Porous Glass with Layered Morphology Prepared by Phase Separation
Abstract
:1. Introduction
2. Experiment
2.1. Glass Preparation
2.2. Phase Separation, Acid Leaching
2.3. Loading V2O5
2.4. Characterization Techniques
3. Results and Discussion
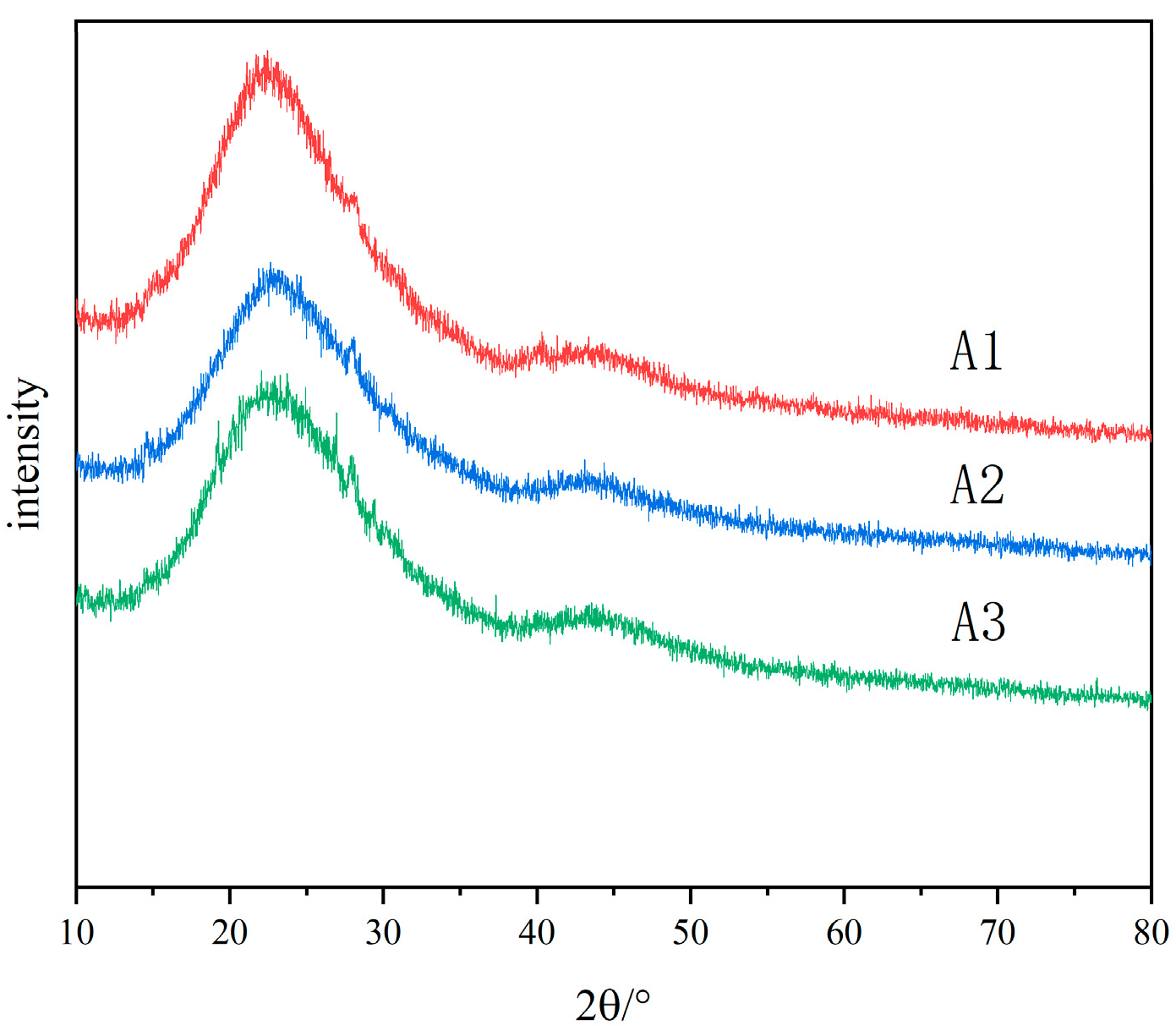
3.1. Phase Separation of Glass
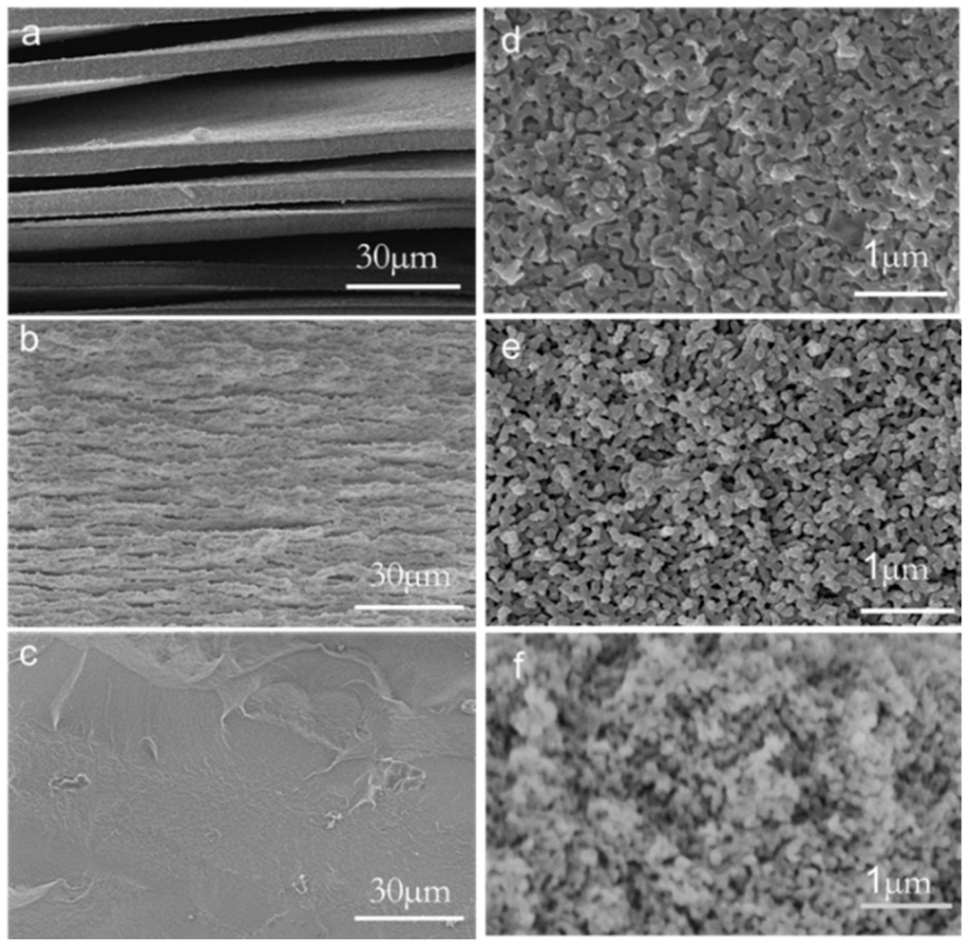
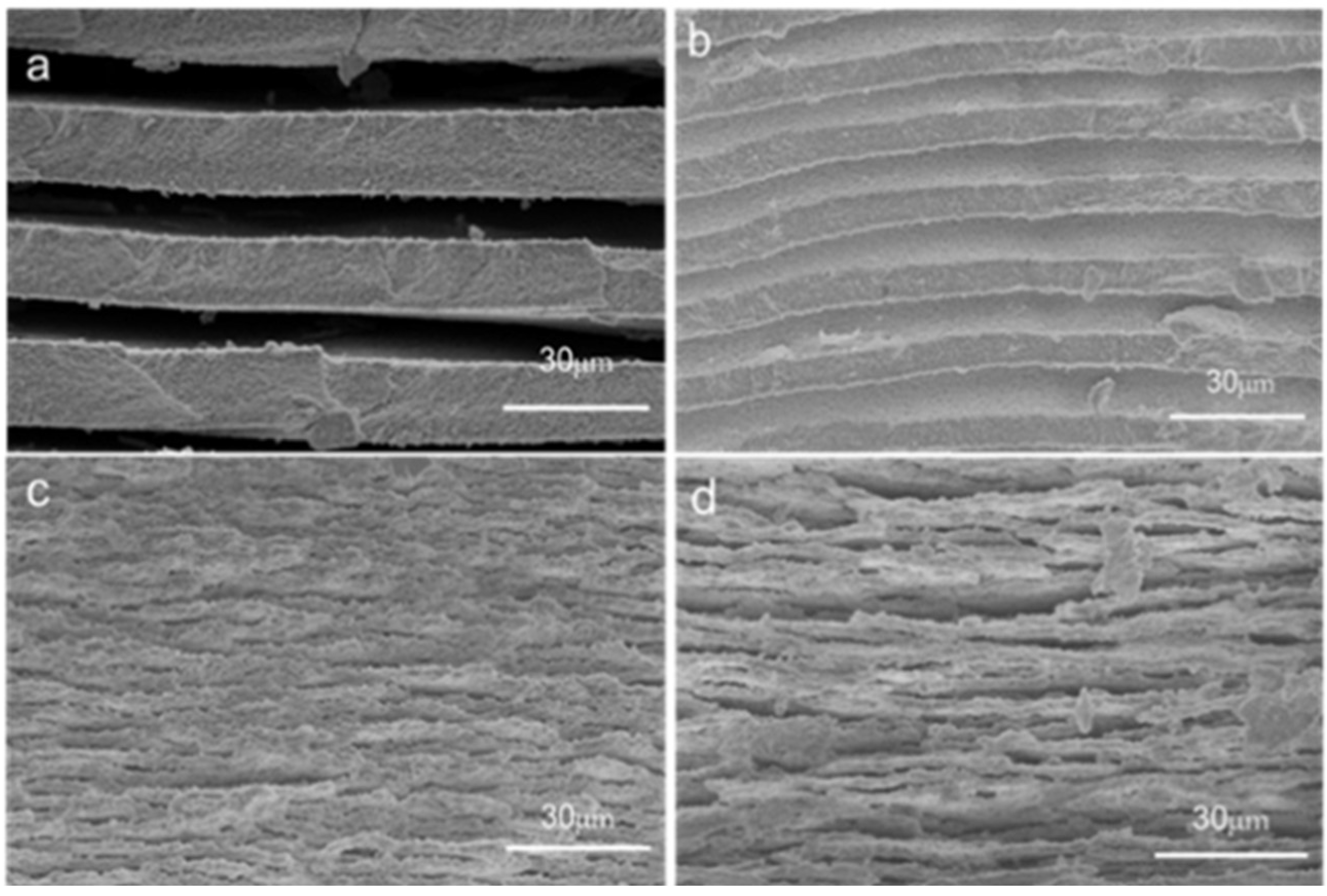
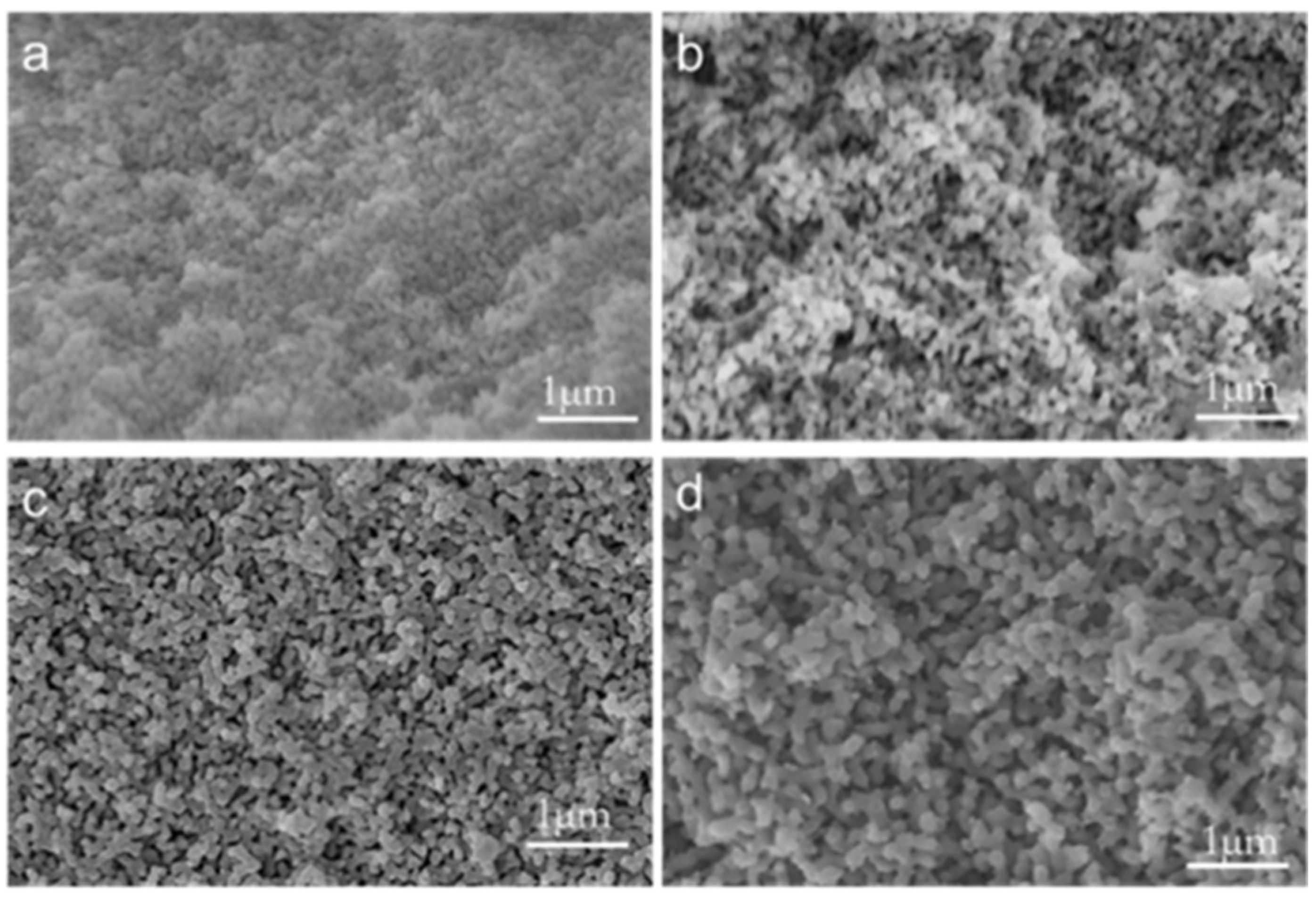
3.2. Morphology of Phase Separation
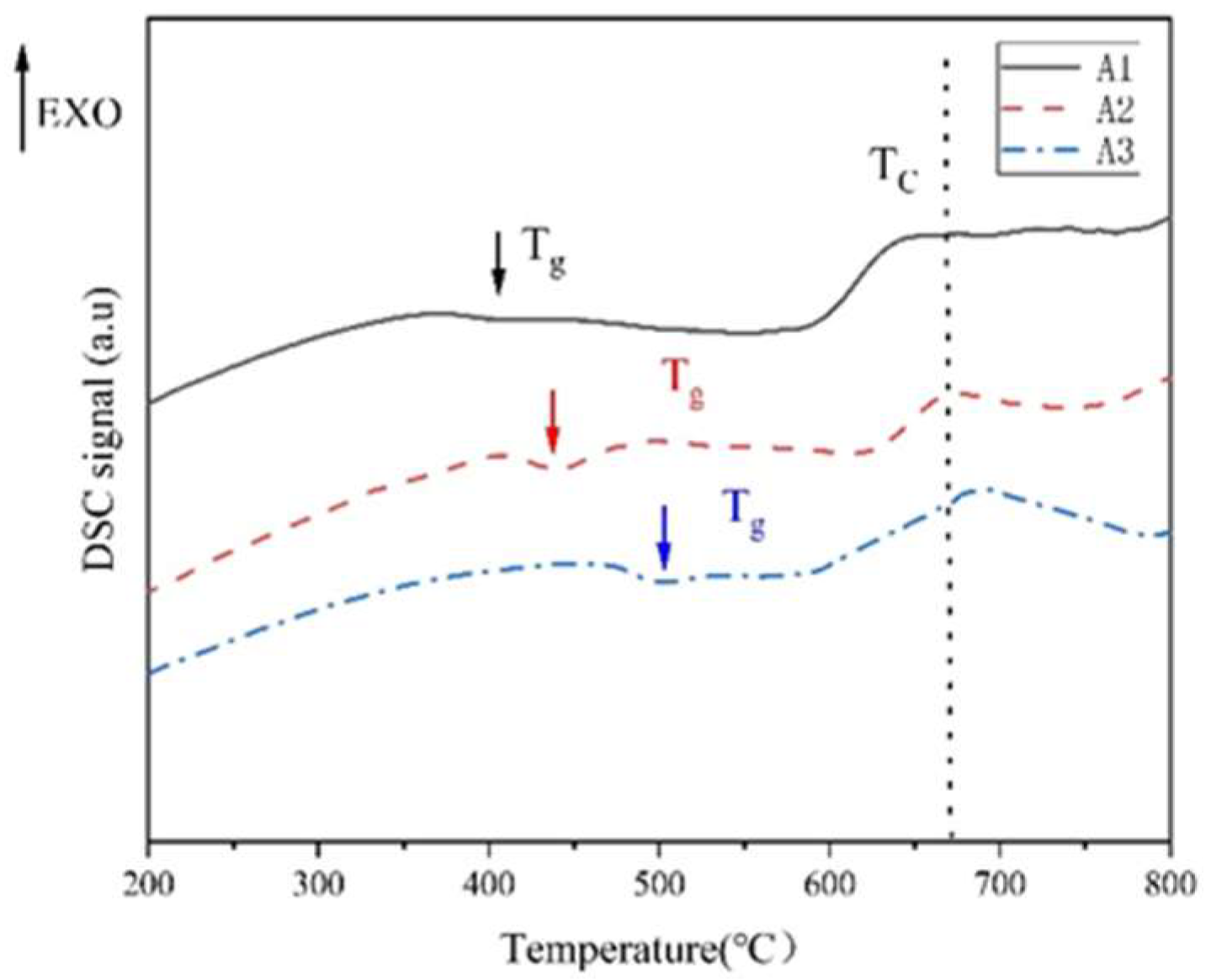
3.3. Thermal Stability of Glass
3.4. Catalyst Loading Morphology
3.5. Mechanism Analysis
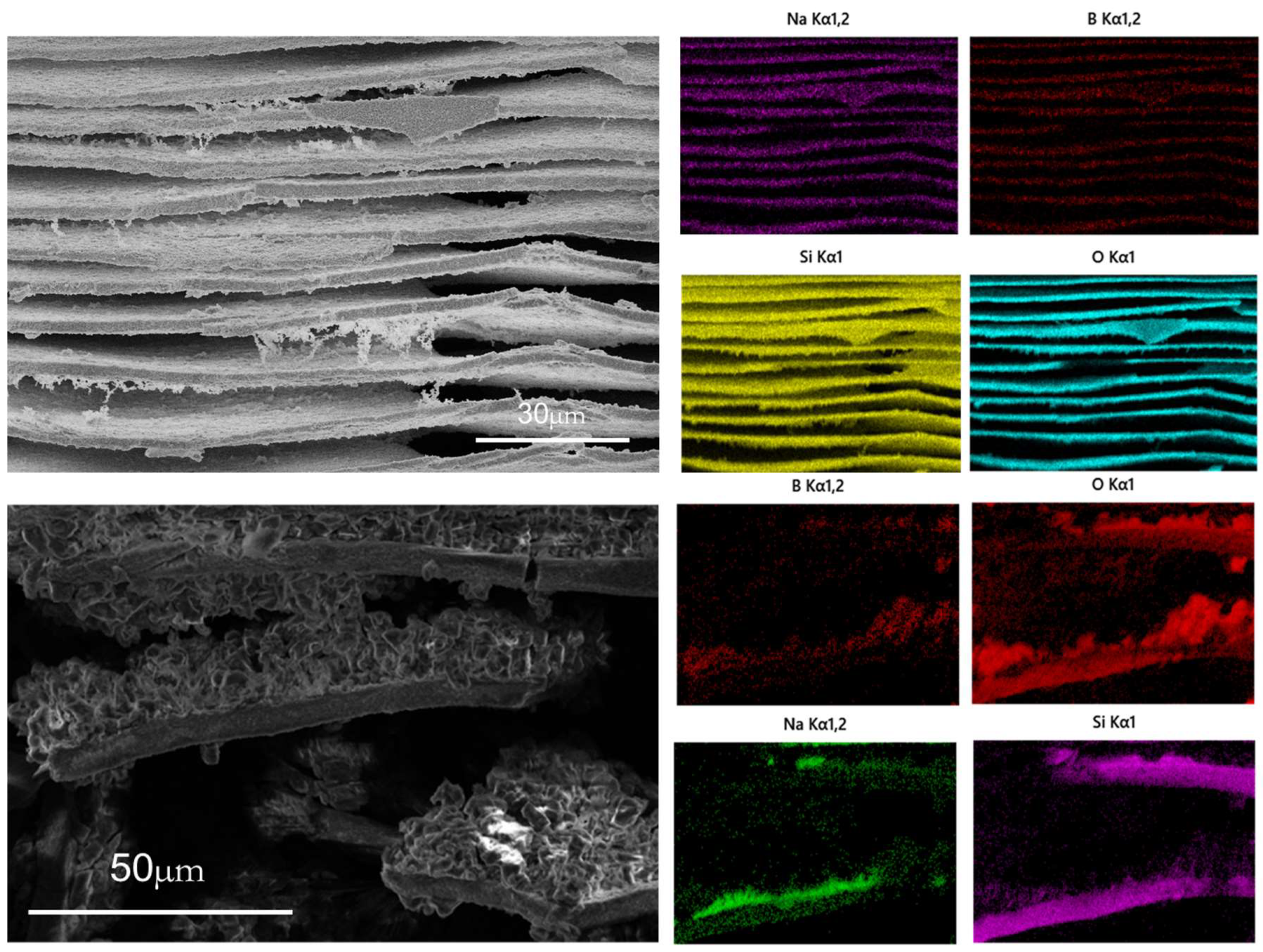
3.5.1. Structural Analysis
3.5.2. Evolution of Phase-Separation Structure with Time
3.5.3. Mechanism of Layered Phase Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, Y.; Liu, Y.; Lv, Q.; Wang, B.; Che, D. Review on the selective catalytic reduction of NO with H2 by using novel catalysts. J. Environ. Chem. Eng. 2021, 9, 106770. [Google Scholar] [CrossRef]
- Inomata, Y.; Mino, M.; Hata, S.; Kiyonaga, E.; Morita, K.; Hikino, K.; Yoshida, K.; Haruta, M.; Murayama, T. Low-temperature NH3-SCR Activity of Nanoparticulate Gold Supported on a Metal Oxide. J. Jpn. Pet. Inst. 2019, 62, 234–243. [Google Scholar] [CrossRef]
- Ding, X.; Liang, Y.; Zhang, H.; Zhao, M.; Wang, J.; Che, Y. Preparation of Reduced Pt-Based Catalysts with High Dispersion and Their Catalytic Performances for NO Oxidation. Acta Phys. Chim. Sin. 2020, 38, 2005009. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Guo, R.-T.; Zhen, W.-L.; Zhou, Y.; Pan, W.G.; Xu, H.-J.; Jin, Q.; Ding, C.-G.; Guo, S.