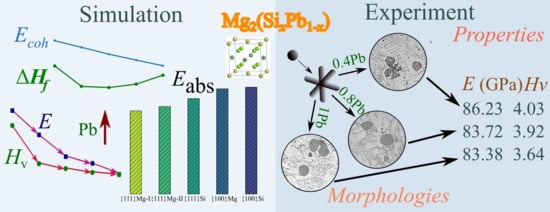
Morphology and Properties of Mg2Si Phase Modified by Pb in As-Cast Mg-2.5Si-xPb Alloys
Abstract
:1. Introduction
2. Theoretical Model, Calculation Method, and Experimental Procedures
2.1. Theoretical Model and Calculation Method
2.2. Experimental Method
3. Results
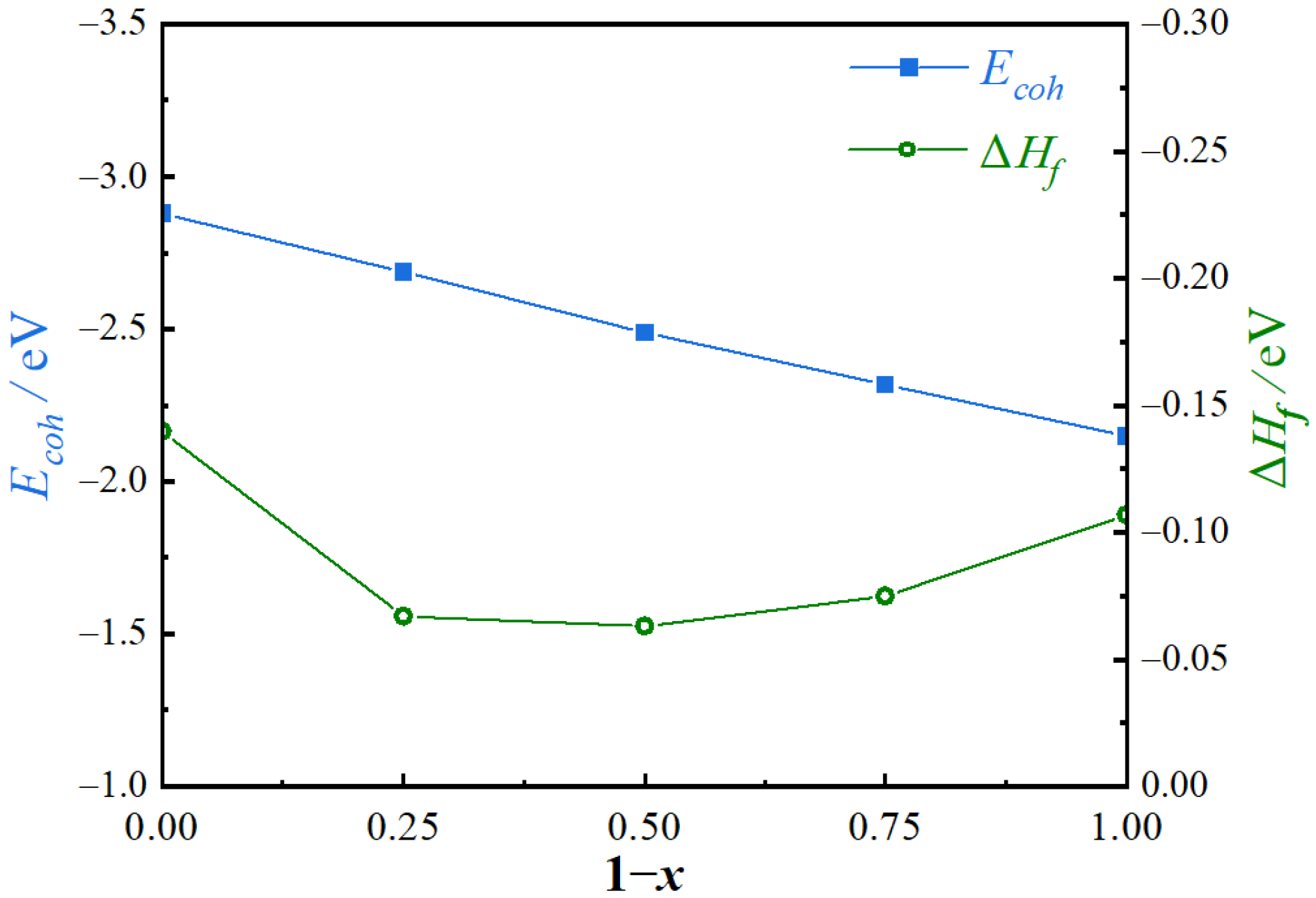
3.1. Structural Stability of the Mg2(SixPb1−x) Phase
3.2. Elastic Constants and Mechanical Properties of the Mg2(SixPb1−x) Phase
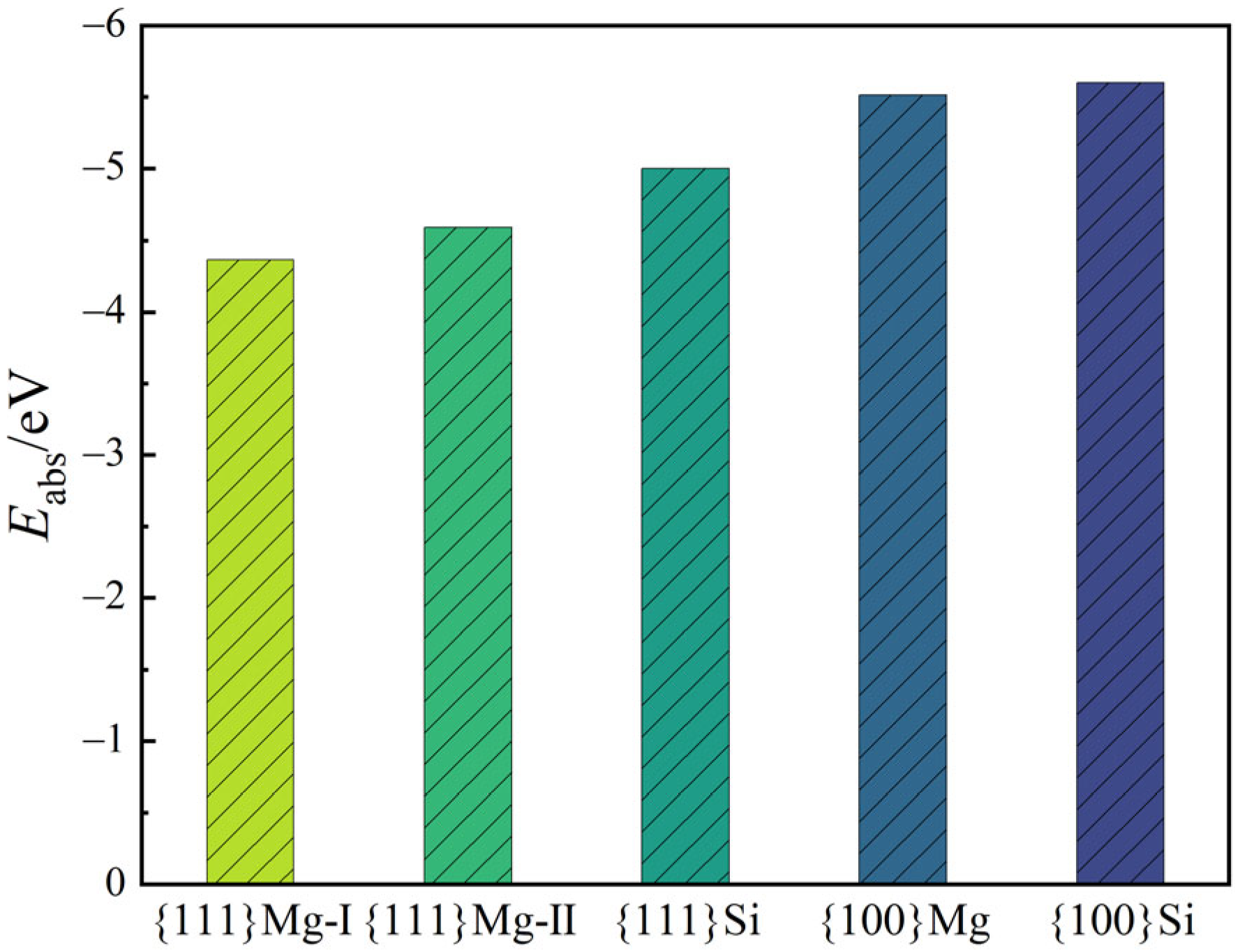
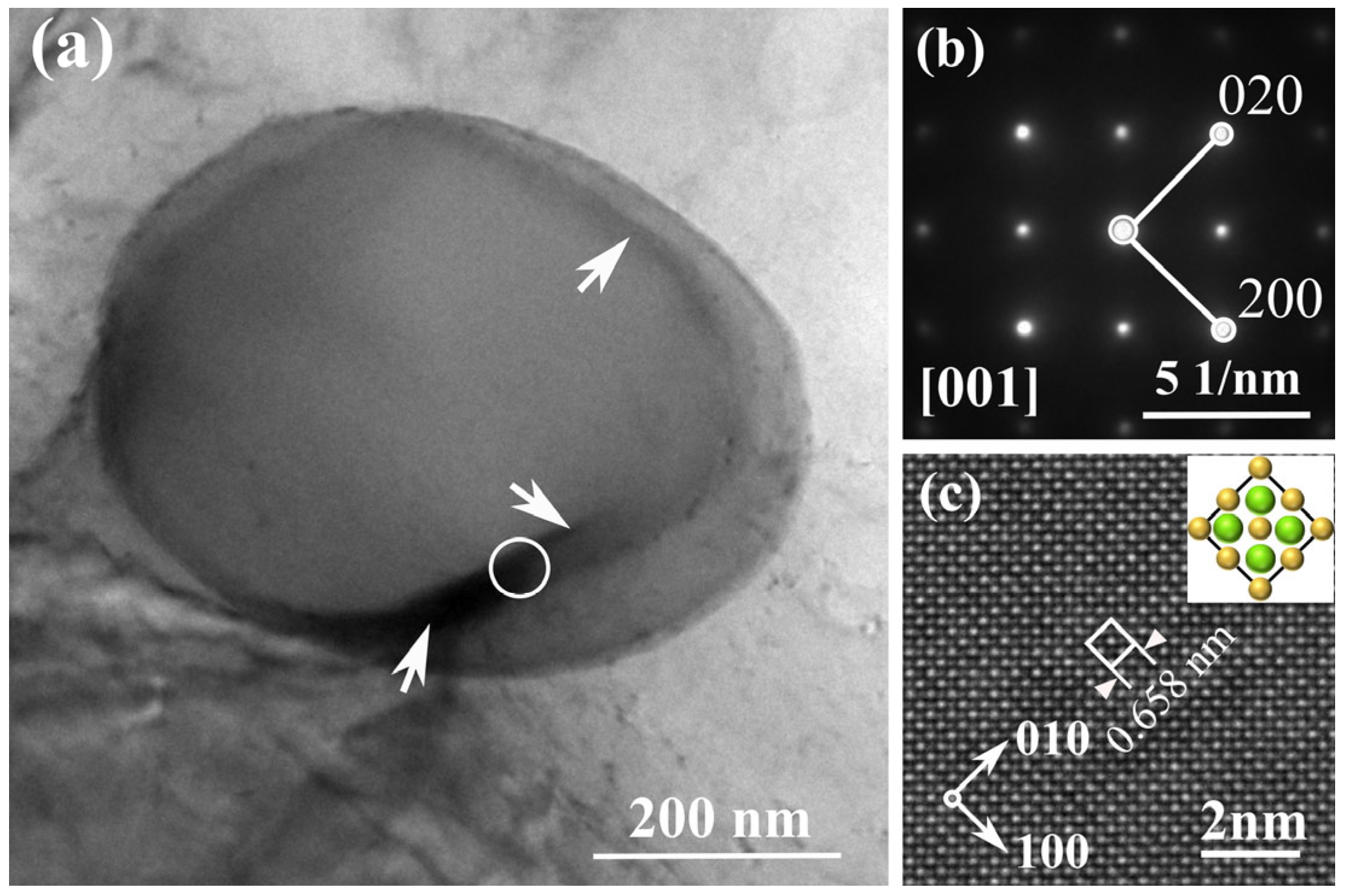
3.3. Growth Morphology of Mg2(SixPb1−x) Crystal
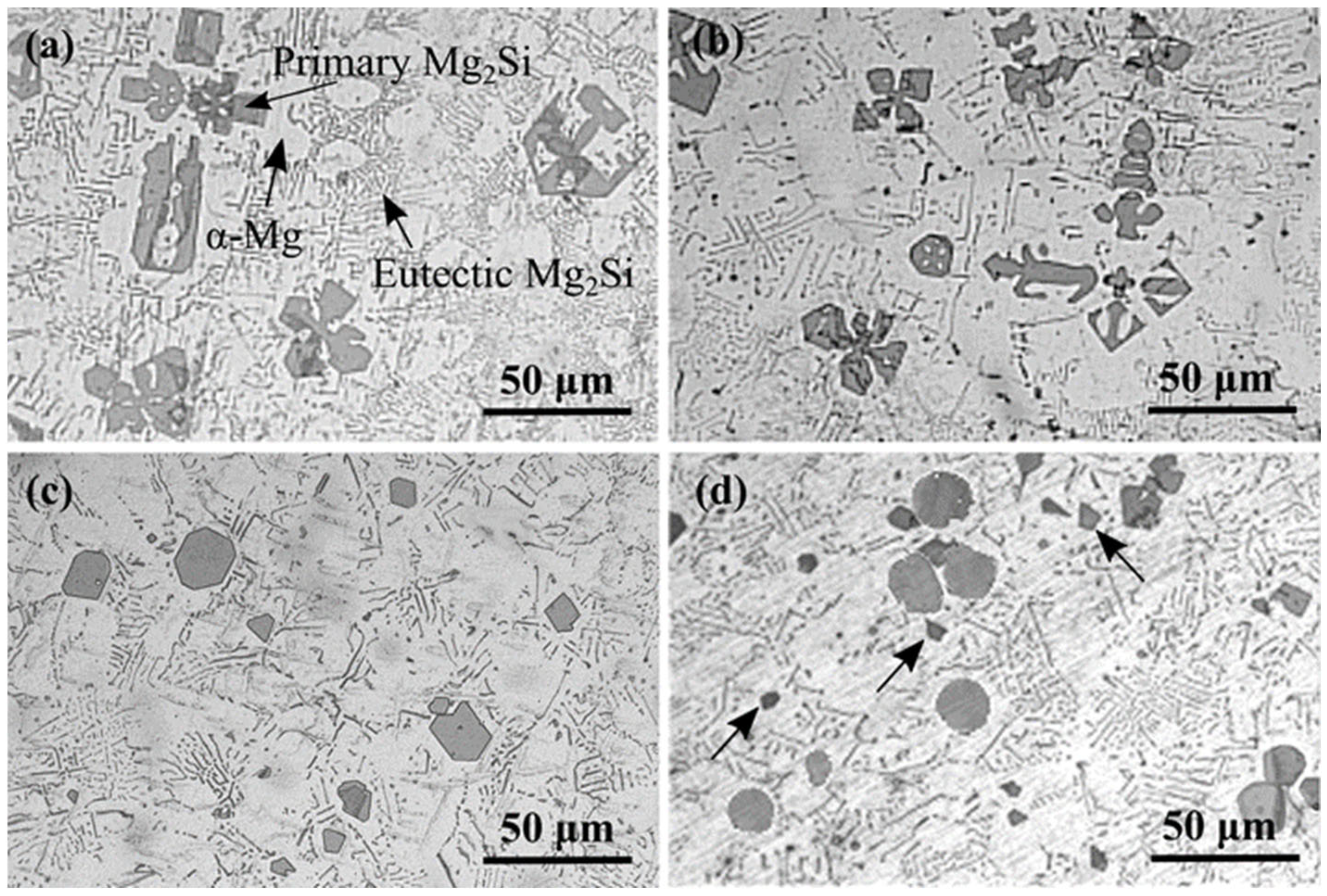
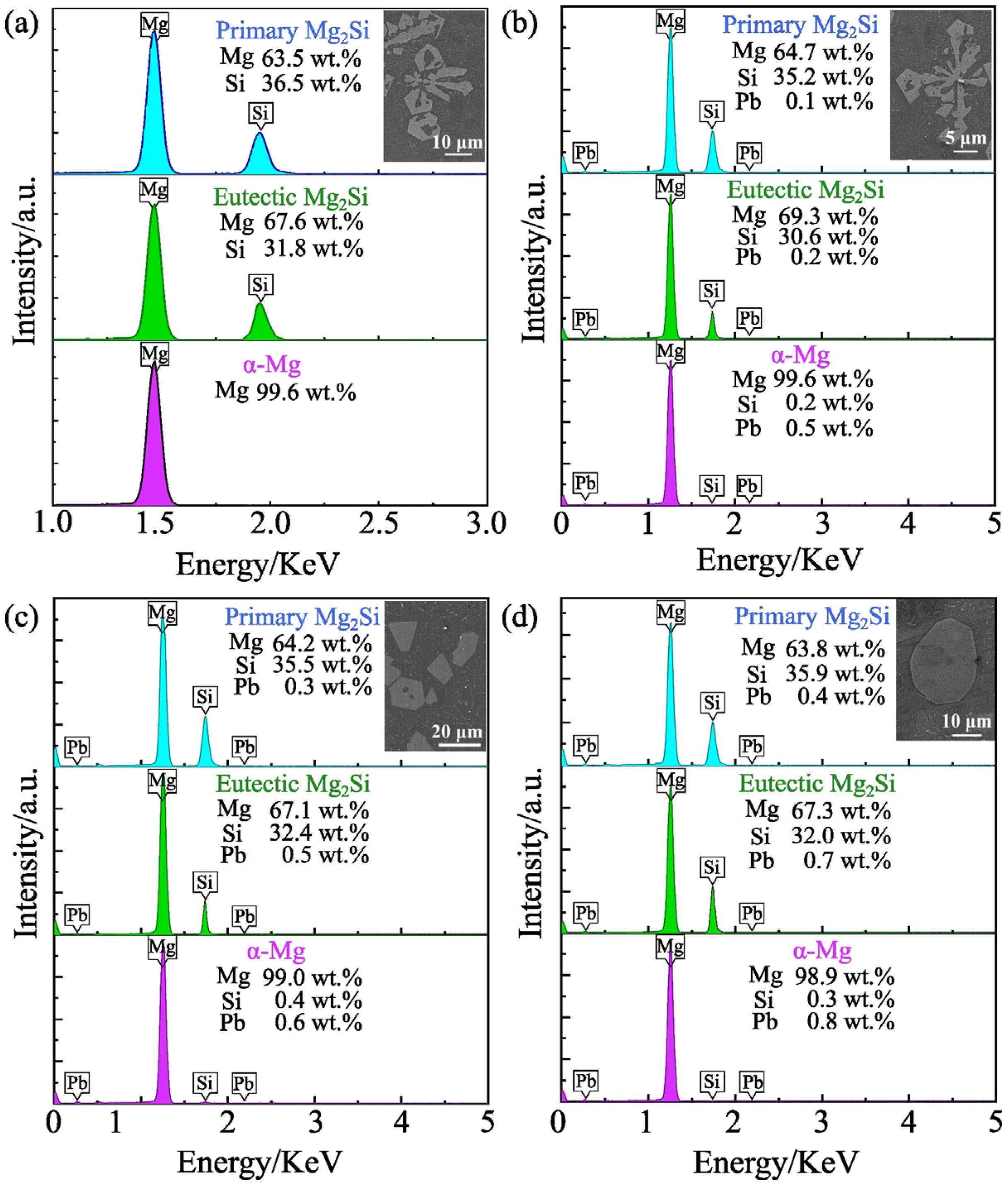
3.4. Microstructure of Mg2(SixPb1−x) in Mg-Si-Pb Alloy
3.5. Nanomechanical Properties of the Mg2(SixPb1−x) Phase
4. Discussion
4.1. Morphology
4.2. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froes, F.H. Advanced metals for aerospace and automotive use. Mater. Sci. Eng. A 1994, 184, 119–133. [Google Scholar] [CrossRef]
- Schumann, S.; Friedrich, H. Current and Future Use of Magnesium in the Automobile Industry. Mater. Sci. Forum 2003, 419–422, 51–56. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Westenge, H.; Rashed, H.M.M.A. Magnesium Alloys: Properties and Applications. Encycl. Mater. Met. Alloys 2016, 1, 11–19. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium: Properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Tanhaee, Z.; Mahmudi, R. The microstructure and creep characteristics of cast Mg–3Si and Mg–3Si–1Gd alloys. Mater. Sci. Eng. A 2016, 673, 148–157. