Synthesis and Characterization of Superhydrophobic Epoxy Resin Coating with SiO2@CuO/HDTMS for Enhanced Self-Cleaning, Photocatalytic, and Corrosion-Resistant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.3. Characterization
3. Results
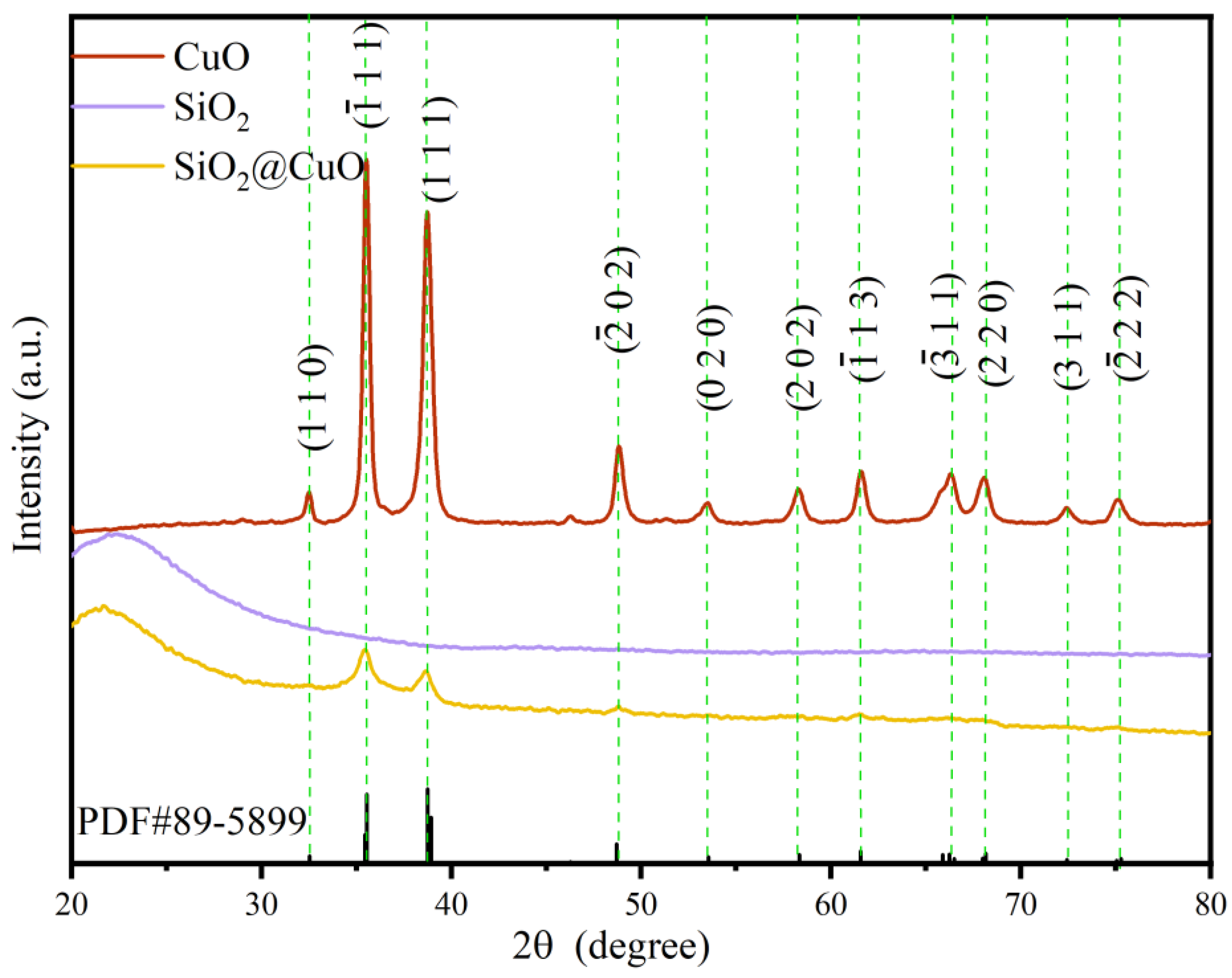
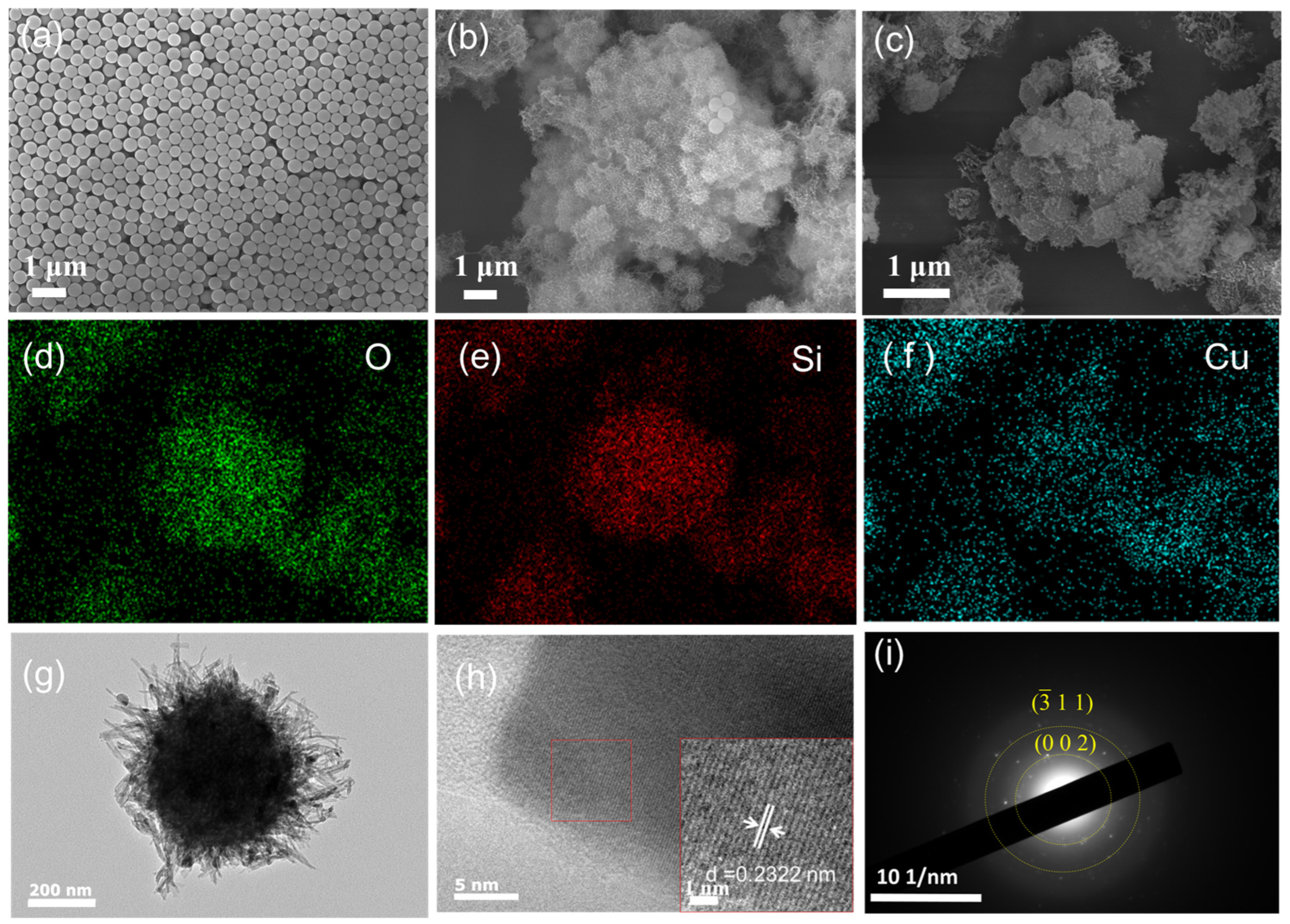
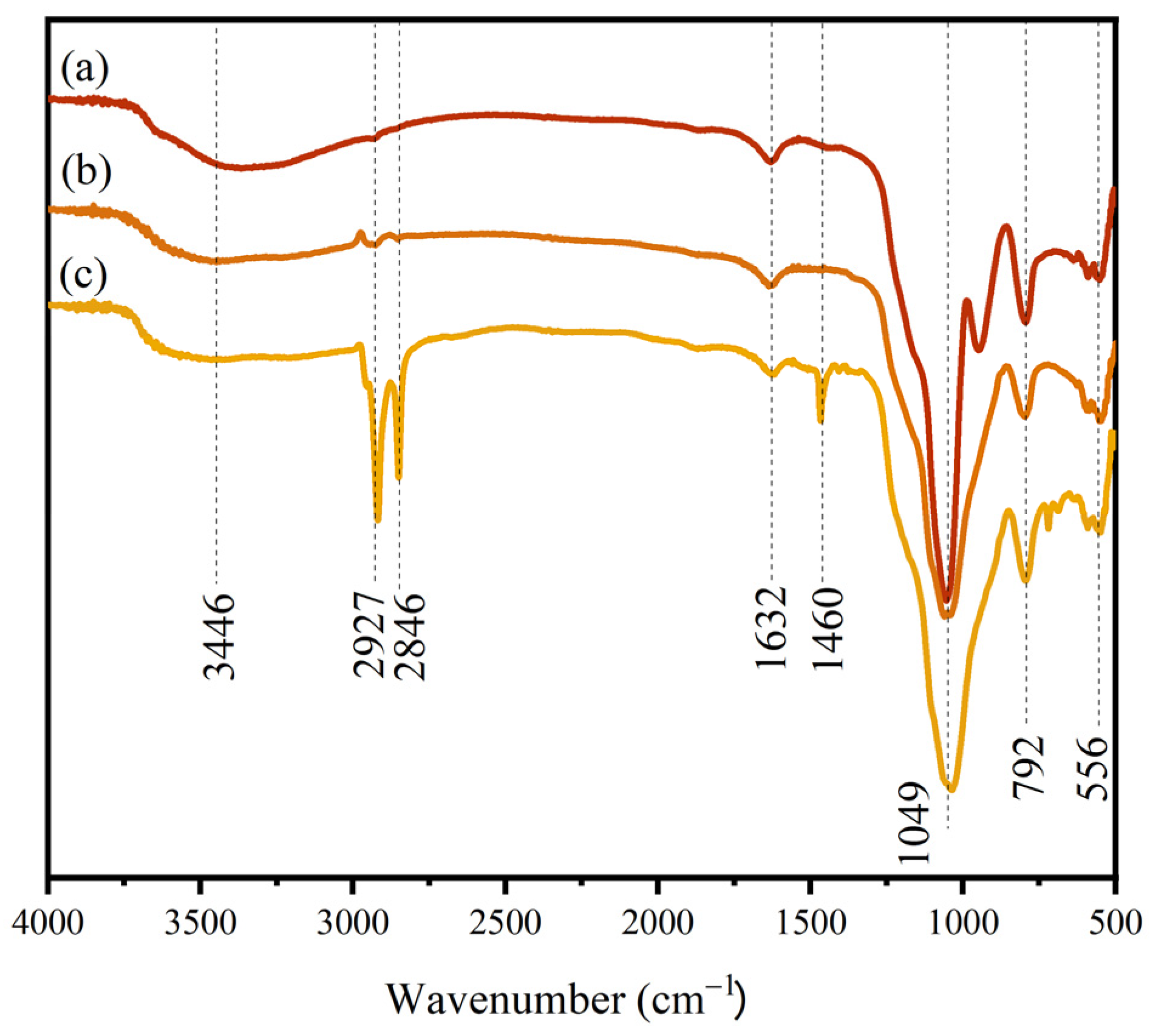
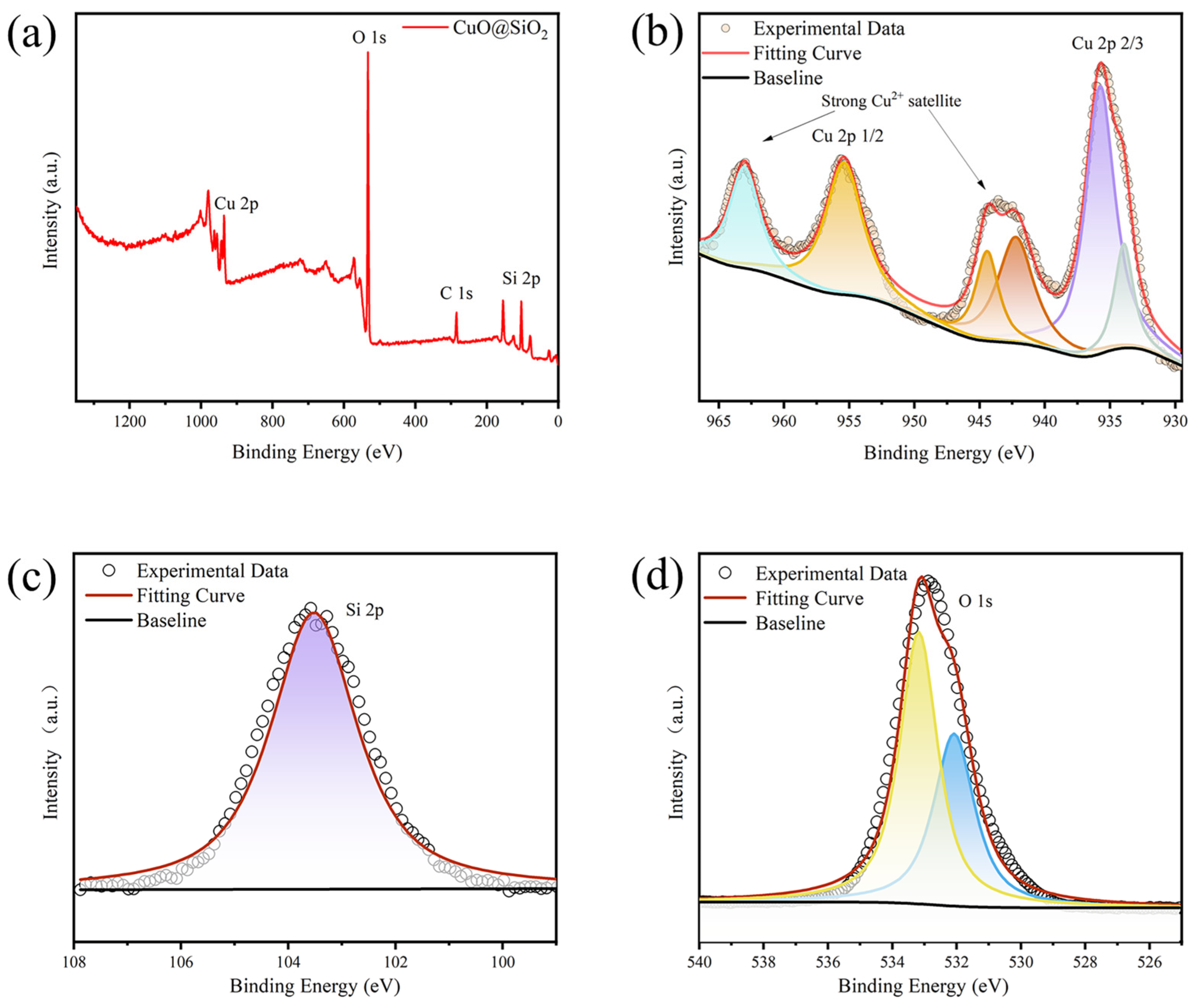
3.1. Characterization of SiO2/CuO Core–Shell Particles
3.1.1. Microstructure and Morphology
3.1.2. Chemical Composition
3.1.3. Bandgap Calculations
3.2. Morphology of SiO2@CuO/HDTMS Coating
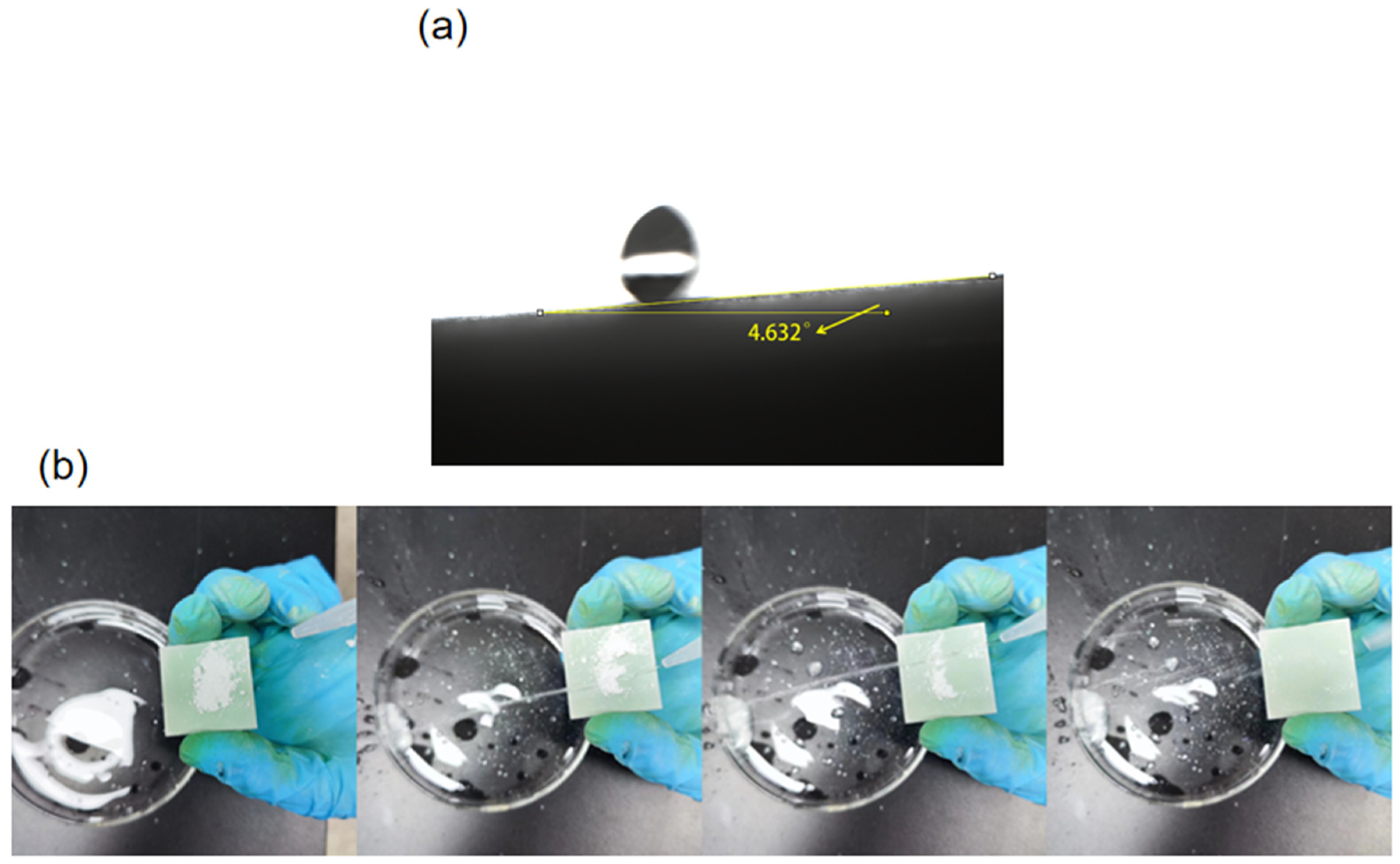
3.3. Liquid Repellent Properties of SiO2@CuO/HDTMS Coating
3.4. Self-Cleaning Property of SiO2@CuO/HDTMS Coating
3.5. Durability of SiO2@CuO/HDTMS Coating
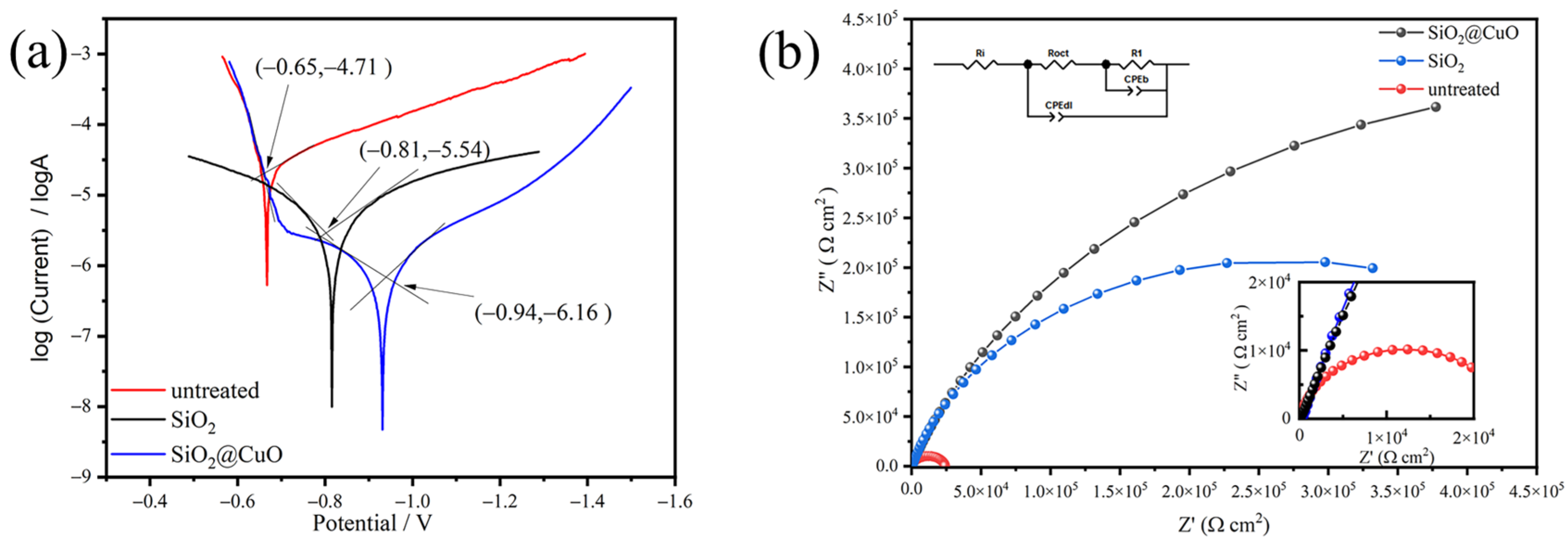
3.6. Corrosion Resistance of the Coating
3.7. Catalytic Degradation Performance
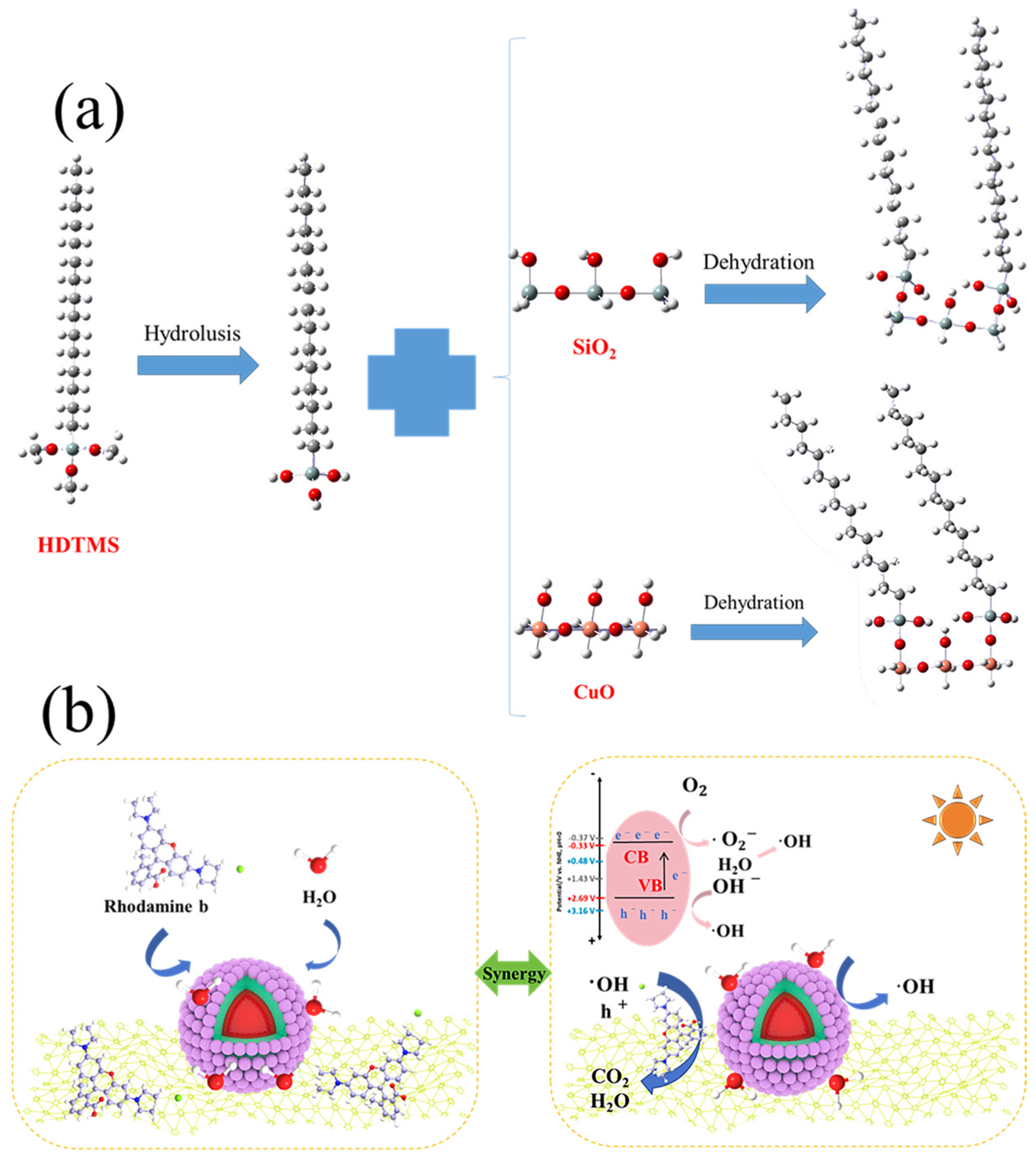
3.8. Superhydrophobic and Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T.; Lovley, D.R. Microbially mediated metal corrosion. Nat. Rev. Microbiol. 2023, 21, 705–718. [Google Scholar] [CrossRef]
- Salzano de Luna, M. Recent Trends in Waterborne and Bio-Based Polyurethane Coatings for Corrosion Protection. Adv. Mater. Interfaces 2022, 9, 2101775. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Thasma Subramanian, B.; Alla, J.P.; Essomba, J.S.; Nishter, N.F. Non-fluorinated superhydrophobic spray coatings for oil-water separation applications: An eco-friendly approach. J. Clean. Prod. 2020, 256, 120693. [Google Scholar] [CrossRef]
- Al Harraq, A.; Bharti, B. Microplastics through the lens of colloid science. ACS Environ. Au 2022, 2, 3–10. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Tao, F.; Zhang, X.; Gai, L.; Liu, L. All-water-based superhydrophobic coating with reversible wettability for oil-water separation and wastewater purification. Prog. Org. Coat. 2022, 165, 106726. [Google Scholar] [CrossRef]
- Xiao, W.; Yan, J.; Gao, S.; Huang, X.; Luo, J.; Wang, L.; Zhang, S.; Wu, Z.; Lai, X.; Gao, J. Superhydrophobic MXene based fabric composite for high efficiency solar desalination. Desalination 2022, 524, 115475. [Google Scholar] [CrossRef]