-Y. Selective Catalytic Reduction of NO by NH3 Over Ceria: Effect of Calcination Temperature on the Activity of Catalysts. Asian J. Chem. 2014, 26, 407–410. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Li, K.; Anwar, M.T.; Mehmood, A.; Gul, M.; Li, D.; Mao, J. Iron oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH+. Rev. Chem. Eng. 2019, 35, 239–264. [Google Scholar] [CrossRef]
- Pan, W.-G.; Zhou, Y.; Guo, R.-T.; Jin, Q.; Ding, C.-G.; Guo, S.-Y. Effect of Cerium Precursor on the Performance of Pure CeO2 Catalysts for Selective Catalytic Reduction of NO with NH3. Asian J. Chem. 2013, 25, 9079–9082. [Google Scholar] [CrossRef]
- Panda, S.; Maity, T.; Sarkar, S.; Manna, A.K.; Mondal, J.; Haldar, R. Diffusion-programmed catalysis in nanoporous material. Nat. Commun. 2025, 16, 1231. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, H.-R.; Cui, X.-Z.; Jiang, W.; Shi, J.-L. Pd Loaded Mesoporous ZrO₂-TiO₂ Composite and Its CO Catalytic Oxidation Property. J. Inorg. Mater. 2013, 28, 992–997. [Google Scholar] [CrossRef]
- Zhang, H.; Ouyang, Q.; Yu, L.; Hu, R.; Wan, J.; Song, B.; Huang, Q.; Yao, Y. Transient and in situ Growth of Nanostructured SiC on Carbon Fibers toward Highly Durable Catalysis. Adv. Funct. Mater. 2023, 33, 2301375. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Li, J.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.; Hao, J. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Kacui, O.R. Deactivating Effects of Lead on the Selective Catalytic Reduction of Nitric Oxide with Ammonia over a V2O5/WO3/TiO2 Catalyst for Waste Incineration Applications. Chem. Res. 1998, 37, 1196–1202. [Google Scholar]
