Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber
Abstract
1. Introduction
| Fiber Type | Strength (MPa) | Toughness (MJ/m3) | Strain (%) | Refs. |
|---|---|---|---|---|
| B. mori cocoon silk 1 | 300–740 | 70 | 70 | [13,14] |
| B. mori reeled silk 2 | 700 | 150 | 18 | [15] |
| L. hespeus 3 | 1400 ± 100 | 243 ± 29 | 30 | [16,17] |
| A. diadematus 4 | 1700 ± 200 | 225 ± 29 | 165 ± 15 | |
| N. edulis 5 | 1200 ± 200 | 215 ± 36 | / | [18,19] |
| Nylon | 950 | 80 | 18 | [20] |
| Kevlar | 3600 | 50 | 2.7 | |
| Carbon fiber | 4000 | 25 | 1.3 | |
| Polypropylene | 490 | / | 23 | [21] |
2. SF Composition
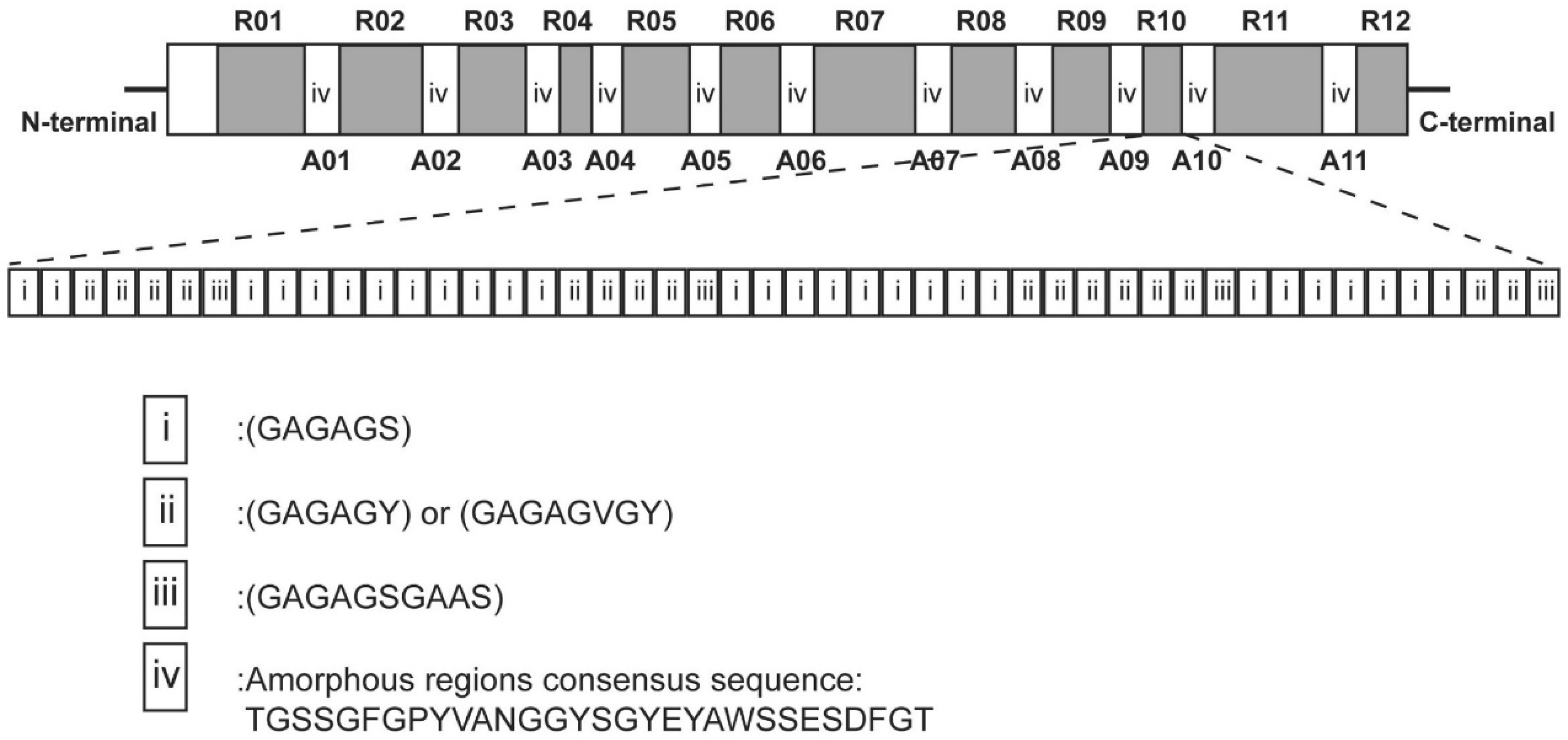
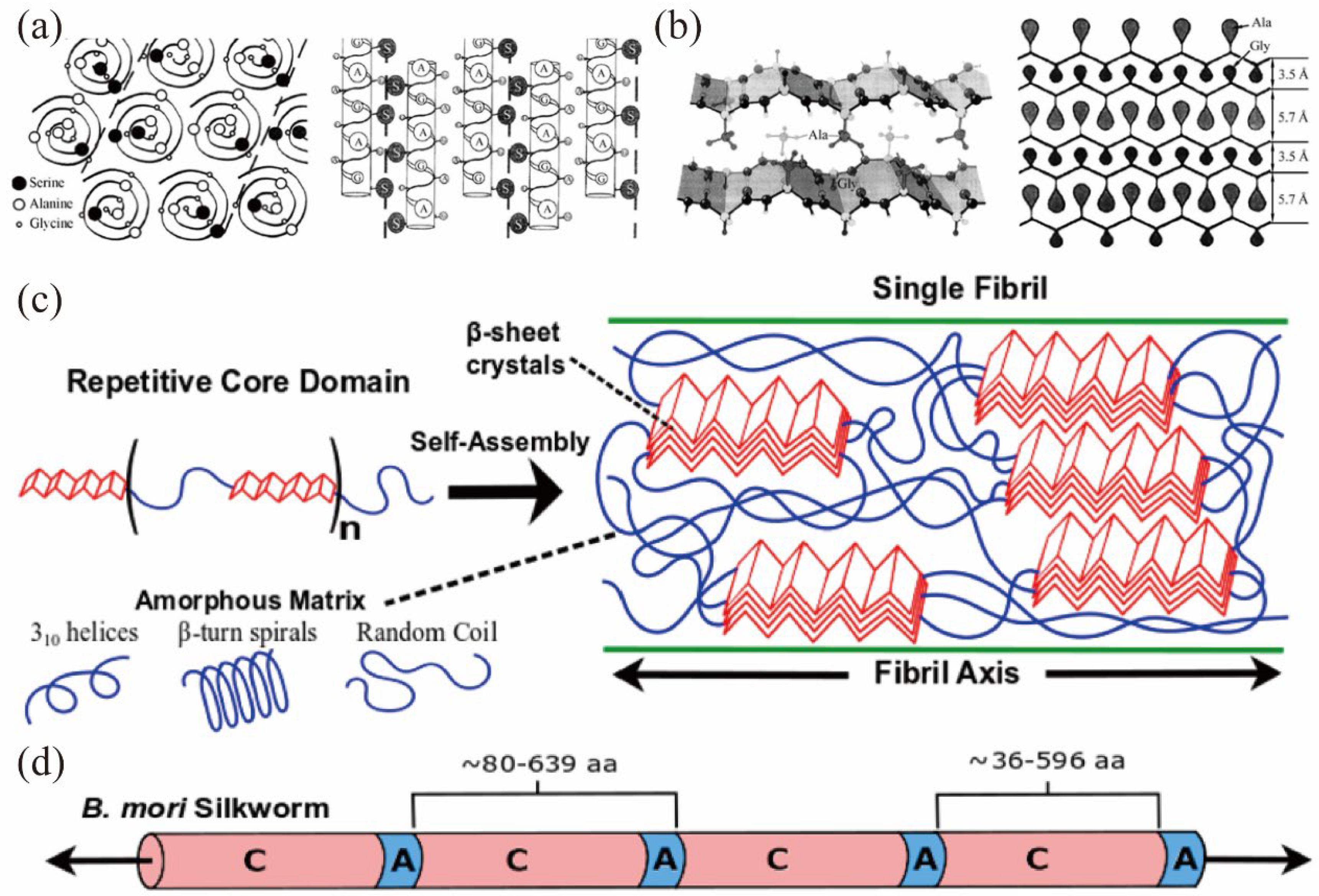
2.1. Primary Structure
2.2. Secondary Structure
2.3. Methods to Resolve SF’s Secondary Structure
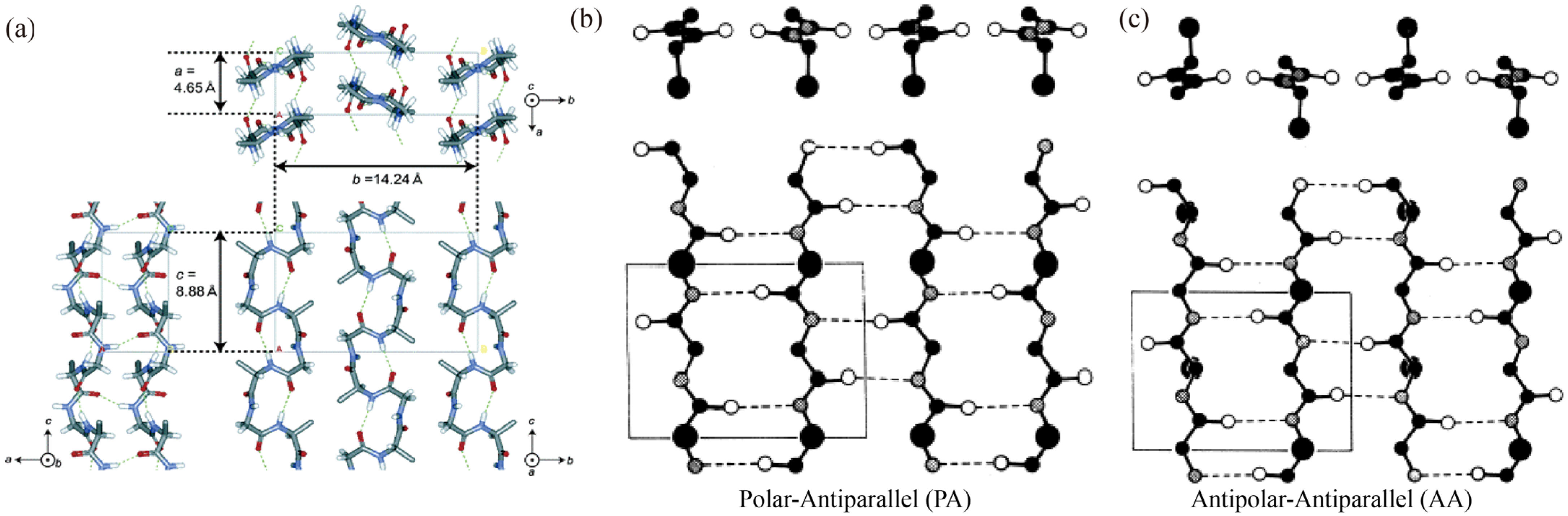
2.4. Spatial Structure
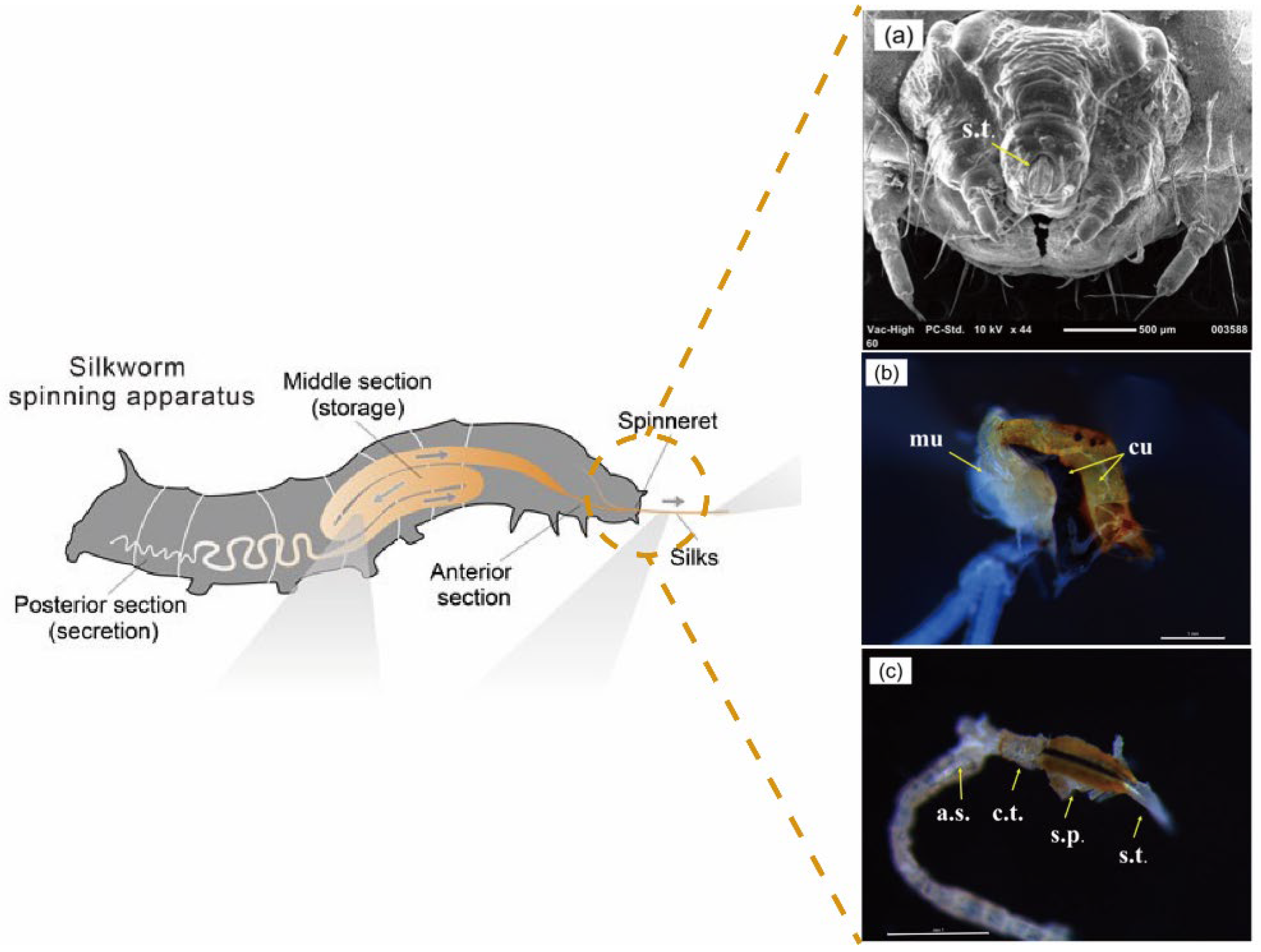
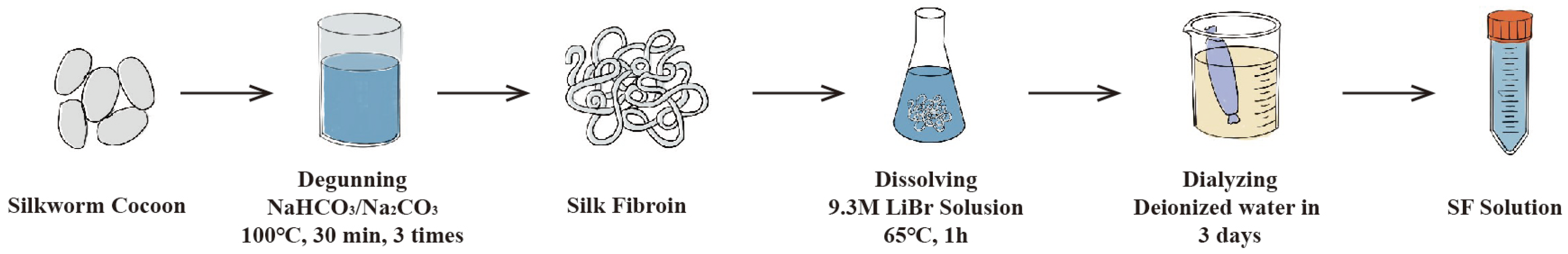
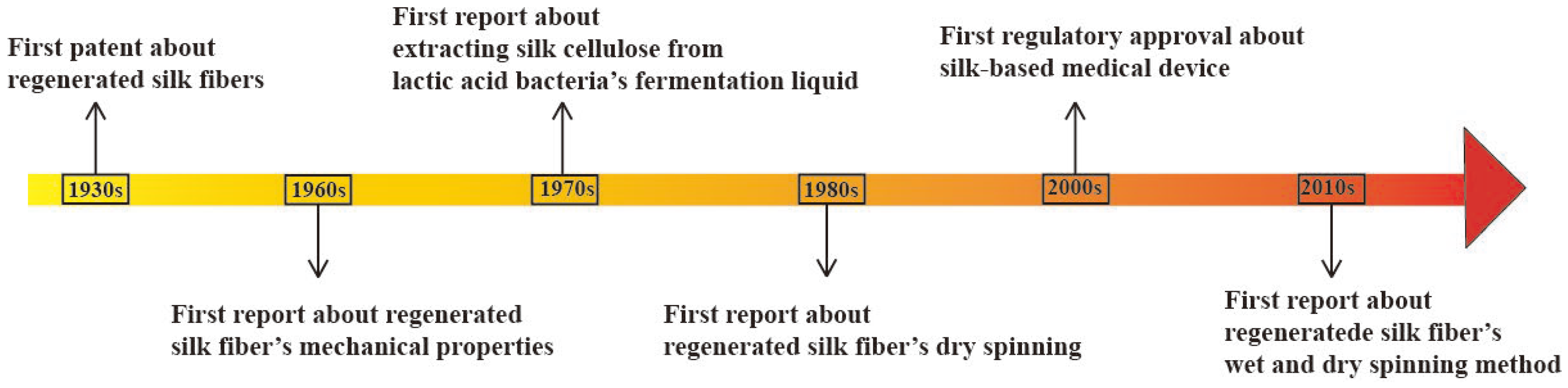
3. Eco-Spinning
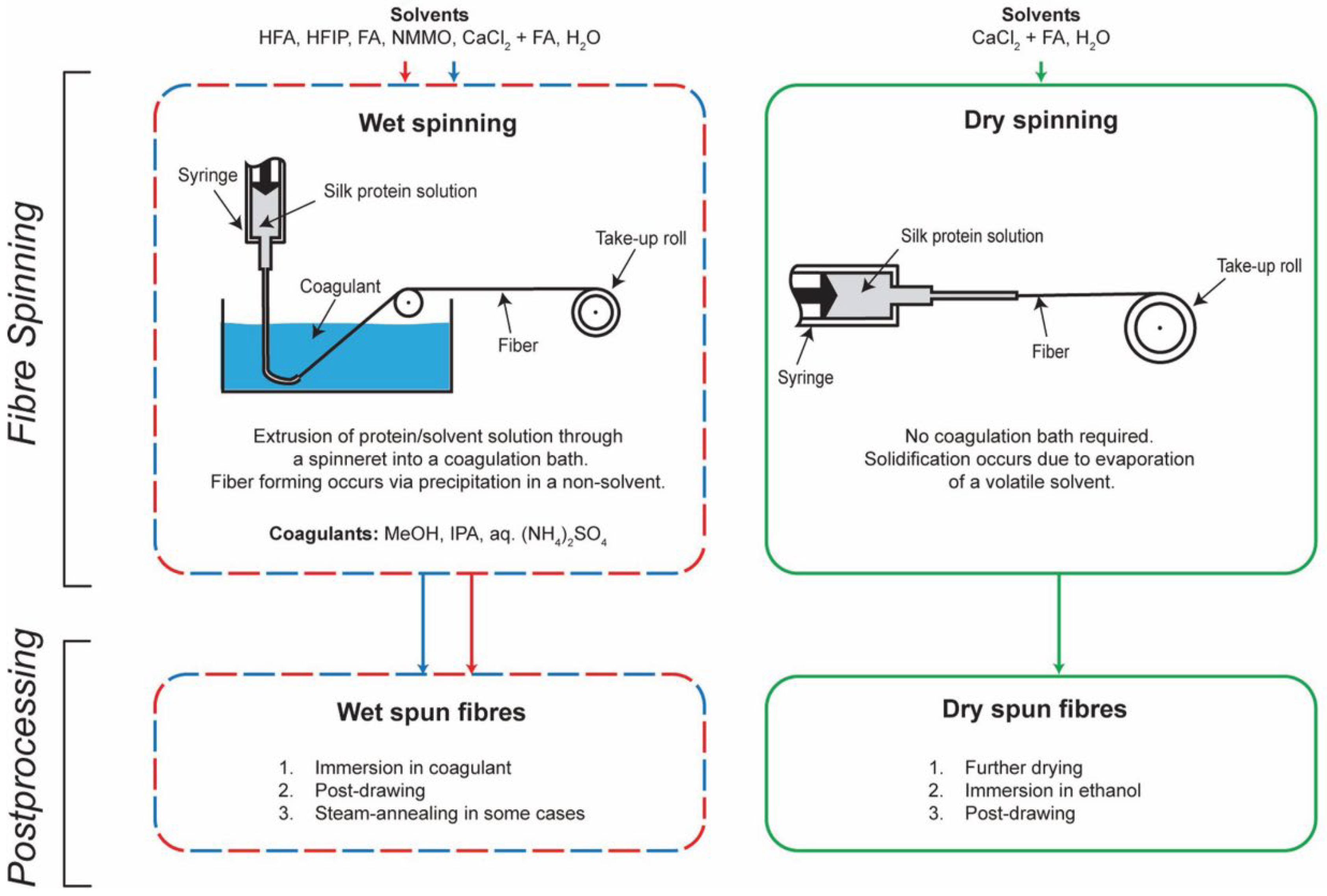
4. Bionic Spinning
4.1. Imitation of Solvents
4.2. Imitation of Behavior
4.3. Possibility of Liquid Crystal Spinning
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trossmann, V.T.; Scheibel, T. Design of Recombinant Spider Silk Proteins for Cell Type Specific Binding. Adv. Healthc. Mater. 2023, 12, 2202660. [Google Scholar] [CrossRef]
- Wang, Q.; McArdle, P.; Wang, S.L.; Wilmington, R.L.; Xing, Z.; Greenwood, A.; Cotten, M.L.; Qazilbash, M.M.; Schniepp, H.C. Protein Secondary Structure in Spider Silk Nanofibrils. Nat. Commun. 2022, 13, 4329. [Google Scholar] [CrossRef]
- Vendrely, C.; Scheibel, T. Biotechnological Production of Spider-Silk Proteins Enables New Applications. Macromol. Biosci. 2007, 7, 401–409. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Huang, J.; Khan, A.Q.; An, B.; Zhou, X.; Liu, Z.; Zhu, M. Spider Silk-Inspired Artificial Fibers. Adv. Sci. 2022, 9, 2103965. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Spider Silk as Archetypal Protein Elastomer. Soft Matter 2006, 2, 377–385. [Google Scholar] [CrossRef]
- Jokisch, S.; Scheibel, T. Spider Silk Foam Coating of Fabric. Pure Appl. Chem. 2017, 89, 1769–1776. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, L.; Day, B.A.; Harris, T.I.; Oliveira, P.; Knittel, C.; Licon, A.L.; Gong, C.; Dion, G.; Lewis, R.V.; et al. CRISPR/Cas9 Initiated Transgenic Silkworms as a Natural Spinner of Spider Silk. Biomacromolecules 2019, 20, 2252–2264. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Jiang, X.; Cao, M.; Wang, S.; Zou, H.; Cao, Y.; Xian, M.; Liu, H. Microbial Production of Amino Acid-Modified Spider Dragline Silk Protein with Intensively Improved Mechanical Properties. Prep. Biochem. Biotechnol. 2016, 46, 552–558. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhang, J.-P.; Chen, S.-L.; Qi, B.; Yao, X.-Y.; Zhang, X.-H.; Li, Y.-T.; Yang, Z.-H. Dry-Spinning of Artificial Spider Silk Ribbons from Regenerated Natural Spidroin in an Organic Medium. Macromol. Rapid Commun. 2023, 44, 2300024. [Google Scholar] [CrossRef]
- Wu, R.; Bae, J.; Jeon, H.; Kim, T. Spider-Inspired Regenerated Silk Fibroin Fiber Actuator via Microfluidic Spinning. Chem. Eng. J. 2022, 444, 136556. [Google Scholar] [CrossRef]
- Huemmerich, D.; Scheibel, T.; Vollrath, F.; Cohen, S.; Gat, U.; Ittah, S. Novel Assembly Properties of Recombinant Spider Dragline Silk Proteins. Curr. Biol. 2004, 14, 2070–2074. [Google Scholar] [CrossRef]
- Fahnestock, S.R.; Bedzyk, L.A. Production of Synthetic Spider Dragline Silk Protein in Pichia Pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. [Google Scholar] [CrossRef]
- Sirichaisit, J.; Brookes, V.L.; Young, R.J.; Vollrath, F. Analysis of Structure/Property Relationships in Silkworm (Bombyx mori) and Spider Dragline (Nephila edulis) Silks Using Raman Spectroscopy. Biomacromolecules 2003, 4, 387–394. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising Strength of Silkworm Silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The Mechanical Design of Spider Silks: From Fibroin Sequence to Mechanical Function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [CrossRef]
- Ko, F.; Jovicic, J. Modeling of Mechanical Properties and Structural Design of Spider Web. Biomacromolecules 2004, 5, 780–785. [Google Scholar] [CrossRef]
- Hermanson, K.D.; Huemmerich, D.; Scheibel, T.; Bausch, A.R. Engineered Microcapsules Fabricated from Reconstituted Spider Silk. Adv. Mater. 2007, 19, 1810–1815. [Google Scholar] [CrossRef]
- Cunniff, P.M.; Fossey, S.A.; Auerbach, M.A.; Song, J.W.; Kaplan, D.L.; Adams, W.W.; Eby, R.K.; Mahoney, D.; Vezie, D.L. Mechanical and Thermal Properties of Dragline Silk from the Spider Nephila Clavipes. Polym. Adv. Technol. 1994, 5, 401–410. [Google Scholar] [CrossRef]
