Corrosion Induced on Aluminum by Biodiesel Components in Non-Oxygen Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mass Loss Assays
2.3. Surface Characterization
2.4. Characterization of Chemical Modifications
2.5. Electrochemical Assays
3. Results and Discussion
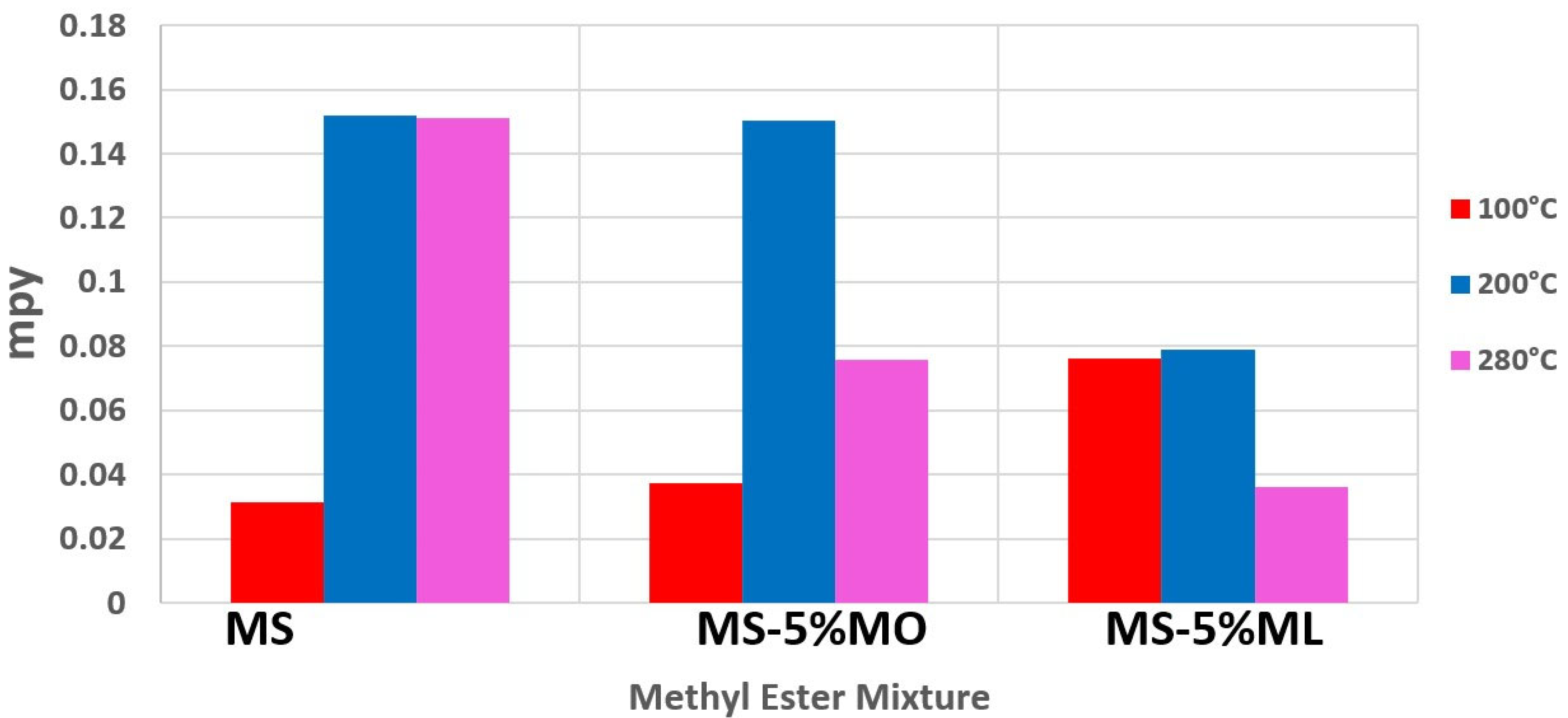
3.1. Mass Loss Measurements
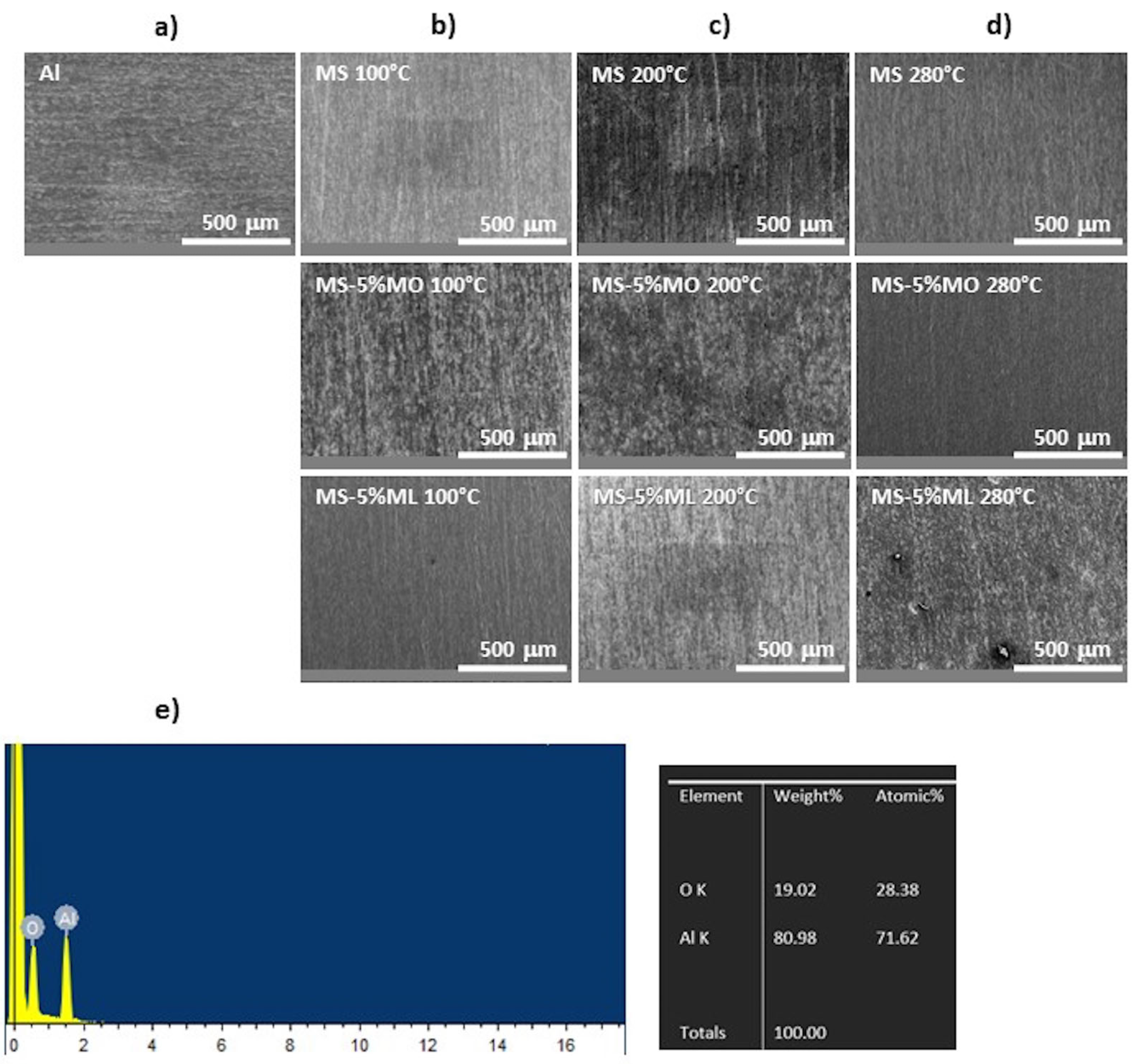
3.2. Surface Morphology
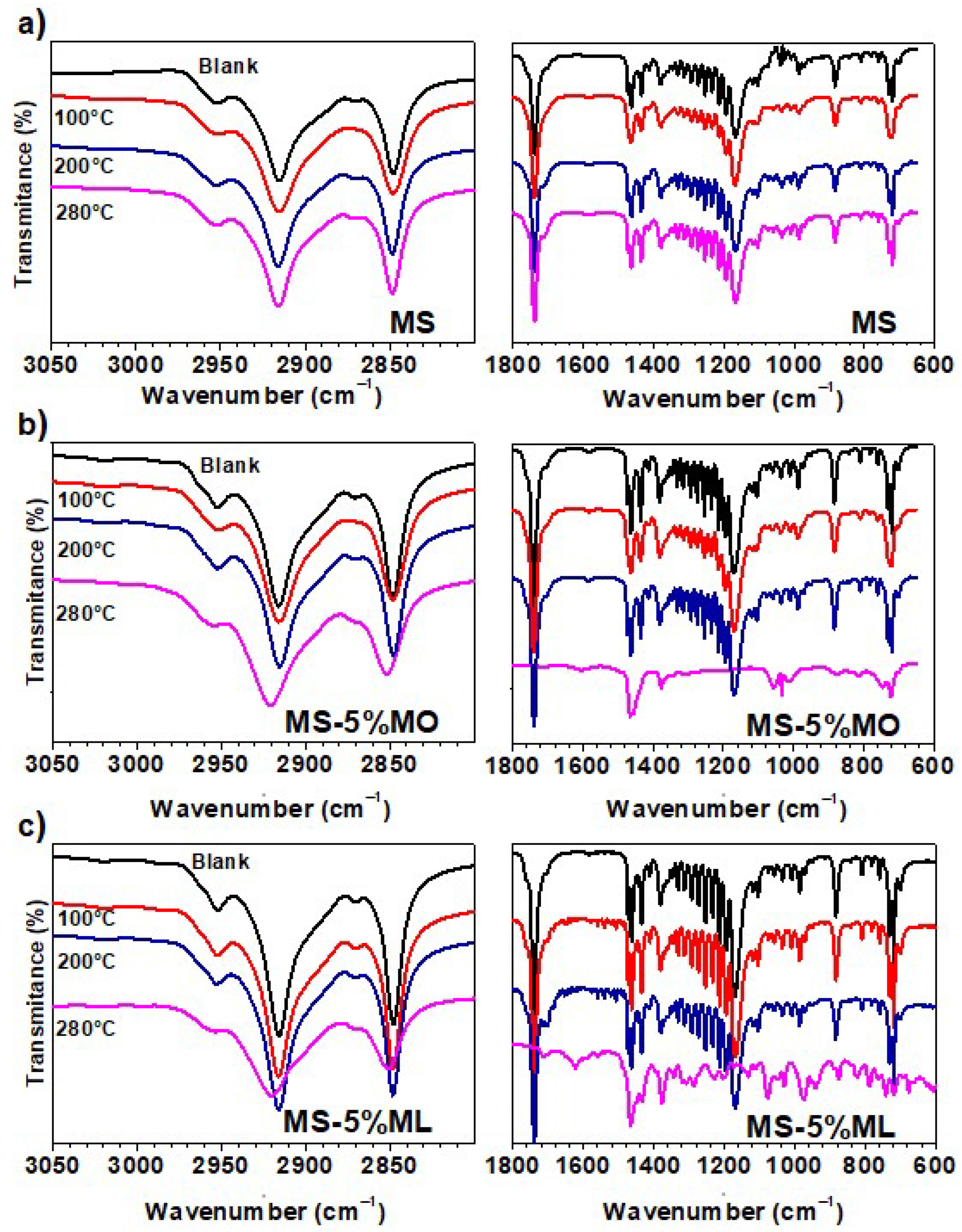
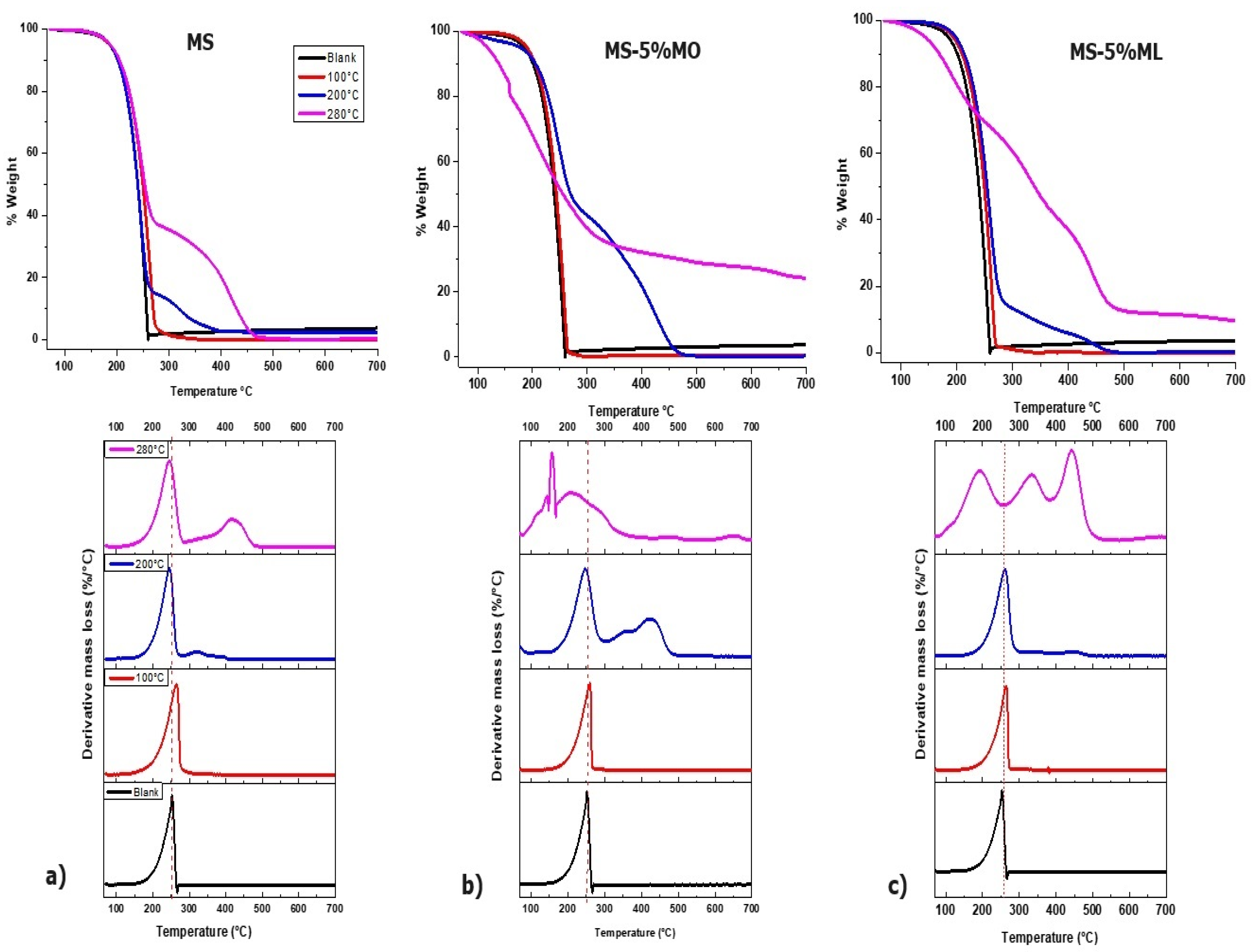
3.3. Characterization of Chemical Changes
3.4. Potentiodynamic Polarization
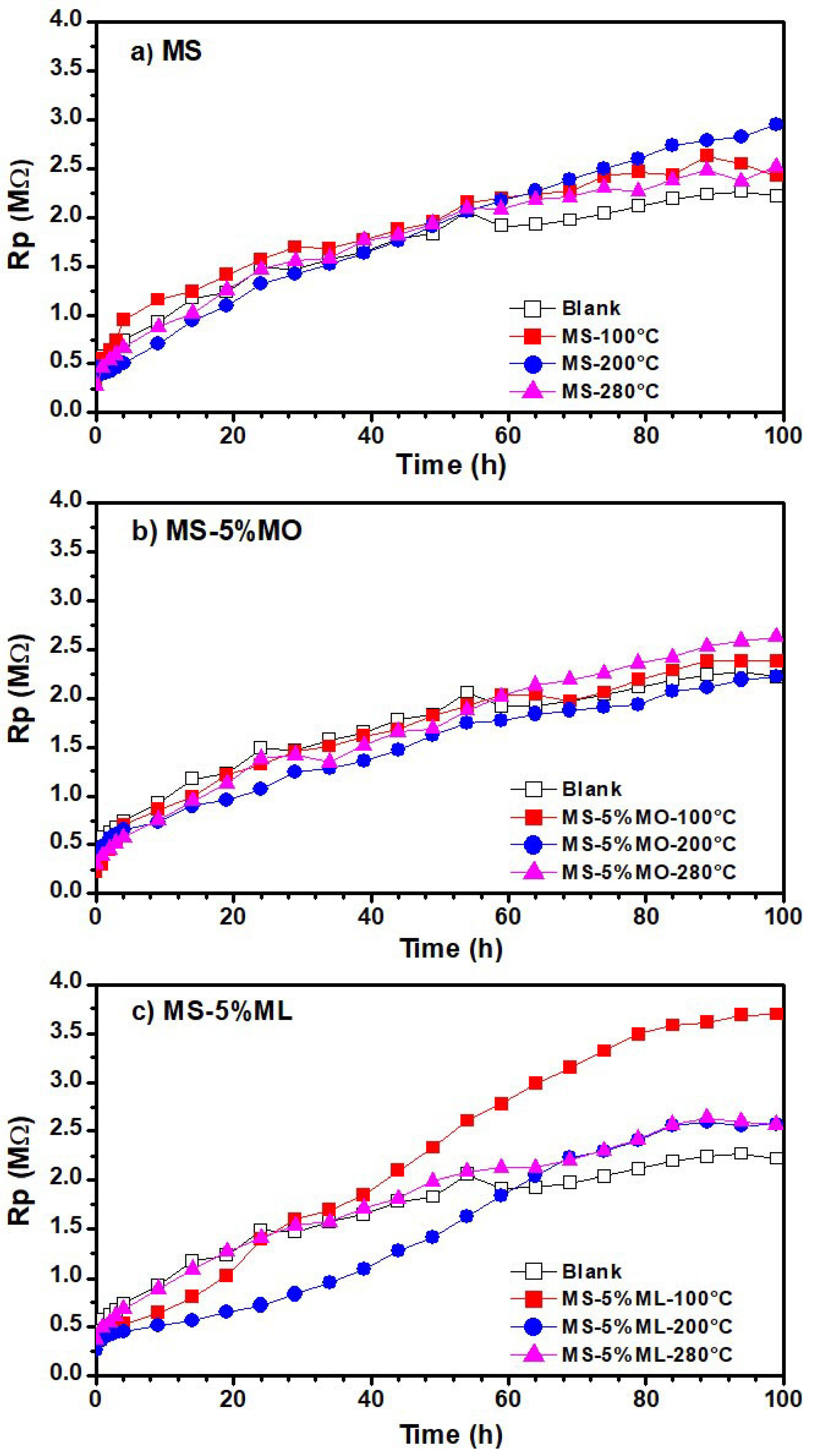
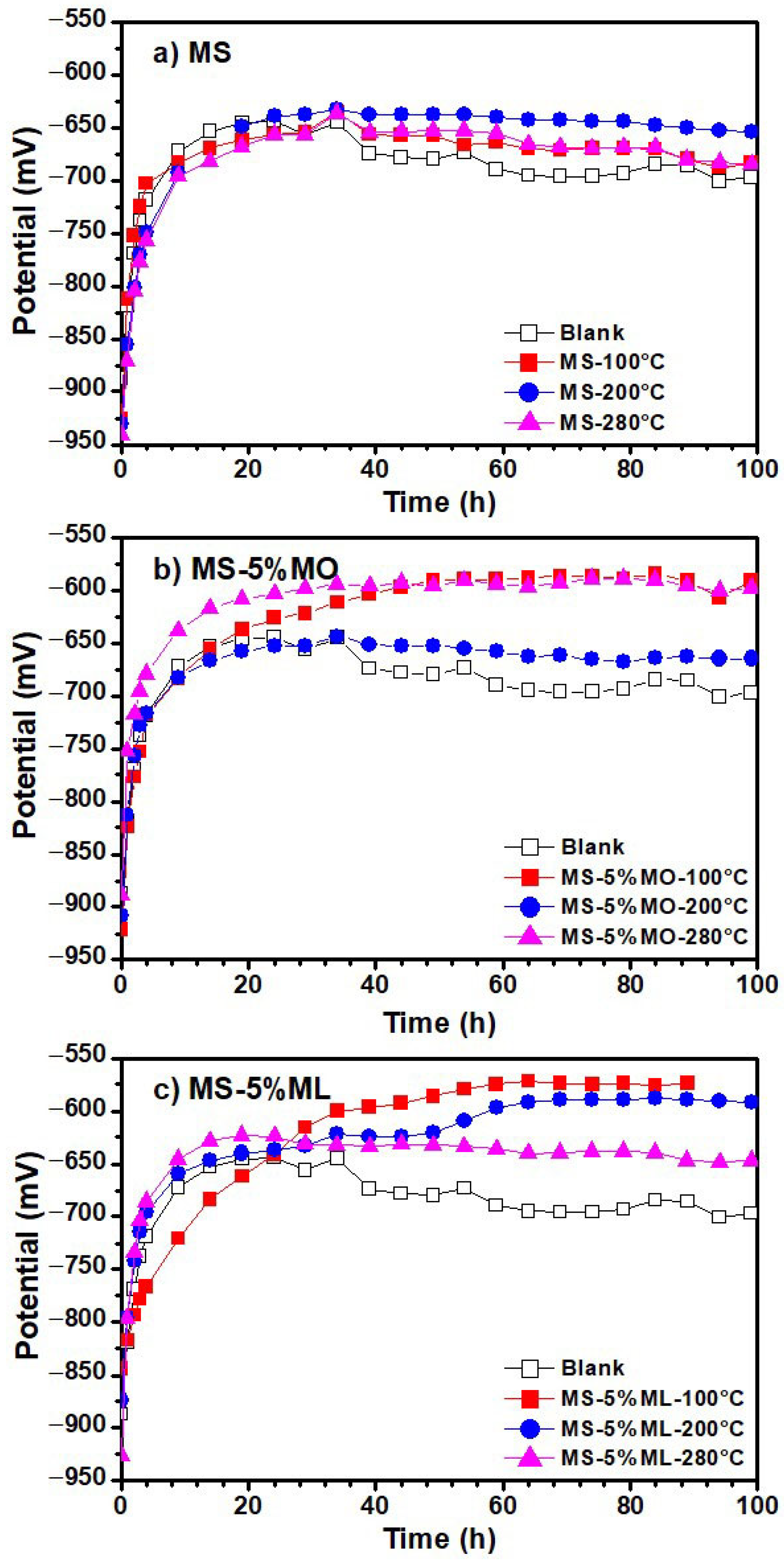
3.5. Linear Polarization Resistance (LPR) Measurements
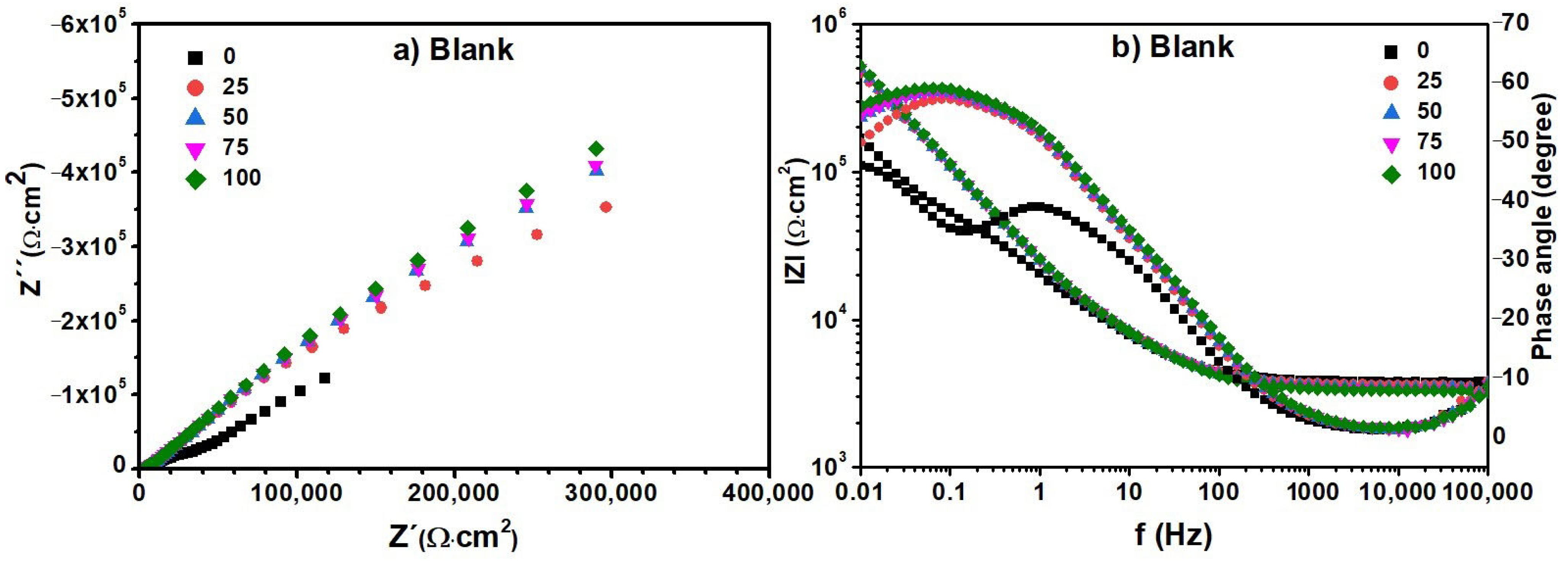
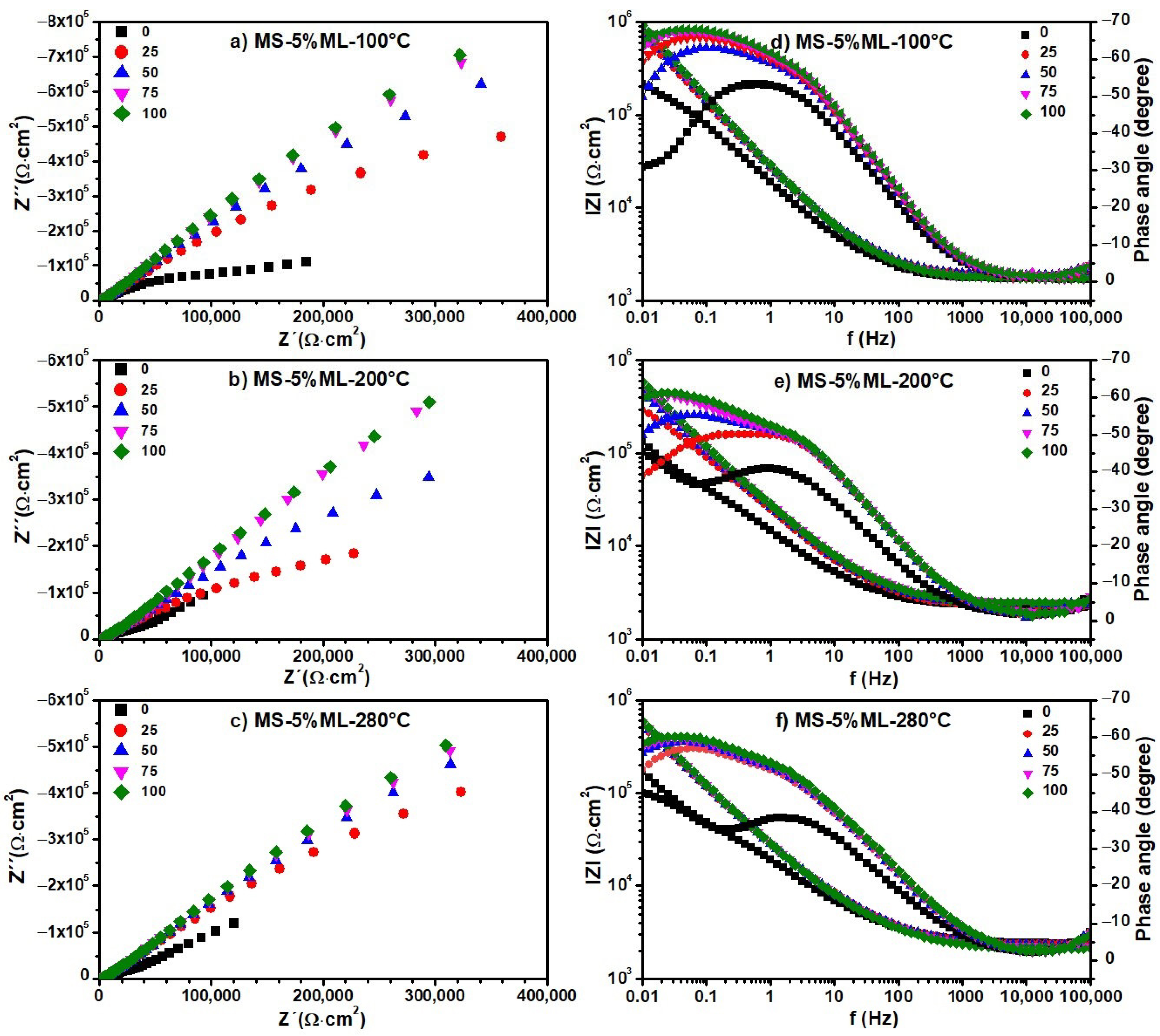
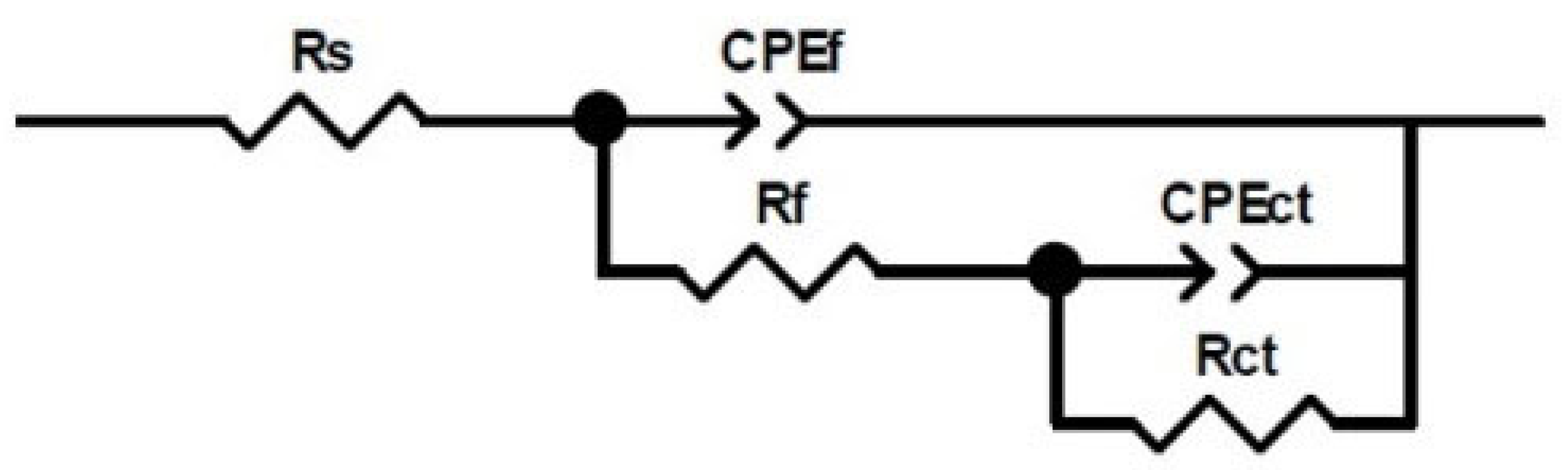
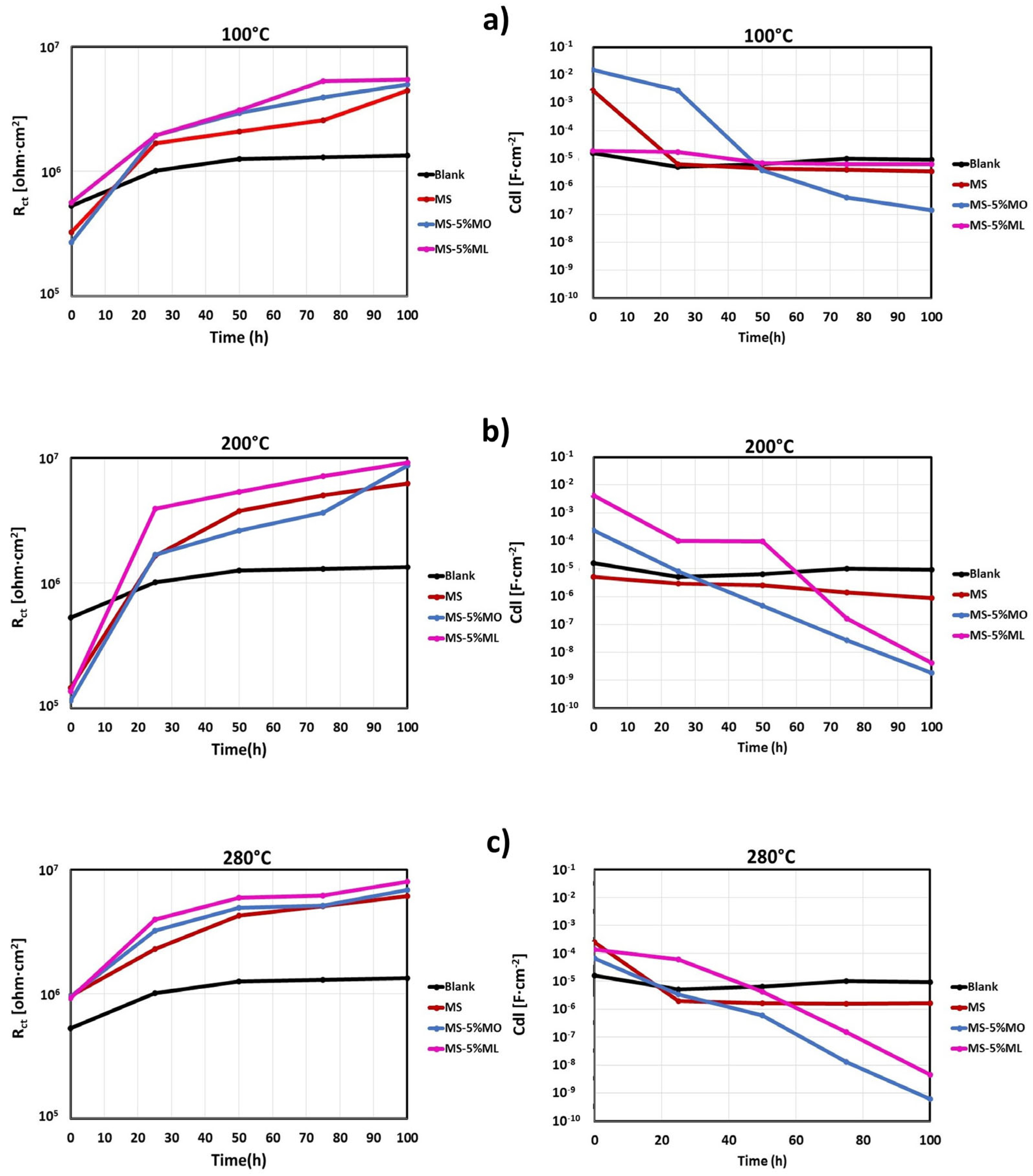
