Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Hydrogels
2.3. Examination of Mechanical Properties
2.4. Drying Kinetics
2.5. FTIR Analysis
2.6. Thermogravimetric Analysis
2.7. SEM Observation
3. Results
3.1. Visual Analysis of Hydrogels
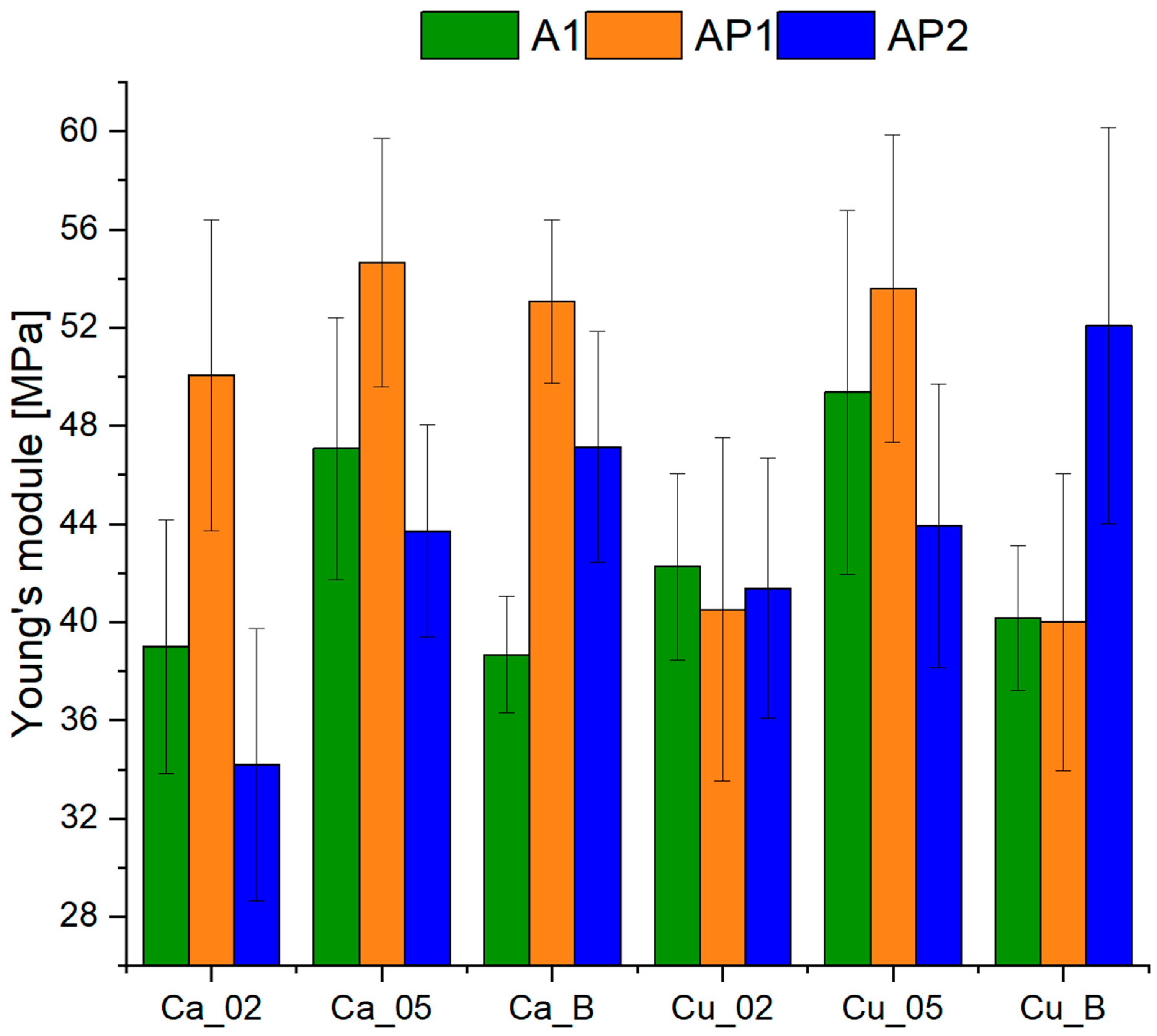
3.2. Examination of Mechanical Properties
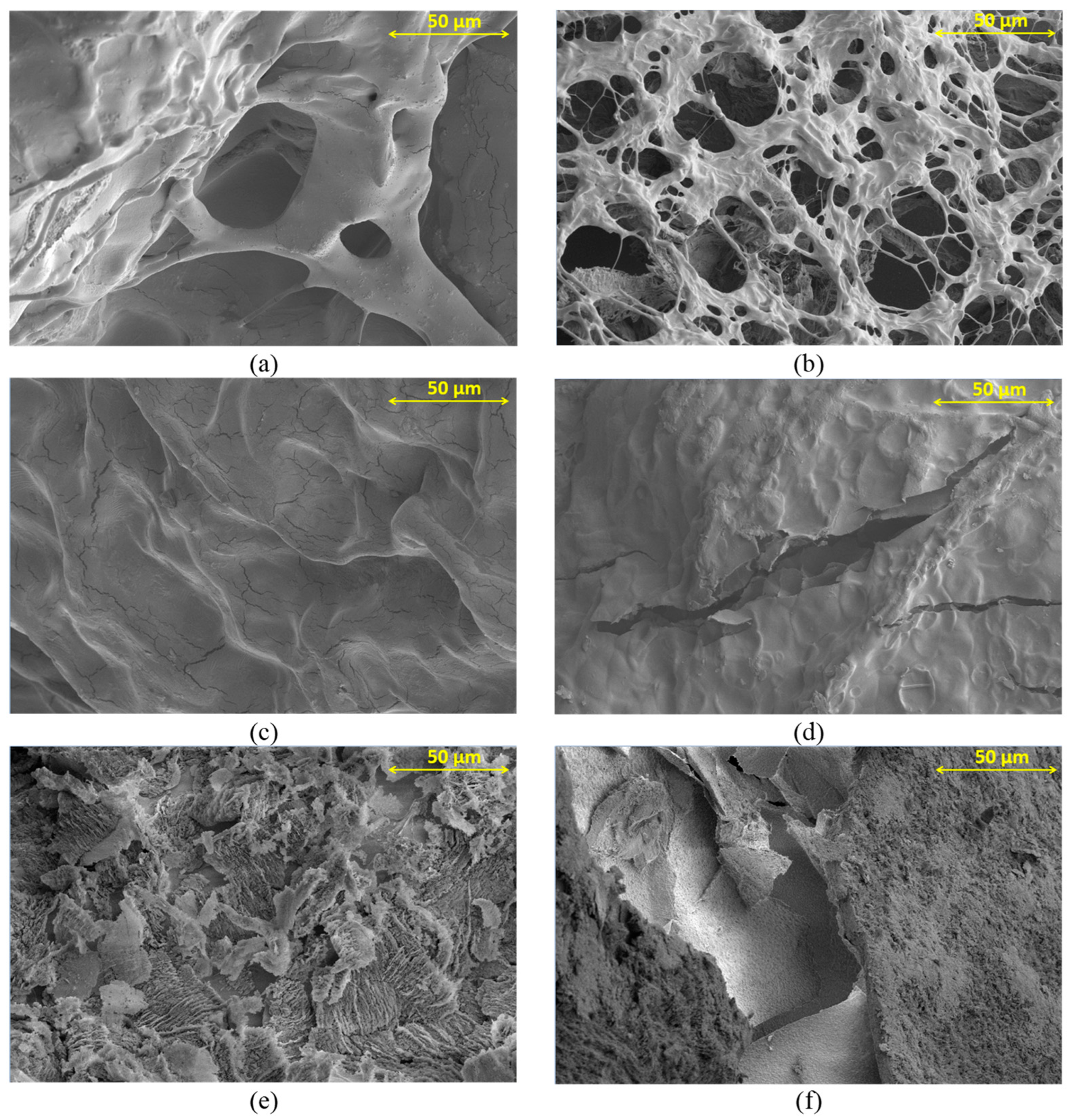
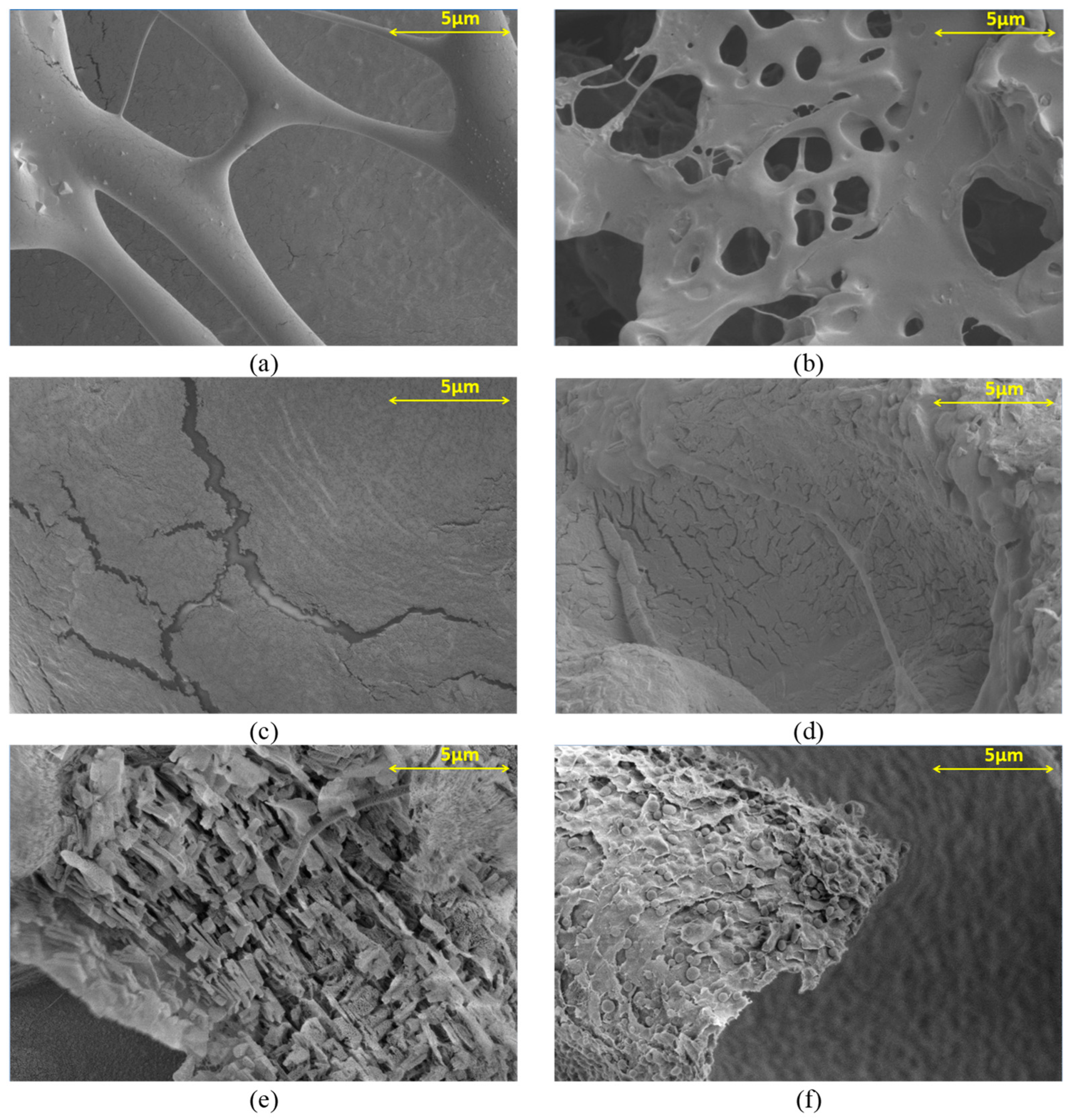
3.3. SEM Observation
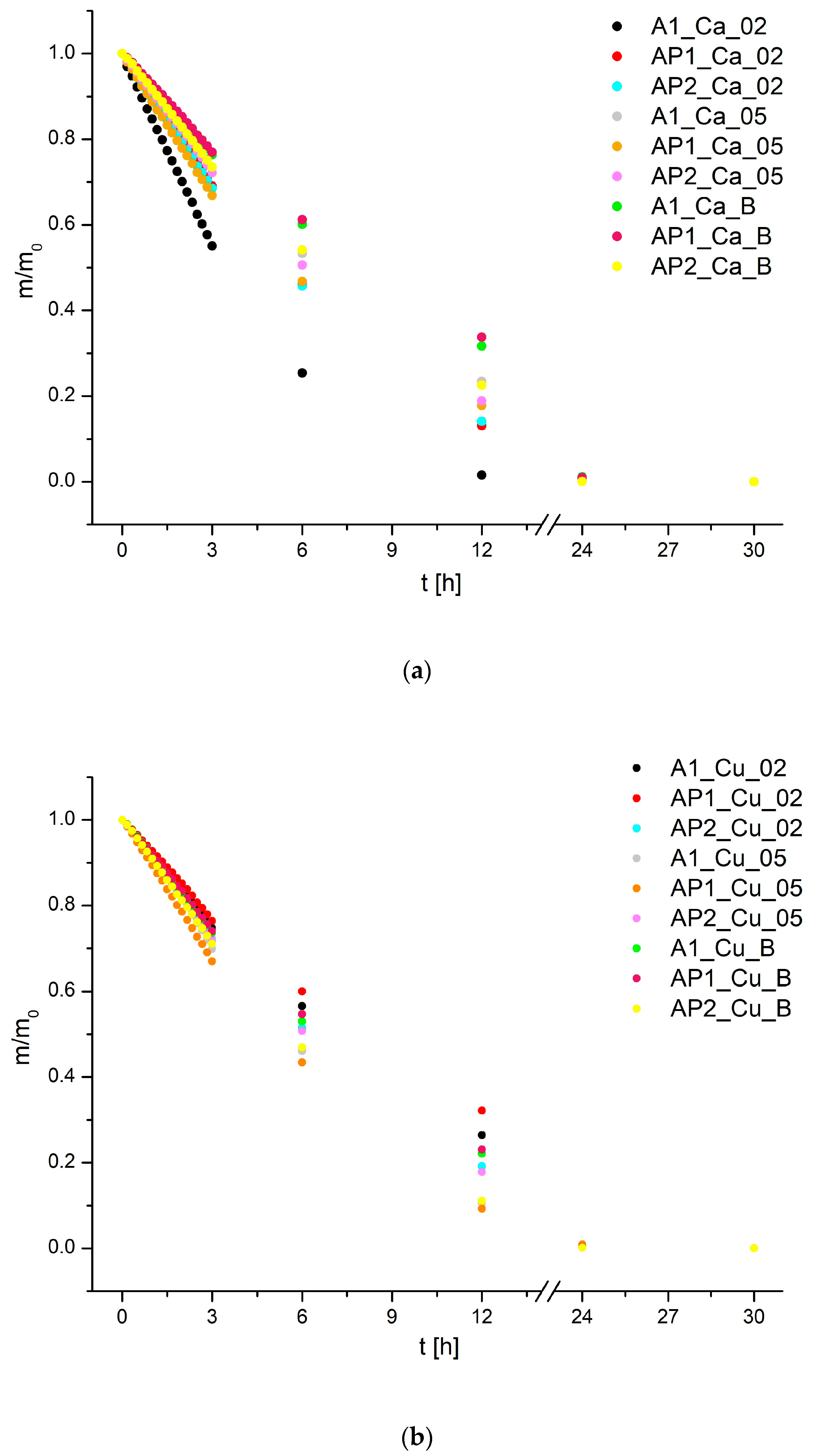
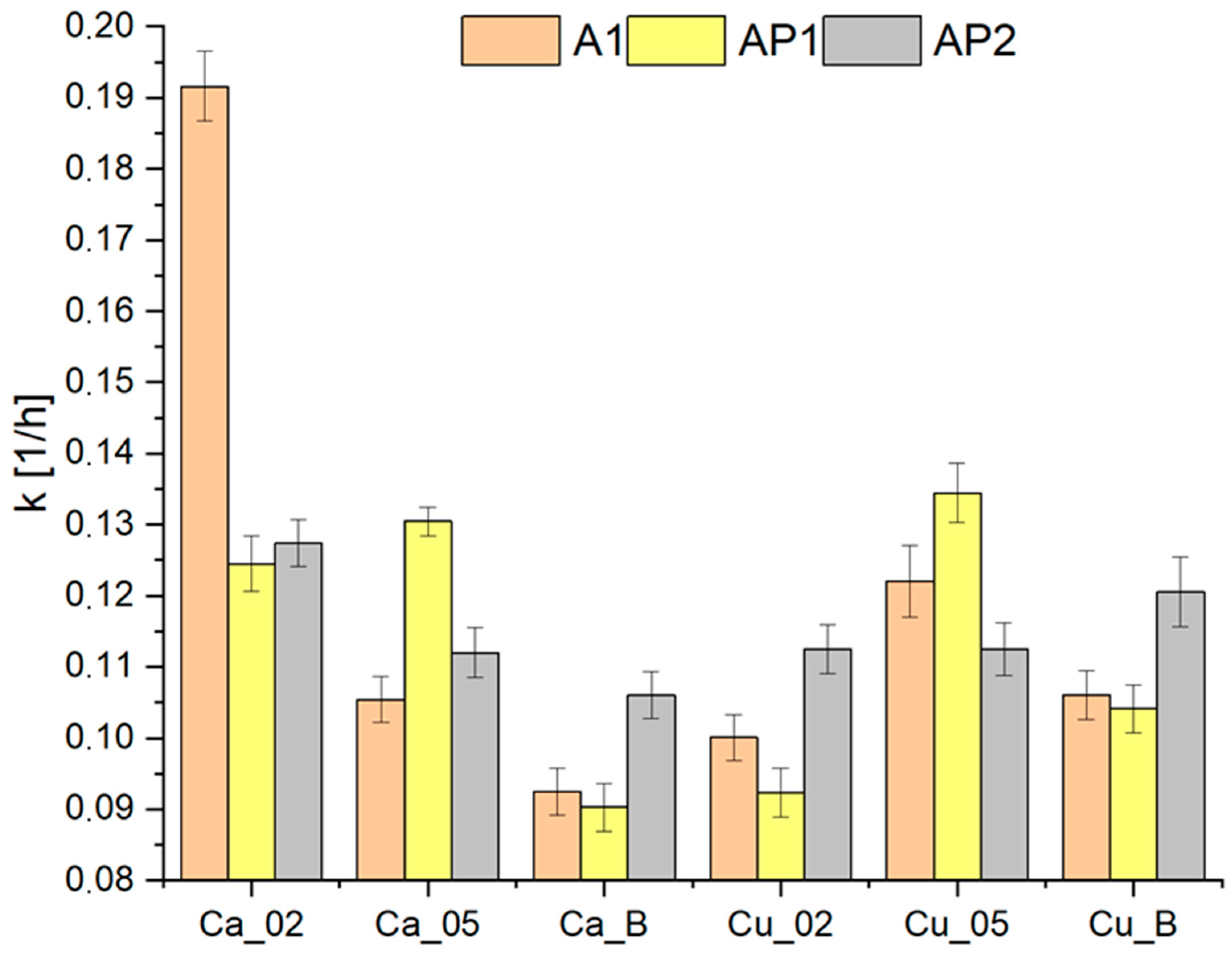
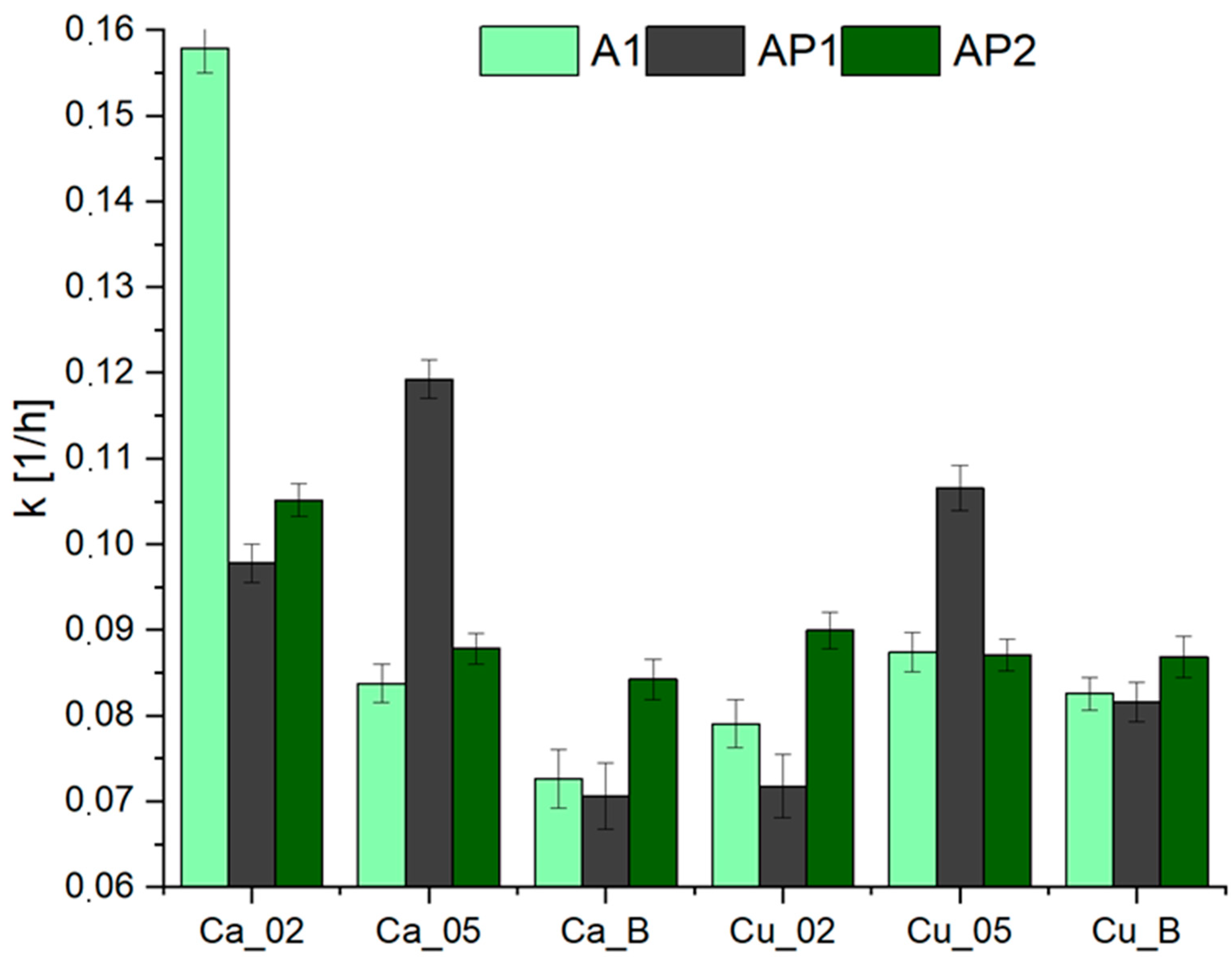
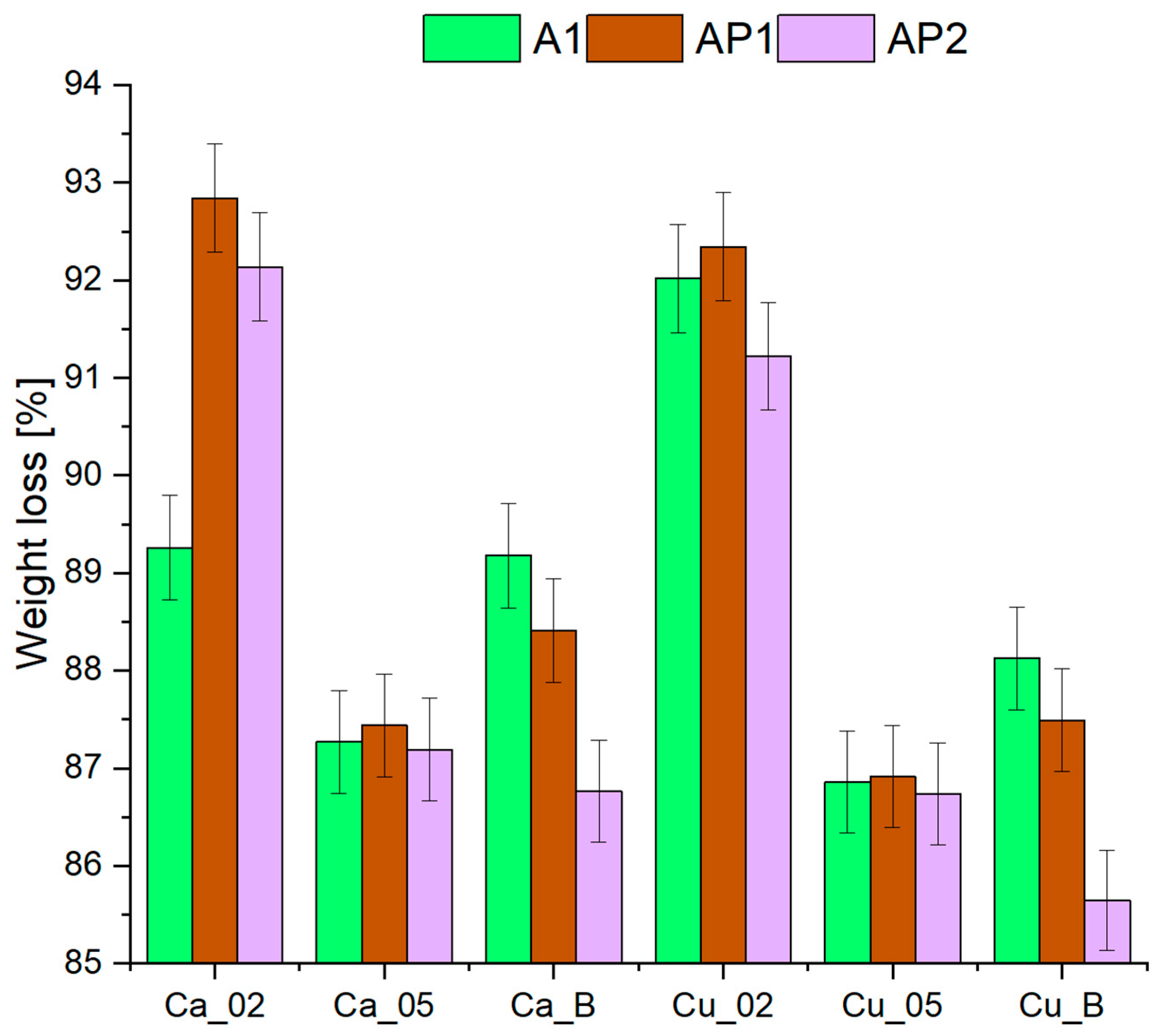
3.4. Drying Kinetics
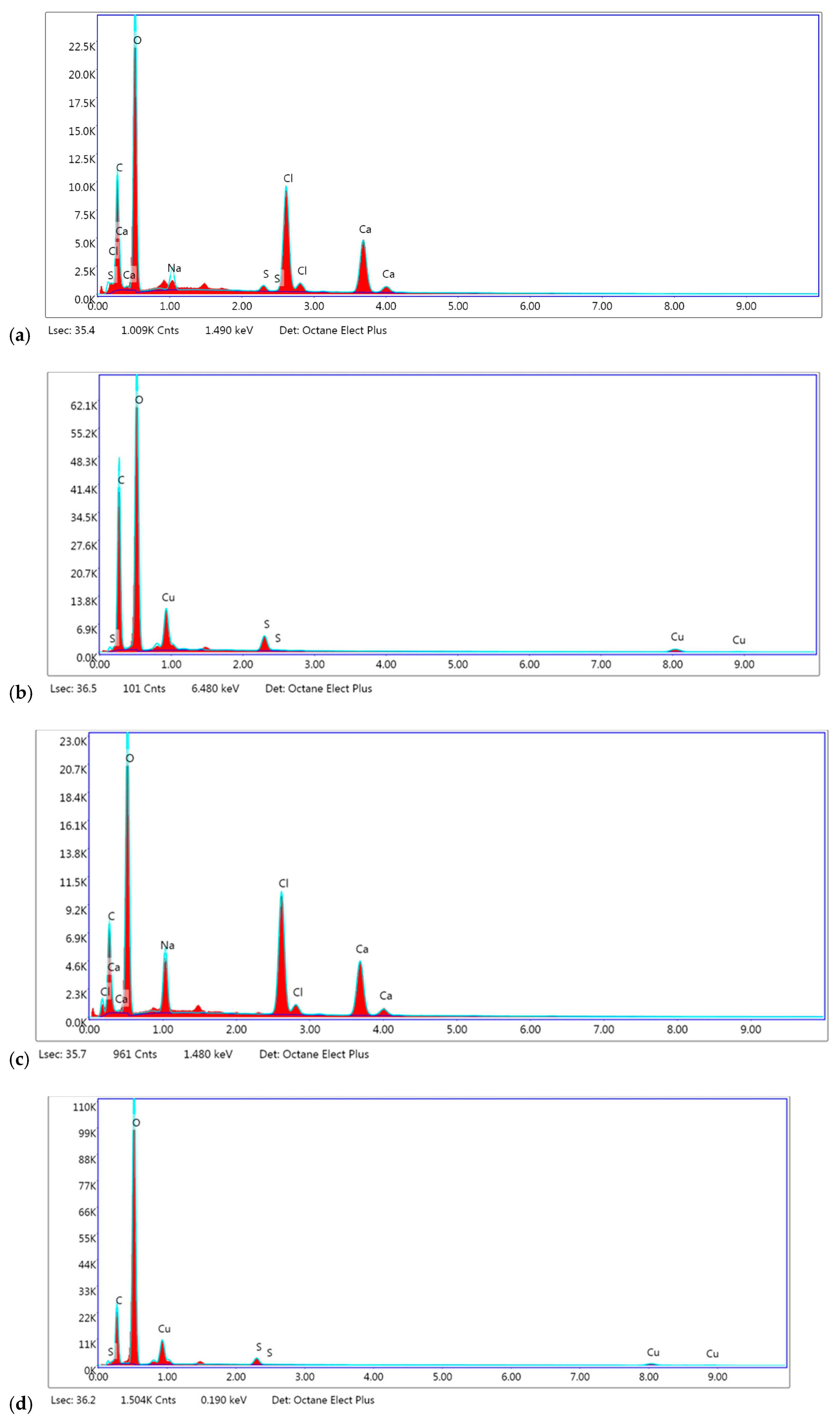
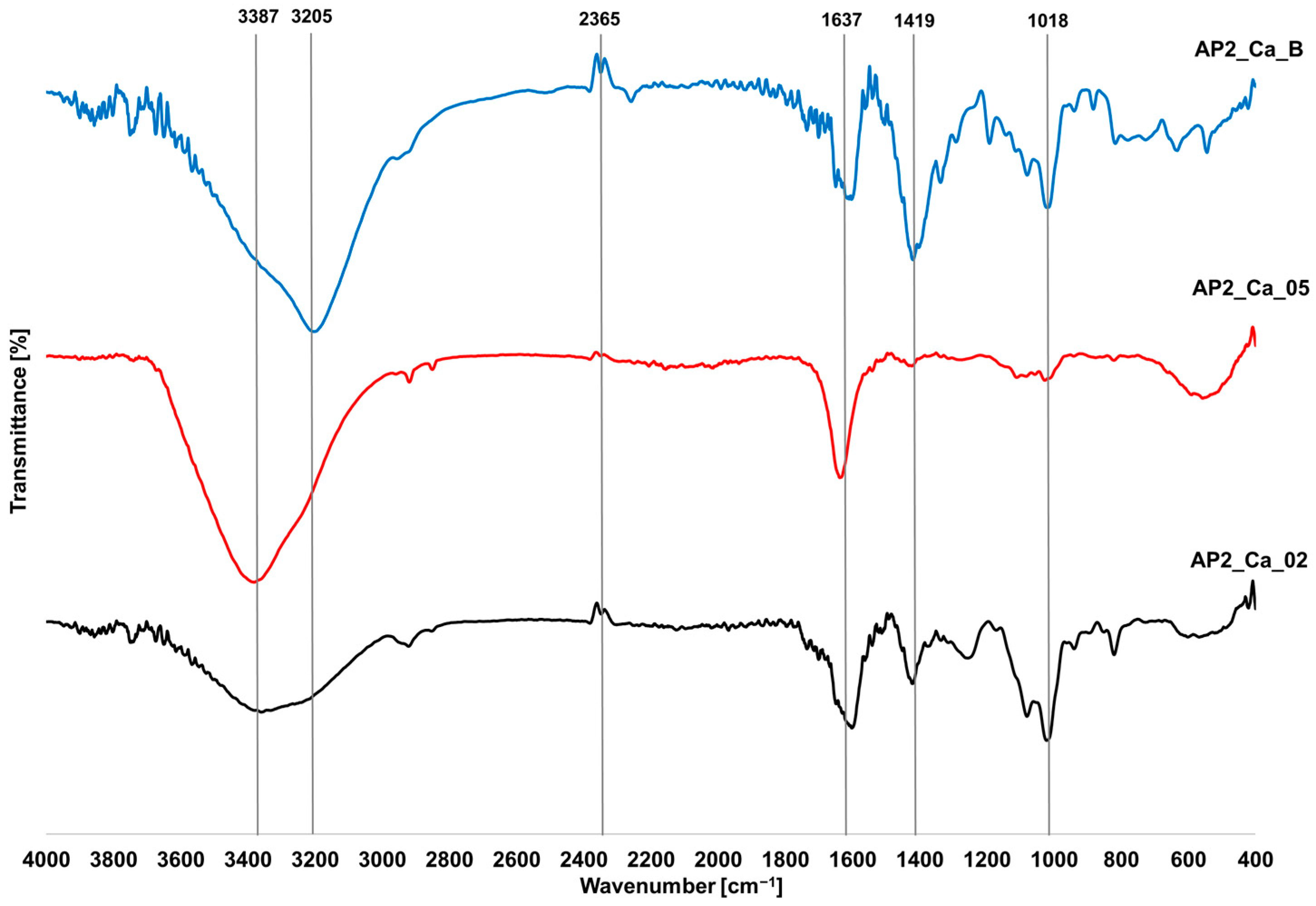
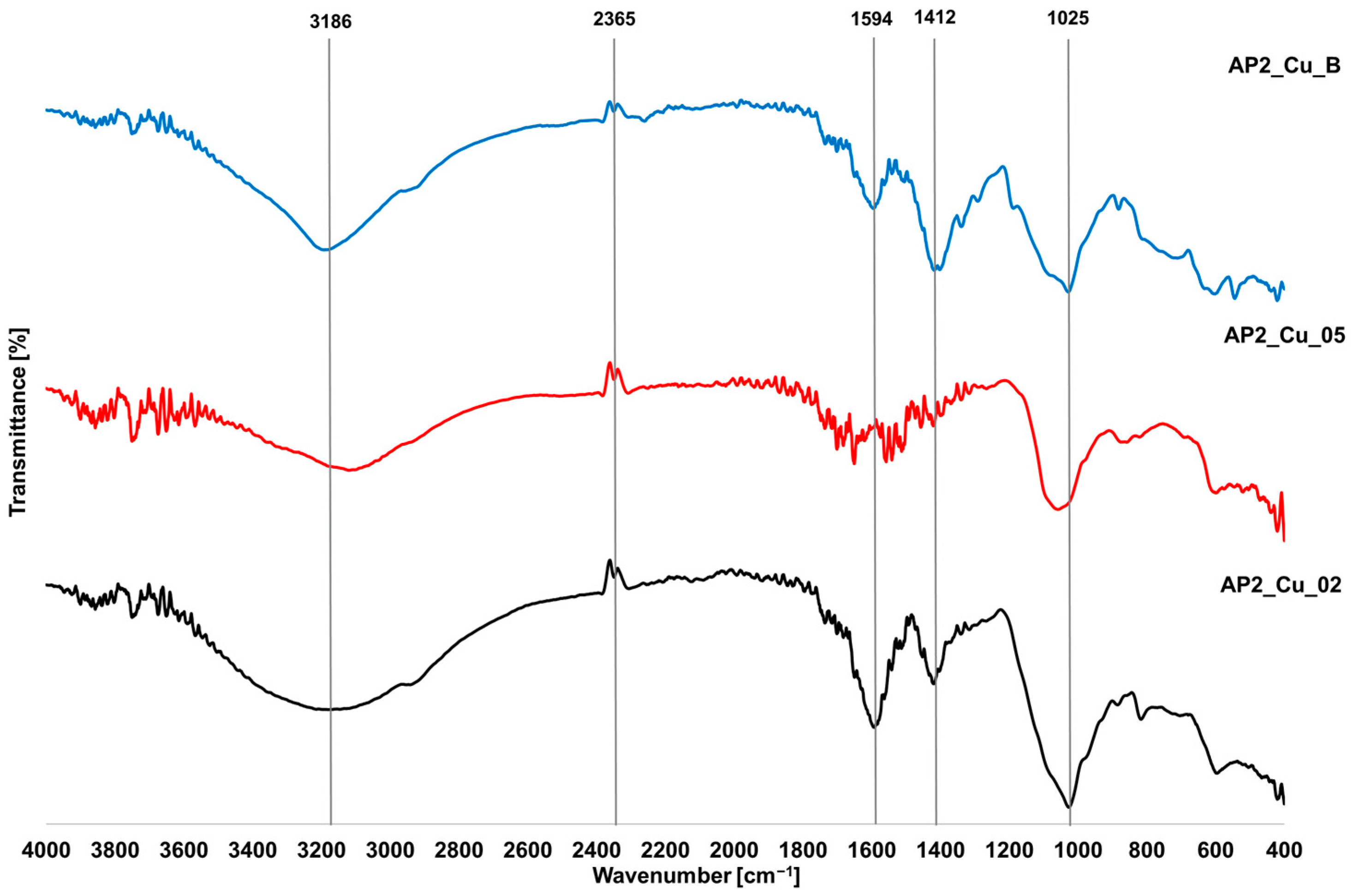
3.5. FTIR Analysis
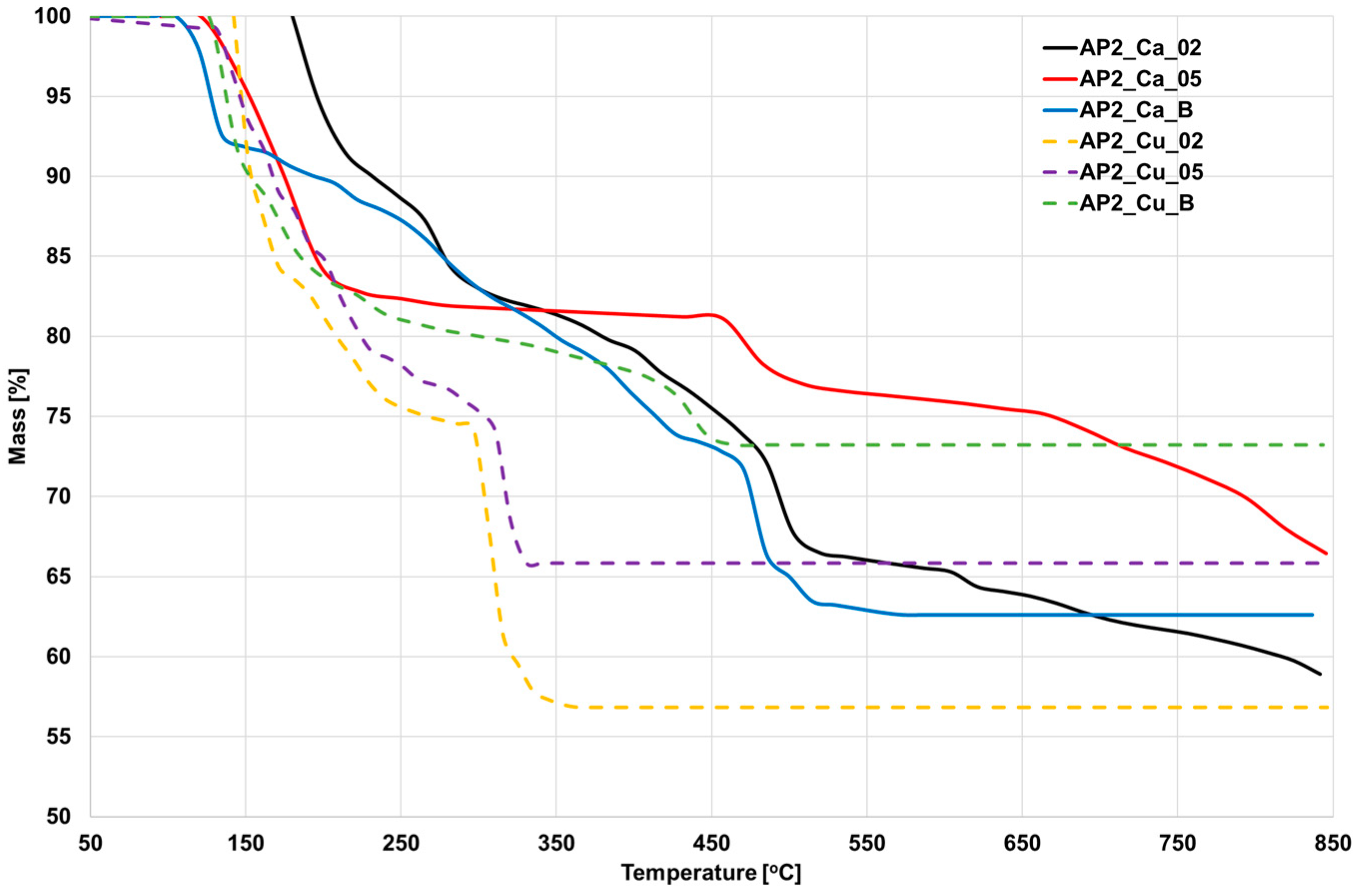
3.6. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.; Zhang, J.; Wei, Z.; Zhang, B.; Weng, X. Advances and Progress in Self-Healing Hydrogel and Its Application in Regenerative Medicine. Materials 2023, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Qi, Y.; You, C. Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety. Foods 2023, 12, 4405. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel Beads for Designing Future Foods: Structures, Mechanisms, Applications, and Challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl Cellulose/Gelatin Hydrogel Films Loaded with Zinc Oxide Nanoparticles for Sustainable Food Packaging Applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, D.; Gil, F.; Izydorczyk, G.; Mikula, K.; Gersz, A.; Hoppe, V.; Chojnacka, K.; Witek-Krowiak, A. Innovative Bio-Waste-Based Multilayer Hydrogel Fertilizers as a New Solution for Precision Agriculture. J. Environ. Manag. 2022, 321, 116002. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, M. Progress in the Preparation of Stimulus-Responsive Cellulose Hydrogels and Their Application in Slow-Release Fertilizers. Polymers 2023, 15, 3643. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D Printing Process and Application of Sodium Alginate Based Hydrogels in Soft Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Witek-Krowiak, A. Encapsulation Efficiency and Survival of Plant Growth-Promoting Microorganisms in an Alginate-Based Matrix—A Systematic Review and Protocol for a Practical Approach. Ind. Crops. Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V.; et al. Strategies in the Preparation of Conductive Polyvinyl Alcohol Hydrogels for Applications in Flexible Strain Sensors, Flexible Supercapacitors, and Triboelectric Nanogenerator Sensors: An Overview. Adv. Compos. Hybrid. Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/Thawed Polyvinyl Alcohol Hydrogels: Present, Past and Future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(Vinyl Alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The Synthesis, Mechanisms, and Additives for Bio-Compatible Polyvinyl Alcohol Hydrogels: A Review on Current Advances, Trends, and Future Outlook. J. Vinyl Addit. Technol. 2023, 29, 939–959. [Google Scholar] [CrossRef]
- Shu, Z.; Cao, Q.; Muhammad, U.; Zhang, T.; Ji, W.; Chen, J.; Liu, C.; Wei, Y. Fabrication of High-Strength Magnetically Responsive Hydrogels by Synergistic Salting-out and Freezing–Thawing and Application of Their Shape Deformation and Swimming. Polym. Eng. Sci. 2023, 63, 1567–1578. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Jiang, D.; Guo, N.; Wang, Y.; Ding, T.; Weng, L. A Highly Stretchable, Sensing Durability, Transparent, and Environmentally Stable Ion Conducting Hydrogel Strain Sensor Built by Interpenetrating Ca2+-SA and Glycerol-PVA Double Physically Cross-Linked Networks. Adv. Compos. Hybrid. Mater. 2022, 5, 1712–1729. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Q. Alginate Hydrogel-Mediated Crystallization of Calcium Carbonate. J. Solid. State Chem. 2011, 184, 1008–1015. [Google Scholar] [CrossRef]