- Phiri, I.; Eum, K.Y.; Kim, J.W.; Choi, W.S.; Kim, S.H.; Ko, J.M.; Jung, H. Simultaneous complementary oil-water separation and water desalination using functionalized woven glass fiber membranes. J. Ind. Eng. Chem. 2019, 73, 78–86. [Google Scholar] [CrossRef]
- Gupta, R.; Verma, R.; Kango, S.; Constantin, A.; Kharia, P.; Saini, R.; Kudapa, V.K.; Mittal, A.; Prakash, J.; Chamoli, P. A critical review on recent progress, open challenges, and applications of corrosion-resistant superhydrophobic coating. Mater. Today Commun. 2023, 34, 105201. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Hashjin, R.R.; Ranjbar, Z.; Yari, H.; Momen, G. Tuning up sol-gel process to achieve highly durable superhydrophobic coating. Surf. Interfaces 2022, 33, 102282. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z. Recent advances in self-healing superhydrophobic coatings. Nano Today 2023, 51, 101933. [Google Scholar] [CrossRef]
- Elhaddad, F.; Luna, M.; Gemelli, G.M.C.; Almoraima Gil, M.L.; Mosquera, M.J. Effectiveness and durability assessment, under extreme environmental conditions, of a superhydrophobic coating applied onto sandstone from Carteia roman archaeological site. Chem. Eng. Sci. 2023, 265, 118236. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Syafiq, A.; Rahim, N.A.; Balakrishnan, V.; Pandey, A.K. Development of self-cleaning polydimethylsiloxane/nano-calcium carbonate-titanium dioxide coating with fog-resistance response for building glass. Pigment. Resin Technol. 2024, 53, 249–260. [Google Scholar] [CrossRef]
- Celik, N.; Torun, I.; Ruzi, M.; Esidir, A.; Onses, M.S. Fabrication of robust superhydrophobic surfaces by one-step spray coating: Evaporation driven self-assembly of wax and nanoparticles into hierarchical structures. Chem. Eng. J. 2020, 396, 125230. [Google Scholar] [CrossRef]
- Sharma, K.; Malik, M.K.; Hooda, A.; Pandey, K.; Sharma, J.; Goyat, M.S. Triethoxyoctylsilane-modified SiO2 nanoparticle-based superhydrophobic coating for corrosion resistance of mild steel. J. Mater. Eng. Perform. 2023, 32, 6329–6338. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Li, X.-D.; Wang, N.; Liu, Y.-J.; Tian, F.; Wang, C.