- Knight, D.P.; Knight, M.M.; Vollrath, F. Beta Transition and Stress-Induced Phase Separation in the Spinning of Spider Dragline Silk. Int. J. Biol. Macromol. 2000, 27, 205–210. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The Elaborate Structure of Spider Silk. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef]
- Kumar, S.; Doshi, H.; Srinivasarao, M.; Park, J.O.; Schiraldi, D.A. Fibers from Polypropylene/Nano Carbon Fiber Composites. Polymer 2002, 43, 1701–1703. [Google Scholar] [CrossRef]
- Fedič, R.; Žurovec, M.; Sehnal, F. Correlation between Fibroin Amino Acid Sequence and Physical Silk Properties. J. Biol. Chem. 2003, 278, 35255–35264. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, N.; Kojima, K.; Iga, M.; Yukuhiro, F.; Iizuka, T.; Yoshioka, T. Fibroin Heavy Chain Gene Replacement with a Highly Ordered Synthetic Repeat Sequence in Bombyx mori. Insect Biochem. Mol. Biol. 2023, 161, 104002. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk Chemistry and Biomedical Material Designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Q.; Ou, Y.; Tang, Y.; Zeng, W.; Wang, H.; Hu, J.; Xu, H. New Insights into the Proteins Interacting with the Promoters of Silkworm Fibroin Genes. Sci. Rep. 2021, 11, 15880. [Google Scholar] [CrossRef]
- Indrakumar, S.; Joshi, A.; Dash, T.K.; Mishra, V.; Tandon, B.; Chatterjee, K. Photopolymerized Silk Fibroin Gel for Advanced Burn Wound Care. Int. J. Biol. Macromol. 2023, 233, 123569. [Google Scholar] [CrossRef]
- Kostag, M.; Jedvert, K.; El Seoud, O.A. Engineering of Sustainable Biomaterial Composites from Cellulose and Silk Fibroin: Fundamentals and Applications. Int. J. Biol. Macromol. 2021, 167, 687–718. [Google Scholar] [CrossRef]
- De Giorgio, G.; Matera, B.; Vurro, D.; Manfredi, E.; Galstyan, V.; Tarabella, G.; Ghezzi, B.; D’Angelo, P. Silk Fibroin Materials: Biomedical Applications and Perspectives. Bioengineering 2024, 11, 167. [Google Scholar] [CrossRef]
- Park, W.; Yoon, T.; Chang, H.; You, J.; Na, S. An Atomistic Scale Simulation Study of Structural Properties in the Silk–Fibrohexamerin Complex. Nanoscale 2024, 16, 821–832. [Google Scholar] [CrossRef]
- Fu, C.; Shao, Z.; Fritz, V. Animal Silks: Their Structures, Properties and Artificial Production. Chem. Commun. 2009, 43, 6515–6529. [Google Scholar] [CrossRef]
- Zhang, X.; Berghe, I.V.; Wyeth, P. Heat and Moisture Promoted Deterioration of Raw Silk Estimated by Amino Acid Analysis. J. Cult. Herit. 2011, 12, 408–411. [Google Scholar] [CrossRef]
- Hozumi, T. Properties of Amino Acid Composition of the Tryptic Fragments of the Heavy Chain of Myosin Subfragment-1. J. Biochem. 1981, 90, 785–788. [Google Scholar] [CrossRef]
- Ahmad, R.; Kamra, A.; Hasnain, S.E. Fibroin Silk Proteins from the Nonmulberry Silkworm Philosamia Ricini Are Biochemically and Immunochemically Distinct from Those of the Mulberry Silkworm Bombyx mori. DNA Cell Biol. 2004, 23, 149–154. [Google Scholar] [CrossRef]
- Sen, K.; Babu, K.M. Studies on Indian Silk. I. Macrocharacterization and Analysis of Amino Acid Composition. J. Appl. Polym. Sci. 2004, 92, 1080–1097. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F.; Yang, Y.; Thøgersen, H.C. Structure and Behavior of Regenerated Spider Silk. Macromolecules 2003, 36, 1157–1161. [Google Scholar] [CrossRef]
- Omenetto, F.G.; Kaplan, D.L. New Opportunities for an Ancient Material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef]
- Wöltje, M.; Isenberg, K.L.; Cherif, C.; Aibibu, D. Continuous Wet Spinning of Regenerated Silk Fibers from Spinning Dopes Containing 4% Fibroin Protein. Int. J. Mol. Sci. 2023, 24, 13492. [Google Scholar] [CrossRef]
- Lucas, F.; Shaw, J.T.B.; Smith, S.G. The Amino Acid Sequence in a Fraction of the Fibroin of Bombyx mori. Biochem. J. 1957, 66, 468–479. [Google Scholar] [CrossRef]
- Zhang, K.; Si, F.W.; Duan, H.L.; Wang, J. Microstructures and Mechanical Properties of Silks of Silkworm and Honeybee. Acta Biomater. 2010, 6, 2165–2171. [Google Scholar] [CrossRef]
- Lucas, F.; Shaw, J.T.B.; Smith, S.G. Some Amino Acid Sequences in the Amorphous Fraction of the Fibroin of Bombyx mori. Biochem. J. 1962, 83, 164–171. [Google Scholar] [CrossRef]
- Nadiger, G.S.; Bhat, N.V.; Padhye, M.R. Investigation of Amino Acid Composition in the Crystalline Region of Silk Fibroin. J. Appl. Polym. Sci. 1985, 30, 221–225. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kikuchi, Y.; Takagi, T.; Kikuchi, A.; Oyama, F.; Shimura, K.; Mizuno, S. Primary Structure of the Silk Fibroin Light Chain Determined by cDNA Sequencing and Peptide Analysis. J. Mol. Biol. 1989, 210, 127–139. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, K.; Zhao, M.; Tao, X.; Hu, X.; Lu, S. Tunable High-Molecular-Weight Silk Fibroin Polypeptide Materials: Fabrication and Self-Assembly Mechanism. ACS Appl. Bio Mater. 2020, 3, 3248–3259. [Google Scholar] [CrossRef]