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.Y.; Li, H.; Liu, X.F. Morphological evolution and growth mechanism of primary Mg2Si phase in Al-Mg2Si alloys. Acta Mater. 2011, 59, 1058–1067. [Google Scholar] [CrossRef]
- Sang, G.L.; Jeon, J.J.; Park, K.C.; Yong, H.P.; Park, I.M. Investigation on microstructure and creep properties of as-cast and aging-treated Mg–6Sn–5Al–2Si alloy. Mater. Sci. Eng. A 2011, 528, 5394–5399. [Google Scholar]
- Metayer, J.; Ye, B.; Guo, W.; Wang, Q.D.; Zhou, H.; Mollet, F. Microstructure and mechanical properties of Mg-Si alloys processed by cyclic closed-die forging. Trans. Nonferrous Met. Soc. China 2014, 24, 66–75. [Google Scholar] [CrossRef]
- Moussa, M.E.; Waly, M.A.; El-Sheikh, A.M. Effect of Ca addition on modification of primary Mg2Si, hardness and wear behavior in Mg-Si hypereutectic alloys. J. Magnes. Alloys 2014, 2, 230–238. [Google Scholar] [CrossRef]
- Cong, M.; Li, Z.; Liu, J.; Li, S. Effect of Sr on microstructure, tensile properties and wear behavior of as-cast Mg–6Zn–4Si alloy. Mater. Des. 2014, 53, 430–434. [Google Scholar] [CrossRef]
- Kumar, K.; Viswanath, A.; Pillai, U.; Pai, B.C.; Chakraborty, M. Influence of Neodymium Addition on the Microstructure, Mechanical and Thermal Properties of Mg-Si Alloys. Procedia Eng. 2013, 55, 103–108. [Google Scholar] [CrossRef]
