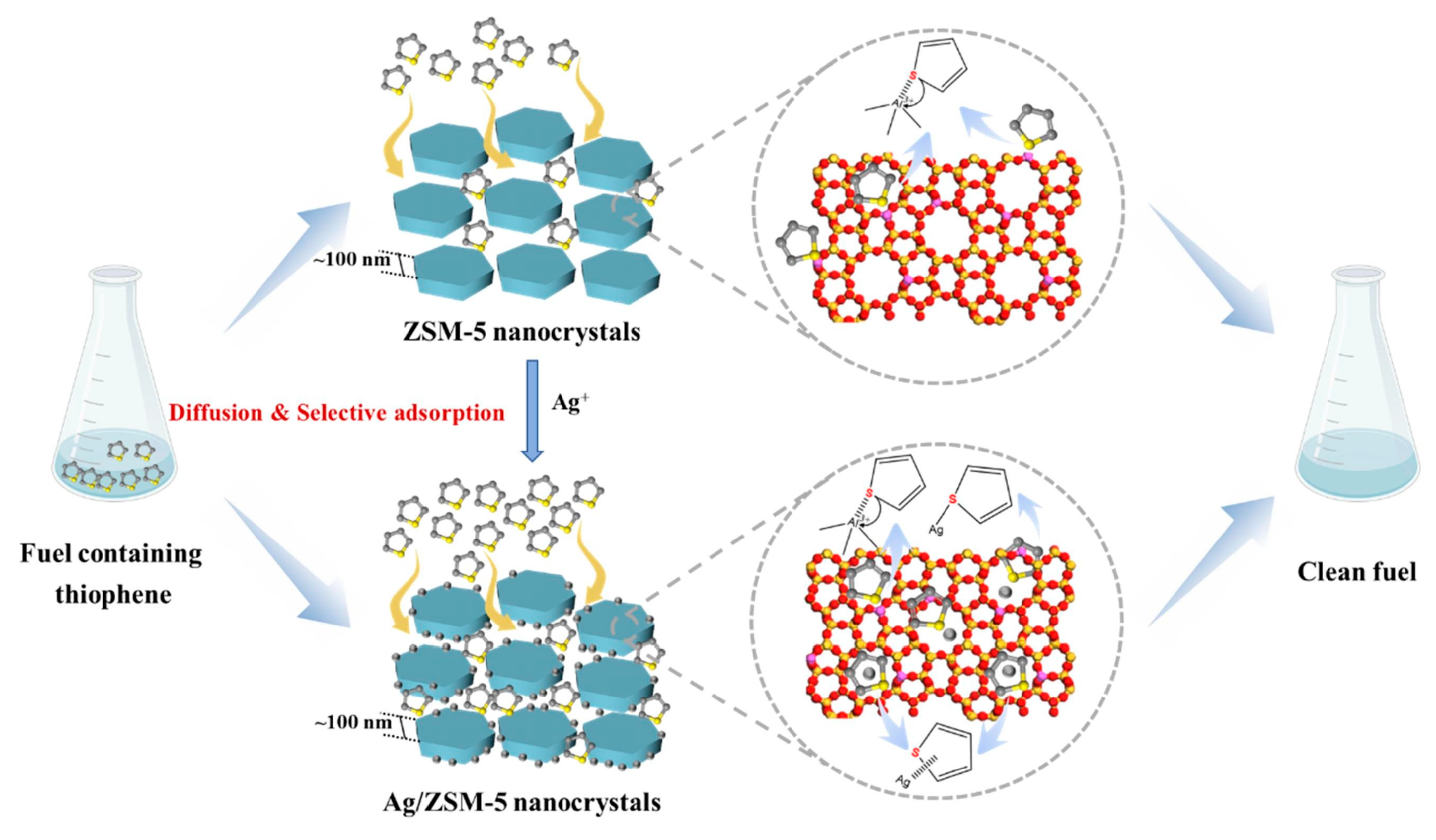
3.2. Zeolite Molecular Sieve Adsorbent
3.3. Metal-Organic Frameworks
3.4. Metal Oxide-Based Adsorbent
3.5. Other Adsorption Materials
4. Regeneration Performance
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haruna, A.; Merican, Z.M.A.; Musa, S.G.; Abubakar, S. Sulfur removal technologies from fuel oil for safe and sustainable environment. Fuel 2022, 329, 125370. [Google Scholar] [CrossRef]
- Crandall, B.S.; Zhang, J.Y.; Stavila, V.; Allendorf, M.D.; Li, Z.L. Desulfurization of Liquid Hydrocarbon Fuels with Microporous and Mesoporous Materials: Metal-Organic Frameworks, Zeolites, and Mesoporous Silicas. Ind. Eng. Chem. Res. 2019, 58, 19322–19352. [Google Scholar] [CrossRef]
- Cui, X.L.; Yang, Q.W.; Krishna, R.; Wu, H.; Zhou, W.; Chen, B.L.; Xing, H.B. Ultrahigh and selective SO2 uptake in inorganic anion-pillared hybrid porous materials. Adv. Mater. 2017, 29, 1606929. [Google Scholar] [CrossRef] [PubMed]
- Shoja, S.M.R.; Abdouss, M.; Saeedirad, R. Synthesis, characterization, and application of N-CNT/1-(2-Hydroxyethyl)-3-methylimidazolium dicyanamide as a green nanocatalyst for the sulfur removal from light oils. Heliyon 2024, 10, e24073. [Google Scholar] [CrossRef]
- Wang, L.; Lv, Y.C.; Deng, C.C.; Ma, Y.Y.; Li, S.Z.; Yaseen, M. Designing Al-SiO2 nanospheres with co-doped Ce/Cd for the removal of 4,6-dimethyldibenzothiophene of fuel oil. J. Alloys Compd. 2023, 968, 171989. [Google Scholar] [CrossRef]
- Azeez, M.O.; Ganiyu, S.A. Review of biomass derived-activated carbon for production of clean fuels by adsorptive desulfurization: Insights into processes, modifications, properties, and performances. Arab. J. Chem. 2023, 16, 105182. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Lateef, S.A. Review of adsorptive desulfurization process: Overview of the non-carbonaceous materials, mechanism and synthesis strategies. Fuel 2021, 294, 120273. [Google Scholar] [CrossRef]
- Sánchez-Hervás, J.; Ortiz, I.; Martí, V.; Andray, A. Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc-Nickel-Oxide Sorbent for Integration in Biofuel Production. Catalysts 2023, 13, 1089. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.H.; Deng, C.C.; Lv, Y.C.; Wang, Y.; Ma, G.X.; Ma, Y.Y.; Yaseen, M.; Li, S.Z. Designing oxygen vacancy rich mesoporous CeO2 nanorods with co-doped Cd and Ni for synergistic oxidative-hydrogenation desulfurization of transportation fuel oils. Fuel Process. Technol. 2023, 250, 107899. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wen, L.Y.; Liao, S.Y.; Zeng, X.Y.; Zhou, R.J.; Zeng, Y. Controllable synthesis of Bi2O3-MoO3 binary system metal composite oxides and structure-activity relationships for aerobic oxidative desulfurization. Chem. Eng. J. 2023, 474, 145473. [Google Scholar] [CrossRef]
- Bi, M.L.; Guo, Y.; Wang, S.Y.; Zhang, B.X.; Jiang, Y.N.; Li, H.A.; Xu, Q.X.; Chen, L.D.; Zhao, Q.; Han, N. Titanium dioxide-modified nanosized TS-1 zeolite-supported phosphotungstic acid as a catalyst for deep catalytic oxidative desulfurization of fuel oil. New J. Chem. 2023, 47, 12027–12036. [Google Scholar] [CrossRef]
- Boshagh, F.; Rahmani, M.; Zhu, W.S. Recent Advances and Challenges in Developing Technological Methods Assisting Oxidative Desulfurization of Liquid Fuels: A Review. Energy Fuels 2022, 36, 12961–12985. [Google Scholar] [CrossRef]
- Hamad, K.I.; Humadi, J.I.; Abdulkareem, H.A.; Al-Salihi, S.; Farhan, O.I. Efficient immobilization of acids into activated carbon for high durability and continuous desulfurization of diesel fuel. Energy Sci. Eng. 2023, 11, 3662–3679. [Google Scholar] [CrossRef]
- Ali, I.S.; Yasin Thayee Al-Janabi, O.; Al-Tikrity, E.T.B.; Foot, P.J.S. Adsorptive desulfurization of model and real fuel via wire-, rod-, and flower-like Fe3O4@MnO2@activated carbon made from palm kernel shells as newly designed magnetic nanoadsorbents. Fuel 2023, 340, 127523. [Google Scholar] [CrossRef]
- Al-Hammadi, S.A.; Al-Amer, A.M.; Saleh, T.A. Alumina-carbon nanofiber composite as a support for MoCo catalysts in hydrodesulfurization reactions. Chem. Eng. J. 2018, 345, 242–251. [Google Scholar] [CrossRef]
- Singh, R.; Kunzru, D.; Sivakumar, S. Co-promoted MoO3 nanoclusters for hydrodesulfurization. Catal. Sci. Technol. 2016, 6, 5949–5960. [Google Scholar] [CrossRef]