-X. Robust superhydrophobic SiO2/epoxy composite coating prepared by one-step spraying method for corrosion protection of aluminum alloy: Experimental and theoretical studies. Mater. Des. 2023, 228, 111833. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Ly, N.H.; Tran, H.N.; Son, S.J.; Joo, S.-W.; Vasseghian, Y.; Osman, S.M.; Luque, R. Transparent oil–water separating spiky SiO2 nanoparticle supramolecular polymer superhydrophobic coatings. Small Methods 2023, 7, 2201257. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Wang, Y.; Ma, H.; Li, H. The preparation of cerium nitrate and attapulgite based superhydrophobic epoxy coatings for the corrosion protection of Q355 mild steel surface. Surf. Coat. Technol. 2023, 473, 129977. [Google Scholar] [CrossRef]
- Ratnam, D.; Bhaumik, S.K. Functionalized borosilicate-silica-epoxy nanocomposite superhydrophobic coating for corrosion inhibition under harsh environment. Prog. Org. Coat. 2024, 188, 108264. [Google Scholar] [CrossRef]
- Talinungsang; Upadhaya, D.; Kumar, P.; Purkayastha, D.D. Superhydrophilicity of photocatalytic ZnO/SnO2 heterostructure for self-cleaning applications. J. Sol-Gel Sci. Technol. 2019, 92, 575–584. [Google Scholar] [CrossRef]
- Bao, Y.; Chang, J.; Zhang, Y.; Chen, L. Robust superhydrophobic coating with hollow SiO2/PAA-b-PS Janus microspheres for self-cleaning and oil–water separation. Chem. Eng. J. 2022, 446, 136959. [Google Scholar] [CrossRef]
- Azmoon, P.; Farhadian, M.; Pendashteh, A.; Tangestaninejad, S. Adsorption and photocatalytic degradation of oilfield produced water by visible-light driven superhydrophobic composite of MIL-101(Cr)/Fe3O4-SiO2: Synthesis, characterization and optimization. Appl. Surf. Sci. 2023, 613, 155972. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Wei, D.; Zheng, Z.; Han, Z.; Liu, Y. Fabrication of a fluorine-free photocatalytic superhydrophobic coating and its long-lasting anticorrosion and excellent antibacterial abilities. Prog. Org. Coat. 2023, 184, 107806. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, N.; Rasheed, S.; Nabeel, M.I.; Mohyuddin, A.; Riaz, M.T.; Hussain, D. Silica-Based superhydrophobic and superoleophilic cotton fabric with enhanced self-cleaning properties for oil–water separation and methylene blue degradation. Langmuir 2024, 40, 5639–5650. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Nouri, N.M. A one step self-cleaning surface with robust superhydrophobic and photocatalytic properties: Electrostatic sprayed fluorinated ethylene propylene mixed with nano TiO2–SiO2 particles. Ceram. Int. 2023, 49, 57–66. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Khan, M.A.J.R.; Al-Mamun, M.R.; Islam, S.Z.; Khaleque, M.A.; Hossain, M.I.; Khan, M.Z.H.; Islam, M.S.; Marwani, H.M.; et al. Toxic dye removal, remediation, and mechanism with doped SnO2-based nanocomposite photocatalysts: A critical review. J. Water Process Eng. 2023, 54, 104069. [Google Scholar] [CrossRef]
- Dhull, P.; Sudhaik, A.; Raizada, P.; Thakur, S.; Nguyen, V.-H.; Van Le, Q.; Kumar, N.; Parwaz Khan, A.A.; Marwani, H.M.; Selvasembian, R.; et al. An overview on ZnO-based sonophotocatalytic mitigation of aqueous phase pollutants. Chemosphere 2023, 333, 138873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, S.; Li, M.; He, P.; Xiao, Y.; Chen, J.; Zhou, Y.; Ren, T. Boosting the photocatalytic hydrogen evolution performance by fabricating the NiO/Zn3In2S6 p-n heterojunction. Appl. Surf. Sci. 2024, 642, 158622. [Google Scholar] [CrossRef]
- Budi, S.; Syafei, D.I.; Yusmaniar; Khasanah, Q.F.; Laxmianti, D. Electrodeposition of Cu2O films at room temperature for methylene blue photodegradation. J. Phys. Conf. Ser. 2022, 2377, 012004. [Google Scholar] [CrossRef]
- Rehman, A.; Aadil, M.; Zulfiqar, S.; Agboola, P.O.; Shakir, I.; Aly Aboud, M.F.; Haider, S.; Warsi, M.F. Fabrication of binary metal doped CuO nanocatalyst and their application for the industrial effluents treatment. Ceram. Int. 2021, 47, 5929–5937. [Google Scholar] [CrossRef]
- Madona, J.; Sridevi, C.; Velraj, G.; Dhayal Raj, A.; George, A. Surfactant assisted morphology controlled CuO nanostructures for enhanced photocatalytic performance and bacterial growth inhibition. Mater. Sci. Eng. B 2023, 294, 116562. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, C.; Xiang, Q.; Xie, J.; Li, X. Surface and interface engineering of hierarchical photocatalysts. Appl. Surf. Sci. 2019, 471, 43–87. [Google Scholar] [CrossRef]