- Lin, D.; Zhang, L.; Liu, Z.; Wang, B.; Han, Y. Progress of selective catalytic reduction denitrification catalysts at wide temperature in carbon neutralization. Front. Chem. 2022, 10, 946133. [Google Scholar] [CrossRef] [PubMed]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO2− nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B Environ. 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, Z.; Liu, Q. The Latest Research Progress of NH3-SCR in the SO2 Resistance of the Catalyst in Low Temperatures for Selective Catalytic Reduction of NOx. Catalysts 2020, 10, 1034. [Google Scholar] [CrossRef]
- Bo, W.; Qiaowen, Y. Optimization of roasting parameters for recovery of vanadium and tungsten from spent SCR catalyst with composite roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Sun, J.; Du, X.; Li, Z.; Yu, H.; Hong, Y.; Pan, M.; Nie, G.; Fan, W.; Gao, F.; Wu, F.; et al. Rational design of poisoning-resistant catalyst based on γ-Al₂O₃ for hydrolysis of carbonyl sulfide. Chem. Eng. Sci. 2024, 295, 120150. [Google Scholar] [CrossRef]
- Inayat, A.; Reinhardt, B.; Uhlig, H.; Einicke, W.-D.; Enke, D. Silica monoliths with hierarchical porosity obtained from porous glasses. Chem. Soc. Rev. 2013, 42, 3753–3764. [Google Scholar] [CrossRef] [PubMed]
- Enke, D.; Janowski, F.; Schwieger, W.J.M. Porous glasses in the 21st century—A short review. Microporous Mesoporous Mater. 2003, 60, 19–30. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, J.; Liu, J.; Mu, J. Fe-containing mesoporous silicates with macro-lamellar morphology. Microporous Mesoporous Mater. 2007, 106, 28–34. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Wang, L.; Tang, K.; Maier, J.; Müllen, K. Graphene-based nanosheets with a sandwich structure. Angew. Chem. 2010, 49, 4795–4799. [Google Scholar] [CrossRef]