- Zhang, M.J.; Wang, C.; Han, Z.N.; Wang, K.J.; Zhang, Z.G.; Guan, G.Q.; Abudula, A.; Xu, G.W. Catalyst Ni-Mo/Al2O3 promoted with infrared heating calcination for hydrodesulfurization of shale oil. Fuel 2021, 305, 121537. [Google Scholar] [CrossRef]
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2021, 214, 106685. [Google Scholar] [CrossRef]
- Majid, M.F.; Mohd Zaid, H.F.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J. Mol. Liq. 2020, 306, 112870. [Google Scholar] [CrossRef]
- Hirschler, A.; Carapito, C.; Maurer, L.; Zumsteg, J.; Villette, C.; Heintz, D.; Dahl, C.; Al-Nayal, A.; Sangal, V.; Mahmoud, H.; et al. Biodesulfurization Induces Reprogramming of Sulfur Metabolism in Rhodococcus qingshengii IGTS8: Proteomics and Untargeted Metabolomics. Microbiol. Spectr. 2021, 9, e0069221. [Google Scholar] [CrossRef]
- Sar, T.; Ozturk, M.; Stark, B.C.; Akbas, M.Y. Improvement in desulfurization of dibenzothiophene and dibenzothiophene sulfone by Paenibacillus strains using immobilization or nanoparticle coating. J. Appl. Microbiol. 2022, 133, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, A.; Sobati, M.A. Extraction of sulfur compounds from middle distillate fuels using ionic liquids and deep eutectic solvents: A critical review. Fuel 2022, 310, 122279. [Google Scholar] [CrossRef]
- Abro, R.; Kiran, N.; Ahmed, S.; Muhammad, A.; Jatoi, A.S.; Mazari, S.A.; Salma, U.; Plechkova, N.V. Extractive desulfurization of fuel oils using deep eutectic solvents—A comprehensive review. J. Environ. Chem. Eng. 2022, 10, 107369. [Google Scholar] [CrossRef]
- Khan, N.; Srivastava, V.C. Quaternary Ammonium Salts-Based Deep Eutectic Solvents: Utilization in Extractive Desulfurization. Energy Fuels 2021, 35, 12734–12745. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Wang, Y.; Zhao, L.; Gao, J.; Hao, T.; Xu, C. High efficiency separation of olefin from FCC naphtha: Influence of combined solvents and related extraction conditions. Fuel Process. Technol. 2020, 208, 106497. [Google Scholar] [CrossRef]
- Niu, A.Q.; Xu, H.; Yuan, Q.L.; Wu, F.M.; Wei, X.F. Metal-based Ionic Liquids and Solid-loaded Catalysts in Fuel Oil Desulfurization: A Review. Mini-Rev. Org. Chem. 2024, 21, 704–716. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Sahraei, S. Assessment of Reaction Parameters in the Oxidative Desulfurization Reaction. Energy Fuels 2023, 37, 15373–15393. [Google Scholar] [CrossRef]
- Mushtaq, A.; Fatima, R.; Shehzad, F.K.; Ammar, M.; Shaaban, I.A.; Assiri, M.A.; Sajid, M.; Asif, H.M.; Hussain, S. Synthesis of POM@Porphyrin COF heterogeneous catalyst for oxidative desulfurization of thio compounds. Inorg. Chim. Acta 2023, 558, 121745. [Google Scholar] [CrossRef]
- Liu, Y.; Su, X.P.; Cui, Y.N.; Zhou, X. One-step preparation of deep eutectic solvents/reduced graphene oxide composite materials for the removal of dibenzothiophene in fuel oil. Sci. Rep. 2023, 13, 832. [Google Scholar] [CrossRef]
- Bartoli, M.; Zhu, C.Y.; Asomaning, J.; Chae, M.; Bressler, D.C. Biosolids-based catalyst for oxidative desulphurization of drop-in fuels derived from waste fats. Fuel 2022, 324, 124546. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Zhu, M.; Dai, B. Cu(I) anchoring in MOF-808 as a stable catalyst in ultra-deep oxidation desulfurization. Fuel 2023, 341, 127674. [Google Scholar] [CrossRef]
- Kayedi, N.; Samimi, A.; Asgari Bajgirani, M.; Bozorgian, A. Enhanced oxidative desulfurization of model fuel: A comprehensive experimental study. S. Afr. J. Chem. Eng. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M. Oxidative Desulfurization of Liquid Fuels Using Polyoxometalate-Based Catalysts: A Review. Energy Fuels 2021, 35, 10347–10373. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Chen, H.; Yang, L.; Bai, L.; Wei, D.; Cao, X.; Liang, Y.; Yang, H. V2CTx MXene: A Promising Catalyst for Low-Temperature Aerobic Oxidative Desulfurization. Catal. Lett. 2023, 153, 3103–3110. [Google Scholar] [CrossRef]
- Dou, S.-Y.; Wang, R. High-efficient utilization of gaseous oxidants in oxidative desulfurization based on sulfonated carbon materials. Fuel Process. Technol. 2022, 235, 107372. [Google Scholar] [CrossRef]