- Hu, J.L.; Tang, C.P.; Zhang, X.M.; Deng, Y.L. Modification of Mg2Si in Mg-Si alloys with neodymium. Trans. Nonferrous Met. Soc. China 2013, 23, 3161–3166. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jiang, Q.C.; Ma, B.X.; Wang, Y.; Wang, J.G. Modification of Mg2Si in Mg-Si alloys with K2TiF6, KBF4 and KBF4 + K2TiF6. J. Alloys Compd. 2005, 2005, 105–108. [Google Scholar] [CrossRef]
- Moussa, M.E.; Waly, M.A.; El-Sheikh, A.M. Effect of high-intensity ultrasonic treatment on modification of primary Mg2Si in the hypereutectic Mg-Si alloys. J. Alloys Compd. 2013, 577, 693–700. [Google Scholar] [CrossRef]
- Seth, P.P.; Parkash, O.; Kumar, D. Structure and mechanical behavior of in situ developed Mg2Si phase in magnesium and aluminum alloys—A review. RSC Adv. 2020, 10, 37327–37345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Yang, W.; Zhang, J. Morphology and properties of Mg2Si and Mg2(SixSn1-x) reinforcements in magnesium alloys. Mater. Sci. Technol. 2017, 33, 1–8. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, J.; Xu, S.; Peng, P. Thermal stability and elastic properties of Mg2X (X = Si, Ge, Sn, Pb) phases from first-principle calculations. Comput. Mater. Sci. 2012, 2012, 409–414. [Google Scholar] [CrossRef]
- Fan, T.W.; Ke, J.L.; Fu, L.; Tang, B.Y.; Ding, W.J. Ideal strength of Mg2X (X = Si, Ge, Sn and Pb) from first-principles. J. Magnes. Alloys 2013, 1, 163–168. [Google Scholar] [CrossRef]
- Jung, I.H.; Kim, J. Thermodynamic modeling of the Mg-Ge-Si, Mg-Ge-Sn, Mg-Pb-Si and Mg-Pb-Sn systems. ChemInform 2010, 41, 137–147. [Google Scholar] [CrossRef]