- Cui, X.; Li, M.; Wei, F.; Tang, X.; Xu, W.; Li, M.; Han, X. Biomimetic light-activatable graphene-based nanoarchitecture for synergistic chemophotothermal therapy. Chem. Eng. J. 2021, 420, 127710. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Coombs, N.; Soheilnia, N.; Ozin, G.A. Graphene oxide-periodic mesoporous silica sandwich nanocomposites with vertically oriented channels. ACS Nano 2010, 4, 7437–7450. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, J.; Li, Y.; Li, S.; Yu, M. Enhancing corrosion protection properties of sol-gel coating by pH-responsive amino-silane functionalized graphene oxide-mesoporous silica nanosheets. Prog. Org. Coat. 2019, 135, 228–239. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Peng, K.; Zhao, K.; Bai, M.; Li, H.; Gao, W.; Gong, Z. Amine-impregnated porous carbon–silica sheets derived from vermiculite with superior adsorption capability and cyclic stability for CO₂ capture. Chem. Eng. J. 2023, 464, 142662. [Google Scholar] [CrossRef]
- Möbus, G.; Yang, G.; Saghi, Z.; Xu, X.; Hand, R.; Pankov, A.; Ojovan, M. Electron Irradiation and Electron Tomography Studies of Glasses and Glass Nanocomposites. MRS Proc. 2008, 1107, 239. [Google Scholar] [CrossRef]
- Möbus, G.; Ojovan, M.; Cook, S.; Tsai, J.; Yang, G. Nano-scale quasi-melting of alkali-borosilicate glasses under electron irradiation. J. Nucl. Mater. 2010, 396, 264–271. [Google Scholar] [CrossRef]
- Liu, H.; Smedskjaer, M.M.; Tao, H.; Jensen, L.R.; Zhao, X.; Yue, Y. A medium range order structural connection to the configurational heat capacity of borate-silicate mixed glasses. Phys. Chem. Chem. Phys. 2016, 18, 10887–10895. [Google Scholar] [CrossRef]