- Oyen, M.L. Mechanical Characterisation of Hydrogel Materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. PH-Sensitive Sodium Alginate/Poly(Vinyl Alcohol) Hydrogel Beads Prepared by Combined Ca2+ Crosslinking and Freeze-Thawing Cycles for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, Á.A.A.; Passes, E.D.; De Brito Alves, S.; Silva, G.S.; Higa, O.Z.; Vítolo, M. Alginate-Poly(Vinyl Alcohol) Core-Shell Microspheres for Lipase Immobilization. J. Appl. Polym. Sci. 2006, 102, 1553–1560. [Google Scholar] [CrossRef]
- Voo, W.P.; Ooi, C.W.; Islam, A.; Tey, B.T.; Chan, E.S. Calcium Alginate Hydrogel Beads with High Stiffness and Extended Dissolution Behaviour. Eur. Polym. J. 2016, 75, 343–353. [Google Scholar] [CrossRef]
- Chen, K.N.; Fan, A.; Tan, C.S.; Reif, R. Bonding Parameters of Blanket Copper Wafer Bonding. J. Electron. Mater. 2006, 35, 230–234. [Google Scholar] [CrossRef]
- Ailincai, D.; Gavril, G.; Marin, L. Polyvinyl Alcohol Boric Acid—A Promising Tool for the Development of Sustained Release Drug Delivery Systems. Mater. Sci. Eng. C 2020, 107, 110316. [Google Scholar] [CrossRef] [PubMed]