3.6. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rubaiee, S.; Al-Zahrani, A. Overview of the interactions between automotive materials and biodiesel obtained from different feedstocks. Fuel Process. Technol. 2019, 196, 106178. [Google Scholar] [CrossRef]
- Amelio, A.; Van de Voorde, T.; Creemers, C.; Degrève, J.; Darvishmanesh, S.; Luis, P.; Van der Bruggen, B. Comparison between exergy and energy analysis for biodiesel production. Energy 2016, 98, 135–145. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Oxidation, thermal, and storage stability studies of Jatropha curcas biodiesel. Int. Sch. Res. Not. 2012, 2012, 861293. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines–A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Fazal, M.A.; Jakeria, M.R.; Haseeb, A.; Rubaiee, S. Effect of antioxidants on the stability and corrosiveness of palm biodiesel upon exposure of different metals. Energy 2017, 135, 220–226. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.; Masjuki, H.H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Domingos, A.K.; Saad, E.B.; Vechiatto, W.W.D.; Wilhelm, H.M.; Ramos, L.P. The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J. Braz. Chem. Soc. 2007, 18, 416–423. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process. Technol. 2014, 125, 223–235. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Stability of biodiesel and its blends: A review. Renew. Sustain. Energy Rev. 2010, 14, 667–678. [Google Scholar] [CrossRef]
- Imahara, H.; Minami, E.; Hari, S.; Saka, S. Thermal stability of biodiesel in supercritical methanol. Fuel 2008, 87, 1–6. [Google Scholar] [CrossRef]
- Haseeb, A.; Fazal, M.A.; Jahirul, M.I.; Masjuki, H.H. Compatibility of automotive materials in biodiesel: A review. Fuel 2011, 90, 922–931. [Google Scholar] [CrossRef]
- Akhabue, C.E.; Aisien, F.A.; Ojo, C.O. The effect of Jatropha oil biodiesel on the corrosion rates of aluminium and mild carbon steel. Biofuels 2014, 5, 545–550. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.; Masjuki, H.H. Degradation of automotive materials in palm biodiesel. Energy 2012, 40, 76–83. [Google Scholar] [CrossRef]