- Jabar, A.; Selmani, Y.; Bahmad, L.; Benyoussef, A. Study of Bi2CaX2 (X = Mg and Mn) compounds: A first-principle approach. Comput. Condens. Matter 2023, 34, e770. [Google Scholar] [CrossRef]
- Srinivasan, A.; Pillai, U.T.S.; Pai, B.C. Effect of Pb addition on ageing behavior of AZ91 magnesium alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2007, 452, 87–92. [Google Scholar] [CrossRef]
- Murtaza, G.; Sajid, A.; Rizwan, M.; Takagiwa, Y.; Jibran, M.; Khenata, R.; Omran, S.B. First principles study of Mg2X (X=Si, Ge, Sn, Pb): Elastic, optoelectronic and thermoelectric properties. Mater. Sci. Semicond. Process. 2015, 40, 429–435. [Google Scholar] [CrossRef]
- Gr Bner, J.; Chumak, I.; Schmid-Fetzer, R. Experimental study of ternary Ca–Mg–Si phase equilibria and thermodynamic assessment of Ca–Si and Ca–Mg–Si systems. Intermetallics 2003, 11, 1065–1074. [Google Scholar] [CrossRef]
- Balasubramani, N.; Srinivasan, A.; Pillai, U.T.S.; Pai, B.C. Effect of Pb and Sb additions on the precipitation kinetics of AZ91 magnesium alloy. Mater. Sci. Eng. A 2007, 457, 275–281. [Google Scholar] [CrossRef]
- Tang, P.Y.; Wen, L.; Tong, Z.F.; Tang, B.Y.; Peng, L.M.; Ding, W.J. Stacking faults in B2-structured magnesium alloys from first principles calculations. Comput. Mater. Sci. 2011, 50, 3198–3207. [Google Scholar] [CrossRef]
- Nakanishi, R.; Sueoka, K.; Shiba, S.; Fukutani, S.; Hino, M.; Murakami, K. First-Principles Calculation on the Stable Structure and Adhesive Strength of Ni/Fe(100) or Cu/Fe(100) Interfaces. J. Jpn. Inst. Met. 2007, 71, 1024–1031. [Google Scholar] [CrossRef]