- Eseva, E.A.; Akopyan, A.V.; Sinikova, N.A.; Anisimov, A.V. In Situ Generated Organic Peroxides in Oxidative Desulfurization of Naphtha Reformate. Pet. Chem. 2021, 61, 472–482. [Google Scholar] [CrossRef]
- Deng, C.; Wu, P.; Li, H.; Zhu, H.; Chao, Y.; Tao, D.; Chen, Z.; Hua, M.; Liu, J.; Liu, J.; et al. Engineering polyhedral high entropy oxide with high-index facets via mechanochemistry-assisted strategy for efficient oxidative desulfurization. J. Colloid Interface Sci. 2023, 629, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Boshagh, F.; Rahmani, M.; Rostami, K.; Yousefifar, M. Key Factors Affecting the Development of Oxidative Desulfurization of Liquid Fuels: A Critical Review. Energy Fuels 2022, 36, 98–132. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, J.T.; Wu, P.W.; Liu, J.X.; Ji, H.Y.; Cui, P.; Chao, Y.H.; Zhu, W.S.; Liu, H.Y.; Liu, Z.C. 3D printing of the multifunctional fixed-bed reactor and matched 3D-CeO2/ATP monolithic adsorbent for adsorption desulfurization. Chem. Eng. J. 2023, 468, 143590. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, W.; Xiao, Y.; Zhao, Q.; Li, C.; Dong, X.; Lu, S. Construction of novel super microporous silica adsorbents using pluronic triblock copolymer as template towards desulfurization from fuel. Fuel 2023, 334, 126657. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.L.; Song, C.S. Adsorptive desulfurization of jet fuels over TiO2-CeO2 mixed oxides: Role of surface Ti and Ce cations. Catal. Today 2021, 371, 265–275. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Ko, C.H.; Kim, S.H.; Kim, J.-N. Removal of Refractory Sulfur Compounds in Diesel Using Activated Carbon with Controlled Porosity. Energy Fuels 2009, 23, 2537–2543. [Google Scholar] [CrossRef]
- Xiong, J.; Luo, J.; Yang, L.; Pang, J.; Zhu, W.; Li, H. Boron defect engineering in boron nitride nanosheets with improved adsorptive desulfurization performance. J. Ind. Eng. Chem. 2018, 64, 383–389. [Google Scholar] [CrossRef]
- Khan, N.A.; Jun, J.W.; Jeong, J.H.; Jhung, S.H. Remarkable adsorptive performance of a metal-organic framework, vanadium-benzenedicarboxylate (MIL-47), for benzothiophene. Chem. Commun. 2011, 47, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Firdous, A.; Zaman, M.S.; Izhar, F.; Riaz, M.; Haider, S.; Majeed, M.; Tariq, S. MOFs for desulfurization of fuel oil: Recent advances and future insights. J. Chin. Chem. Soc. 2023, 70, 789–824. [Google Scholar] [CrossRef]
- Khidr, A.S.A.; Soliman, F.S.; Zaki, T.; Sabry, D.Y.; Al-Sabagh, A.M. Adsorptive removal of thiophene by using water based silver nanofluid. Fuel 2023, 334, 126584. [Google Scholar] [CrossRef]
- Salonikidou, E.D.; Giannakoudakis, D.A.; Deliyanni, E.A.; Triantafyllidis, K.S. Deep desulfurization of model fuels by metal-free activated carbons: The impact of surface oxidation and antagonistic effects by mono- and poly-aromatics. J. Mol. Liq. 2022, 351, 118661. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, R.T. Desulfurization of liquid fuels by adsorption on carbon-based sorbents and ultrasound-assisted sorbent regeneration. Langmuir 2007, 23, 3825–3831. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.S.; Li, H.P.; Yang, L.; Chao, Y.H.; Wu, P.W.; Xun, S.H.; Jiang, W.; Zhang, M.; Li, H.M. Carbon-doped porous boron nitride: Metal-free adsorbents for sulfur removal from fuels. J. Mater. Chem. A 2015, 3, 12738–12747. [Google Scholar] [CrossRef]
- Deng, L.L.; Lu, B.Q.; Li, J.L.; Lv, G.Q.; Du, S.J.; Shi, J.Y.; Yang, Y.X. Effect of pore structure and oxygen-containing groups on adsorption of dibenzothiophene over activated carbon. Fuel 2017, 200, 54–61. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, J.L.; Guan, T.T.; Zhang, G.L.; Wang, N.; Li, K.X. Tailored C-N bond toward defect-rich hierarchically porous carbon from coal tar pitch for high-efficiency adsorptive desulfurization. Fuel 2021, 292, 120251. [Google Scholar] [CrossRef]
- Luo, J.; Wang, C.; Liu, J.X.; Wei, Y.C.; Chao, Y.H.; Zou, Y.R.; Mu, L.P.; Huang, Y.; Li, H.M.; Zhu, W.S. High-performance adsorptive desulfurization by ternary hybrid boron carbon nitride aerogel. AIChE J. 2021, 67, e17280. [Google Scholar] [CrossRef]