- Topsøe, N.Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cai, Z.; Liang, J.; Song, S.; Cao, W. Research progress on deactivation mechanism and preventive measures of V₂O₅-WO₃/TiO₂ catalyst. Iron Steel Vanadium Titan. 2024, 45, 95–104. [Google Scholar]
- Huang, F.P.; Cui, M.L.; Zhang, S.; Guo, Y.-Y.; Wang, S.; Li, Y. Preparation and Properties of Nano Dy/TiO₂ Films Supported on High Silica Fiber. J. Mater. Eng. 2017, 45, 66–70. [Google Scholar]
- Möncke, D.; Ehrt, D.; Varsamis, C.P.; Kamitsos, E.I.; Kalampounias, A.G. Thermal history of a low alkali borosilicate glass probed by infrared and Raman spectroscopy. Glass Technol. Eur. J. Glas. Sci. Technol. Part A 2006, 47, 133–137. [Google Scholar]
- Galeener, F.L.; Lucovsky, G. Longitudinal Optical Vibrations in Glasses: GeO2 and SiO2. Phys. Rev. Lett. 1976, 37, 1474–1478. [Google Scholar] [CrossRef]
- Konijnendijk, W.L.; Stevels, J.M. The structure of borate glasses studied by Raman scattering. J. Non-Cryst. Solids 1975, 18, 307–331. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Karakassides, M.A.; Chrysikos, G.D. Vibrational Spectra of Magnesium-Sodium-Borate Glasses. 2. Raman and Midinfrared Investigation of the Network Structure. J. Phys. Chem. 1987, 91, 1073–1079. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Patsis, A.P.; Karakassides, M.A.; Chryssikos, G.D. Infrared Reflectance Spectra of Lithium Borate Glasses. J. Non-Cryst. Solids 1990, 126, 52–67. [Google Scholar] [CrossRef]
- Kamitsos, E.I. Infrared Studies of Borate Glasses. Phys. Chem. Glas. 2003, 44, 79–87. [Google Scholar]