- Ganeshan, S.; Shang, S.L.; Wang, Y.; Liu, Z.K. Temperature dependent elastic coefficients of Mg2X (X=Si, Ge, Sn, Pb) compounds from first-principles calculations. J. Alloys Compd. 2010, 498, 191–198. [Google Scholar] [CrossRef]
- Milstein, F.; Zhao, J.; Chantasiriwan, S.; Maroudas, D. Applicability of Born’s stability criterion to face-centered-cubic crystals in [111] loading. Appl. Phys. Lett. 2005, 87, 160–1279. [Google Scholar] [CrossRef]
- Guo, X.; Lei, L.; Liu, Z.; Yu, D.; He, J.; Liu, R.; Bo, X.; Tian, Y.; Wang, H.T. Hardness of covalent compounds: Roles of metallic component and d valence electrons. J. Appl. Phys. 2008, 104, 15502. [Google Scholar] [CrossRef]
- Tian, W.; Chen, H. Insight into the mechanical, thermodynamics and superconductor properties of NbRuB via first-principles calculation. Sci. Rep. 2016, 6, 19055. [Google Scholar] [CrossRef]
- Nie, J.; Wu, Y.; Li, P.; Li, H.; Liu, X. Morphological evolution of TiC from octahedron to cube induced by elemental nickel. Crystengcomm 2012, 14, 2213–2221. [Google Scholar] [CrossRef]
- Ghandvar, H.; Jabbar, K.A.; Idris, M.H.; Ahmad, N.; Jahare, M.H.; Rahimian Koloor, S.S.; Petrů, M. Influence of barium addition on the formation of primary Mg2Si crystals from Al–Mg–Si melts. J. Mater. Res. Technol. 2021, 11, 448–465. [Google Scholar] [CrossRef]
- Gomez, J.A.; Larkin, I.; Schwingenschloegl, U. First-principles calculations of the YBa2Cu3O7/PrBa2Cu3O7 interface. Math. Comput. Simul. 2010, 80, 1499–1508. [Google Scholar] [CrossRef]