- Liu, W.F.; Qin, L.; An, Z.L.; Shi, W.P.; Chen, L.; Liu, X.G.; Yang, Y.Z. Selective adsorption and separation of dibenzothiophene by molecularly imprinted polymer on the surface of porous magnetic carbon nanospheres. Fuller. Nanotub. Carbon. Nanostruct. 2019, 27, 14–22. [Google Scholar] [CrossRef]
- Othman, C.S.; Salih, Y.M.; Hamasalih, L.O.; Saleem, H.J. Influence of different nanocomposite carbon-based adsorbers on the adsorption desulfurization of dibenzothiophene in model oil and diesel fuel: A comparative study. React. Kinet. Mech. Catal. 2023, 136, 919–936. [Google Scholar] [CrossRef]
- Yaseen, M.; Ullah, S.; Ahmad, W.; Subhan, S.; Subhan, F. Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel 2021, 284, 119102. [Google Scholar] [CrossRef]
- Li, W.W.; Chen, J.W.; Cong, G.J.; Tang, L.; Cui, Q.; Wang, H.Y. Solvent desulfurization regeneration process and analysis of activated carbon for low-sulfur real diesel. RSC Adv. 2016, 6, 20258–20268. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, J.; Wu, P.; Liu, J.; Ji, H.; Huang, Y.; Chao, Y.; Liu, H.; Zhu, W.; Liu, Z. 3D printing of hierarchically porous lightweight activated carbon/alumina monolithic adsorbent for adsorptive desulfurization of hydrogenated diesel. Sep. Purif. Technol. 2024, 330, 125334. [Google Scholar] [CrossRef]
- Saleem, H.J.; Salih, Y.M.; Hamasalih, L.O.; Othman, C.S. Application of green synthesis nanocomposite adsorbents in the adsorption desulfurization of dibenzothiophene in model oil. J. Sulfur. Chem. 2023, 44, 416–431. [Google Scholar] [CrossRef]
- Azeez, M.O.; Tanimu, A.; Alhooshani, K.; Ganiyu, S.A. Synergistic effect of nitrogen and molybdenum on activated carbon matrix for selective adsorptive Desulfurization: Insights into surface chemistry modification. Arab. J. Chem. 2022, 15, 103454. [Google Scholar] [CrossRef]
- Bai, R.S.; Song, Y.; Bai, R.B.; Yu, J.H. Creation of Hierarchical Titanosilicate TS-1 Zeolites. Adv. Mater. Interfaces 2021, 8, 2001095. [Google Scholar] [CrossRef]
- Cha, Y.H.; Mun, S.; Lee, K.B. Development of modified zeolite for adsorption of mixed sulfur compounds in natural gas by combination of ion exchange and impregnation. Appl. Surf. Sci. 2023, 619, 156634. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Zhu, K.; Zhu, D.A.; Zhu, L.H.; Zhu, W.S.; Li, H.M.; Jiang, W. Modification of cobalt doped mesoporous sieve by grafting N, N-dihydroxypyromellitimide to enhance desulfurization catalyst stability. J. Mol. Liq. 2023, 390, 122970. [Google Scholar] [CrossRef]
- Nezhad, A.B.; Bastan, F.; Panjehshahin, A.; Zamani, M. Adsorptive Desulfurization of Condensate Contains Aromatic Compounds Using a Commercial Molecular Sieve. ACS Omega 2023, 8, 10365–10372. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.M.; Alalwan, H.A.; Alminshid, A.; Hussein, S.A.M.; Mohammed, M.F. Desulfurization of heavy naphtha by oxidation-adsorption process using iron-promoted activated carbon and Cu2+-promoted zeolite 13X. Catal. Commun. 2022, 169, 106473. [Google Scholar] [CrossRef]
- Bulut, B.; Isbilen, E.; Atakül, H.; Tantekin-Ersolmaz, S.B. Adsorptive removal of dimethyl disulfide and thiophene from liquefied petroleum gas by zeolite-based adsorbents. Microporous Mesoporous Mater. 2022, 337. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Adesina, A.; Li, P.; Wang, H.L.; Zhou, R.J. Enhanced adsorptive-oxidative desulfurization of dibenzothiophene over Ti-MWW using cumene hydroperoxide as oxidant. Korean J. Chem. Eng. 2022, 39, 96–108. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Fu, J.H.; Zhu, H.Y.; Zhao, Q.D.; Zhou, L. Facile and controllable preparation of nanocrystalline ZSM-5 and Ag/ZSM-5 zeolite with enhanced performance of adsorptive desulfurization from fuel. Sep. Purif. Technol. 2022, 288, 120698. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mohammadian, M.; Khosravi-Nikou, M.R.; Baghban, A. Experimental, kinetic, and thermodynamic studies of adsorptive desulfurization and denitrogenation of model fuels using novel mesoporous materials. J. Hazard. Mater. 2019, 374, 129–139. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Wang, C.Y.; Zhao, J.Q.; Sun, H.Z.; Sun, C.Y. The synthesis and oxidation desulfurization performance of Ti-modified hierarchical cheese-like ZSM-5 zeolite. J. Chem. Res. 2022, 46, 17475198211068663. [Google Scholar] [CrossRef]