- Eliaz, D.; Paul, S.; Benyamin, D.; Cernescu, A.; Cohen, S.R.; Rosenhek-Goldian, I.; Brookstein, O.; Miali, M.E.; Solomonov, A.; Greenblatt, M.; et al. Micro and Nano-Scale Compartments Guide the Structural Transition of Silk Protein Monomers into Silk Fibers. Nat. Commun. 2022, 13, 7856. [Google Scholar] [CrossRef]
- Zafar, M.S.; Belton, D.J.; Hanby, B.; Kaplan, D.L.; Perry, C.C. Functional Material Features of Bombyx mori Silk Light versus Heavy Chain Proteins. Biomacromolecules 2015, 16, 606–614. [Google Scholar] [CrossRef]
- Kojima, K.; Kuwana, Y.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Tamada, Y. A New Method for the Modification of Fibroin Heavy Chain Protein in the Transgenic Silkworm. Biosci. Biotechnol. Biochem. 2007, 71, 2943–2951. [Google Scholar] [CrossRef]
- Long, D.; Lu, W.; Zhang, Y.; Guo, Q.; Xiang, Z.; Zhao, A. New Insight into the Mechanism Underlying Fibroin Secretion in Silkworm, Bombyx mori. FEBS J. 2015, 282, 89–101. [Google Scholar] [CrossRef]
- Pérez-Rigueiro, J.; Elices, M.; Llorca, J.; Viney, C. Tensile Properties of Silkworm Silk Obtained by Forced Silking. J. Appl. Polym. Sci. 2001, 82, 1928–1935. [Google Scholar] [CrossRef]
- Asakura, T.; Ohgo, K.; Ishida, T.; Taddei, P.; Monti, P.; Kishore, R. Possible Implications of Serine and Tyrosine Residues and Intermolecular Interactions on the Appearance of Silk I Structure of Bombyx mori Silk Fibroin-Derived Synthetic Peptides: High-Resolution 13C Cross-Polarization/Magic-Angle Spinning NMR Study. Biomacromolecules 2005, 6, 468–474. [Google Scholar] [CrossRef]
- Zhou, C.-Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.-G.; Janin, J. Silk Fibroin: Structural Implications of a Remarkable Amino Acid Sequence. Proteins Struct. Funct. Bioinform. 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical Applications of Chemically-Modified Silk Fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Ha, S.W.; Tonelli, A.E.; Hudson, S.M. Structural Studies of Bombyx mori Silk Fibroin during Regeneration from Solutions and Wet Fiber Spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef]
- Takahashi, Y.; Gehoh, M.; Yuzuriha, K. Crystal Structure of Silk (Bombyx mori). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 889–891. [Google Scholar] [CrossRef]
- Asakura, T.; Ogawa, T.; Naito, A.; Williamson, M.P. Chain-Folded Lamellar Structure and Dynamics of the Crystalline Fraction of Bombyx mori Silk Fibroin and of (Ala-Gly-Ser-Gly-Ala-Gly) Model Peptidesn. Int. J. Biol. Macromol. 2020, 164, 3974–3983. [Google Scholar] [CrossRef]
- Valluzzi, R.; Gido, S.P. The Crystal Structure of Bombyx mori Silk Fibroin at the Air–Water Interface. Biopolymers 1997, 42, 705–717. [Google Scholar] [CrossRef]
- Sarkar, A.; Connor, A.J.; Koffas, M.; Zha, R.H. Chemical Synthesis of Silk-Mimetic Polymers. Materials 2019, 12, 4086. [Google Scholar] [CrossRef]
- Taketani, I.; Nakayama, S.; Nagare, S.; Senna, M. The Secondary Structure Control of Silk Fibroin Thin Films by Post Treatment. Appl. Surf. Sci. 2005, 244, 623–626. [Google Scholar] [CrossRef]
- Mu, X.; Amouzandeh, R.; Vogts, H.; Luallen, E.; Arzani, M. A Brief Review on the Mechanisms and Approaches of Silk Spinning-Inspired Biofabrication. Front. Bioeng. Biotechnol. 2023, 11, 1252499. [Google Scholar] [CrossRef]
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the Site of Disulfide Linkage between Heavy and Light Chains of Silk Fibroin Produced by Bombyx mori. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1999, 1432, 92–103. [Google Scholar] [CrossRef]
- Marsh, R.E.; Corey, R.B.; Pauling, L. An Investigation of the Structure of Silk Fibroin. Biochim. Et Biophys. Acta 1955, 16, 1–34. [Google Scholar] [CrossRef]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic Interaction of P25, Containing Asn-Linked Oligosaccharide Chains, with the H-L Complex of Silk Fibroin Produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Zhou, C.-Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine Organization of Bombyx mori Fibroin Heavy Chain Gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Ambrose, E.J.; Bamford, C.H.; Elliott, A.; Hanby, W.E. Water-Soluble Silk: An α-Protein. Nature 1951, 167, 264–265. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z. Microstructure Transitions and Dry-Wet Spinnability of Silk Fibroin Protein from Waste Silk Quilt. Polymers 2019, 11, 1622. [Google Scholar] [CrossRef]