- Ciriza, J.; Rodríguez-Romano, A.; Nogueroles, I.; Gallego-Ferrer, G.; Cabezuelo, R.M.; Pedraz, J.L.; Rico, P. Borax-Loaded Injectable Alginate Hydrogels Promote Muscle Regeneration in Vivo after an Injury. Mater. Sci. Eng. C 2021, 123, 112003. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, J.; Xia, Y. Effect of SiO2, PVA and Glycerol Concentrations on Chemical and Mechanical Properties of Alginate-Based Films. Int. J. Biol. Macromol. 2018, 107, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.T.B.; Pereira, P.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. Development of Light-Degradable Poly(Urethane-Urea) Hydrogel Films. Mater. Sci. Eng. C 2021, 131, 112520. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene Oxide Reinforced Alginate/PVA Double Network Hydrogels for Efficient Dye Removal. Polymers 2018, 10, 835. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Wattanakornsiri, A.; Wachirawongsakorn, P.; Kaewpirom, S. Effects of Crosslinking Degree of Poly(Vinyl Alcohol) Hydrogel in Aqueous Solution: Kinetics and Mechanism of Copper(II) Adsorption. Polym. Bull. 2014, 71, 1081–1100. [Google Scholar] [CrossRef]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of Hydrogel Composites Using Ca2+ and Cu2+ Ions as Crosslinking Agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, M. Design and in Vitro Investigation of Nanocomposite Hydrogel Based in Situ Spray Dressing for Chronic Wounds and Synthesis of Silver Nanoparticles Using Green Chemistry. J. Appl. Polym. Sci. 2016, 133, 43260. [Google Scholar] [CrossRef]
- Kumari, P.; Khatkar, B.S. Nutritional Composition and Drying Kinetics of Aonla Fruits. J. Food Sci. Technol. 2018, 55, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.S.C.; Poh, P.E.; Pasbakhsh, P.; Tey, B.T.; Yeoh, H.K.; Chan, E.S. Physicochemical Characterization of Halloysite/Alginate Bionanocomposite Hydrogel. Appl. Clay Sci. 2014, 101, 444–454. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Skrodzka, M.; Sicińska, H.; Michalak, I.; Detyna, J. Influence of Cross-Linking Conditions on Drying Kinetics of Alginate Hydrogel. Gels 2023, 9, 63. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.J.; Liu, R.H.; You, L.J. Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharides from Prunella Vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef]
- Dartois, E.; Schmitt, B. Carbon Dioxide Clathrate Hydrate FTIR Spectrum. Astron. Astrophys. 2009, 504, 869–873. [Google Scholar] [CrossRef][Green Version]