- Dursun, S.; Koyuncu, S.N.; Kaya, İ.C.; Kaya, G.G.; Kalem, V.; Akyildiz, H. Production of CuO–WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Process Eng. 2020, 36, 101390. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Pasa, A.A.; Alcântara, C.C.J.; Acuña, J.J.S.; Bilmes, S.A.; Martínez Ricci, M.L.; Landers, R.; Fermino, T.Z.; Rodrigues-Filho, U.P. Enhanced photocatalytic properties of core@shell SiO2@TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 179, 333–343. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmed, R.M.; Taha, M.; Soliman, T.S. Fe3O4 nanoparticles and Fe3O4 @SiO2 core-shell: Synthesize, structural, morphological, linear, and nonlinear optical properties. J. Alloys Compd. 2023, 947, 169639. [Google Scholar] [CrossRef]
- Hou, Z.; Chu, J.; Liu, C.; Wang, J.; Li, A.; Lin, T.; François-Xavier, C.P. High efficient photocatalytic reduction of nitrate to N2 by Core-shell Ag/SiO2@cTiO2 with synergistic effect of light scattering and surface plasmon resonance. Chem. Eng. J. 2021, 415, 128863. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zare, M.; Moradi, L. Preparation of hollow mesoporous boron nitride spheres with surface decorated by CuO: A bifunctional acid-base catalyst for the green synthesis of some heterocyclic [3,3,3] propellane derivatives in water media. Appl. Surf. Sci. 2022, 582, 152454. [Google Scholar] [CrossRef]
- Faheem, M.; Jiang, X.; Wang, L.; Shen, J. Synthesis of Cu2O-CuFe2O4 microparticles from Fenton sludge and its application in the Fenton process: The key role of Cu2O in the catalytic degradation of phenol. RSC Adv. 2018, 8, 5740–5748. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Li, Z.; Yu, Z.; Singh, S.; Guo, C. Superhydrophobic Al Surfaces with Properties of Anticorrosion and Reparability. ACS Omega 2018, 3, 17425–17429. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Geng, L.; Wu, S.; Yan, W.; Liu, G. Highly-efficient cocatalyst-free H2-evolution over silica-supported CdS nanoparticle photocatalysts under visible light. Chem. Commun. 2015, 51, 10676–10679. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Jian, W.; Jing, P.; Zhang, W.; Yan, W.; Bai, F.-Q.; Liu, G. Crystal phase effect of iron oxides on the aerobic oxidative coupling of alcohols and amines under mild conditions: A combined experimental and theoretical study. J. Catal. 2019, 377, 145–152. [Google Scholar] [CrossRef]