- Kratky, O.; Schauenstein, E. X-Ray and U.-V. Spectrographic Investigations of Fibrous and Globular Modifications of Silk Fibroin. Discuss. Faraday Soc. 1951, 11, 171–178. [Google Scholar] [CrossRef]
- Koga, N.; Tatsumi-Koga, R.; Liu, G.; Xiao, R.; Acton, T.B.; Montelione, G.T.; Baker, D. Principles for Designing Ideal Protein Structures. Nature 2012, 491, 222–227. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Beaulieu, L.; Lefèvre, T.; Paradis, J.; Asakura, T.; Pézolet, M. Characterization by Raman Microspectroscopy of the Strain-Induced Conformational Transition in Fibroin Fibers from the Silkworm Samia Cynthia Ricini. Biomacromolecules 2006, 7, 2512–2521. [Google Scholar] [CrossRef]
- Drummy, L.F.; Phillips, D.M.; Stone, M.O.; Farmer, B.L.; Naik, R.R. Thermally Induced α-Helix to β-Sheet Transition in Regenerated Silk Fibers and Films. Biomacromolecules 2005, 6, 3328–3333. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C. Natural Flavonoid-Functionalized Silk Fiber Presenting Antibacterial, Antioxidant, and UV Protection Performance. ACS Sustain. Chem. Eng. 2017, 5, 10518–10526. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Fossey, S.A.; Némethy, G.; Gibson, K.D.; Scheraga, H.A. Conformational Energy Studies of β-Sheets of Model Silk Fibroin Peptides. I. Sheets of Poly(Ala-Gly) Chains. Biopolymers 1991, 31, 1529–1541. [Google Scholar] [CrossRef]
- Cheng, K.; Tao, X.; Qi, Z.; Yin, Z.; Kundu, S.C.; Lu, S. Highly Absorbent Silk Fibroin Protein Xerogel. ACS Biomater. Sci. Eng. 2021, 7, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kameda, T.; Tashiro, K.; Ohta, N.; Schaper, A.K. Transformation of Coiled α-Helices into Cross-β-Sheets Superstructure. Biomacromolecules 2017, 18, 3892–3903. [Google Scholar] [CrossRef]
- Athiyarath, V.; Madhusudhanan, M.C.; Kunnikuruvan, S.; Sureshan, K.M. Secondary Structure Tuning of a Pseudoprotein Between β-Meander and α-Helical Forms in the Solid-State. Angew. Chem. Int. Ed. 2022, 61, e202113129. [Google Scholar] [CrossRef]
- Lazo, N.D.; Downing, D.T. Crystalline Regions of Bombyx mori Silk Fibroin May Exhibit β-Turn and β-Helix Conformations. Macromolecules 1999, 32, 4700–4705. [Google Scholar] [CrossRef]
- Asakura, T.; Watanabe, Y.; Itoh, T. NMR of Silk Fibroin. 3. Assignment of Carbonyl Carbon Resonances and Their Dependence on Sequence and Conformation in Bombyx mori Silk Fibroin Using Selective Isotopic Labeling. Macromolecules 1984, 17, 2421–2426. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, P.; Jiang, T.; Zhou, P. Environment-Induced Silk Fibroin Conformation Based on the Magnetic Resonance Spectroscopy. In On Biomimetics; IntechOpen: London, UK, 2011; ISBN 978-953-307-271-5. [Google Scholar]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the Structure of Bombyx mori Silk Fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar] [CrossRef]
- Lotz, B.; Keith, H.D. The Crystal Structures of Poly(LAla-Gly-Gly-Gly)II and Poly(LAla-Gly-Gly)II. J. Mol. Biol. 1971, 61, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Burghammer, M.; Davies, R.J.; Riekel, C. Thermal Behavior of Bombyx mori Silk: Evolution of Crystalline Parameters, Molecular Structure, and Mechanical Properties. Biomacromolecules 2007, 8, 3548–3556. [Google Scholar] [CrossRef]
- Inouye, H.; Fraser, P.E.; Kirschner, D.A. Structure of Beta-Crystallite Assemblies Formed by Alzheimer Beta-Amyloid Protein Analogues: Analysis by x-Ray Diffraction. Biophys. J. 1993, 64, 502–519. [Google Scholar] [CrossRef]
- Saitoh, H.; Ohshima, K.; Tsubouchi, K.; Takasu, Y.; Yamada, H. X-Ray Structural Study of Noncrystalline Regenerated Bombyx mori Silk Fibroin. Int. J. Biol. Macromol. 2004, 34, 259–265. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, Y.; Li, M.; Zuo, B.; Lu, S.; Wang, J.; Zhu, H.; Kaplan, D.L. Silk Fibroin Electrogelation Mechanisms. Acta Biomater. 2011, 7, 2394–2400. [Google Scholar] [CrossRef]
- Wilson, D.; Valluzzi, R.; Kaplan, D. Conformational Transitions in Model Silk Peptides. Biophys. J. 2000, 78, 2690–2701. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Lefèvre, T.; Beaulieu, L.; Asakura, T.; Pézolet, M. Study of Protein Conformation and Orientation in Silkworm and Spider Silk Fibers Using Raman Microspectroscopy. Biomacromolecules 2004, 5, 2247–2257. [Google Scholar] [CrossRef]
- Lefèvre, T.; Paquet-Mercier, F.; Rioux-Dubé, J.-F.; Pézolet, M. Structure of Silk by Raman Spectromicroscopy: From the Spinning Glands to the Fibers. Biopolymers 2012, 97, 322–336. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Cabiaux, V.; Ruysschaert, J.-M. Secondary Structure and Dosage of Soluble and Membrane Proteins by Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy on Hydrated Films. Eur. J. Biochem. 1990, 193, 409–420. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Jung, C. Insight into Protein Structure and Protein–Ligand Recognition by Fourier Transform Infrared Spectroscopy. J. Mol. Recognit. 2000, 13, 325–351. [Google Scholar] [CrossRef]