- Varsamis, C.P.; Kamitsos, E.I.; Chrysikos, G.D. Structure of fast-ion-conducting Ag I-doped borate glasses. Phys. Rev. B 1999, 60, 3885–3898. [Google Scholar] [CrossRef]
- Möncke, D.; Ehrt, D.; Eckert, H.; Mertens, V. Influence of melting and annealing conditions on the structure of borosilicate glasses. Phys. Chem. Glas. 2003, 44, 113–116. [Google Scholar]
- Agarwal, A.; Tomozawa, M. Correlation of silica glass properties with the infrared spectra. J. Non-Cryst. Solids 1997, 209, 166–174. [Google Scholar] [CrossRef]
- Osipov, A.; Osipova, L.; Zainullina, R. Raman Spectroscopy and Statistical Analysis of the Silicate Species and Group Connectivity in Cesium Silicate Glass Forming System. Int. J. Spectrosc. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Osipov, A.A.; Osipova, L.M.; Eremyashev, V.E. Structure of alkali borosilicate glasses and melts according to Raman spectroscopy data. Glass Phys. Chem. 2013, 39, 105–112. [Google Scholar] [CrossRef]
- Manara, D.; Grandjean, A.; Neuville, D.R. Advances in understanding the structure of borosilicate glasses: A Raman spectroscopy study. Am. Mineral. 2009, 94, 777–784. [Google Scholar] [CrossRef]
- Kwinda, T.I.; Enke, D.; Koppka, S. Effect of TiO₂ on microphase development during phase separation and crystallization in Na₂O–B₂O₃–SiO₂ glass system. J. Am. Ceram. Soc. 2021, 105, 3261–3278. [Google Scholar] [CrossRef]
- Möncke, D.; Tricot, G.; Winterstein, A.; Kamitsos, E.I. Preferential bonding in low alkali borosilicate glasses. Phys. Chem. Glas. 2017, 58, 171–179. [Google Scholar] [CrossRef]