- ALMisned, G.; Susoy, G.; Sen Baykal, D.; Alkarrani, H.; Güler, Ö.; Tekin, H.O. A closer-look on W and Pb alloys: In-depth evaluation in elastic modulus, gamma-ray, and neutron attenuation for critical applications. Nucl. Eng. Des. 2024, 420, 113063. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A.; Stylianopoulos, T. A simplified approach for the determination of fitting constants in Oliver–Pharr method regarding biological samples. Phys. Biol. 2019, 16, 56003. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.Y.; Li, Y.J.; Zha, M.; Jiang, Q.C. Morphology and size control of octahedral and cubic primary Mg2Si in an Mg-Si system by regulating Sr contents. CrystEngComm 2014, 2014, 448–454. [Google Scholar] [CrossRef]
- Swaminathan, R.; Willard, M.A.; Mchenry, M.E. Critical Nucleus and Growth Shapes of Cuboctahedrally Truncated Nanoparticles. Acta Mater. 2006, 54, 807. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Cui, H.; Fan, G.; Guo, X. PrimaryMg2Si phase and Mg2Si/α-Mg interface modified by Sn and Sb elements in a Mg-5Sn-2Si-1.5Al-1Zn-0.8Sb alloy. J. Magnes. Alloys 2022, 10, 3234–3249. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Lee, C.; Jo, H.; Hong, H.; Chung, J.; Lee, B.H. Strengthening mechanisms of solid solution and precipitation at elevated temperature in fire-resistant steels and the effects of Mo and Nb addition. J. Mater. Res. Technol. 2021, 15, 5095–5105. [Google Scholar] [CrossRef]