- Mester, L.; Govyadinov, A.A.; Chen, S.; Goikoetxea, M.; Hillenbrand, R. Subsurface Chemical Nanoidentification by Nano-FTIR Spectroscopy. Nat. Commun. 2020, 11, 3359. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and Applications in Nanoscale Infrared Spectroscopy and Chemical Imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Lansford, J.L.; Vlachos, D.G. Infrared Spectroscopy Data- and Physics-Driven Machine Learning for Characterizing Surface Microstructure of Complex Materials. Nat. Commun. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Hutařová Vařeková, I.; Hutař, J.; Midlik, A.; Horský, V.; Hladká, E.; Svobodová, R.; Berka, K. 2DProts: Database of Family-Wide Protein Secondary Structure Diagrams. Bioinformatics 2021, 37, 4599–4601. [Google Scholar] [CrossRef]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Zheng, J.H.; Shao, J.Z.; Liu, J.Q. Studies on Distribution of Amino Acids in Silk Fibroi. Acta Polym. Sin. 2002, 6, 818–823. [Google Scholar]
- Lefèvre, T.; Rousseau, M.-E.; Pézolet, M. Protein Secondary Structure and Orientation in Silk as Revealed by Raman Spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, Y.; Ren, J.; Tang, Y.; Qi, Z.; Zhou, X.; Chen, X.; Shao, Z.; Chen, M.; Kaplan, D.L.; et al. Understanding Secondary Structures of Silk Materials via Micro- and Nano-Infrared Spectroscopies. ACS Biomater. Sci. Eng. 2019, 5, 3161–3183. [Google Scholar] [CrossRef]
- Paquet-Mercier, F.; Lefèvre, T.; Auger, M.; Pézolet, M. Evidence by Infrared Spectroscopy of the Presence of Two Types of β-Sheets in Major Ampullate Spider Silk and Silkworm Silk. Soft Matter 2013, 9, 208–215. [Google Scholar] [CrossRef]
- Koperska, M.A.; Pawcenis, D.; Bagniuk, J.; Zaitz, M.M.; Missori, M.; Łojewski, T.; Łojewska, J. Degradation Markers of Fibroin in Silk through Infrared Spectroscopy. Polym. Degrad. Stab. 2014, 105, 185–196. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Dynamic Protein−Water Relationships during β-Sheet Formation. Macromolecules 2008, 41, 3939–3948. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, H.; Shao, H.; Hu, X. Effect of Shearing on Formation of Silk Fibers from Regenerated Bombyx mori Silk Fibroin Aqueous Solution. Int. J. Biol. Macromol. 2006, 38, 284–288. [Google Scholar] [CrossRef]
- Stair, P.C. Advances in Raman Spectroscopy Methods for Catalysis Research. Curr. Opin. Solid. State Mater. Sci. 2001, 5, 365–369. [Google Scholar] [CrossRef]
- Ji, D.; Deng, Y.-B.; Zhou, P. Folding Process of Silk Fibroin Induced by Ferric and Ferrous Ions. J. Mol. Struct. 2009, 938, 305–310. [Google Scholar] [CrossRef]
- Monti, P.; Taddei, P.; Freddi, G.; Asakura, T.; Tsukada, M. Raman Spectroscopic Characterization of Bombyx mori Silk Fibroin: Raman Spectrum of Silk I. J. Raman Spectrosc. 2001, 32, 103–107. [Google Scholar] [CrossRef]
- Monti, P.; Freddi, G.; Bertoluzza, A.; Kasai, N.; Tsukada, M. Raman Spectroscopic Studies of Silk Fibroin from Bombyx mori. J. Raman Spectrosc. 1998, 29, 297–304. [Google Scholar] [CrossRef]
- Takahashi, Y.; Gehoh, M.; Yuzuriha, K. Structure Refinement and Diffuse Streak Scattering of Silk (Bombyx mori). Int. J. Biol. Macromol. 1999, 24, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Naito, A. Structure of Silk I (Bombyx mori Silk Fibroin before Spinning) in the Dry and Hydrated States Studied Using 13C Solid-State NMR Spectroscopy. Int. J. Biol. Macromol. 2022, 216, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of Silk III at the Air-Water Interface. Int. J. Biol. Macromol. 1999, 24, 237–242. [Google Scholar] [CrossRef]
- Lotz, B.; Keith, H.D. Crystal Structure of Poly(L-Ala-Gly)II: A Model for Silk I. J. Mol. Biol. 1971, 61, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Kurokawa, M. The Structure of Silk Fibroin-α. Sen-I Gakkaishi 1968, 24, 550–554. [Google Scholar] [CrossRef]
- Asakura, T.; Ashida, J.; Yamane, T.; Kameda, T.; Nakazawa, Y.; Ohgo, K.; Komatsu, K. A Repeated β-Turn Structure in Poly(Ala-Gly) as a Model for Silk I of Bombyx mori Silk Fibroin Studied with Two-Dimensional Spin-Diffusion NMR under off Magic Angle Spinning and Rotational Echo Double Resonance. J. Mol. Biol. 2001, 306, 291–305. [Google Scholar] [CrossRef]
- Landau, E.M.; Popovitz-biro, R.; Levanon, M.; Leiserowitz, L.; Lahav, M.; Sagiv, J. Langmuir Monolayers Designed for the Oriented Growth of Glycine and Sodium Chloride Crystals at Air/Water Interfaces. Mol. Cryst. Liq. Cryst. 1986, 134, 323–335. [Google Scholar] [CrossRef]