- Dashtpeyma, G.; Shabanian, S.R.; Ahmadpour, J.; Nikzad, M. The investigation of adsorption desulphurization performance using bimetallic CuCe and NiCe mesoporous Y zeolites: Modification of Y zeolite by H4EDTA-NaOH sequential treatment. Fuel Process. Technol. 2022, 235, 107379. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Liu, Z.; Etim, U.J.; Wadood, A.; Ullah, R. Confinement of mesopores within ZSM-5 and functionalization with Ni NPs for deep desulfurization. Chem. Eng. J. 2018, 354, 706–715. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Xue, Q.Q.; Zhu, K.R.; He, G.H. Enhanced Performance of Adsorptive Removal of Thiophene from Model Fuel over Micro-Mesoporous Binderless ZSM-5 Prepared by In Situ Crystallization. Energy Fuels 2020, 34, 5623–5633. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.F.; Yaseen, M. Highly dispersive Cu species constructed in mesoporous silica derived from ZSM-5 for batch and continuous adsorptive desulfurization of thiophene. Fuel Process. Technol. 2022, 235, 107351. [Google Scholar] [CrossRef]
- Song, H.; Yang, G.; Song, H.L.; Wang, D.; Wang, X.Q. Preparation of Ag/TiO2-Zeolite Adsorbents, Their Desulfurization Performance, and Benzothiophene Adsorption Isotherms. Russ. J. Phys. Chem. A 2017, 91, 390–397. [Google Scholar] [CrossRef]
- Cerutti, M.; Hackbarth, F.V.; Maass, D.; Chiaro, S.S.X.; Pinto, R.R.C.; Cardoso, M.J.B.; Arroyo, P.A.; de Souza, A.A.U.; de Souza, S. Copper-exchanged Y zeolites for gasoline deep-desulfurization. Adsorpt. J. Int. Adsorpt. Soc. 2019, 25, 1595–1609. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, L.H.; Wen, Z.H.; Zhao, J.C.; Wei, X.J.; Liao, J.J.; Chang, L.P.; Xie, K.C. Insight into the relationship between effective active sites and ultra-deep adsorption desulfurization performance of CuCeY with different Cu precursors. Fuel Process. Technol. 2023, 250, 107930. [Google Scholar] [CrossRef]
- Saleh, H.A.M.; Khan, S.; Kumar, M.; Ansari, A.; Shahid, M.; Sama, F.; Qasem, K.M.A.; Khan, M.Y.; Mehtab, M.; Ahmad, M.; et al. Fabrication of Unique Mixed-Valent CoICoII and CuICuII Metal-Organic Frameworks (MOFs) for Desulfurization of Fuels: A Combined Experimental and Theoretical Approach toward Green Fuel. Inorg. Chem. 2024, 63, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, Y.P.; Chen, Y.F.; Liu, C.J.; Tu, X. Synthesis, characterization and application of defective metal-organic frameworks: Current status and perspectives. J. Mater. Chem. A 2020, 8, 21526–21546. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Polyzoidis, A.; Schwarzer, M.; Loebbecke, S. Stability of UiO-66 under acidic treatment: Opportunities and limitations for post-synthetic modifications. Microporous Mesoporous Mater. 2015, 208, 30–35. [Google Scholar] [CrossRef]
- Mondol, M.M.H.; Ahmed, I.; Lee, H.J.; Morsali, A.; Jhung, S.H. Metal-organic frameworks and metal-organic framework-derived materials for denitrogenation of liquid fuel via adsorption and catalysis. Coord. Chem. Rev. 2023, 495, 215382. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, G.; Kaur, J.; Singh, P.P. First transition series metal-organic frameworks: Synthesis, properties and applications. Mater. Adv. 2021, 2, 7308–7335. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, K.; Guo, R.L.; Wei, Z. Ionic Liquids Functionalized MOFs for Adsorption. Chem. Rev. 2023, 123, 10432–10467. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.Q.; Wang, R. Functionally decorated metal-organic frameworks in environmental remediation. Chem. Eng. J. 2023, 455, 140741. [Google Scholar] [CrossRef]
- Lee, G.; Yoo, D.K.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks composed of nitro groups: Preparation and applications in adsorption and catalysis. Chem. Eng. J. 2023, 451, 138538. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Chung, Y.G.; Snurr, R.Q. Progress toward the computational discovery of new metal-organic framework adsorbents for energy applications. Nat. Energy 2024, 9, 121–133. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Mguni, L.L.; Yao, Y.; Ren, J.; Liu, X.; Hildebrandt, D. Modulated synthesized Ni-based MOF with improved adsorptive desulfurization activity. J. Clean. Prod. 2021, 323, 129196. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Y.; Guo, K.; Yin, Y.; Wang, J.; Zeng, Y. Hierarchical-pore UiO-66 modified with Ag+ for π-complexation adsorption desulfurization. J. Hazard. Mater. 2021, 418, 126247. [Google Scholar] [CrossRef]