- Kac, M.; Âkova, A.; Capek, P.; Sasinkova, Â.A.V.; Wellner, N.; Ebringerova, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar]
- Ahmad, J.U.; Räisänen, M.T.; Leskelä, M.; Repo, T. Copper Catalyzed Oxidation of Benzylic Alcohols in Water with H2O2. Appl. Catal. A Gen. 2012, 411–412, 180–187. [Google Scholar] [CrossRef]
- Zhang, J.; Kamdem, D.P. FTIR Characterization of Copper Ethanolamine—Wood Interaction for Wood Preservation. Holzforschung 2000, 54, 119–122. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J. Alginate Composite Hydrogel Bead with Multilayer Flake Structure for Dye Adsorptions. J. Renew. Mater. 2019, 7, 983–996. [Google Scholar] [CrossRef]
- Gholamali, I.; Asnaashariisfahani, M.; Alipour, E. Silver Nanoparticles Incorporated in PH-Sensitive Nanocomposite Hydrogels Based on Carboxymethyl Chitosan-Poly (Vinyl Alcohol) for Use in a Drug Delivery System. Regen. Eng. Transl. Med. 2020, 6, 138–153. [Google Scholar] [CrossRef]
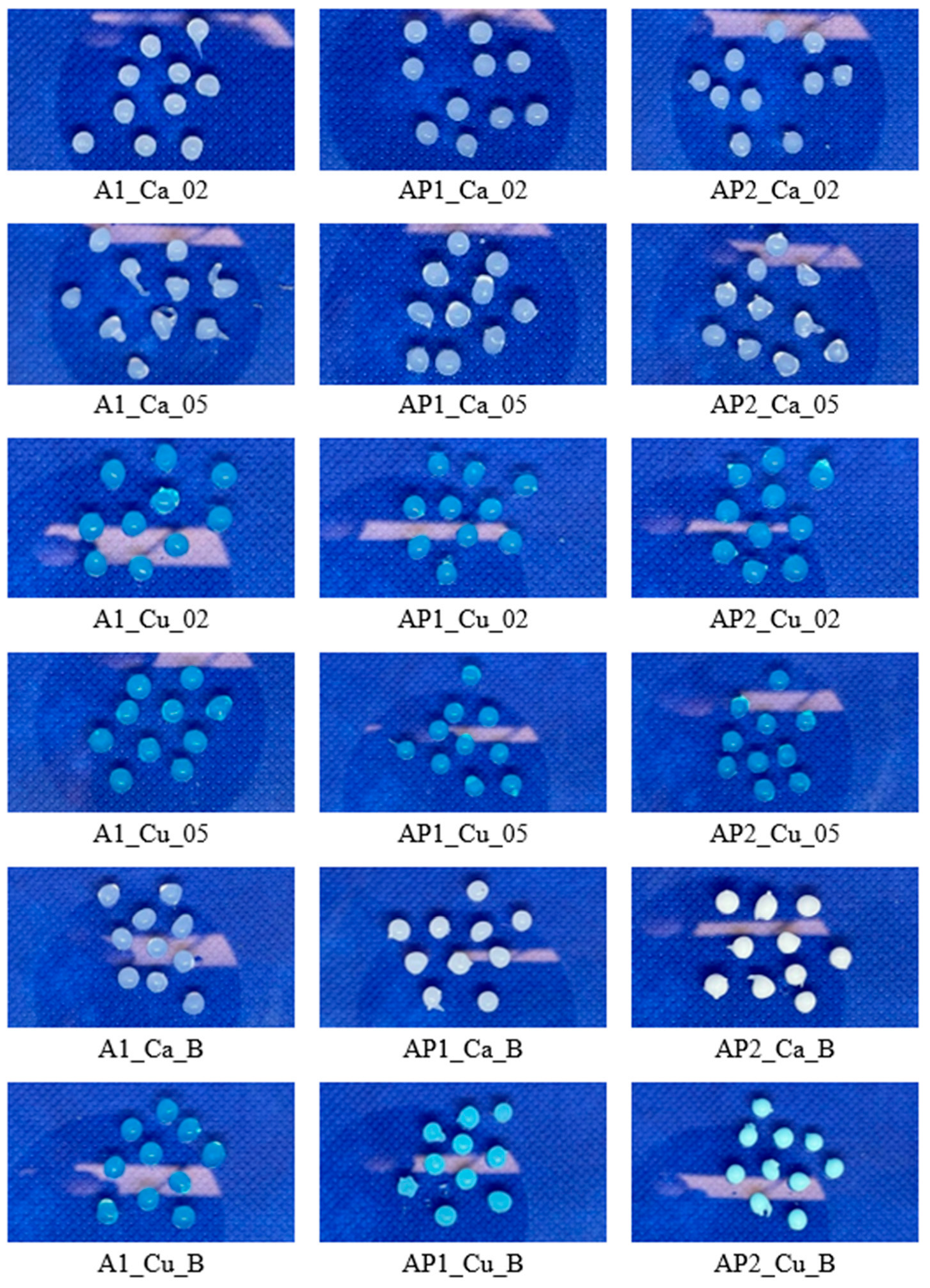
| Solution | SA (%) | PVA1 (%) | PVA2 (%) |
|---|---|---|---|
| A1 | 3.2 | 0 | 0 |
| AP1 | 3.2 | 0.4 | 0 |
| AP2 | 3.2 | 0 | 2 |
| Crosslinking Solution | ||||||
|---|---|---|---|---|---|---|