- Termonia, Y. Molecular Modeling of Spider Silk Elasticity. Macromolecules 1994, 27, 7378–7381. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Silks as Ancient Models for Modern Polymers. Polymer 2009, 50, 5623–5632. [Google Scholar] [CrossRef]
- Porter, D.; Vollrath, F. The Role of Kinetics of Water and Amide Bonding in Protein Stability. Soft Matter 2008, 4, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Vollrath, F.; Shao, Z. Predicting the Mechanical Properties of Spider Silk as a Model Nanostructured Polymer. Eur. Phys. J. E 2005, 16, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Ohgo, K.; Komatsu, K.; Kanenari, M.; Okuyama, K. Refinement of Repeated β-Turn Structure for Silk I Conformation of Bombyx mori Silk Fibroin Using 13C Solid-State NMR and X-Ray Diffraction Methods. Macromolecules 2005, 38, 7397–7403. [Google Scholar] [CrossRef]
- Guo, N.; Lu, K.; Cheng, L.; Li, Z.; Wu, C.; Liu, Z.; Liang, S.; Chen, S.; Chen, W.; Jiang, C.; et al. Structure Analysis of the Spinneret from Bombyx mori and Its Influence on Silk Qualities. Int. J. Biol. Macromol. 2019, 126, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Kaplan, D.L. Mechanism of Silk Processing in Insects and Spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Guo, J.; Dai, X.; Yu, S.; Zhong, B. Modeling the 3-Dimensional Structure of the Silkworm’s Spinning Apparatus in Silk Production. Acta Biomater. 2024, 174, 217–227. [Google Scholar] [CrossRef]
- Koeppel, A.; Holland, C. Progress and Trends in Artificial Silk Spinning: A Systematic Review. ACS Biomater. Sci. Eng. 2017, 3, 226–237. [Google Scholar] [CrossRef]
- Zhang, F.; You, X.; Dou, H.; Liu, Z.; Zuo, B.; Zhang, X. Facile Fabrication of Robust Silk Nanofibril Films via Direct Dissolution of Silk in CaCl2–Formic Acid Solution. ACS Appl. Mater. Interfaces 2015, 7, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Liu, Z.; Bie, S.; Zhang, F.; Zuo, B. Novel Silk Fibroin Films Prepared by Formic Acid/Hydroxyapatite Dissolution Method. Mater. Sci. Eng. C 2014, 37, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Earland, C.; Raven, D.J. A New Solvent for Silk. Nature 1954, 174, 461. [Google Scholar] [CrossRef]
- Brown, J.; Lu, C.-L.; Coburn, J.; Kaplan, D.L. Impact of Silk Biomaterial Structure on Proteolysis. Acta Biomater. 2015, 11, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Degradation Behavior of Silk Nanoparticles—Enzyme Responsiveness. ACS Biomater. Sci. Eng. 2018, 4, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, T.; Cheng, G.; Huang, L.; Chen, L.; Wu, D. Dissolution Behavior of Silk Fibroin in a Low Concentration CaCl2-Methanol Solvent: From Morphology to Nanostructure. Int. J. Biol. Macromol. 2018, 113, 458–463. [Google Scholar] [CrossRef]
- Gobin, A.S.; Froude, V.E.; Mathur, A.B. Structural and Mechanical Characteristics of Silk Fibroin and Chitosan Blend Scaffolds for Tissue Regeneration. J. Biomed. Mater. Res. Part. A 2005, 74A, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, C.; Yang, Y.; Ren, J.; Ling, S. The Fractal Network Structure of Silk Fibroin Molecules and Its Effect on Spinning of Silkworm Silk. ACS Nano 2023, 17, 7662–7673. [Google Scholar] [CrossRef]
- Yin, J.; Chen, E.; Porter, D.; Shao, Z. Enhancing the Toughness of Regenerated Silk Fibroin Film through Uniaxial Extension. Biomacromolecules 2010, 11, 2890–2895. [Google Scholar] [CrossRef]
- Hirlekar, S.; Ray, D.; Aswal, V.K.; Prabhune, A.; Nisal, A.; Ravindranathan, S. Silk Fibroin–Sodium Dodecyl Sulfate Gelation: Molecular, Structural, and Rheological Insights. Langmuir 2019, 35, 14870–14878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, Mechanical Properties and Bio-Applications of Silk Fibroin-Based High-Strength Hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Pal, R.K.; Maniglio, D.; Quaranta, A.; Mulloni, V.; Motta, A.; Yadavalli, V.K. Fabrication of Nanoscale Patternable Films of Silk Fibroin Using Benign Solvents. Macromol. Mater. Eng. 2017, 302, 1700110. [Google Scholar] [CrossRef]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; Salas-de la Cruz, D. Impact of Ionic Liquid Type on the Structure, Morphology and Properties of Silk-Cellulose Biocomposite Materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Xu, W.; Liu, H.; Ouyang, C. Blend Films of Silk Fibroin and Water-Insoluble Polyurethane Prepared from an Ionic Liquid. Mater. Lett. 2011, 65, 2489–2491. [Google Scholar] [CrossRef]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; De Long, H.C.; Mantz, R.A. Dissolution and Regeneration of Bombyx mori Silk Fibroin Using Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, H.; Watanabe, Y.; ishida KAnd fukumoto, O. Regenerated silk prepeared from ortho phosphoric acid solution of fibroin. J. Sericultural Sci. Jpn. 1989, 58, 87–95. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kim, H.-S. Structure and Characteristic of Chitosan/Bombyx mori Silk Fibroin Blend Filems. Polymer 2005, 29, 408–412. [Google Scholar]