- Lu, S.N.; Xiao, Y.H.; Zhao, Q.D.; Zhao, W.K.; He, G.H. Construction of rational defective CPO-27-Ni using N, N-dimethyloctadecylamine as template towards enhanced adsorptive desulfurization from fuel. Sep. Purif. Technol. 2023, 306, 122665. [Google Scholar] [CrossRef]
- Huo, Q.; Li, J.; Qi, X.; Liu, G.; Zhang, X.; Zhang, B.; Ning, Y.; Fu, Y.; Liu, J.; Liu, S. Cu, Zn-embedded MOF-derived bimetallic porous carbon for adsorption desulfurization. Chem. Eng. J. 2019, 378, 122106. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Chen, J.; Liu, Y.; Li, L.; Du, G. Investigation of attapulgite modified Fe3O4/MOF-199 and its adsorptive desulfurization performance. J. Mater. Sci. 2022, 57, 4528–4540. [Google Scholar] [CrossRef]
- Bagheri, M.; Masoomi, M.Y.; Morsali, A. High organic sulfur removal performance of a cobalt based metal-organic framework. J. Hazard. Mater. 2017, 331, 142–149. [Google Scholar] [CrossRef]
- Khan, N.A.; Kim, C.M.; Jhung, S.H. Adsorptive desulfurization using Cu-Ce/metal-organic framework: Improved performance based on synergy between Cu and Ce. Chem. Eng. J. 2017, 311, 20–27. [Google Scholar] [CrossRef]
- Habimana, F.; Huo, Y.X.; Jiang, S.; Ji, S.F. Synthesis of europium metal-organic framework (Eu-MOF) and its performance in adsorptive desulfurization. Adsorpt. J. Int. Adsorpt. Soc. 2016, 22, 1147–1155. [Google Scholar] [CrossRef]
- Chen, M.Y.; Wang, Z.Y.; Liu, Y.C.; Chen, J.; Liu, J.; Gan, D. Effect of ligands of functional magnetic MOF-199 composite on thiophene removal from model oil. J. Mater. Sci. 2021, 56, 2979–2993. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lin, Q.J.; Gao, D.L.; Liu, Y.X.; Qin, N.; Xue, Y.X.; Dai, F.F.; Chen, J.H.; Yang, Q. The introduction of nitrogen-containing heterocyclic compounds (NHC) and Cu2+ in Co-MOF further improves the removal of dibenzothiophene from model fuels. Sep. Purif. Technol. 2024, 335, 126096. [Google Scholar] [CrossRef]
- Zuhra, Z.; Mu, C.C.; Tang, F.; Zhou, Y.S.; Zhang, L.J.; Zhao, Z.P.; Qin, L.B. Enhanced adsorptive desulfurization by iso-structural amino bearing IRMOF-3 and IRMOF-3@Al2O3 versus MOF-5 and MOF-5@Al2O3 revealing the predominant role of hydrogen bonding. Dalton Trans. 2019, 48, 14792–14800. [Google Scholar] [CrossRef]
- Zhu, L.J.; Jia, X.Y.; Bian, H.; Huo, T.; Duan, Z.B.; Xiang, Y.Z.; Xia, D.H. Structure and adsorptive desulfurization performance of the composite material MOF-5@AC. New J. Chem. 2018, 42, 3840–3850. [Google Scholar] [CrossRef]
- Khan, N.A.; Bhadra, B.N.; Jhung, S.H. Heteropoly acid-loaded ionic liquid@metal-organic frameworks: Effective and reusable adsorbents for the desulfurization of a liquid model fuel. Chem. Eng. J. 2018, 334, 2215–2221. [Google Scholar] [CrossRef]
- Huang, L.; Wang, G.; Qin, Z.; Dong, M.; Du, M.; Ge, H.; Li, X.; Zhao, Y.; Zhang, J.; Hu, T.; et al. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO. Appl. Catal. B Environ. 2011, 106, 26–38. [Google Scholar] [CrossRef]
- Ullah, R.; Tuzen, M. Interactions of Ni/ZnO with alumina support and their influence on deep reactive adsorption desulfurization. J. Mol. Liq. 2022, 365, 120082. [Google Scholar] [CrossRef]
- Liu, B.W.; Bai, P.; Wang, Y.; Dong, Z.; Wu, P.P.; Mintova, S.; Yan, Z.F. Highly stable Ni/ZnO-Al2O3 adsorbent promoted by TiO2 for reactive adsorption desulfurization. Ecomat 2021, 3, e12114. [Google Scholar] [CrossRef]
- Ullah, R.; Tuzen, M.; Ullah, S.; Haroon, M.; Khattak, R.; Saleh, T.A. Acidic sites enhanced ultra-deep desulfurization performance of novel NiZnO-based mixed oxides mesoporous adsorbents. Surf. Interfaces 2023, 36, 102566. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, X.; Liao, J.; Guo, J.; Bao, W.; Chang, L. Desulfurization mechanism of an excellent Cu/ZnO sorbent for ultra-deep removal of thiophene in simulated coke oven gas. Chem. Eng. J. 2022, 446, e12114. [Google Scholar] [CrossRef]
- Li, L.; Ju, F.; Ling, H. Reactivation of NiSO4/ZnO-Al2O3-SiO2 adsorbent for reactive adsorption desulfurization. Fuel 2023, 339, 127411. [Google Scholar] [CrossRef]