- Cursaru, D.-L.; Brănoiu, G.; Ramadan, I.; Miculescu, F. Degradation of automotive materials upon exposure to sunflower biodiesel. Ind. Crop. Prod. 2014, 54, 149–158. [Google Scholar] [CrossRef]
- Samuel, O.D.; Gulum, M. Mechanical and corrosion properties of brass exposed to waste sunflower oil biodiesel-diesel fuel blends. Chem. Eng. Commun. 2019, 206, 682–694. [Google Scholar] [CrossRef]
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Haseeb, A.; Masjuki, H.H.; Ann, L.J.; Fazal, M.A. Corrosion characteristics of copper and leaded bronze in palm biodiesel. Fuel Process. Technol. 2010, 91, 329–334. [Google Scholar] [CrossRef]
- Román, A.S.; Barrientos, M.S.; Noceras, M.Á.; Méndez, C.M.; Ares, A.E. Resistance to corrosion of Al-Cu alloy in biodiesel. Matéria 2018, 23, e12052. [Google Scholar]
- Rocabruno-Valdés, C.I.; Hernández, J.A.; Juantorena, A.U.; Arenas, E.G.; Lopez-Sesenes, R.; Salinas-Bravo, V.M.; González-Rodriguez, J.G. An electrochemical study of the corrosion behaviour of metals in canola biodiesel. Corros. Eng. Sci. Technol. 2018, 53, 153–162. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.; Masjuki, H.H. Effect of temperature on the corrosion behavior of mild steel upon exposure to palm biodiesel. Energy 2011, 36, 3328–3334. [Google Scholar] [CrossRef]
- Jin, D.; Zhou, X.; Wu, P.; Jiang, L.; Ge, H. Corrosion behavior of ASTM 1045 mild steel in palm biodiesel. Renew. Energy 2015, 81, 457–463. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Chandran, D.; Gan, S.; Lau, H.L.N.; Raviadaran, R.; Salim, M.; Khalid, M. Critical relationship between biodiesel fuel properties and degradation of fuel delivery materials of a diesel engine. Therm. Sci. Eng. Prog. 2018, 7, 20–26. [Google Scholar] [CrossRef]
- Díaz-Ballote, L.; López-Sansores, J.F.; Maldonado-López, L.; Garfias-Mesias, L.F. Corrosion behavior of aluminum exposed to a biodiesel. Electrochem. Commun. 2009, 11, 41–44. [Google Scholar] [CrossRef]
- Yeşilyurt, M.K.; Öner, İ.V.; Yilmaz, E.C. Biodiesel induced corrosion and degradation. Pamukkale Üniversitesi Mühendislik Bilim. Derg. 2019, 25, 60–70. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Vu, H.N. Corrosion of the metal parts of diesel engines in biodiesel-based fuels. Int. J. Renew. Energy Dev. 2019, 8, 119. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A review of the effect of biodiesel on the corrosion behavior of metals/alloys in diesel engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2923–2943. [Google Scholar] [CrossRef]
- Singh, B.; Korstad, J.; Sharma, Y.C. A critical review on corrosion of compression ignition (CI) engine parts by biodiesel and biodiesel blends and its inhibition. Renew. Sustain. Energy Rev. 2012, 16, 3401–3408. [Google Scholar] [CrossRef]
- ASTM G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2021.