- Jhi, S.H.; Louie, S.G.; Cohen, M.L.; Ihm, J. Vacancy hardening and softening in transition metal carbides and nitrides. Phys. Rev. Lett. 2001, 86, 3348–3351. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; He, J.; Wu, E.; Liu, S.; Yu, D.; Li, D.; Zhang, S.; Tian, Y. Hardness of covalent crystals. Phys. Rev. Lett. 2003, 91, 15502. [Google Scholar] [CrossRef] [PubMed]





| x | 1.00 | 0.75 | 0.50 | 0.25 | 0.00 | |
|---|---|---|---|---|---|---|
| a (nm) | Theoretical | 0.6365 | 0.6623 | 0.6704 | 0.6775 | 0.6938 |
| Experimental | 0.6338 [26] | - | - | - | 0.6933 [27] |
| x | C11, GPa | C44, GPa | C12, GPa | B, GPa | G, GPa | E, GPa | G/B | |
|---|---|---|---|---|---|---|---|---|
| 1 | 114.04 | 41.50 | 21.76 | 52.52 | 43.35 | 102.00 | 0.1763 | 0.83 |
| 0.75 | 92.29 | 35.21 | 19.32 | 43.64 | 35.72 | 84.19 | 0.1785 | 0.82 |
| 0.5 | 75.78 | 28.45 | 16.65 | 37.93 | 28.46 | 68.29 | 0.1999 | 0.75 |
| 0.25 | 70.93 | 26.99 | 23.09 | 39.04 | 25.76 | 63.35 | 0.2295 | 0.66 |
| 0 | 59.00 | 24.85 | 20.33 | 33.22 | 22.64 | 55.36 | 0.2223 | 0.68 |
| Crystals | Bone Types | Number | D (Å) | P | V (Å3) | Hv (GPa) |
|---|---|---|---|---|---|---|
| Mg2Si | Mg-Si | 32 | 2.7562 | 0.36 | 257.8875 | 4.17 |
| Mg2(Si0.75Pb0.25) | Mg-Si | 24 | 2.8163 | 0.38 | 279.6076 | 3.03 |
| Mg-Pb | 8 | 2.8780 | 0.03 | |||
| Mg2(Si0.5Pb 0.5) | Mg-Si | 16 | 2.8178 | 0.40 | 291.9998 | 2.84 |
| Mg-Pb | 16 | 2.9284 | 0.10 | |||
| Mg2(Si0.25Pb0.75) | Mg-Si | 8 | 2.8501 | 0.42 | 311.0440 | 2.69 |