- Fritzsche, J.O.; Rüdinger, B.; Deubener, J. Slow coarsening of tetragonal zirconia nanocrystals in a phase-separated sodium borosilicate glass. J. Non-Cryst. Solids 2023, 606, 122206. [Google Scholar] [CrossRef]
- Winterstein-Beckmann, A.; Möncke, D.; Palles, D.; Kamitsos, E.; Wondraczek, L. Raman spectroscopic study of structural changes induced by micro-indentation in low alkali borosilicate glasses. J. Non-Cryst. Solids 2014, 401, 110–114. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Chou, K. Structure of CaO–B₂O₃–SiO₂–TiO₂ glasses: A Raman spectral study. Iron Steel Inst. Jpn. Int. 2011, 51, 1021–1027. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Chou, K. Structural Studies of CaO-B₂O₃-TiO₂ Glass System by Raman Spectroscopy. High Temp. Mater. Process. 2011, 30, 233–239. [Google Scholar] [CrossRef]
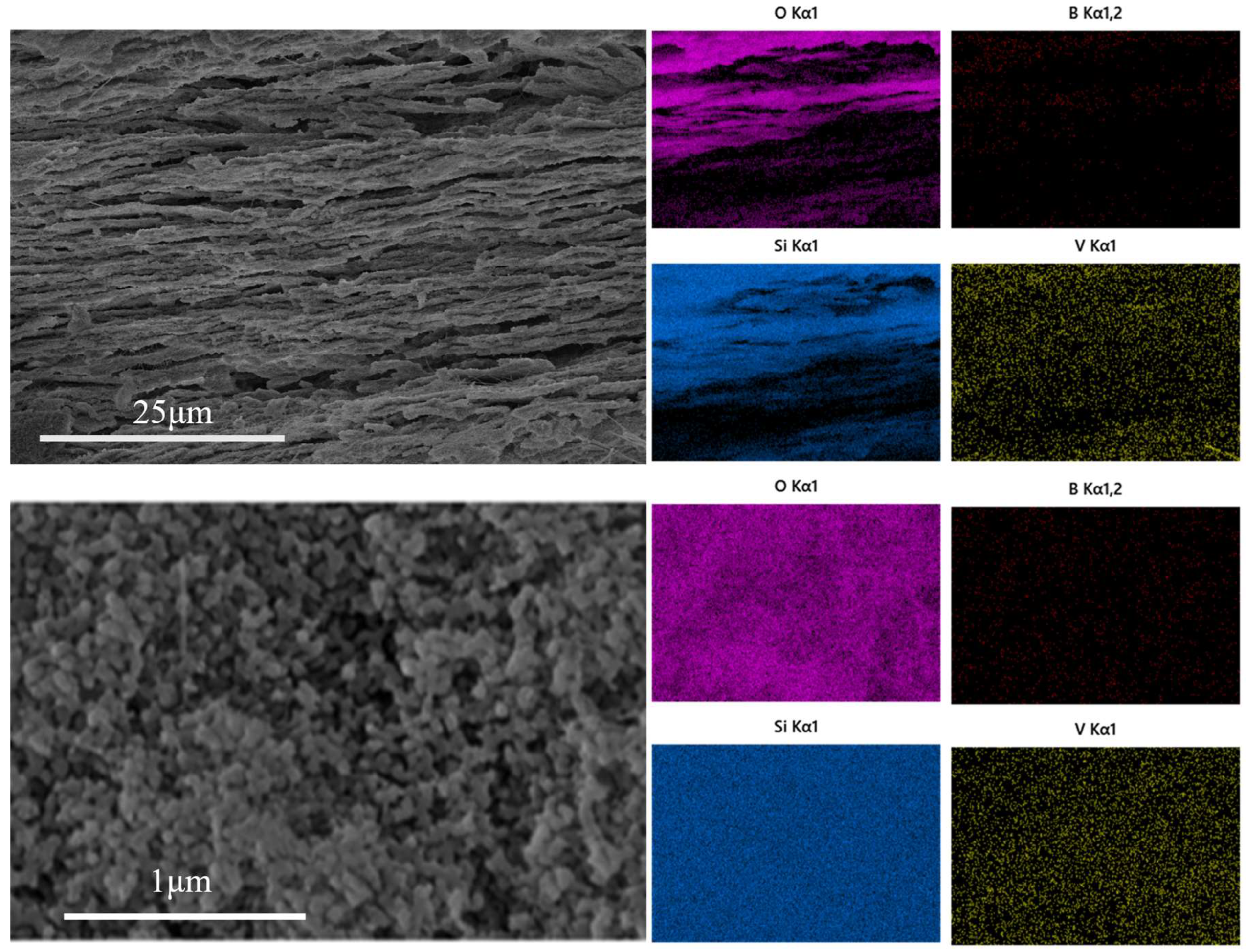


| Glass | B2O3 | SiO2 | Na2O | R = (Na2O/B2O3) | K = (SiO2/B2O3) |
|---|---|---|---|---|---|
| A1 | 50.25 | 44.72 | 5.03 | 0.10 | 0.89 |
| A2 | 48.00 | 42.80 | 9.20 | 0.20 | 0.89 |
| A3 | 45.66 | 40.64 | 13.70 | 0.30 | 0.89 |
| Samples | Average Pore Size of Sample (nm) | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| PGA1 | 7.11 ± 0.35 | 68.76 ± 1.38 | 0.12 ± 0.0012 |
| PGA2 | 8.88 ± 0.44 | 149.06 ± 2.98 | 0.33 ± 0.0033 |
| PGA2-600 | 8.82 ± 0.44 | 146.99 ± 2.94 | 0.32 ± 0.0012 |
| PGA3 | 3.97 ± 0.20 | 218.59 ± 4.37 | 0.21 ± 0.0022 |
| Wavenumber (cm−1) | Vibration Types |
|---|---|
| 400–500 | Si-O-Si bending vibration [32,33,34,35] |
| 670 | Si-O-B bending vibration [34,35,36] |
| 700 | [BO3] bending vibration [32,33,34,35,36] |
| 800 | Si-O-Si symmetric stretching vibration [32,33,34,35,36,37,38,39] |
| 900–1000 | Asymmetric stretching vibration of [BO4] [32,33,34,35,36,37,38,39,40] |