- Kulkarni, A.S.; Nemade, H.B.; Swaminathan, R. Replacement of conventional reference electrode with platinum electrode for electronic tongue based analysis of dairy products. Results Chem. 2023, 6, 101185. [Google Scholar] [CrossRef]
- Zhang, J.K.; Zhao, D.; Yang, H.; Li, C.; Xu, M.; Wu, D.P. Stability study of Pt as reference electrode for ISFETs operating in reference electrolyte. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012042. [Google Scholar] [CrossRef]
- Zuñiga-Díaz, J.; Reyes-Dorantes, E.; Quinto-Hernandez, A.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Biodiesel from “Morelos” Rice: Synthesis, Oxidative Stability, and Corrosivity. J. Chem. 2018, 2018, 4595130. [Google Scholar] [CrossRef]
- Kasem, B.K.K.; Jones, S. Platinum as a reference electrode in electrochemical measurements. Platin. Met. Rev. 2008, 52, 100–106. [Google Scholar] [CrossRef]
- Lou, X.; Yang, D.; Singh, P.M. Film breakdown and anodic dissolution during stress corrosion cracking of carbon steel in bioethanol. J. Electrochem. Soc. 2009, 157, C86. [Google Scholar] [CrossRef]
- Nie, X.; Li, X.; Northwood, D.O. Corrosion Behavior of metallic materials in ethanol-gasoline alternative fuels. Mater. Sci. Forum 2007, 546–549, 1093–1100. [Google Scholar] [CrossRef]
- Baena, L.M.; Vásquez, F.A.; Calderón, J.A. Corrosion assessment of metals in bioethanol-gasoline blends using electrochemical impedance spectroscopy. Heliyon 2021, 7, e07585. [Google Scholar] [CrossRef]
- Baena, L.M.; Gómez, M.; Calderón, J.A. Aggressiveness of a 20% bioethanol–80% gasoline mixture on autoparts: I behavior of metallic materials and evaluation of their electrochemical properties. Fuel 2012, 95, 320–328. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Rocha, J.G.; dos Santos, M.D.R.; Madeira, F.B.; Rocha, S.F.L.S.; Bauerfeldt, G.F.; da Silva, W.L.G.; Salomão, A.A.; Tubino, M. Influence of fatty acid methyl ester composition, acid value, and water content on metallic copper corrosion caused by biodiesel. J. Braz. Chem. Soc. 2019, 30, 1751–1761. [Google Scholar] [CrossRef]
- Pillar, R.; Ginic-Markovic, M.; Clarke, S.; Matisons, J. Effect of alkyl chain unsaturation on methyl ester thermo-oxidative decomposition and residue formation. J. Am. Oil Chem. Soc. 2009, 86, 363–373. [Google Scholar] [CrossRef]
- Conceição, M.M.; Fernandes, V.J.; Araújo, A.S.; Farias, M.F.; Santos, I.M.G.; Souza, A.G. Thermal and oxidative degradation of castor oil biodiesel. Energy Fuels 2007, 21, 1522–1527. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Strakšys, A.; Bražinskienė, D.; Mačiulytė, S. Thermal degradation behavior of thin films of fatty acid methyl ester. Chem. Pap. 2021, 75, 2695–2701. [Google Scholar] [CrossRef]
- Sazzad, B.S.; Fazal, M.A.; Haseeb, A.; Masjuki, H.H. Retardation of oxidation and material degradation in biodiesel: A review. RSC Adv. 2016, 6, 60244–60263. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Gong, Y.; Liu, X. Analysis of the oxidative degradation of biodiesel blends using FTIR, UV–Vis, TGA and TD-DES methods. Fuel 2017, 202, 23–28. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lim, S.-M.; Bae, S.-Y.; Oh, S.C. Thermal decomposition and stability of fatty acid methyl esters in supercritical methanol. J. Anal. Appl. Pyrolysis 2011, 92, 332–338. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.; Masjuki, H.H. Corrosion mechanism of copper in palm biodiesel. Corros. Sci. 2013, 67, 50–59. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z. EIS and potentiodynamic polarization studies of arc-sprayed aluminum coating on Q235 steel surface. Int. J. Electrochem. Sci. 2023, 18, 100058. [Google Scholar] [CrossRef]
- Calderón, J.P.; Reyes Barragán, J.L.; Barraza Fierro, J.I.; Cruz Mejía, H.; Arrieta González, C.D.; Ravelero Vázquez, V.; Sánchez, K.P.; Torres-Mancera, M.T.; Retes-Mantilla, R.F.; Rodríguez-Díaz, R.A. Corrosion behavior of Al modified with Zn in chloride solution. Materials 2022, 15, 4229. [Google Scholar] [CrossRef]
- Wang, D.; Wu, M.; Ming, J.; Shi, J. Inhibitive effect of sodium molybdate on corrosion behaviour of AA6061 aluminium alloy in simulated concrete pore solutions. Constr. Build. Mater. 2021, 270, 121463. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, H.; Ma, H.; Zhang, J.; Da, B.; Zhu, H. Rebar corrosion in coral aggregate concrete: Determination of chloride threshold by LPR. Corros. Sci. 2020, 163, 108238. [Google Scholar] [CrossRef]
- Deyab, M.A.; Corrêa, R.G.C.; Mazzetto, S.E.; Dhmees, A.S.; Mele, G. Improving the sustainability of biodiesel by controlling the corrosive effects of soybean biodiesel on aluminum alloy 5052 H32 via cardanol. Ind. Crop. Prod. 2019, 130, 146–150. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Taheri Shoja, S.; Heydari Zebardast, B.; Mohamadian Samim, P. An electrochemical impedance spectroscopic study of the passive state on AISI 304 stainless steel. Int. J. Electrochem. 2011, 2011, 152143. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Chafki, L.; Galai, M.; Rifi, E.H.; Touir, R.; Touhami, M.E.; Hatim, Z.; Tazouti, A. Corrosion inhibition of mild steel in 1 M HCl Solution by hydroxyapatite. J. Chem. Pharm. Res. 2015, 7, 44–53. [Google Scholar]