- Chen, X.; Knight, D.P.; Shao, Z.; Vollrath, F. Regenerated Bombyx Silk Solutions Studied with Rheometry and FTIR. Polymer 2001, 42, 09969–09974. [Google Scholar] [CrossRef]
- Junghans, F.; Morawietz, M.; Conrad, U.; Scheibel, T.; Heilmann, A.; Spohn, U. Preparation and Mechanical Properties of Layers Made of Recombinant Spider Silk Proteins and Silk from Silk Worm. Appl. Phys. A 2006, 82, 253–260. [Google Scholar] [CrossRef]
- Freddi, G.; Pessina, G.; Tsukada, M. Swelling and Dissolution of Silk Fibroin (Bombyx mori) in N-Methyl Morpholine N-Oxide. Int. J. Biol. Macromol. 1999, 24, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Madurga, R.; Gañán-Calvo, A.M.; Mariscal, T.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Pérez-Rigueiro, J. Production of Regenerated Silkworm Silk Fibers from Aqueous Dopes through Straining Flow Spinning. Text. Res. J. 2019, 89, 4554–4567. [Google Scholar] [CrossRef]
- Porter, D.; Guan, J.; Vollrath, F. Spider Silk: Super Material or Thin Fibre? Adv. Mater. 2013, 25, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, B.; Drodge, D.R.; Dragnevski, K.I.; Siviour, C.R.; Holland, C. In Situ Tensile Tests of Single Silk Fibres in an Environmental Scanning Electron Microscope (ESEM). J. Mater. Sci. 2013, 48, 5055–5062. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, G.; Knight, D.P.; Shao, Z.; Chen, X. Wet-Spinning of Regenerated Silk Fiber from Aqueous Silk Fibroin Solution: Discussion of Spinning Parameters. Biomacromolecules 2010, 11, 1–5. [Google Scholar] [CrossRef]
- Domigan, L.J.; Andersson, M.; Alberti, K.A.; Chesler, M.; Xu, Q.; Johansson, J.; Rising, A.; Kaplan, D.L. Carbonic Anhydrase Generates a pH Gradient in Bombyx mori Silk Glands. Insect Biochem. Mol. Biol. 2015, 65, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Q.; Yue, X.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Regeneration of High-Quality Silk Fibroin Fiber by Wet Spinning from CaCl2–Formic Acid Solvent. Acta Biomater. 2015, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Masuda, H.; Zhao, C.; Asakura, T. Artificial Spinning and Characterization of Silk Fiber from Bombyx m Ori Silk Fibroin in Hexafluoroacetone Hydrate. Macromolecules 2002, 35, 6–9. [Google Scholar] [CrossRef]
- Ki, C.S.; Lee, K.H.; Baek, D.H.; Hattori, M.; Um, I.C.; Ihm, D.W.; Park, Y.H. Dissolution and Wet Spinning of Silk Fibroin Using Phosphoric Acid/Formic Acid Mixture Solvent System. J. Appl. Polym. Sci. 2007, 105, 1605–1610. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.; Lee, K.G.; Ihm, D.W.; Lee, J.-H.; Park, Y.H. Wet Spinning of Silk Polymer: I. Effect of Coagulation Conditions on the Morphological Feature of Filament. Int. J. Biol. Macromol. 2004, 34, 89–105. [Google Scholar] [CrossRef]
- Arafat, M.T.; Tronci, G.; Yin, J.; Wood, D.J.; Russell, S.J. Biomimetic Wet-Stable Fibres via Wet Spinning and Diacid-Based Crosslinking of Collagen Triple Helices. Polymer 2015, 77, 102–112. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-Based Biomaterials in Biomedical Textiles and Fiber-Based Implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef]
- Qiu, W.; Teng, W.; Cappello, J.; Wu, X. Wet-Spinning of Recombinant Silk-Elastin-Like Protein Polymer Fibers with High Tensile Strength and High Deformability. Biomacromolecules 2009, 10, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Zhao, Y.; Luo, J.; Shao, H.; Hu, X. Bio-Inspired Capillary Dry Spinning of Regenerated Silk Fibroin Aqueous Solution. Mater. Sci. Eng. C 2011, 31, 1602–1608. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Shao, H.; Hu, X. Posttreatment of the Dry-Spun Fibers Obtained from Regenerated Silk Fibroin Aqueous Solution in Ethanol Aqueous Solution. J. Mater. Res. 2011, 26, 1100–1106. [Google Scholar] [CrossRef]
- Canetti, M.; Seves, A.; Secundo, F.; Vecchio, G. CD and Small-Angle X-ray Scattering of Silk Fibroin in Solution. Biopolymers 1989, 28, 1613–1624. [Google Scholar] [CrossRef]
- Lee, K.H.; Baek, D.H.; Ki, C.S.; Park, Y.H. Preparation and Characterization of Wet Spun Silk Fibroin/Poly(Vinyl Alcohol) Blend Filaments. Int. J. Biol. Macromol. 2007, 41, 168–172. [Google Scholar] [CrossRef]
- Magoshi, J. Physical Properties and Structure of Silk: 4. Spherulites Grown from Aqueous Solution of Silk Fibroin. Polymer 1977, 18, 643–646. [Google Scholar] [CrossRef]