- Gong, B.; Ma, L.; Guan, Q.; Tan, R.; Wang, C.; Wang, Z.; Wang, K.; Liu, C.; Deng, C.; Song, W.; et al. Preparation and particle size effects study of sustainable self-cleaning and durable silicon materials with superhydrophobic surface performance. J. Environ. Chem. Eng. 2022, 10, 107884. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatt, V.; Nayal, O.S.; Sharma, S.; Kumar, V.; Thakur, M.S.; Kumar, N.; Bal, R.; Singh, B.; Sharma, U. CuI nanoparticles as recyclable heterogeneous catalysts for C–N bond formation reactions. Catal. Sci. Technol. 2017, 7, 2857–2864. [Google Scholar] [CrossRef]
- Xiang, T.; Han, Y.; Guo, Z.; Wang, R.; Zheng, S.; Li, S.; Li, C.; Dai, X. Fabrication of Inherent Anticorrosion Superhydrophobic Surfaces on Metals. ACS Sustain. Chem. Eng. 2018, 6, 5598–5606. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Q.; Quan, X.; Feng, W.; Wang, Q. Fluorine-free and hydrophobic hexadecyltrimethoxysilane-TiO2 coated mesh for gravity-driven oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124189. [Google Scholar] [CrossRef]
- Shirke, Y.M.; Yu, Y.J.; Chung, J.-W.; Cho, S.-J.; Kwon, S.J.; Hong, S.U.; Jeon, J.-D. Hydrophobic hollow fiber composite membranes based on hexadecyl-modified SiO2 nanoparticles for toluene separation. J. Environ. Chem. Eng. 2024, 12, 111819. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Zhu, Y.; Li, Z.; Sheng, T.; Hu, Y.M.; Yang, D.-Q. A study of the mechanical and chemical durability of Ultra-Ever Dry Superhydrophobic coating on low carbon steel surface. Colloids Surf. A 2016, 497, 16–27. [Google Scholar] [CrossRef]
- Hermann, M.; Agrawal, P.; Koch, I.; Oleschuk, R. Organic-free, versatile sessile droplet microfluidic device for chemical separation using an aqueous two-phase system. Lab Chip 2019, 19, 654–664. [Google Scholar] [CrossRef]
- Oomen, P.E.; Mulder, J.P.S.H.; Verpoorte, E.; Oleschuk, R.D. Controlled, synchronized actuation of microdroplets by gravity in a superhydrophobic, 3D-printed device. Anal. Chim. Acta 2017, 988, 50–57. [Google Scholar] [CrossRef]