| 1050–1150 | Si-O-Si asymmetric stretching vibration [35,36,37,38,39,40] |
| 1260–1310 | The stretching vibration of B-O-B [33,34,35,36,37,38,39] |
| 1400 | Asymmetric stretching vibration of [BO3] [32,33,34,35,36,37,38,39,40] |
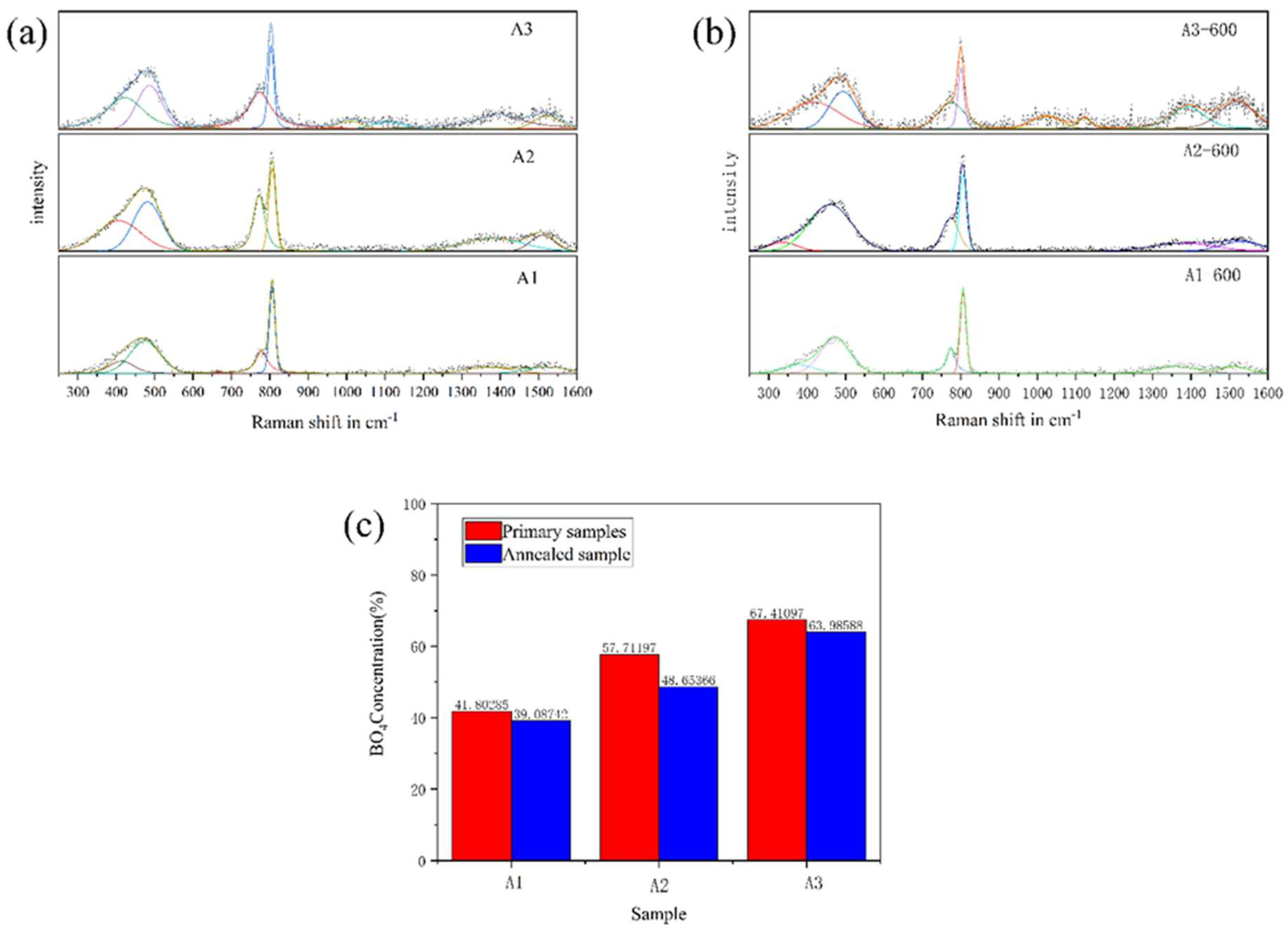
| Raman Shift (cm−1) | Raman Assignment |
|---|---|
| 300–600 | Mixed bending and stretching of bridged Si-O-Si, Si-O-B and B-O-B in three-dimensional network [41,42,43] |
| 780 | Vibration of four-coordinated boron in an asymmetric boroxol ring [44,45,46,47] |
| 808 | Vibration of three-coordinated boron in symmetrical boroxol ring [44,45,46,47] |
| 900–1200 | Tensile vibration of Si-O-Si (containing 2–4 bridge oxygen) [45,46,47,48] |
| 1360 | Stretching of non-annular [BO3] linked to [BO4] [48,49] |
| 1515 | Stretching of three-coordinated boron in boron oxide ring [44,45,46,47,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Chen, Y.; Wang, Z.; Zhou, Y.; Li, C. Porous Glass with Layered Morphology Prepared by Phase Separation. Materials 2025, 18, 1133. https://doi.org/10.3390/ma18051133
Chen Q, Chen Y, Wang Z, Zhou Y, Li C. Porous Glass with Layered Morphology Prepared by Phase Separation. Materials. 2025; 18(5):1133. https://doi.org/10.3390/ma18051133
Chicago/Turabian StyleChen, Qiang, Yihong Chen, Zhe Wang, Yao Zhou, and Changjiu Li. 2025. "Porous Glass with Layered Morphology Prepared by Phase Separation" Materials 18, no. 5: 1133. https://doi.org/10.3390/ma18051133
APA StyleChen, Q., Chen, Y., Wang, Z., Zhou, Y., & Li, C. (2025). Porous Glass with Layered Morphology Prepared by Phase Separation. Materials, 18(5), 1133. https://doi.org/10.3390/ma18051133