- Nkomzwayo, T.; Mguni, L.; Hildebrandt, D.; Yao, Y.L. Adsorptive desulfurization using period 4 transition metals oxide: A study of Lewis acid strength derived from the adsorbent ionic-covalent parameter. Chem. Eng. J. 2022, 444, 136484. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, H.; Ye, Z.; Huang, Q.; Chen, X. Adsorption desulfurization performance and adsorption-diffusion study of B2O3 modified Ag-CeOx/TiO2-SiO2. J. Hazard. Mater. 2019, 362, 424–435. [Google Scholar] [CrossRef]
- Yin, L.X.; Xu, J.C.; Zhang, B.; Wang, L.G.; Tao, W.Y.; Teng, X.; Ning, W.S.; Zhang, Z.K. A facile fabrication of highly dispersed CeO2/SiO2 aerogel composites with high adsorption desulfurization performance. Chem. Eng. J. 2022, 428, 132581. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Lin, J.; Yuan, X.H.; Song, T.; Yu, C.; Liu, Z.Y.; He, X.; Liang, J.L.; Tang, C.C.; Huang, Y. Desulfurization of Model Oil by Selective Adsorption over Porous Boron Nitride Fibers with Tailored Microstructures. Sci. Rep. 2017, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chao, Y.; Tang, Z.; Hua, M.; Li, X.; Wei, Y.; Ji, H.; Xiong, J.; Zhu, W.; Li, H. Design of Lewis Acid Centers in Bundlelike Boron Nitride for Boosting Adsorptive Desulfurization Performance. Ind. Eng. Chem. Res. 2019, 58, 13303–13312. [Google Scholar] [CrossRef]
- Li, X.Y.; Shi, J.J.; Wang, J.A.; Xi, L.; Sun, R.; Zhang, F.; Wu, X.; Zhou, Z.Y.; Ren, Z.Q. Preparation of CeVO4/BNNS catalyst and its application in oxidation desulfurization of diesel oil. Fuel 2023, 337, 126875. [Google Scholar] [CrossRef]
- Sales, R.V.; Moura, H.; Silva, S.R.B.; de Souza, M.A.F.; Campos, L.M.A.; Rodríguez-Castellón, E.; de Carvalho, L.S. Experimental and theoretical study of adsorptive interactions in diesel fuel desulfurization over Ag/MCM-41 adsorbent. Adsorpt. J. Int. Adsorpt. Soc. 2020, 26, 189–201. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, X.; Feng, Y.R.; An, J.Y.; Chi, Y.X.; Liu, Y.T.; Zhang, L.F.; Zhao, Y.B.; Zeng, X.F.; Wang, Z.B.; et al. Synthesis of nanofluids composed of deep eutectic solvents and metal-modified MCM-41 particles as multifunctional promoters for fuel oil desulfurization. Front. Chem. Sci. Eng. 2023, 17, 1776–1787. [Google Scholar] [CrossRef]
- Kaur, P.; Chopra, H.K. MCM-41 supported S-alkyl/aryl-substituted 2-mercaptobenzothiazolium--based ionic liquids: Synthesis, characterization, and application in the fuel desulfurization. Fuel 2023, 332, 126009. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, J.; Chang, L.; Bao, W. Ag modification of SBA-15 and MCM-41 mesoporous materials as sorbents of thiophene. Fuel 2022, 311, 122537. [Google Scholar] [CrossRef]
- Shi, C.W.; Wu, Y.F.; Chen, W.Y.; Wu, W.Y.; Bian, X.; Zhao, S.L.; Pei, M.Y. Synthesis and characteristics of Ce modified HY/SBA-15 and its desulfurization performance for simulated oil. J. Porous Mater. 2017, 24, 381–388. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, J.; Lv, G.Q.; Zhang, L.M. Novel method to synthesize Ni2P/SBA-15 adsorbents for the adsorptive desulfurization of model diesel fuel. J. Alloys Compd. 2018, 745, 467–476. [Google Scholar] [CrossRef]
- Zhang, J.L.; Qiu, G.H.; Fan, L.; Meng, X.R.; Cai, Q.H.; Wang, Y.H. Enhanced sulfur capacity of durable and regenerable mesoporous sorbents for the deep desulfurization of diesel. Fuel 2015, 153, 578–584. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Cata Saady, N.M. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100635. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Zhen, L.; Ahmad, A.; Naeem, M.; Zeng, J.; Ullah, R.; Etim, U.J. Facile functionalization of 3-D ordered KIT-6 with cuprous oxide for deep desulfurization. Chem. Eng. J. 2017, 330, 372–382. [Google Scholar] [CrossRef]
- Aslam, S.; Subhan, F.; Yan, Z.; Peng, P.; Qiao, K.; Xing, W.; Bai, P.; Ullah, R.; Etim, U.J.; Zeng, J.; et al. Facile fabrication of Ni-based KIT-6 for adsorptive desulfurization. Chem. Eng. J. 2016, 302, 239–248. [Google Scholar] [CrossRef]