- Hu, W.-H.; Yang, D.-Q.; Sacher, E. Improving the Mechanical Durability of Superhydrophobic Coating by Deposition onto a Mesh Structure. Mater. Res. Express 2018, 5, 065521. [Google Scholar] [CrossRef]
- Salahuddin, M.; Uddin, M.N.; Hwang, G.; Asmatulu, R. Superhydrophobic PAN nanofibers for gas diffusion layers of proton exchange membrane fuel cells for cathodic water management. Int. J. Hydrogen Energy 2018, 43, 11530–11538. [Google Scholar] [CrossRef]
- Samah, W.; Clain, P.; Rioual, F.; Fournaison, L.; Delahaye, A. Experimental investigation on the wetting behavior of a superhydrophobic surface under controlled temperature and humidity. Colloids Surf. A 2023, 656, 130451. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Y.; Zhang, S.-W. A universal, multifunctional, high-practicability superhydrophobic paint for waterproofing grass houses. NPG Asia Mater. 2021, 13, 47. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.-T.; Xu, Y.-Y. A sprayable superhydrophobic dental protectant with photo-responsive anti-bacterial, acid-resistant, and anti-fouling functions. Nano Res. 2022, 15, 5245–5255. [Google Scholar] [CrossRef]
- Keshavarzi, R.; Molabahrami, N.; Afzali, N. Improving Efficiency and Stability of Carbon-Based Perovskite Solar Cells by a Multifunctional Triple-Layer System: Antireflective, UV-Protective, Superhydrophobic, and Self-Cleaning. Sol. RRL 2020, 4, 2000491. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Z.-C.; Wang, X.-Q. A compound of ZnO/PDMS with photocatalytic, self-cleaning and antibacterial properties prepared via two-step method. Appl. Surf. Sci. 2021, 550, 149286. [Google Scholar] [CrossRef]
- Bai, X.; Yang, S.-Q.; Tan, C.-H. Synthesis of TiO2 based superhydrophobic coatings for efficient anti-corrosion and self-cleaning on stone building surface. J. Clean. Prod. 2022, 380, 134975. [Google Scholar] [CrossRef]
- Rahman, A.; Rahman, A. Silver Oxide-Decorated Silica Nanoparticles for Visible-Light-Driven Photolytic Pollutant Degradation and Water-Oil Separation. ACS Appl. Nano Mater. 2022, 5, 939–947. [Google Scholar] [CrossRef]