| Solution | CaCl2 (0.2 M) | CaCl2 (0.5 M) | CuSO4 (0.2 M) | CuSO4 (0.5 M) | CaCl2 (0.2 M) + H3BO3 | CuSO4 (0.2 M) + H3BO3 |
| A1 | A1_Ca_02 | A1_Ca_05 | A1_Cu_02 | A1_Cu_05 | A1_Ca_B | A1_Cu_B |
| AP1 | AP1_Ca_02 | AP1_Ca_05 | AP1_Cu_02 | AP1_Cu_05 | AP1_Ca_B | AP1_Cu_B |
| AP2 | AP2_Ca_02 | AP2_Ca_05 | AP2_Cu_02 | AP2_Cu_05 | AP2_Ca_B | AP2_Cu_B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niewiadomski, K.; Szopa, D.; Pstrowska, K.; Wróbel, P.; Witek-Krowiak, A. Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels. Materials 2024, 17, 1816. https://doi.org/10.3390/ma17081816
Niewiadomski K, Szopa D, Pstrowska K, Wróbel P, Witek-Krowiak A. Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels. Materials. 2024; 17(8):1816. https://doi.org/10.3390/ma17081816
Chicago/Turabian StyleNiewiadomski, Konrad, Daniel Szopa, Katarzyna Pstrowska, Paulina Wróbel, and Anna Witek-Krowiak. 2024. "Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels" Materials 17, no. 8: 1816. https://doi.org/10.3390/ma17081816
APA StyleNiewiadomski, K., Szopa, D., Pstrowska, K., Wróbel, P., & Witek-Krowiak, A. (2024). Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels. Materials, 17(8), 1816. https://doi.org/10.3390/ma17081816