- Li, Y.-d.; Zhang, Y.; Li, S.-m.; Zhao, P.-z. Influence of adipic acid on anodic film formation and corrosion resistance of 2024 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 492–500. [Google Scholar] [CrossRef]



| Methyl Stearate (MS) | ||||
|---|---|---|---|---|
| Temperature | No. | Product | No. | Product |
| 200 | 1 | Eicosanoic acid, methyl ester | 3 | Hexadecanoic acid, methyl ester |
| 2 | Octadecanoic acid, methyl ester | |||
| 280 | 2 | Octadecanoic acid, methyl ester | 5 | Decanedioic acid, dibutyl ester |
| 4 | Heptadecanoic acid, methyl ester | 6 | n-Heptadecane | |
| 5% Methyl Oleate in Methyl Stearate (MS-5% MO) | ||||
| 1 | Eicosanoic acid, methyl ester | 8 | Octadecanoic acid, octyl ester | |
| 200 | 7 | Nonadecanoic acid, methyl ester | 4 | Heptadecanoic acid, methyl ester |
| 2 | Octadecanoic acid, methyl ester | 3 | Hexadecanoic acid, methyl ester | |
| 280 | 9 | n-Hexatriacontane | 13 | n-Nonadecane |
| 10 | n-Pentatriacontane | 14 | n-Octadecane | |
| 11 | n-Heneicosane | 15 | Oxiraneoctanoic acid, 9-oxo methyl ester | |
| 12 | n-Eicosane | |||
| 5%Methyl Linoleate in Methyl Stearate (MS-5% ML) | ||||
| 200 | 1 | Eicosanoic acid, methyl ester | 3 | Hexadecanoic acid, methyl ester |
| 2 | Octadecanoic acid, methyl ester | 6 | n-Heptadecane | |
| 8 | Octadecanoic acid, octyl ester | 16 | Bis(2-ethylhexyl)phthalate | |
| 280 | 10 | n-Pentatriacontane | 18 | n-Pentadecane |
| 14 | n-Octadecane | 2 | Octadecanoic acid, methyl ester | |
| 6 | n-Heptadecane | 5 | Decanedioic, dibutyl ester | |
| 17 | n-Hexadecane | |||
| Methyl Ester Mixture | T (°C) | Ecorr (V) | icorr (µA/cm2) | βa (mV/Dec) | βc (mV/Dec) |
|---|---|---|---|---|---|
| Blank | −1.120 | 0.529 | 443.9 | 737.1 | |
| MS | 100 | −1.080 | 0.913 | 304.2 | 738.9 |
| 200 | −1.050 | 0.566 | 421.4 | 624.7 | |
| 280 | −1.130 | 0.472 | 285.3 | 397.5 | |
| MS-5% MO | 100 | −1.050 | 0.895 | 279.8 | 705.9 |
| 200 | −1.060 | 0.476 | 270.9 | 524.1 | |
| 280 | −0.989 | 0.185 | 196.7 | 376.4 | |
| MS-5% ML | 100 | −1.003 | 1.260 | 273.5 | 695.8 |
| 200 | −1.010 | 1.370 | 272.0 | 523.6 | |
| 280 | −1.100 | 0.544 | 292.0 | 457.7 |
| Methyl Ester Mixture | 100 (°C) | 200 (°C) | 280 (°C) | ||||
|---|---|---|---|---|---|---|---|
| Time (h) | Rct × 106 (Ω·cm2) | Cdl × 10−6 (F·cm2) | Rct × 106 (Ω·cm2) | Cdl × 10−6 (F·cm2) | Rct × 106 (Ω·cm2) | Cdl × 10−6 (F·cm2) | |
| Blank | 0 | 0.526 | 16.00 | ||||
| 25 | 1.010 | 5.110 | |||||
| 50 | 1.260 | 6.370 | |||||
| 75 | 1.290 | 9.970 | |||||
| 100 | 1.340 | 9.320 | |||||
| MS | 0 | 0.325 | 2890 | 0.145 | 5.010 | 0.959 | 245 |
| 25 | 1.680 | 6.260 | 1.640 | 2.980 | 2.290 | 2.010 | |
| 50 | 2.090 | 4.350 | 3.760 | 2.510 | 4.270 | 1.670 | |
| 75 | 2.550 | 4.140 | 5.000 | 1.390 | 5.090 | 1.600 | |
| 100 | 4.430 | 3.520 | 6.250 | 0.883 | 6.090 | 1.620 | |
| MS-5% MO | 0 | 0.271 | 15,600 | 0.115 | 240 | 0.951 | 67.20 |
| 25 | 1.950 | 2800 | 1.690 | 8.220 | 3.210 | 3.35 | |
| 50 | 2.940 | 3.810 | 2.630 | 0.471 | 4.890 | 0.588 | |
| 75 | 3.920 | 0.418 | 3.660 | 0.027 | 5.140 | 0.0127 | |
| 100 | 4.950 | 0.143 | 8.680 | 0.002 | 6.860 | 0.001 | |
| MS-5% ML | 0 | 0.561 | 19.400 | 0.137 | 4220 | 0.920 | 136.00 |
| 25 | 1.940 | 17.300 | 3.920 | 101.000 | 3.960 | 60.000 | |
| 50 | 3.082 | 7.050 | 5.320 | 94.500 | 5.950 | 4.350 | |
| 75 | 5.312 | 6.510 | 7.110 | 0.165 | 6.190 | 0.154 | |
| 100 | 5.442 | 6.500 | 9.090 | 0.004 | 7.980 | 0.005 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Juarez, F.; Porcayo-Calderon, J.; Perez-Orozco, J.P.; Acevedo-Quiroz, M.E.; Bustos-Terrones, V.; Quinto-Hernandez, A. Corrosion Induced on Aluminum by Biodiesel Components in Non-Oxygen Environments. Materials 2024, 17, 1821. https://doi.org/10.3390/ma17081821
Vergara-Juarez F, Porcayo-Calderon J, Perez-Orozco JP, Acevedo-Quiroz ME, Bustos-Terrones V, Quinto-Hernandez A. Corrosion Induced on Aluminum by Biodiesel Components in Non-Oxygen Environments. Materials. 2024; 17(8):1821. https://doi.org/10.3390/ma17081821
Chicago/Turabian StyleVergara-Juarez, Fabiola, Jesus Porcayo-Calderon, Juan Pablo Perez-Orozco, Macdiel Emilio Acevedo-Quiroz, Victoria Bustos-Terrones, and Alfredo Quinto-Hernandez. 2024. "Corrosion Induced on Aluminum by Biodiesel Components in Non-Oxygen Environments" Materials 17, no. 8: 1821. https://doi.org/10.3390/ma17081821
APA StyleVergara-Juarez, F., Porcayo-Calderon, J., Perez-Orozco, J. P., Acevedo-Quiroz, M. E., Bustos-Terrones, V., & Quinto-Hernandez, A. (2024). Corrosion Induced on Aluminum by Biodiesel Components in Non-Oxygen Environments. Materials, 17(8), 1821. https://doi.org/10.3390/ma17081821