- Li, G.; Yu, T. Investigation of the Liquid-Crystal State in Silk Fibroin. Die Makromol. Chem. Rapid Commun. 1989, 10, 387–389. [Google Scholar] [CrossRef]
- Kerkam, K.; Viney, C.; Kaplan, D.; Lombardi, S. Liquid Crystallinity of Natural Silk Secretions. Nature 1991, 349, 596–598. [Google Scholar] [CrossRef]
- Knight, D.P.; Vollrath, F. Liquid Crystals and Flow Elongation in a Spider’s Silk Production Line. Proc. R. Soc. London. Ser. B Biol. Sci. 1999, 266, 519–523. [Google Scholar] [CrossRef]
- Willcox, P.J.; Gido, S.P.; Muller, W.; Kaplan, D.L. Evidence of a Cholesteric Liquid Crystalline Phase in Natural Silk Spinning Processes. Macromolecules 1996, 29, 5106–5110. [Google Scholar] [CrossRef]
- Jenkins, S.; Thammongkol, V.; Polk, M.B. Synthesis and Spinning of a Thermotropic Liquid Crystal Copolyester Containing a Semirigid Cycloaliphatic Spacer. J. Polym. Sci. Part. A Polym. Chem. 1998, 36, 1473–1480. [Google Scholar] [CrossRef]
- Hyde, S. CHAPTER 16 Identification of Lyotropic Liquid Crystalline Mesophases. In Handbook of Applied Surface and Colloid Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rey, A.D.; Herrera-Valencia, E.E. Liquid Crystal Models of Biological Materials and Silk Spinning. Biopolymers 2012, 97, 374–396. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Amino Acid Composition (mol%) | ||||||
|---|---|---|---|---|---|---|---|
| B. Mori | Serotonin Sericin | Mulberry (Bivoltine) | Mulberry (Crossbreed) | ||||
| All | H Chain | L Chain | P25 Glycoprotein Chain | ||||
| Aspartic | 1.9 | 0.4 | 7.4 | 5.9 | 8.22 | 1.64 | 1.49 |
| Glutamic | 1.4 | 0.6 | 6.1 | 3.4 | 2.12 | 1.77 | 1.53 |
| Serine | 12.2 | 12.1 | 9.8 | 6.9 | 12.9 | 10.38 | 10.85 |
| Glycine | 42.9 | 45.9 | 9 | 4.4 | 25.85 | 43.45 | 43.73 |
| Histidine | 0.2 | 0.1 | 2.1 | 5.1 | 1.56 | 0.13 | 0.15 |
| Arginine | 0.5 | 0.3 | 4.1 | 5.9 | 5.13 | 1.13 | 1.16 |
| Threonine | 0.9 | 0.9 | 2.9 | 5.9 | 0.47 | 0.92 | 0.76 |
| Alanine | 30 | 30.3 | 13.5 | 4.4 | 44.1 | 27.56 | 28.36 |
| Proline | 0.5 | 0.3 | 2.9 | 5.9 | 0.47 | 0.79 | 0.76 |
| Tyrosine | 4.8 | 5.3 | 4.1 | 4.9 | 9.1 | 5.58 | 5.76 |
| Valine | 2.5 | 1.8 | 7 | 4.4 | 1.5 | 2.37 | 2.89 |
| Methionine | 0.1 | 0.1 | 0.4 | 0.5 | 0.5 | 0.19 | 0.11 |
| Cystine | 0.1 | 0.1 | 1.2 | 3.9 | 0.23 | 0.13 | 0.12 |
| Lsoleusine | 0.2 | 0.2 | 8.2 | 6.9 | 0.38 | 0.75 | 0.78 |
| Leucine | 0.6 | 0.1 | 7 | 9.4 | 0.4 | 0.73 | 0.75 |
| Phenylalanine | 0.6 | 0.6 | 2.9 | 7.4 | 0.47 | 0.14 | 0.18 |
| Tryptophan | 0.2 | 0.7 | 9.8 | 11.4 | 1.75 | 0.73 | 0.75 |
| Lysine | 0.4 | 0.2 | 1.6 | 3.4 | 0.21 | 0.23 | 0.25 |
| Refs. | [30,31] | [32,33,34] | [35] | [34] | |||
| Assignment | Amide I | Amide II | Amide III | Amide IV |
|---|---|---|---|---|
| α-helix | 1656–1662 | 1530–1540 | 1266 | 669 |
| β-sheet | 1697–1703 1620–1635 | 1525–1530 | 1260–1265 | 900 |
| β-turn | 1663–1668 | 1520–1535 | 1235 | 650 |
| Random | 1647–1655 | 1534–1548 | 1240–1230 | / |
| Assignment | Amide I | Amide III | C-C |
|---|---|---|---|
| α-helix | 1645–1658 | 1264–1310 | 1103–1108 |
| β-sheet | 1665–1680 | 1220–1245 | 1020–1060, 1185 |
| Random | 1660–1665 | 1250 | <1106 |
| Solvent | Ratio of Solvent | Temp. (°C) | Refs. |
|---|---|---|---|
| Calcium chloride/Formic acid | / | 25 | [122] |
| Hydroxyapatite/ Formic acid | Hydroxyapatite content ≤ 5% | 25 | [123] |
| Formic acid | 98% | 25 | [124] |
| Protease XIV | ≥3.5 units per mg protein | 25 | [125] |
| Proteinase K | ≥30 units per mg protein | 25 | [126] |
| Papain (lysosomal-like enzyme) | ≥10 units per mg protein | 25 | [127] |
| Calcium chloride/ Water | 5 M | 80 | [128] |
| Calcium chloride/Ethanol/Water | 1:4:2 | 80 | [129] |
| Lithium bromide/Water | 9.2–9.5 M | 60 | [130,131,132] |
| Lithium thiocyanide/Water | 9 M | 25 | [133] |
| Lithium chloride/DMSO | 1 M lithium chloride dissolved in DMSO | 60 | [134] |
| [AMIM]Cl | 98% | 70 | [135] |
| [BMIM]Cl | 98% | 100 | [136] |
| [BMIM]Br | 95% | 100 | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, G.; Jia, T.; Qi, Z.; Lu, S. Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber. Materials 2024, 17, 1834. https://doi.org/10.3390/ma17081834
Tan G, Jia T, Qi Z, Lu S. Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber. Materials. 2024; 17(8):1834. https://doi.org/10.3390/ma17081834
Chicago/Turabian StyleTan, Guohongfang, Tianshuo Jia, Zhenzhen Qi, and Shenzhou Lu. 2024. "Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber" Materials 17, no. 8: 1834. https://doi.org/10.3390/ma17081834
APA StyleTan, G., Jia, T., Qi, Z., & Lu, S. (2024). Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber. Materials, 17(8), 1834. https://doi.org/10.3390/ma17081834