| Mg-Pb | 24 | 2.9629 | 0.18 | |||
| Mg2Pb | Mg-Pb | 32 | 3.0022 | 0.24 | 333.2925 | 2.61 |
| Mg2Si | {111}Mg-I | {111}Mg-II | {111}Si | {100}Mg | {100}Si |
|---|---|---|---|---|---|
| Esurf (eV) | 0.48 | 1.60 | 1.62 | 1.07 | 1.27 |
| Esurf/Pb (eV) | 1.09 | 3.53 | 1.65 | 1.32 | 2.79 |
| Alloy | Primary Mg2Si (%) | Eutectic Mg2Si (%) | α-Mg (%) | |
|---|---|---|---|---|
| Mg-2.5Si | 17.5 | 3.5 | 78.2 | |
| Mg-2.5Si-0.4Pb | 17.6 | 3.8 | 77.7 | |
| Mg-2.5Si-0.8Pb | 18.6 | 4.8 | 76.3 | |
| Mg-2.5Si-1.0Pb | 19.9 | 4.6 | 75.1 |
| Alloy | Elastic Modulus E (GPa) | Hardness Hv (Gpa) | hmax (nm) | hf (nm) | hf/hmax (%) |
|---|---|---|---|---|---|
| Mg-2.5Si | 94.6 | 4.6 | 66.8 | 38.7 | 57.9 |
| Mg-2.5Si-0.4Pb | 86.2 | 4.0 | 73.9 | 42.9 | 58.1 |
| Mg-2.5Si-0.8Pb | 83.7 | 3.9 | 73.7 | 41.8 | 56.7 |
| Mg-2.5Si-1.0Pb | 83.4 | 3.6 | 77.5 | 45.8 | 59.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yang, W.; Cui, H.; Wang, Y.; Xu, Z. Morphology and Properties of Mg2Si Phase Modified by Pb in As-Cast Mg-2.5Si-xPb Alloys. Materials 2024, 17, 1811. https://doi.org/10.3390/ma17081811
Chen L, Yang W, Cui H, Wang Y, Xu Z. Morphology and Properties of Mg2Si Phase Modified by Pb in As-Cast Mg-2.5Si-xPb Alloys. Materials. 2024; 17(8):1811. https://doi.org/10.3390/ma17081811
Chicago/Turabian StyleChen, Liang, Wenpeng Yang, Hongbao Cui, Ying Wang, and Zhichao Xu. 2024. "Morphology and Properties of Mg2Si Phase Modified by Pb in As-Cast Mg-2.5Si-xPb Alloys" Materials 17, no. 8: 1811. https://doi.org/10.3390/ma17081811