- McCrary, P.D.; Rogers, R.D. 1-Ethyl-3-methylimidazolium hexafluorophosphate: From ionic liquid prototype to antitype. Chem. Commun. 2013, 49, 6011–6014. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Omar, R.A.; Verma, N. Review of Adsorptive Desulfurization of Liquid Fuels and Regeneration Attempts. Ind. Eng. Chem. Res. 2022, 61, 8595–8606. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Tahir, S.; Qazi, U.Y.; Naseem, Z.; Tahir, N.; Zahid, M.; Javaid, R.; Shahid, I. Deep eutectic solvents as alternative green solvents for the efficient desulfurization of liquid fuel: A comprehensive review. Fuel 2021, 305, 121502. [Google Scholar] [CrossRef]
- Song, H.; Li, X.J.; Jiang, B.L.; Gong, M.Y.; Hao, T.Z. Preparation of Novel and Highly Stable Py/MOF and Its Adsorptive Desulfurization Performance. Ind. Eng. Chem. Res. 2019, 58, 19586–19598. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.S.; Ding, W.J.; Wang, P.; Chao, Y.H.; Zhang, M.; Zhu, F.X.; Li, H.M. Controllable synthesis of functionalized ordered mesoporous silica by metal-based ionic liquids, and their effective adsorption of dibenzothiophene. RSC Adv. 2014, 4, 40588–40594. [Google Scholar] [CrossRef]
- Tan, P.; Jiang, Y.; Liu, X.Q.; Zhang, D.Y.; Sun, L.B. Magnetically Responsive Core-Shell Fe3O4@C Adsorbents for Efficient Capture of Aromatic Sulfur and Nitrogen Compounds. ACS Sustain. Chem. Eng. 2016, 4, 2223–2231. [Google Scholar] [CrossRef]
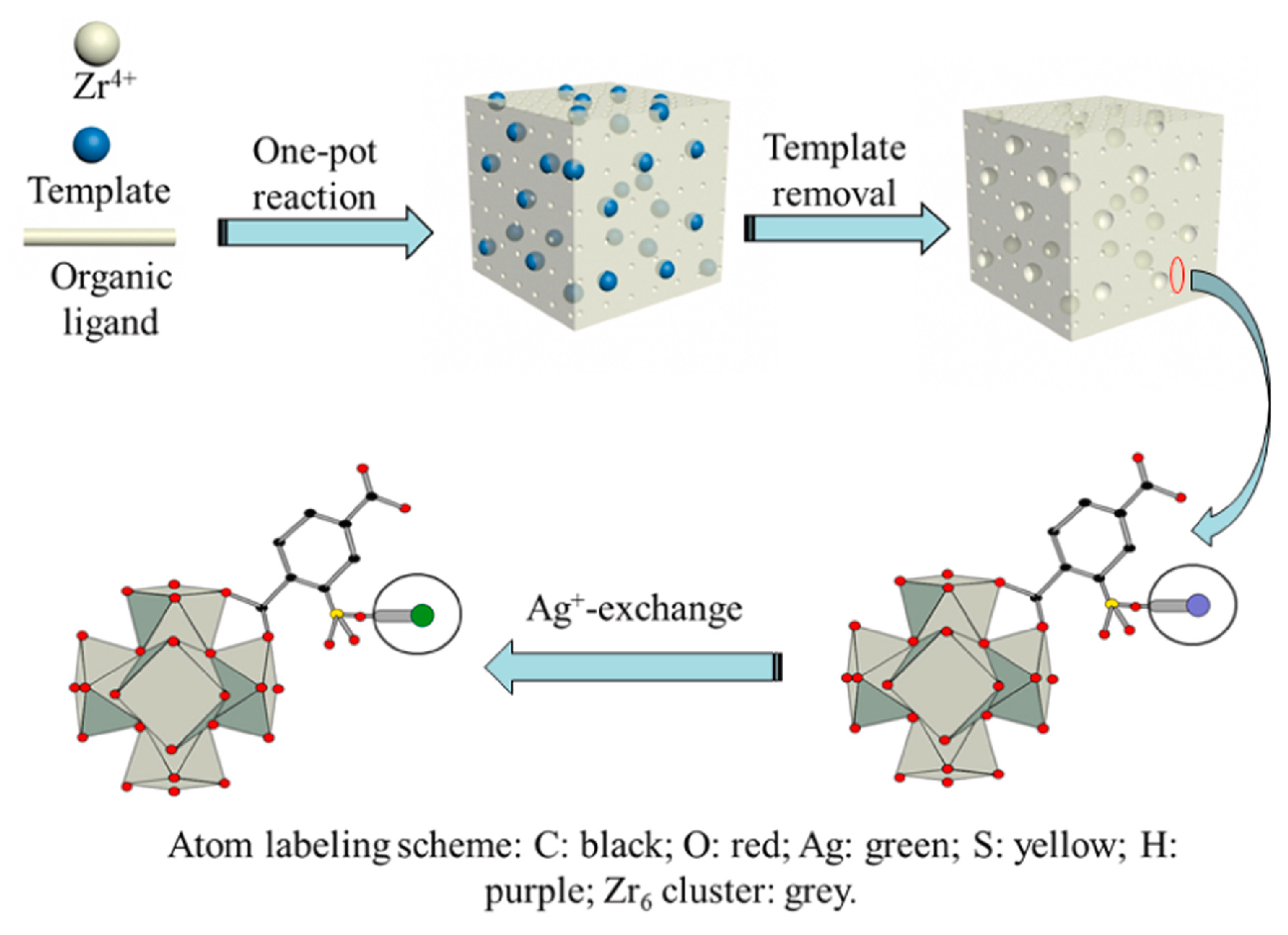
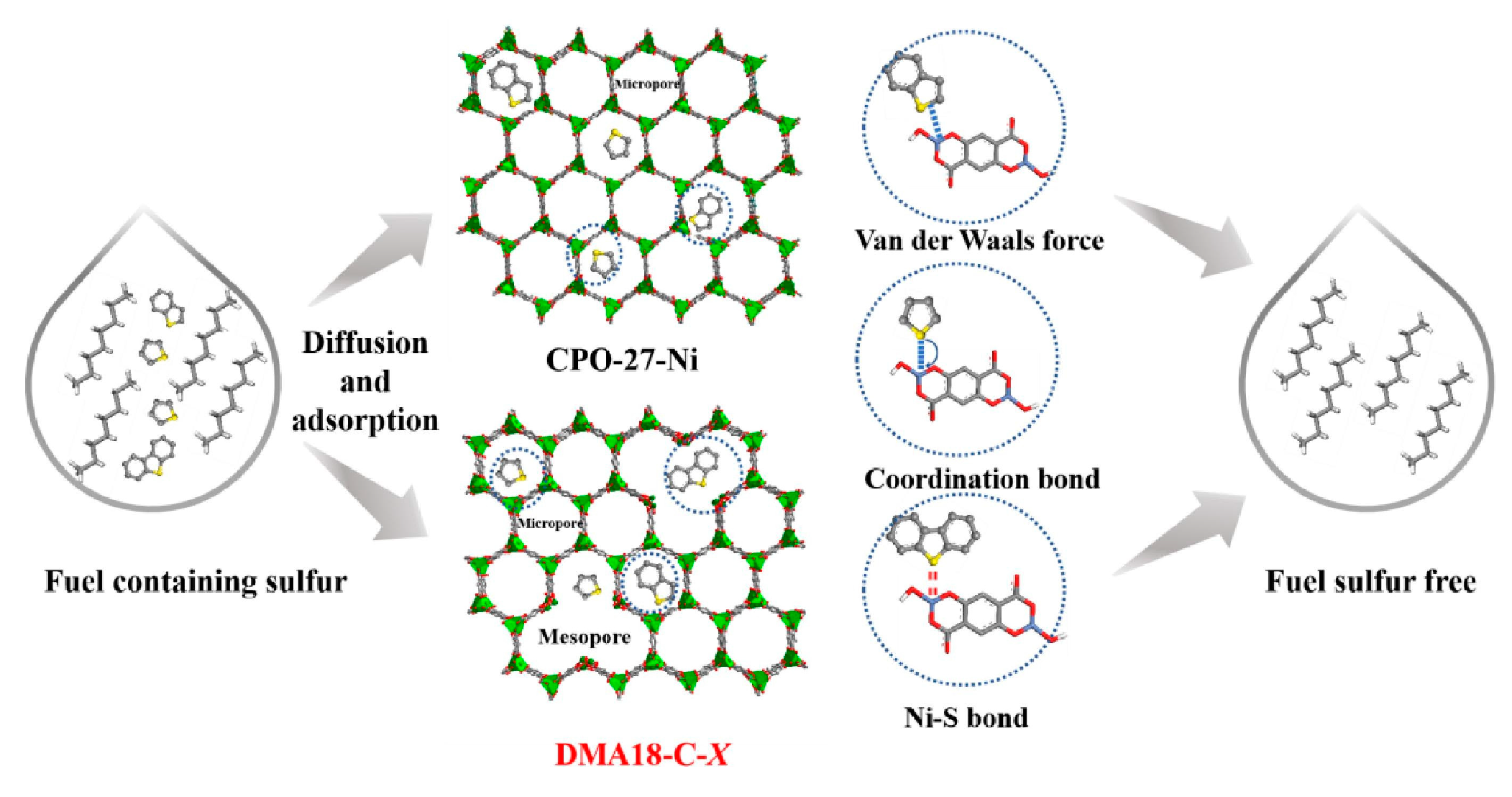
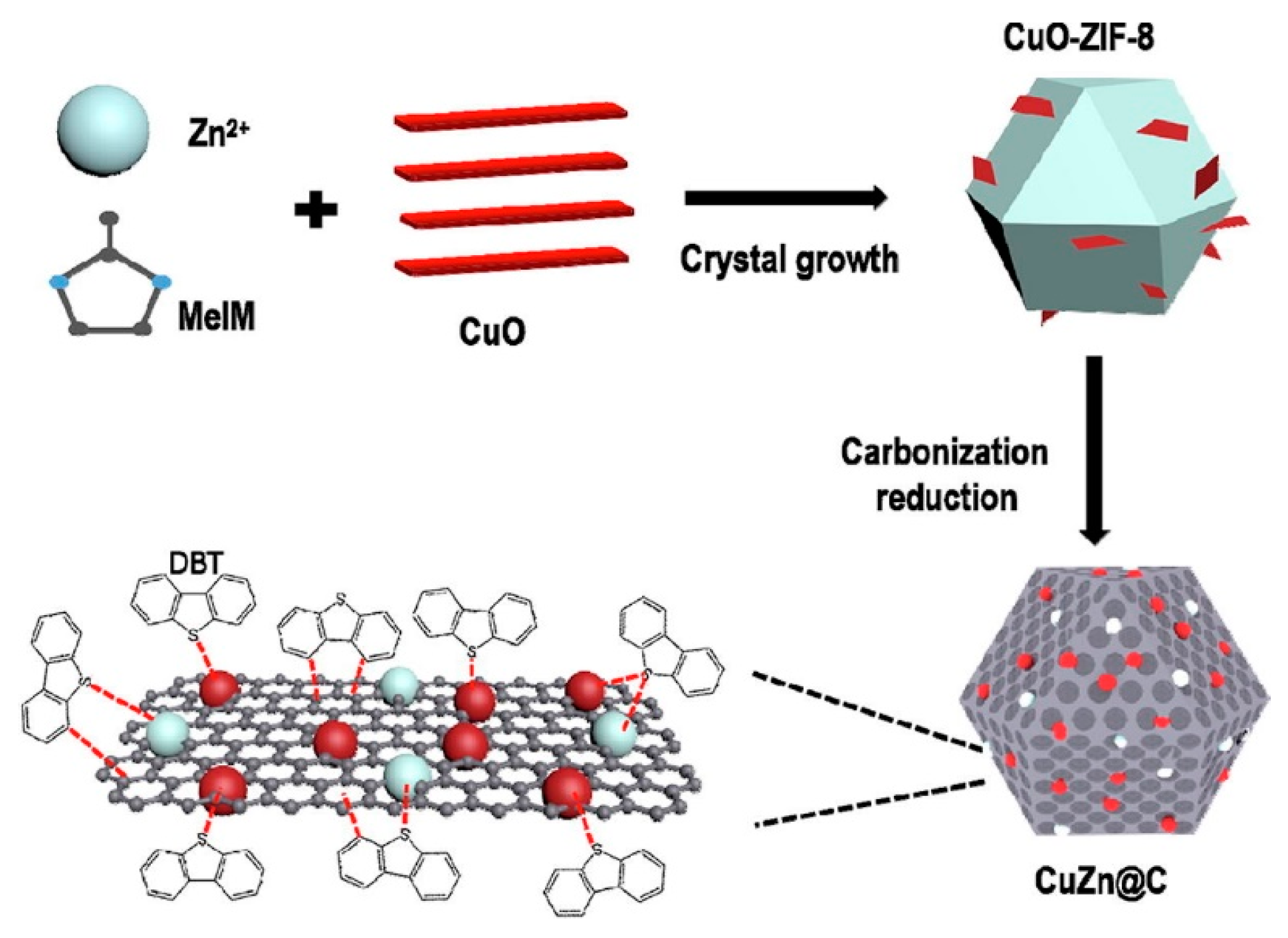
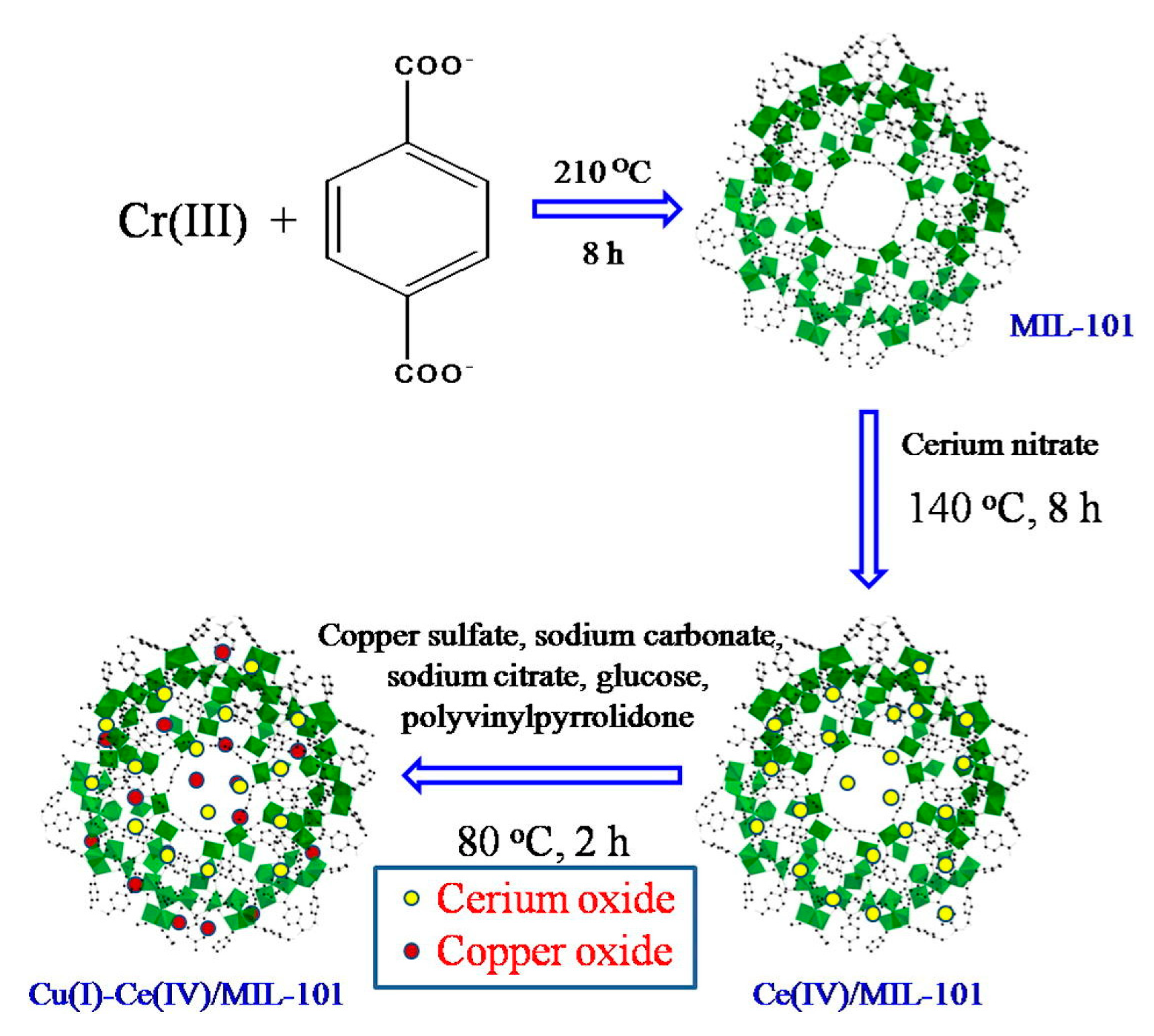
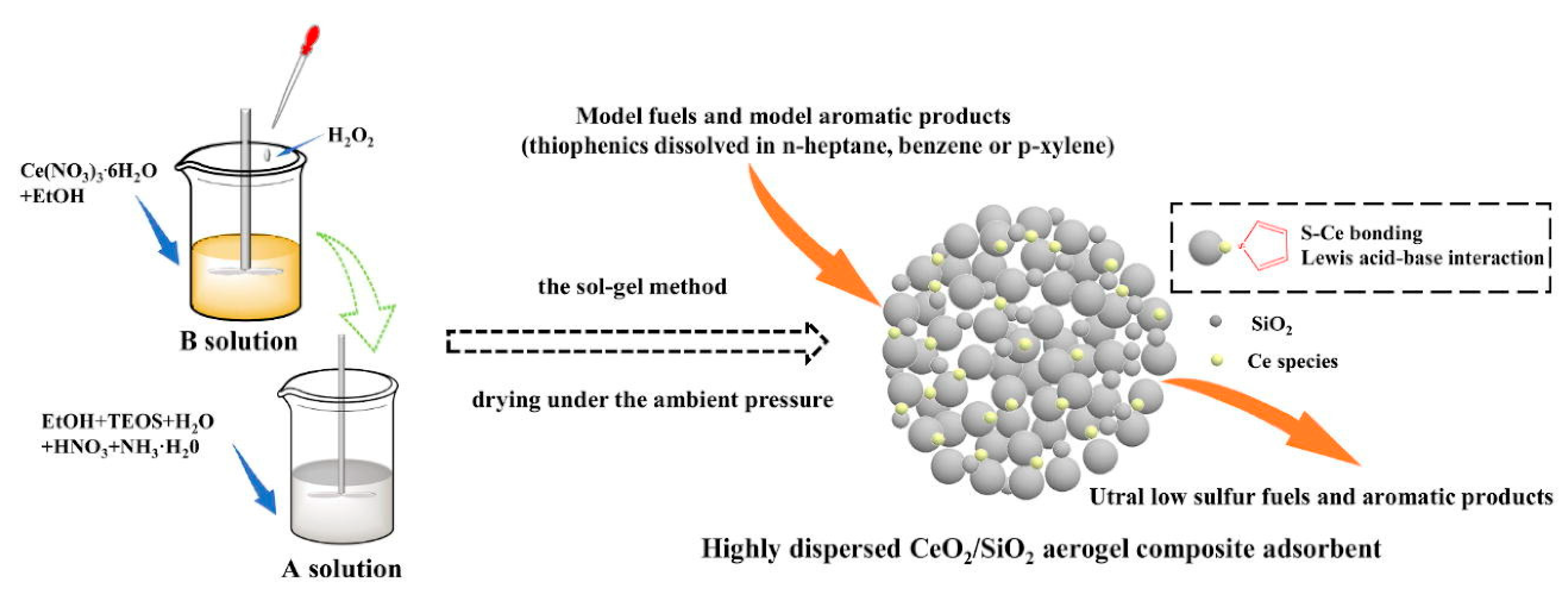
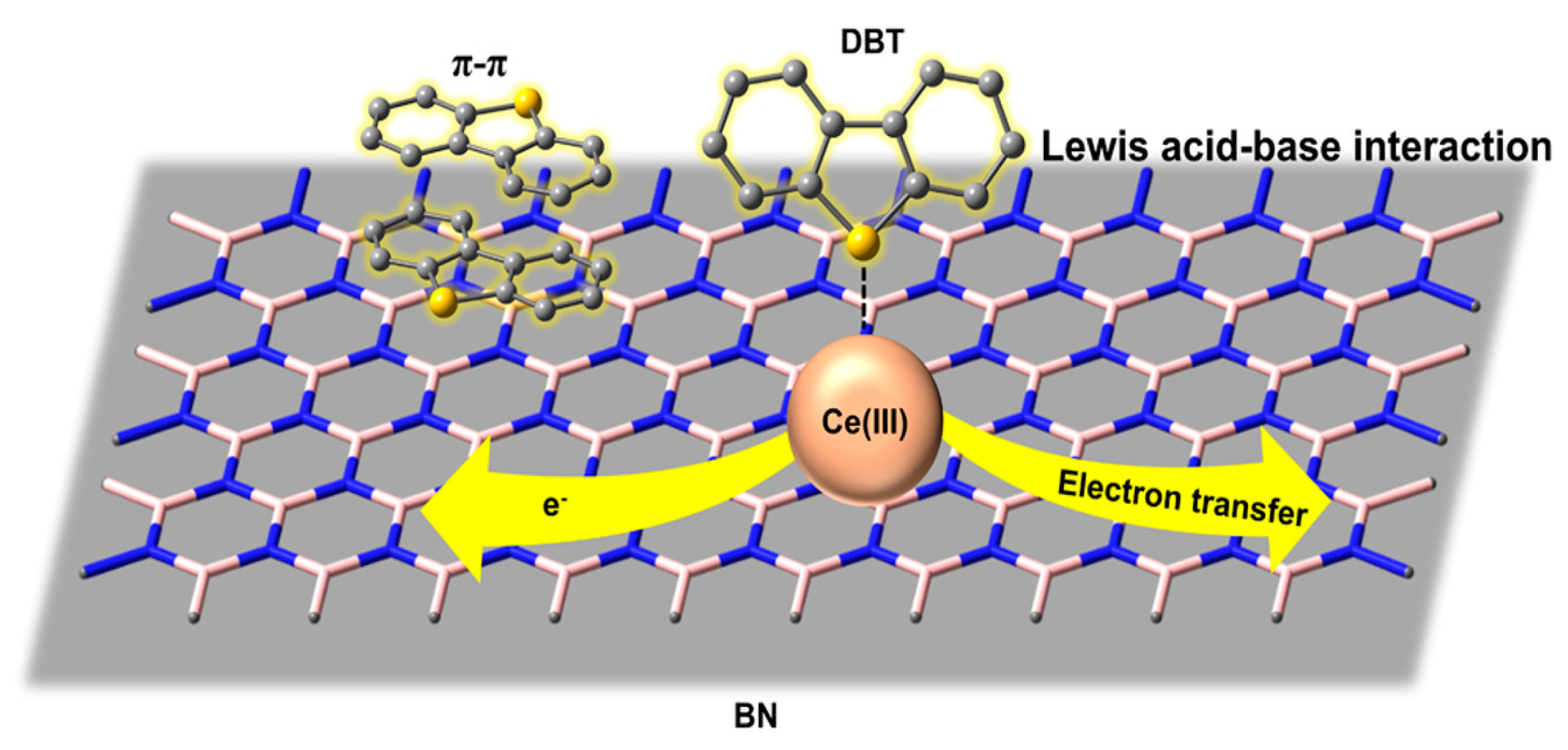
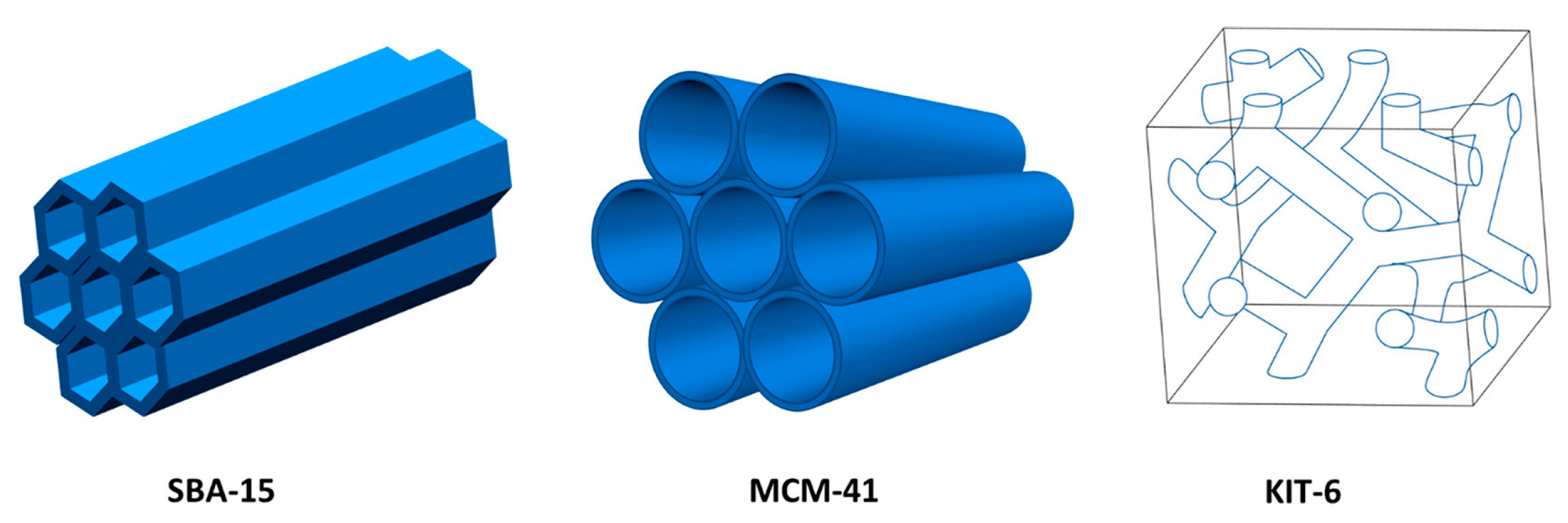
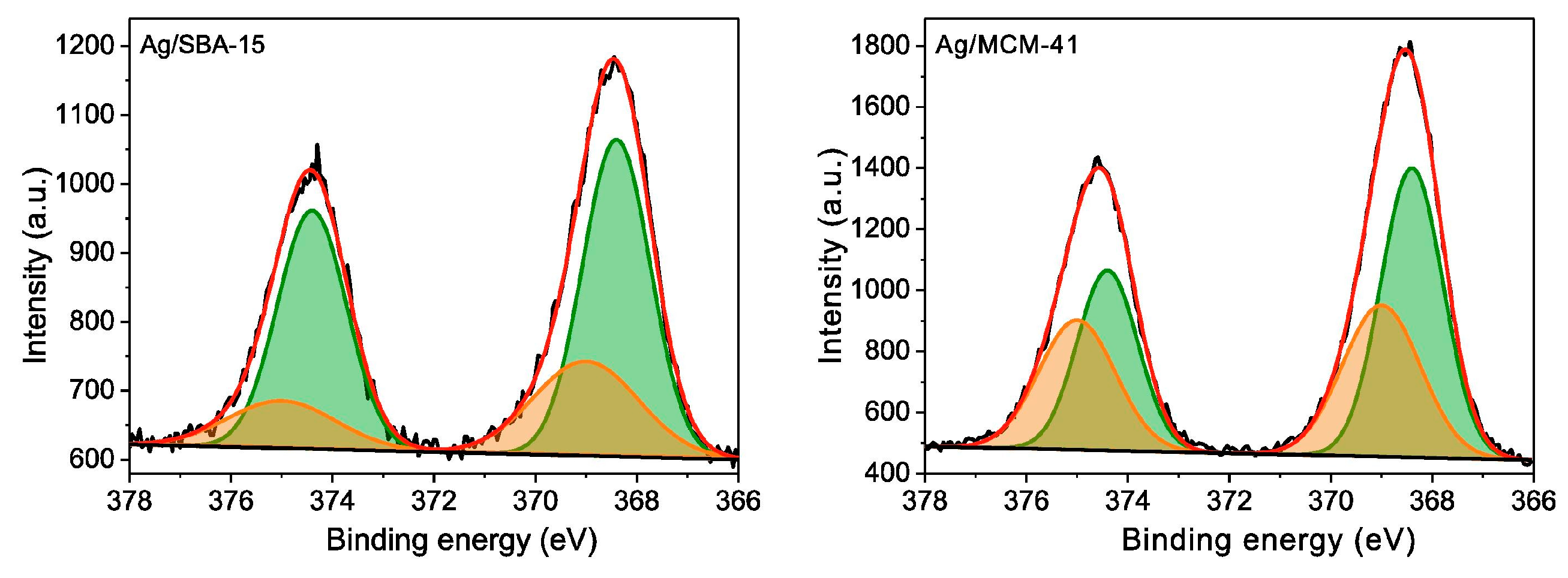
| Absorbents | Sulfur Source | Initial S Contents | Final S Contents | Performance | Ref. |
|---|---|---|---|---|---|
| AC/Mn/Cu | DBT | 250–2500 ppm | 16 ppm | 136.78 mg/g | [55] |
| Mn/AC | DBT | 200 ppm | <20 ppm | removal of 95.7% | [56] |
| AC | S-compounds | 34.83 | <10 ppm | ---- | [57] |
| 3D-AC/Al2O3 | feed oil | 500 ppmw | <20 ppmw | 96.6% sulfur removal | [58] |
| Ag/ZnONPS-AC | DBT | 250 mg/L | ---- | removed 99% | [59] |
| AC-N-Mo | DBT | 50–200 mg-S/L | ----- | 19.94 mg S/g | [60] |
| Absorbents | Sulfur Source | Initial S Contents | Adsorption Capacity | Ref. |
|---|---|---|---|---|
| ZSM-5-based micro-/mesostructures | TP | 50–450 ppm | 14.1 mg/g | [72] |
| ZSM-5 | TP | 500 ppmw | 0.0550 mmol S/g. | [68] |
| BL-ZSM-5 | TP | 100 ppmw | 0.38 mg/g | [73] |
| ZSM-5 | TP | 500 ppm | 0.48 mmol/g | [74] |
| Ag/TiO2-NaY (TY) | BT | 10 mg/L | desulfurization rate of 95% | [75] |
| CuY1 | synthetic gasoline | --- | 4.14 mg S/g | [76] |
| CuCeY | TP | 500 mg/L | 6.25 mg/g | [77] |
| Absorbents | Sulfur Source | Initial S Contents | Performance | Ref. |
|---|---|---|---|---|
| Ni-based MOF | TH | 150 ppm | 4.14 mg/g | [89] |
| Eu-MOF | TP/n-octane | 1000 μg/g | 24.59 mg S/g | [96] |
| MOF-199 | TP | ----- | 72.575 mg/g | [97] |
| NHC/Co-MOF-Cu2+ | DBT | 100 mg/L | 150.4 mg/g | [98] |
| MOF-5 | DBT | 1000 ppmw | 31.9 mg S/g | [99] |
| HP-UiO-66-SO3Ag | BT | 200–1000 ppm | 31.4 mg S/g | [90] |
| MOF-5@AC | TP | 100 μg/g | 11.3 mg S/g | [100] |
| HPA-IL/ZIF-8 | BT | 1000 ppm | 16.25 mg S/g | [101] |
| Absorbents | Sulfur Source | Initial S Contents | Final S Contents | Adsorbent Performance | Ref. |
|---|---|---|---|---|---|
| Ce-HY/SBA-15 | TP | --- | ---- | 5.14 mg S/g | [118] |
| Ni2P/SBA-15 | DBT | 500 ppmw | ---- | 11 mg S/g | [119] |
| Ag-Al/MCM-41 | DBT | 500 ppmw | <80 ppmw | 84.6% DBT adsorbed fom model fuel | [120] |
| Co/MCM-41 | actual diesel fuel | 12,000 ppm | 6700 ppm | reduced the sulfur content from 1.2 wt% to 0.67 wt% | [121] |
| Ce-KIT-6 | TP | 510 ppmw | <200 ppm | 4.5 mg S/g | [122] |
| Ni-KIT-6 | TP | 517 ppmw | ----- | 6.25 mg S/g | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Wang, B.; Wang, R.; Kozhevnikov, I.V. New Adsorption Materials for Deep Desulfurization of Fuel Oil. Materials 2024, 17, 1803. https://doi.org/10.3390/ma17081803
Qiu X, Wang B, Wang R, Kozhevnikov IV. New Adsorption Materials for Deep Desulfurization of Fuel Oil. Materials. 2024; 17(8):1803. https://doi.org/10.3390/ma17081803
Chicago/Turabian StyleQiu, Xiaoyu, Bingquan Wang, Rui Wang, and Ivan V. Kozhevnikov. 2024. "New Adsorption Materials for Deep Desulfurization of Fuel Oil" Materials 17, no. 8: 1803. https://doi.org/10.3390/ma17081803