- Trávníčková, E.; Pijáková, B.; Marešová, D. Antifouling performance of photocatalytic superhydrophobic coatings against Klebsormidium alga. J. Environ. Chem. Eng. 2020, 8, 104153. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.-W.; Chen, R.-R. Fabrication of the pod-like KCC-1/TiO2 superhydrophobic surface on AZ31 Mg alloy with stability and photocatalytic property. Appl. Surf. Sci. 2020, 499, 143933. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, C.; Liu, W.-Q. A water-rich system of constructing durable and fluorine-free superhydrophobic surfaces for oil/water separation. Appl. Surf. Sci. 2020, 507, 145165. [Google Scholar] [CrossRef]



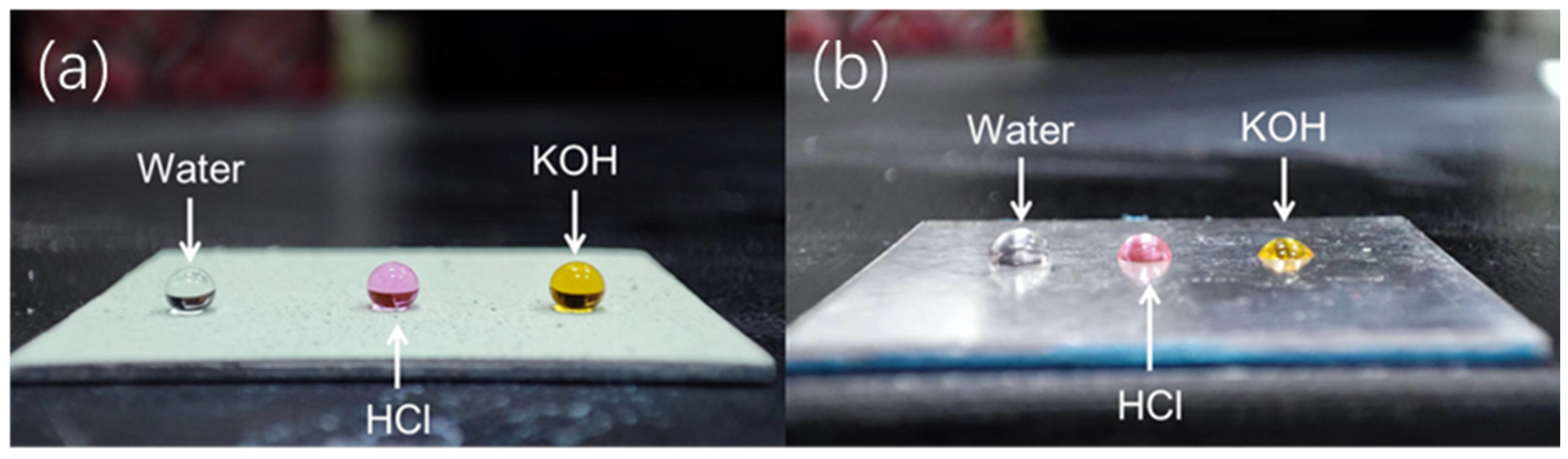


| Different Droplets | Water | HCI | NaOH |
|---|---|---|---|
| untreated Al | 66.8 ± 1.2° | 62.4 ± 1.5° | 58.9 ± 2.3° |
| SiO2 @HDTMS coating | 146.5 ± 3.4° | 140.4 ± 4.1° | 142.6 ± 3.9° |
| SiO2/CuO@HDTMS coating | 157.4 ± 4.7° | 152.5 ± 5.0° | 153.4 ± 5.5° |
| Sample | (V) | (A/cm2) | CR (mm/year) | CIE/% |
|---|---|---|---|---|
| untreated | −0.65 | 1.95 × 10−5 | 0.2124 | 0 |
| SiO2 | −0.81 | 2.88 × 10−6 | 0.0314 | 85.2 |
| SiO2@CuO | −0.94 | 6.92 × 10−7 | 0.0075 | 96.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, X.; Shang, Y.; Wang, B.; Lu, K.; Gan, W.; Lai, H.; Wang, J.; Huang, C.; Chen, Z.; et al. Synthesis and Characterization of Superhydrophobic Epoxy Resin Coating with SiO2@CuO/HDTMS for Enhanced Self-Cleaning, Photocatalytic, and Corrosion-Resistant Properties. Materials 2024, 17, 1849. https://doi.org/10.3390/ma17081849
Wang Z, Zhou X, Shang Y, Wang B, Lu K, Gan W, Lai H, Wang J, Huang C, Chen Z, et al. Synthesis and Characterization of Superhydrophobic Epoxy Resin Coating with SiO2@CuO/HDTMS for Enhanced Self-Cleaning, Photocatalytic, and Corrosion-Resistant Properties. Materials. 2024; 17(8):1849. https://doi.org/10.3390/ma17081849
Chicago/Turabian StyleWang, Zhongmin, Xiaoyu Zhou, Yongwei Shang, Bingkui Wang, Kecheng Lu, Weijiang Gan, Huajun Lai, Jiang Wang, Caimin Huang, Zongning Chen, and et al. 2024. "Synthesis and Characterization of Superhydrophobic Epoxy Resin Coating with SiO2@CuO/HDTMS for Enhanced Self-Cleaning, Photocatalytic, and Corrosion-Resistant Properties" Materials 17, no. 8: 1849. https://doi.org/10.3390/ma17081849






